Abstract
The rodent model of folic acid (FA)-induced acute kidney injury (AKI) provides a useful model for studying human AKI, but little is known about longitudinal changes in renal hemodynamics and evolution of renal fibrosis in vivo. In this work, we aimed to longitudinally assess renal structural and functional changes using multiparametric magnetic resonance imaging (MRI). Ten adult mice were injected with FA, after which a multiparametric MRI was used to measure kidney volume, hypoxia index R2*, magnetization transfer ratio (MTR), perfusion, T1, and glomerular filtration rate (GFR) at 2 wk posttreatment. Then five mice were euthanized for histology, and the other five underwent MRI again at 4 wk, followed by histology. Control mice (n = 5) were injected with vehicle and studied with MRI at 2 wk. Trichrome and hematoxylin-eosin staining were performed to assess FA-induced tissue injuries. Whereas kidney size and oxygenation showed progressive deterioration, a transient impairment in renal perfusion and normalized GFR slightly improved by 4 wk. Kidney fluid content, as reflected by T1, was prominent at 2 wk and tended to regress at 4 wk, consistent with observed tubular dilation. Trichrome staining revealed patchy necrosis and mild interstitial fibrosis at 2 wk, which exacerbated at 4 wk. MTR detected increased fibrosis at 4 wk. In conclusion, multiparametric MRI captured the longitudinal progression in kidney damage evolving within the first month after treatment with folic acid and may provide a useful tool for assessment of therapeutic strategies.
Keywords: folic acid-induced nephropathy, multiparametric magnetic resonance imaging, renal fibrosis, renal structure and function
INTRODUCTION
Acute kidney injury (AKI) is characterized by a sudden loss of renal function, leading to the accumulation of end products of nitrogen metabolism, such as urea, creatinine, and other waste products (1). It has been well recognized that AKI is an important risk factor for loss of kidney function, incident chronic kidney disease, and accelerated progression to end-stage renal disease, leading to high morbidity, mortality, and cost (16). These outcomes are linked to the development of renal fibrosis, which replaces the functional kidney tissue (19). AKI affects ~20% of hospitalized patients, 10% of whom require kidney transplantation (17), stimulating avid investigation into the mechanisms responsible for subsequent adverse remodeling of the kidney.
The rodent model of folic acid (FA)-induced AKI is nonsurgical and mimics important aspects of human AKI, as characterized by acute tubular cell necrosis, proliferation, inflammatory cell infiltration, and mild fibrosis in the chronic phase (4, 6, 8, 21). FA-induced AKI may also eventuate in chronic or end-stage kidney diseases (15). Therefore, this rodent model provides a useful model for preclinical research on AKI and its aftermath. As an essential vitamin, FA is necessary for the production and maintenance of new cells and helps in synthesizing genetic materials, including DNA and RNA. However, a high dose of FA induces toxicity in the kidney, as it rapidly crystalizes in renal tubules, blocks renal tubules, and induces tubular injury. Coadministration of sodium bicarbonate alleviates crystal formation but not tubular lesions, indicating the direct toxicity of FA to tubular epithelial cells (26). Rodent models of FA-induced renal injury have been very useful to investigate the pathophysiology of AKI (2, 8, 9, 20, 21, 27) but have mostly focused on the assessment of fibrogenic pathways activated in the tissue, as detected in ex vivo studies. In these studies, the assessment of renal function was typically based on levels of serum creatinine and blood urea nitrogen (BUN). However, given the limited resolution of these measurements, little is known about longitudinal changes in renal hemodynamics in vivo during the evolution of tissue fibrosis.
Renal magnetic resonance imaging (MRI) has undergone several technical developments and shows promise for a reliable renal structural and functional assessment. In addition to the three-dimensional (3D) volumetric measurement of kidney size, blood-oxygenation-dependent MRI (BOLD-MRI) can be used to assess tissue hypoxia, which is indexed by the tissue transverse relaxation rate R2* (1/T2*) (22). Arterial spin labeling, which uses magnetically labeled arterial blood water protons as an endogenous tracer, offers a noncontrast-enhanced technique for measuring tissue perfusion (7, 18). In addition, an increase in the longitudinal relaxation time T1 has been shown to be associated with increased tissue water content (10, 24), which results from fluid accumulation. Recent technical developments in dynamic contrast-enhanced MRI (DCE-MRI) and magnetization transfer imaging (MTI) also provide the opportunity for measuring murine glomerular filtration rate (GFR) (13) and renal fibrosis (11, 12), respectively.
In this study, we aimed to test the ability of multiparametric MRI techniques to longitudinally evaluate the evolution of FA-induced nephropathy in mice in vivo. Specifically, kