Abstract
Experimental data and observational studies in adults suggest that even subtle changes in acid-base balance, indicative of a higher systemic proton load, are related to higher blood pressure (BP) levels and an increased hypertension risk. However, these associations have not been investigated during growth. The kidney is the central organ in regulating excretion of nonvolatile acids, and renal citrate excretion has been shown to be a sensitive, noninvasive marker of changes in systemic acid balance. We thus analyzed the prospective relation of 24-h citrate excretion, as well as net acid excretion capacity (NAEC; a noninvasive indicator of the renal ability to excrete protons), during adolescence (boys: 10–15 yr; girls: 9–14 yr) with BP levels in young adulthood (18–30 yr) in 374 healthy participants of the Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study. In linear-regression analyses adjusted for age, sex, 24-h urinary excretions of sodium and potassium, as well as further relevant confounders, a 1-mmol/1.73 m2/day higher adolescent citrate excretion was related to 1.2 mmHg lower systolic BP (P = 0.02) but not to diastolic BP (P = 0.6). A 10-mEq higher NAEC during adolescence was related to 1.7 mmHg lower systolic BP in young men, but this association was statistically nonsignificant (P = 0.07) after multivariable adjustment. Additional adjustment for adult body mass index did not alter these findings. To conclude, subtle changes in systemic acid-base balance during adolescence are already indicative for later BP. Potential sex differences in these associations should be investigated in further studies.
Keywords: acid excretion, adolescence, blood pressure, citrate, epidemiology
INTRODUCTION
Regarding the worldwide impact of elevated blood pressure (BP) on cardiovascular morbidity and mortality (18), there is great interest in identifying potentially modifiable influences on BP. A link between acid-base status and BP has been reported in several cross-sectional, as well as prospective, studies (1, 13, 27, 49, 50). Supporting these findings, experimental studies demonstrated associations between blood levels of bicarbonate and intracellular pH with BP in animal models of hypertension (4, 15, 24), as well as lower arterial pH and plasma bicarbonate in humans with salt-sensitive BP (44).
One aspect influencing human acid-base status is diet, as has been demonstrated in several recent studies (3, 38). Consequently, a number of observational analyses have shown that the acid load produced by dietary intakes may be relevant for BP levels and hypertension risk (2, 22, 32, 57), although these associations are not consistently observed (11, 25). As is clearly shown in manifest kidney disease, renal excretion of acids is a second important determinant of acid-base status apart from diet. A recent study (35), demonstrating associations between renal hyperfiltration as an early marker of abnormal kidney function and lower bicarbonate levels even within a relatively healthy population not suffering from chronic kidney disease (CKD), supports the notion that milder forms of renal dysfunction may be relevant as well.
Apart from its importance in maintaining acid-base balance, it has been known for a long time that kidney function is also central for regulation of BP (16). Interestingly, higher concentrations of the renal marker cystatin C have been related to higher systolic BP and pulse pressure even in a population with a normal glomerular filtration rate (GFR) (36). However, to date, the kidneys’ ability to excrete acid loads has not been explicitly investigated with respect to BP. It has been shown that renal ammonia excretion (which is, to a great part, responsible for excretion of protons) is associated with mortality and terminal kidney disease, as well as onset of acidosis independent of GFR in patients with CKD (40), demonstrating that the GFR and the ability to excrete acids are not completely interchangeable in characterizing kidney function. To estimate the renal ability to excrete acids, the net acid excretion capacity (NAEC), depicting the amount of acids excreted at a certain stimulus (i.e., at a certain level of free protons), has been suggested as a noninvasive tool (28). Studies in recent years have already shown that the NAEC decreases with age and that it relates to blood pH (5, 6), but its relevance for BP has not been investigated so far.
Apart from the NAEC as a surrogate parameter for renal functioning with respect to acid-base status, 24-h urinary citrate excretion represents a more integrated and sensitive, noninvasive marker of human acid-base balance (45, 56) that has been associated not only with BP in adults (50) but also with bone quality in healthy children and adolescents (12). However, the relevance of citrate excretion for BP at a younger age has not been investigated so far. Thus our aim was to examine the prospective relation of 24-h citrate excretion and NAEC with BP levels in a healthy, young population, while concurrently controlling for important diet-dependent BP influences, such as sodium (Na) and potassium (K). In this regard, the timeframe of adolescence may be of special interest, because major changes in average BP values occur at this age (54).
MATERIALS AND METHODS
Study population.
For our analyses on prospective associations of citrate and NAEC with BP, healthy, young adults (18–30 yr) were selected from the Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study. This ongoing, open-cohort study was initiated in 1985 and gathers information on diet, metabolism, and development from infancy until young adulthood in healthy volunteers living in the area of Dortmund (Germany). Regular examinations start at 3 mo of age and comprise 3-day weighed dietary records, 24-h urine collections (starting in 3- to 4-yr-old participants), anthropometric and medical assessments, as well as interviews on health, behavior, and environment. Assessment of parental characteristics [including body mass index (BMI) and BP] is also repeatedly scheduled during study participation (21). The DONALD Study was approved by the Ethics Committee of the University of Bonn (Germany), and all assessments were performed with parental and later, children’s written consent.
Of 580 participants without endocrine or renal disorders that might affect BP, 374 individuals could finally be selected who had BP measured in young adulthood (18–30 yr) until March 2017, did not take antihypertensive medication, and had also provided at least three complete (24-h creatinine excretion ≥ 0.1 mg/kg body weight) (43) urine collections during adolescence (boys: 10–15 yr; girls: 9–14 yr). If a participant had collected more than three, 24-h urine samples during this time frame, then three samples were randomly selected for determination of citrate excretion and NAEC. Because 24-h urine sampling usually takes place once a year, time between the single urine samples for each individual thus varies between 1 and 4 yr. To additionally control for “baseline” BP in our analyses, we also used information on adolescent BP. For 368 out of the 374 participants, BP was measured at least twice during adolescence, and the first two available measurements were selected for each participant.
Urine sampling and urinary analyses.
Annually scheduled 24-h urine sample collections were performed, according to standard procedures, after detailed instructions were given to children and parents (43). During the collection period at home, all micturitions were immediately stored in preservative-free, Extran-cleaned (Extran, MA03; Merck, Darmstadt, Germany), 1-liter plastic containers at less than −12°C for the time of collection and then, at −22°C until thawed and analyzed (42). Determination of 24-h creatinine excretion was performed with a creatinine analyzer (Beckman-2; Beckman Instruments, Fullerton, CA) using the kinetic Jaffé procedure. Urinary Na and K, as well as magnesium and calcium, were quantified by flame atomic absorption spectrometry (Perkin Elmer 1100 spectrometer; Perkin Elmer, Überlingen, Germany). For measurement of urinary citrate, an enzymatic method (citrate kit from Boehringer Mannheim, Ingelheim am Rhein, Germany) was used, which was based on the conversion of citrate to L-malate and L-lactate via oxaloacetate, as described by Moellering and Gruber (31). For calculation of NAEC, the components of the net acid excretion (NAE), i.e., titratable acid (TA), ammonium (NH4), and bicarbonate, as well as urine pH, were directly measured in freshly thawed aliquots of the 24-h urine samples, according to the titration method of Lüthy et al. (26), and NAE was subsequently determined as follows: TA + NH4 – HCO3. NAEC was calculated as residuals of NAE on urine pH. Since the relationship of NAE with pH is slightly convex, residuals were calculated within two urine-pH groups to satisfy model assumption of linearity, as has been described by Berkemeyer et al. (6), using the median urine pH of 6.25 in the study population as a cutoff. All urinary variables were standardized to a body surface area (BSA) of 1.73 m2 to reduce influence of individual body size on excretion rates.
BP measurements.
Sphygmomanometric BP measurements are usually performed every 2 yr in DONALD participants, beginning at 4 yr of age. Systolic and diastolic BP was measured in the seated position by trained nurses, according to standard procedures with legs uncrossed, feet at the floor, and the arm supported at heart level. At each measurement occasion, two consecutive BP readings were taken after the participant had sat quiet for ~5 min, and different cuff sizes were used according to arm circumferences. The arithmetic mean of both measurements is used in our analyses. Before 1994, a random zero sphygmomanometer was used for the measurements, whereas a standard mercury sphygmomanometer was used from 1994 onward. Because random zero sphygmomanometers yield systematically lower BP values (55), internally derived conversion factors were applied for BP measurements before 1994 (six out of 374 measurements in young adulthood) to harmonize both measurement methods. As BP physiologically rises during childhood and adolescence and also depends on the individual’s height, we calculated age-, sex-, and height-independent BP-SD score (SDS) values for the adolescent BP measurements, according to German reference values for children aged 3–17 yr (34).
Anthropometric and additional variables.
Anthropometric assessments are usually performed at each visit at the study center. Measurements were made by trained nurses, according to standard procedures. Standing height was determined to the nearest 0.1 cm using a digital stadiometer (Harpenden; Holtain, Crymych, UK), and weight was measured to the nearest 0.1 kg with an electronic scale (Seca 753 E; Seca Weighing and Measuring Systems, Hamburg, Germany) in participants dressed in underwear only and without shoes. From these data, BMI was calculated as weight/height2 (in kg/m2), and age- and sex-independent BMI-SDS values, as well as height SDS values were determined for adolescent measurements (34). Body surface area was also calculated from height and weight data as 0.007184 × height (cm)0.725 × weight (kg)0.425, according to the formula of DuBois and DuBois (10). To obtain information on body fat in adolescence, triceps and subscapular skinfolds were measured with a Holtain calliper to the nearest 0.1 mm and were used to calculate percentage body fat, according to the method of Slaughter et al. (46). Fat mass index (FMI) was subsequently calculated as fat mass (in kg) divided by height (in m2).
On their child’s admission to the DONALD Study, family characteristics were assessed by questionnaires and maternal anthropometric data, and BP was measured with the same methods as used for study participants. Duration of (exclusive) breast feeding was assessed during the first study visits until complementary feeding was initiated. Information on birth characteristics was abstracted from the “Mutterpass.”
Statistical analyses.
Procedures of SAS (version 9.2; SAS Institute, Cary, NC) were used for analyses, and P < 0.05 was considered significant in all statistical tests. Characteristics during adolescence are presented as arithmetic means of three repeated measurements for each individual. The proportion of between- and within-person variation for these three repeated measurements was estimated using intraclass correlation coefficients (17). Differences between boys/men and girls/women in adolescence and young adulthood were tested using the unpaired t-test or the Wilcoxon rank sum test for normally and non-normally distributed continuous variables and the χ2 test for categorical variables. Associations between citrate excretion and NAEC with systolic and diastolic BP were evaluated in linear-regression models in which the means of citrate or NAEC from three urine samples per individual were used as main predictors. In basic models, tests for interaction were performed with respect to sex and BMI-SDS (positive vs. negative SDS values) during puberty. Because these tests indicated significant differences in the association of NAEC with systolic BP between boys/men and girls/women (P for interaction: 0.03), all analyses for NAEC are presented in sex-stratified models. Thus basic models for citrate were adjusted for age and sex, whereas sex-stratified basic models for NAEC included adult age as the only covariate. Biological plausible confounders were tested in these basic models and were retained in the adjusted models if they were either independently associated with the outcome or modified the β-coefficient of the main predictor by >10%. Additionally, 24-h excretions of Na and K were included in all final models because of their known influence on BP and their potential association with renal markers of acid-base status. For reasons of comparability, confounder selection for the predictor citrate was based on the model for systolic BP, and the same adjustment was made for diastolic BP as the outcome. Similarly, for the predictor NAEC, confounders were selected in the model for systolic BP in men and applied to the model for women, as well as to those for diastolic BP. In a separate model, additional adjustment was made for adult BMI as an important determinant of BP levels and a potential mediator of the effects of adolescent NAEC and citrate excretion on adult BP. Regression models for systolic and diastolic BP visually conformed to model assumptions of linearity, normal distribution of residuals, and homoscedasticity. There was no evidence for multicollinearity.
For illustration of mean differences in adult BP, according to levels of adolescent citrate excretion and NAEC, we additionally calculated least-squares means of systolic and diastolic BP in tertiles of the respective predictor. In these models, the same adjustment was used as in the linear-regression analyses, and the least-squares means for the predictor NAEC are presented separately for men and women.
Because NH4 excretion is the most variable component of NAE in different situations, such as metabolic acidosis (14, 41), we additionally calculated the residuals of NH4 on urine pH to obtain an estimate of “ammonia excretion capacity” (NH4C) as an alternative predictor to NAEC in our linear-regression models.
RESULTS
Descriptive data.
Regarding the characteristics of the study population during adolescence (Table 1), mean age was ~1 yr lower in girls, due to the predefined age ranges. Most 24-h urinary excretion rates differed between girls and boys even after correction for individual BSA: urinary citrate and urine pH were higher in girls, whereas urinary creatinine, Na, urinary potential renal acid load (uPRAL), NAE, and NAEC were higher in boys. In contrast, BSA-standardized potassium excretion was similar between the sexes. Girl and boy participants displayed similar height- and BMI-SDS values, but systolic BP-SDS was in trend (P = 0.09) and diastolic BP-SDS significantly (P = 0.002) lower in girls during adolescence. Mean systolic and diastolic BP in women remained significantly lower in young adulthood (Table 2). Additionally, the prevalence of high-normal or hypertensive BP values (i.e., ≥130/85 mmHg) was almost 30% in men, whereas it was only 10% in women. These findings are comparable with those reported in the German representative German Health Interview and Examination Survey (or DEGS1) Study for a similar age group (33). Young women in our study also had a lower mean BMI compared with young men. Regarding parental and early-life characteristics, only birth weight was significantly higher in men. Intraclass correlation coefficients for the repeated urinary measurements indicated that 46% of the variation in citrate and 25% of the variation in NAEC were between persons.
Table 1.
Characteristics of the DONALD Study population (n = 374) in adolescence (girls: 9–14 yr; boys: 10–15 yr)
| All (n = 374) | Female Subjects (n = 190) | Male Subjects (n = 184) | Pdifference | |
|---|---|---|---|---|
| Age, yr | 11.9 (±1.0)* | 11.4 (±0.8) | 12.4 (±0.8) | <0.0001 |
| Urinary data | ||||
| Volume, ml/day | 921 (720, 1,124)† | 905 (708, 1,114) | 932 (749, 1,159) | 0.2 |
| pH | 6.22 (±0.36) | 6.28 (±0.34) | 6.16 (±0.37) | 0.0006 |
| Citrate, mmol/1.73 m2 | 2.80 (2.17, 3.54) | 3.08 (2.46, 3.76) | 2.50 (1.91, 3.30) | <0.0001 |
| Creatinine, mmol/1.73 m2 | 9.43 (8.53, 10.38) | 8.73 (8.08, 9.54) | 9.99 (9.39, 10.99) | <0.0001 |
| Sodium, mmol/1.73 m2 | 133.9 (±33.7) | 129.7 (±34.8) | 138.3 (±32.0) | 0.01 |
| NAE, mEq/1.73 m2 | 55.4 (±16.3) | 49.9 (±14.9) | 61.1 (±15.7) | <0.0001 |
| NAEC,‡ mEq/1.73 m2 | 0.0 (±9.9) | −3.0 (±9.5) | 3.1 (±9.4) | <0.0001 |
| Potassium, mmol/1.73 m2 | 62.6 (±14.9) | 62.1 (±13.9) | 63.2 (±15.9) | 0.5 |
| uPRAL, mEq/1.73 m2 | 10.84 (±16.84) | 6.42 (±17.30) | 15.37 (±15.11) | <0.0001 |
| Anthropometric data | ||||
| Height, m | 1.55 (±0.95) | 1.52 (±0.83) | 1.59 (±0.93) | <0.0001 |
| Height-SDS | 0.35 (±0.99) | 0.30 (±1.01) | 0.40 (±0.96) | 0.3 |
| Weight, kg | 44.4 (38.5, 52.0) | 42.1 (35.4, 47.8) | 48.1 (41.7, 54.9) | <0.0001 |
| BMI, kg/m2 | 18.2 (16.7, 20.1) | 17.6 (16.5, 20.1) | 18.7 (17.0, 20.4) | 0.002 |
| BMI-SDS | −0.13 (±0.85) | −0.18 (±0.84) | −0.08 (±0.86) | 0.3 |
| BSA, m2 | 1.41 (±0.19) | 1.35 (±0.17) | 1.48 (±0.19) | <0.0001 |
| FMI, kg/m2 | 3.15 (2.40, 4.71) | 3.33 (2.72, 4.84) | 2.99 (2.07, 4.47) | 0.001 |
| Systolic BP-SDS§ | −0.61 (±0.96) | −0.69 (±0.95) | −0.52 (±0.96) | 0.09 |
| Diastolic BP-SDS§ | −0.47 (±1.16) | −0.66 (±1.17) | −0.28 (±1.13) | 0.002 |
Values are means (±SD).
Values are medians (Q1, Q3).
NAEC was calculated as residuals of NAE on urine pH within 2 urine pH groups (above and below median pH of the study population).
Data represent the average of the first 2 available measurements in adolescence; data available for n = 368 (186 girls, 182 boys); missing data were amended using population median values for the regression analyses.
BMI, body mass index; BP, blood pressure; BSA, body surface area; DONALD, Dortmund Nutritional and Anthropometric Longitudinally Designed Study; FMI, fat mass index; NAE, net acid excretion; NAEC, net acid excretion capacity; SDS, SD score; uPRAL, urinary potential renal acid load (data available for n = 370 participants).
Table 2.
Early life, parental, and adult data (18–30 yr) of the study population (n = 374)
| All (n = 374) | Female Subjects (n = 190) | Male Subjects (n = 184) | Pdifference | |
|---|---|---|---|---|
| Adult data | ||||
| Age, yr | 21.1 (18.1, 25.0)* | 21.3 (18.2, 25.2) | 20.9 (18.1, 24.6) | 0.04 |
| Systolic BP, mmHg | 116.3 (±11.0)† | 112.4 (±9.2) | 120.3 (±11.1) | <0.0001 |
| Diastolic BP, mmHg | 74.3 (±9.1) | 72.4 (±8.8) | 76.3 (±9.0) | <0.0001 |
| BP ≥ 130/85 mmHg,‡ n, % | 73 (19.5) | 19 (10.0) | 54 (29.4) | <0.0001 |
| BMI, kg/m2 | 22.8 (20.7, 24.9) | 22.0 (20.2, 24.2) | 23.4 (21.1, 26.4) | 0.0001 |
| Early life/parental data | ||||
| Birth weight,§ g | 3448 (±493) | 3315 (±468) | 3584 (±481) | <0.0001 |
| Gestational age,§ wk | 40 (39, 41) | 40 (39, 41) | 40 (39, 41) | 0.3 |
| Breast fed > 2 wk,§ n, % | 284 (76.1) | 149 (78.4) | 135 (73.8) | 0.3 |
| Maternal overweight,§ n, % | 109 (29.5) | 53 (27.9) | 56 (31.1) | 0.5 |
| Maternal school education ≥ 12 yr, n, % | 192 (51.3) | 92 (48.4) | 100 (54.4) | 0.3 |
| Smoking in the household, n, % | 112 (30.0) | 59 (31.1) | 53 (28.8) | 0.6 |
| Maternal systolic BP,§ mmHg | 113.6 (±12.4) | 112.9 (±12.4) | 114.3 (±12.4) | 0.3 |
| Maternal diastolic BP,§ mmHg | 72.8 (±9.4) | 72.5 (±9.5) | 73.0 (±9.4) | 0.6 |
Values are medians (Q1, Q3).
Values are means (±SD).
High normal or hypertensive BP values, according to the European Society of Cardiology/European Society of Hypertension guidelines.
10 missing values for maternal BP, 4 missing values for maternal overweight, 3 missing values for birth weight, 2 missing values for gestational age, and 1 missing value for breast feeding. Missing data were amended using population median values for the regression analyses.
BMI, body mass index; BP, blood pressure.
Linear-regression analyses.
In basic models adjusted for adult age and sex, a 1-mmol/1.73 m2/day higher citrate excretion during adolescence was associated with a 1.2-mmHg lower systolic BP in young adulthood (Table 3). Additional adjustment for adolescent height- and BP-SDS, as well as urinary excretions of Na and K and maternal BP, did not change this association. Additional inclusion of BMI in young adulthood in the adjusted models attenuated these results only marginally. In contrast to the findings for systolic BP, diastolic BP in adulthood was not related to adolescent citrate excretion, neither in basic nor in adjusted models.
Table 3.
Linear-regression analyses on the association of adolescent citrate excretion with adult BP
| Predictor: Citrate, mmol·1.73 m−2·day−1 | β (95% CI) | P | R2 (%) |
|---|---|---|---|
| Systolic BP | |||
| Basic model* | −1.172 (−2.179, −0.166) | 0.02 | 16.2 |
| Adjusted model† | −1.213 (−2.235, −0.191) | 0.02 | 25.9 |
| Adjusted model + adult BMI‡ | −1.092 (−2.100, −0.084) | 0.03 | 28.5 |
| Diastolic BP | |||
| Basic model* | −0.457 (−1.321, 0.408) | 0.3 | 11.4 |
| Adjusted model† | −0.253 (−1.136, 0.630) | 0.6 | 20.8 |
| Adjusted model + adult BMI‡ | −0.204 (−1.086, 0.678) | 0.7 | 21.4 |
Citrate excretion standardized to a body surface area of 1.73 m2; model adjusted for sex and adult age.
Basic model additionally adjusted for adolescent height-SD score and basic adolescent BP-SD score, excretion of sodium and potassium (both standardized to body surface area of 1.73 m2), as well as maternal systolic BP.
Adjusted model additionally adjusted for adult BMI.
BMI, body mass index; BP, blood pressure; CI, confidence interval.
Owing to a significant sex interaction in the association between adolescent NAEC and adult systolic BP, models are presented, stratified for men and women (Table 4). In age-adjusted basic models, a higher NAEC was significantly related to lower systolic BP in men (β = −0.178, P = 0.04) but not in women. Adjustment for adolescent FMI, height-SDS, and BP-SDS, as well as 24-h excretion rates of Na, K, and magnesium, moderately attenuated the NAEC-BP relation in men so that it was no longer significant (β = −0.173, P = 0.07). In contrast, a significant inverse association between NAEC and systolic BP in men was seen again after additional adjustment for adult BMI. Our analyses showed no associations between NAEC and diastolic BP in either sex, as well as between NAEC and systolic BP in young women.
Table 4.
Sex-stratified linear-regression analyses on the association of adolescent net acid excretion capacity with adult BP
| Female Subjects (n = 190) |
Male Subjects (n = 184) |
|||||
|---|---|---|---|---|---|---|
| Predictor: NAEC* | β (95% CI) | P Value | R2(%) | β (95% CI) | P Value | R2 (%) |
| Systolic BP | ||||||
| Basic model† | 0.054 (−0.086, 0.194) | 0.4 | 1.3 | −0.178 (−0.348, −0.008) | 0.04 | 5.6 |
| Adjusted model‡ | 0.033 (−0.123, 0.190) | 0.7 | 12.2 | −0.173 (−0.364, 0.018) | 0.07 | 15.7 |
| Adjusted model + adult BMI§ | 0.013 (−0.142, 0.168) | 0.9 | 15.2 | −0.196 (−0.381, −0.011) | 0.04 | 21.7 |
| Diastolic BP | ||||||
| Basic model† | −0.007 (−0.139, 0.126) | 0.9 | 3.7 | −0.094 (−0.228, 0.039) | 0.2 | 11.8 |
| Adjusted model‡ | −0.062 (−0.210, 0.086) | 0.4 | 14.1 | −0.016 (−0.164, 0.133) | 0.8 | 22.4 |
| Adjusted model + adult BMI§ | −0.072 (−0.221, 0.077) | 0.3 | 14.7 | −0.024 (−0.172, 0.125) | 0.8 | 23.3 |
NAEC was calculated as residuals of net acid excretion (standardized to a body surface area of 1.73 m2) on urine pH.
Model adjusted for adult age.
Basic model additionally adjusted for adolescent height-SD score (SDS), fat mass index, and basic adolescent BP-SDS, as well as excretion of sodium, potassium, and magnesium (all standardized to body surface area of 1.73 m2).
Adjusted model additionally adjusted for adult BMI.
BMI, body mass index; BP, blood pressure; CI, confidence interval; NAEC, net acid excretion capacity.
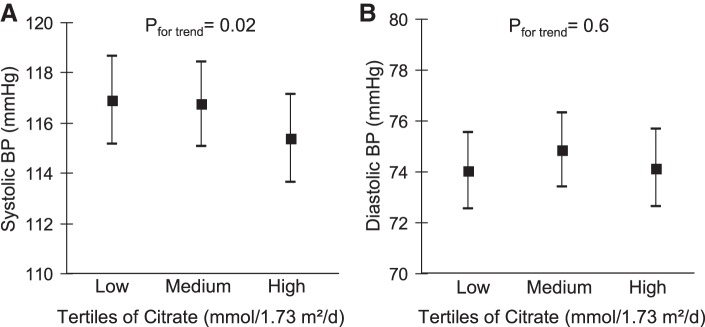
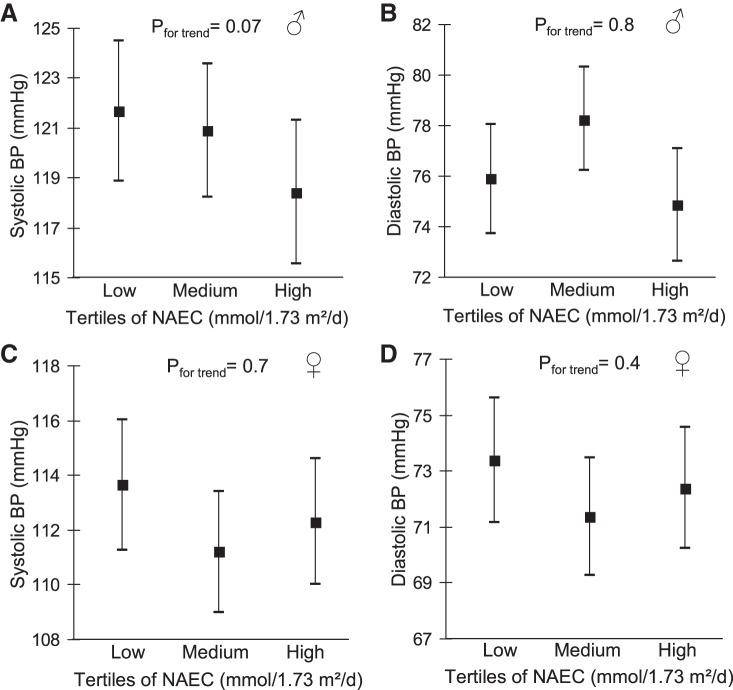
Comparison of adjusted least-squares means of BP between tertiles of adolescent citrate excretion demonstrated a 1.5-mmHg lower mean systolic BP in the highest vs. the lowest citrate tertile, whereas no clear trend was discernible for diastolic BP (Fig. 1). Regarding NAEC, men in the highest tertile had a 3.3-mmHg lower mean systolic BP compared with those with the lowest NAEC. Again, no clear trends across NAEC tertiles were observable for means of diastolic BP in men, as well as for means of systolic or diastolic BP in women (Fig. 2).
Fig. 1.
Adjusted (Table 3, Model 2) means (95% confidence interval) of young adult systolic (A) and diastolic (B) blood pressure (BP) in tertiles of adolescent citrate excretion. Pfor trend refers to P values obtained in linear-regression models with citrate excretion and BP as continuous variables. Data for n = 374 participants.
Fig. 2.
Adjusted (Table 4, Model 2) means (95% confidence interval) of young adult systolic (A and C) and diastolic (B and D) blood pressure (BP) in tertiles of adolescent net acid excretion capacity (NAEC; standardized to a body surface area of 1.73 m2). Pfor trend refers to P values obtained in linear-regression models with NAEC and BP as continuous variables. Data for n = 184 men (A and B) and n = 190 women (C and D).
Additional/sensitivity analyses.
When we conducted our analyses with ammonia excretion capacity (NH4C) instead of NAEC as the predictor, results were generally similar compared with those for NAEC but did not reach statistical significance (P = 0.07 and P = 0.1 for the association between NH4C and systolic BP in basic and adjusted models in men, respectively; data not shown). If urinary variables were included as absolute daily excretion rates instead of BSA-standardized excretions, then adjusted results were very similar to those presented in Tables 3 and 4. Additional adjustment for 24-h creatinine excretion also did not change the observed associations.
DISCUSSION
In our prospective analyses, we show that a higher adolescent citrate excretion, indicative of a lower systemic acid load, is associated with lower systolic BP in young, healthy adults. This association was independent of adolescent Na and K excretion and was also not explained by a higher adult BMI. Concurrently, a higher adolescent NAEC, as an estimate of a better renal ability to excrete acids, was prospectively related to lower systolic BP, although this association seemed to be less robust and was discernible in men only.
In contrast to our findings for systolic BP, we were not able to show associations between NAEC or citrate and diastolic BP. These results are in accordance with the studies of Taylor et al. (49), as well as Abramowitz et al. (1), who reported significant associations of lower serum bicarbonate and higher anion gap with higher systolic, but not diastolic BP in adults, indicating that diastolic BP may probably be less responsive to subclinical changes in acid-base balance. According to our adjusted linear-regression models, a 1-mmol/1.73 m2/day higher citrate excretion was associated with a 1.2-mmHg lower systolic BP, whereas a 10-mEq higher NAEC was related to a 1.7-mmHg lower systolic BP in men. Although these associations seem to be of rather moderate strength, it has to be kept in mind that the examined population is characterized by low-normal BP values, and stronger effects can be expected in a hypertensive population. However, an advantage of our investigation in this young, healthy population is that no major confounding effects of BP influencing medication or chronic diseases have to be expected.
Prior evidence on the relationship of urinary citrate excretion with BP and hypertension prevalence comes predominantly from studies in kidney-stone formers (20, 23), in whom lower urinary citrate excretion was observed in hypertensive patients independent of age, BMI (20), and titratable acid excretion (23). The associations between citrate excretion and hypertension prevalence seem, however, not to be clearly different between adults with and without kidney stones (50).
Testing for sex interactions in our basic linear-regression models revealed differences in the relationship of NAEC with systolic BP between men and women (P for interaction: 0.03), with a significant inverse association found in men only. We have no definite explanation for the sex differences observed in our analyses, but it has been shown that mean adolescent increase in systolic and diastolic BP is much more pronounced in boys compared with girls (34). These differing BP slopes for men and women during growth were also observed in the present analysis (see Tables 1 and 2) and probably contribute to a higher influenceability of male adolescent BP. In contrast, sex differences in kidney function do not seem to be a major explanation for our findings, since GFR does not seem to be different between healthy men and women until ~50 yr of age, when corrected for BSA (39). In our study population, urinary pH was lower, and NAE (as well as NAEC) was higher in adolescent boys even after correction for BSA, indicating that a higher stimulus of the renal capacity to excrete acids in men may contribute to our findings. More generally, the observation that NAEC-BP associations seemed to be less robust than citrate-BP associations in our study might also be related to the fact that acid excretion capacity was noninvasively estimated from urinary data under normal living conditions in the DONALD adolescents and was not experimentally tested with NH4Cl− loading, such as used in identifying patients with incomplete distal renal tubular acidosis (37). As a consequence, NAEC, used in our analyses, might only partly reflect the ability to excrete acids under maximal renal stimulation.
There are several discussed mechanisms that might explain direct associations between the body’s proton load (indicated by lower urinary citrate excretion) and BP: First, administration of alkali has been shown to reduce glucocorticoid secretion in healthy adults (8, 29). Since higher cortisol levels, such as observed in subclinical hypercortisolism, frequently relate to higher BP levels (9), this aspect may be partially responsible for the observed citrate-BP associations in our study. A second aspect that has been related to both acid-base balance and BP is serum levels of uric acid (UA). Higher levels of UA have shown consistent associations with an increased hypertension risk (52), whereas alkalization may increase renal excretion of UA and thus reduce serum UA (19). As an additional point, renal function itself might also link a higher acid load to higher BP levels, probably explained by higher intrarenal ammonia concentrations during (subclinical) acidosis that lead to complement activation and tubular injury (51). It has also been shown that acid retention in patients with moderate CKD is related to higher plasma levels of endothelin and aldosterone (53), both of which are involved in BP regulation. Interestingly, apart from these rather indirect mechanisms, a recent animal study (48) suggested that a proton-sensing receptor might be involved in BP regulation as well. It should also be kept in mind that a higher citrate excretion is related to fruit- and vegetable-rich diets (30) and that high consumption of fruits and vegetables has been consistently associated with lower hypertension risk (7). Moreover, a lower citrate excretion might be a surrogate parameter for a higher dietary acid intake. Adjusting our models for K excretion, which reasonably reflects fruit and vegetable intake (30, 47), did not, however, change the observed citrate-BP relationship. Citrate-BP associations also remained stable after additional adjustment for uPRAL, a marker for dietary acid load (data not shown).
In our observational, noninvasive study, we were not able to further explore these suggested mechanisms. The noninvasive design of the DONALD Study, which includes no blood sampling until the age of 18 yr, also accounts for another limitation of our analyses, in that we had no information on plasma acid-base parameters to better characterize adolescent acid-base balance. Relatedly, we could not control for GFR during adolescence, due to lack of serum creatinine measurements. However, we excluded participants with known physician-diagnosed kidney impairment from our analyses, and of the 272 participants with available information on serum creatinine parallel to the adult BP measurement, only six had creatinine values above the sex-specific reference range. Additional adjustment for serum creatinine in the subgroup with available data (n = 272) did not change the findings for citrate or NAEC. Since some of the urine samples were stored frozen for several years before measurement of citrate and NAE, stability of the respective analytes might also be a concern. It has, however, been shown that storage stability and recovery are good for urinary citrate and at least moderate for NAE after 12–15 yr (42). Finally, the single office BP measurement used as an outcome measure in our analyses can be seen as an additional limitation, and ambulatory BP measurements could be useful in further examining in more detail the observed associations between citrate excretion, NAEC, and BP.
Strengths of the current study include its prospective design and the availability of three repeated, 24-h urine samples in healthy subjects during adolescence to characterize habitual acid-base status. Moreover, we could benefit from information on baseline BP and further important covariates, such as 24-h excretions of Na and K. As has been mentioned above, the fact that no disturbing antihypertensive medication was taken in this young, healthy population allowed us to examine the independent associations between adolescent acid-base parameters and adult BP.
To conclude, our analyses demonstrate that a higher citrate excretion during adolescence (indicative of a lower systemic proton load) might be beneficial for systolic BP in young adulthood and that a better renal ability to excrete acids could also be related to lower systolic BP at least in men. The mechanisms behind these observations, as well as potential sex differences, need further investigation.
GRANTS
The DONALD Study is supported by the Ministry of Innovation, Science, Research and Technology of the State of North Rhine-Westphalia, Germany.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.K. and T.R. conceived and designed research; D.K. and J.E. analyzed data; T.H.W., J.E., and T.R. interpreted results of experiments; D.K. prepared figures; D.K. drafted manuscript; T.H.W., J.E., and T.R. edited and revised manuscript; T.H.W., J.E., and T.R. approved final version of manuscript.
REFERENCES
- 1.Abramowitz MK, Hostetter TH, Melamed ML. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney Int 81: 1033–1042, 2012. doi: 10.1038/ki.2011.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akter S, Eguchi M, Kurotani K, Kochi T, Pham NM, Ito R, Kuwahara K, Tsuruoka H, Mizoue T, Kabe I, Nanri A. High dietary acid load is associated with increased prevalence of hypertension: the Furukawa Nutrition and Health Study. Nutrition 31: 298–303, 2015. doi: 10.1016/j.nut.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Amodu A, Abramowitz MK. Dietary acid, age, and serum bicarbonate levels among adults in the United States. Clin J Am Soc Nephrol 8: 2034–2042, 2013. doi: 10.2215/CJN.03600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batlle D, Redon J, Gutterman C, LaPointe M, Saleh A, Sharma A, Rombola G, Ye M, Alsheikha W, Gomez L, Sobrero M. Acid-base status and intracellular pH regulation in lymphocytes from rats with genetic hypertension. J Am Soc Nephrol 5, Suppl 1: S12–S22, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Berkemeyer S. Net acid excretion capacity is related to blood hydrogen ion and serum carbon dioxide. Metabolism 59: 338–342, 2010. doi: 10.1016/j.metabol.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Berkemeyer S, Vormann J, Günther AL, Rylander R, Frassetto LA, Remer T. Renal net acid excretion capacity is comparable in prepubescence, adolescence, and young adulthood but falls with aging. J Am Geriatr Soc 56: 1442–1448, 2008. doi: 10.1111/j.1532-5415.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 7.Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Müller MJ, Oberritter H, Schulze M, Stehle P, Watzl B. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr 51: 637–663, 2012. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buehlmeier J, Remer T, Frings-Meuthen P, Maser-Gluth C, Heer M. Glucocorticoid activity and metabolism with NaCl-induced low-grade metabolic acidosis and oral alkalization: results of two randomized controlled trials. Endocrine 52: 139–147, 2016. doi: 10.1007/s12020-015-0730-7. [DOI] [PubMed] [Google Scholar]
- 9.Di Dalmazi G, Pasquali R, Beuschlein F, Reincke M. Subclinical hypercortisolism: a state, a syndrome, or a disease? Eur J Endocrinol 173: M61–M71, 2015. doi: 10.1530/EJE-15-0272. [DOI] [PubMed] [Google Scholar]
- 10.DuBois D, DuBois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic) XVII: 863–871, 1916. doi: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
- 11.Engberink MF, Bakker SJ, Brink EJ, van Baak MA, van Rooij FJ, Hofman A, Witteman JC, Geleijnse JM. Dietary acid load and risk of hypertension: the Rotterdam Study. Am J Clin Nutr 95: 1438–1444, 2012. doi: 10.3945/ajcn.111.022343. [DOI] [PubMed] [Google Scholar]
- 12.Esche J, Johner S, Shi L, Schönau E, Remer T. Urinary citrate, an index of acid-base status, predicts bone strength in youths and fracture risk in adult females. J Clin Endocrinol Metab 101: 4914–4921, 2016. doi: 10.1210/jc.2016-2677. [DOI] [PubMed] [Google Scholar]
- 13.Forman JP, Rifas-Shiman SL, Taylor EN, Lane K, Gillman MW. Association between the serum anion gap and blood pressure among patients at Harvard Vanguard Medical Associates. J Hum Hypertens 22: 122–125, 2008. doi: 10.1038/sj.jhh.1002286. [DOI] [PubMed] [Google Scholar]
- 14.Garibotto G, Verzola D, Sofia A, Saffioti S, Menesi F, Vigo E, Tarroni A, Deferrari G, Gandolfo MT. Mechanisms of renal ammonia production and protein turnover. Metab Brain Dis 24: 159–167, 2009. doi: 10.1007/s11011-008-9121-6. [DOI] [PubMed] [Google Scholar]
- 15.Gröger N, Vitzthum H, Fröhlich H, Krüger M, Ehmke H, Braun T, Boettger T. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet 21: 1025–1036, 2012. doi: 10.1093/hmg/ddr533. [DOI] [PubMed] [Google Scholar]
- 16.Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman L, Stawski RS. Persons as contexts: evaluating between-person and within-person effects in longitudinal analysis. Res Hum Dev 6: 97–120, 2009. doi: 10.1080/15427600902911189. [DOI] [Google Scholar]
- 18.Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K, Mente A, Yusuf S. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res 121: 677–694, 2017. doi: 10.1161/CIRCRESAHA.117.308903. [DOI] [PubMed] [Google Scholar]
- 19.Kanbara A, Miura Y, Hyogo H, Chayama K, Seyama I. Effect of urine pH changed by dietary intervention on uric acid clearance mechanism of pH-dependent excretion of urinary uric acid. Nutr J 11: 39, 2012. doi: 10.1186/1475-2891-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YJ, Park MS, Kim WT, Yun SJ, Kim WJ, Lee SC. Hypertension influences recurrent stone formation in nonobese stone formers. Urology 77: 1059–1063, 2011. doi: 10.1016/j.urology.2010.07.492. [DOI] [PubMed] [Google Scholar]
- 21.Kroke A, Manz F, Kersting M, Remer T, Sichert-Hellert W, Alexy U, Lentze MJ. The DONALD Study. History, current status and future perspectives. Eur J Nutr 43: 45–54, 2004. doi: 10.1007/s00394-004-0445-7. [DOI] [PubMed] [Google Scholar]
- 22.Krupp D, Shi L, Remer T. Longitudinal relationships between diet-dependent renal acid load and blood pressure development in healthy children. Kidney Int 85: 204–210, 2014. doi: 10.1038/ki.2013.331. [DOI] [PubMed] [Google Scholar]
- 23.Losito A, Nunzi EG, Covarelli C, Nunzi E, Ferrara G. Increased acid excretion in kidney stone formers with essential hypertension. Nephrol Dial Transplant 24: 137–141, 2009. doi: 10.1093/ndt/gfn468. [DOI] [PubMed] [Google Scholar]
- 24.Lucas PA, Lacour B, McCarron DA, Drüeke T. Disturbance of acid-base balance in the young spontaneously hypertensive rat. Clin Sci (Lond) 73: 211–215, 1987. doi: 10.1042/cs0730211. [DOI] [PubMed] [Google Scholar]
- 25.Luis D, Huang X, Riserus U, Sjögren P, Lindholm B, Arnlöv J, Cederholm T, Carrero JJ. Estimated dietary acid load is not associated with blood pressure or hypertension incidence in men who are approximately 70 years old. J Nutr 145: 315–321, 2015. doi: 10.3945/jn.114.197020. [DOI] [PubMed] [Google Scholar]
- 26.Lüthy C, Moser C, Oetliker O. [Acid-base determination of urine in 3 steps]. Med Lab (Stuttg) 30: 174–181, 1977. [PubMed] [Google Scholar]
- 27.Mandel EI, Forman JP, Curhan GC, Taylor EN. Plasma bicarbonate and odds of incident hypertension. Am J Hypertens 26: 1405–1412, 2013. doi: 10.1093/ajh/hpt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manz F, Wentz A, Lange S. Factors affecting renal hydrogen ion excretion capacity in healthy children. Pediatr Nephrol 16: 443–445, 2001. doi: 10.1007/s004670100566. [DOI] [PubMed] [Google Scholar]
- 29.Maurer M, Riesen W, Muser J, Hulter HN, Krapf R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol 284: F32–F40, 2003. doi: 10.1152/ajprenal.00212.2002. [DOI] [PubMed] [Google Scholar]
- 30.Meschi T, Maggiore U, Fiaccadori E, Schianchi T, Bosi S, Adorni G, Ridolo E, Guerra A, Allegri F, Novarini A, Borghi L. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int 66: 2402–2410, 2004. doi: 10.1111/j.1523-1755.2004.66029.x. [DOI] [PubMed] [Google Scholar]
- 31.Moellering H, Gruber W. Determination of citrate with citrate lyase. Anal Biochem 17: 369–376, 1966. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- 32.Murakami K, Sasaki S, Takahashi Y, Uenishi K; Japan Dietetic Students’ Study for Nutrition and Biomarkers Group . Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br J Nutr 100: 642–651, 2008. doi: 10.1017/S0007114508901288. [DOI] [PubMed] [Google Scholar]
- 33.Neuhauser H, Thamm M, Ellert U. [Blood pressure in Germany 2008–2011: results of the German Health Interview and Examination Survey for Adults (DEGS1)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 56: 795–801, 2013. doi: 10.1007/s00103-013-1669-6. [DOI] [PubMed] [Google Scholar]
- 34.Neuhauser HK, Thamm M, Ellert U, Hense HW, Rosario AS. Blood pressure percentiles by age and height from nonoverweight children and adolescents in Germany. Pediatrics 127: e978–e988, 2011. doi: 10.1542/peds.2010-1290. [DOI] [PubMed] [Google Scholar]
- 35.Park M, So R, Joo KW, Yoon HJ. Association between lower serum bicarbonate and renal hyperfiltration in the general population with preserved renal function: a cross-sectional study. BMC Nephrol 17: 3, 2016. doi: 10.1186/s12882-015-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peralta CA, Whooley MA, Ix JH, Shlipak MG. Kidney function and systolic blood pressure new insights from cystatin C: data from the Heart and Soul Study. Am J Hypertens 19: 939–946, 2006. doi: 10.1016/j.amjhyper.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira PC, Miranda DM, Oliveira EA, Simoes e Silva AC. Molecular pathophysiology of renal tubular acidosis. Curr Genomics 10: 51–59, 2009. doi: 10.2174/138920209787581262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pizzorno J, Frassetto LA, Katzinger J. Diet-induced acidosis: is it real and clinically relevant? Br J Nutr 103: 1185–1194, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Pottel H, Delanaye P, Weekers L, Selistre L, Goffin K, Gheysens O, Dubourg L. Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J 10: 545–551, 2017. doi: 10.1093/ckj/sfx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raphael KL, Carroll DJ, Murray J, Greene T, Beddhu S. Urine ammonium predicts clinical outcomes in hypertensive kidney disease. J Am Soc Nephrol 28: 2483–2490, 2017. doi: 10.1681/ASN.2016101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remer T, Manz F. Dietary protein as a modulator of the renal net acid excretion capacity: evidence that an increased protein intake improves the capability of the kidney to excrete ammonium. J Nutr Biochem 6: 431–437, 1995. doi: 10.1016/0955-2863(95)00064-7. [DOI] [Google Scholar]
- 42.Remer T, Montenegro-Bethancourt G, Shi L. Long-term urine biobanking: storage stability of clinical chemical parameters under moderate freezing conditions without use of preservatives. Clin Biochem 47: 307–311, 2014. doi: 10.1016/j.clinbiochem.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr 75: 561–569, 2002. doi: 10.1093/ajcn/75.3.561. [DOI] [PubMed] [Google Scholar]
- 44.Sharma AM, Kribben A, Schattenfroh S, Cetto C, Distler A. Salt sensitivity in humans is associated with abnormal acid-base regulation. Hypertension 16: 407–413, 1990. doi: 10.1161/01.HYP.16.4.407. [DOI] [PubMed] [Google Scholar]
- 45.Simpson DP. Citrate excretion: a window on renal metabolism. Am J Physiol Renal Physiol 244: F223–F234, 1983. [DOI] [PubMed] [Google Scholar]
- 46.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol 60: 709–723, 1988. [PubMed] [Google Scholar]
- 47.Smith-Warner SA, Elmer PJ, Tharp TM, Fosdick L, Randall B, Gross M, Wood J, Potter JD. Increasing vegetable and fruit intake: randomized intervention and monitoring in an at-risk population. Cancer Epidemiol Biomarkers Prev 9: 307–317, 2000. [PubMed] [Google Scholar]
- 48.Sun X, Tommasi E, Molina D, Sah R, Brosnihan KB, Diz D, Petrovic S. Deletion of proton-sensing receptor GPR4 associates with lower blood pressure and lower binding of angiotensin II receptor in SFO. Am J Physiol Renal Physiol 311: F1260–F1266, 2016. doi: 10.1152/ajprenal.00410.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor EN, Forman JP, Farwell WR. Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension 50: 320–324, 2007. doi: 10.1161/HYPERTENSIONAHA.107.092643. [DOI] [PubMed] [Google Scholar]
- 50.Taylor EN, Mount DB, Forman JP, Curhan GC. Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis 47: 780–789, 2006. doi: 10.1053/j.ajkd.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 51.van den Berg E, Hospers FA, Navis G, Engberink MF, Brink EJ, Geleijnse JM, van Baak MA, Gans RO, Bakker SJ. Dietary acid load and rapid progression to end-stage renal disease of diabetic nephropathy in Westernized South Asian people. J Nephrol 24: 11–17, 2011. doi: 10.5301/JN.2010.5711. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Qin T, Chen J, Li Y, Wang L, Huang H, Li J. Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS One 9: e114259, 2014. doi: 10.1371/journal.pone.0114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wesson DE, Simoni J, Broglio K, Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300: F830–F837, 2011. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 54.Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, Benzeval M, Brunner E, Cooper R, Kivimaki M, Kuh D, Muniz-Terrera G, Hardy R. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med 8: e1000440, 2011. doi: 10.1371/journal.pmed.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang W, Gu D, Chen J, Jaquish CE, Rao DC, Wu X, Hixson JE, Duan X, Kelly TN, Hamm LL, Whelton PK, He J; GenSalt Collaborative Research Group . Agreement of blood pressure measurements between random-zero and standard mercury sphygmomanometers. Am J Med Sci 336: 373–378, 2008. doi: 10.1097/MAJ.0b013e31816956ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zacchia M, Preisig P. Low urinary citrate: an overview. J Nephrol 23, Suppl 16: S49–S56, 2010. [PubMed] [Google Scholar]
- 57.Zhang L, Curhan GC, Forman JP. Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension 54: 751–755, 2009. doi: 10.1161/HYPERTENSIONAHA.109.135582. [DOI] [PMC free article] [PubMed] [Google Scholar]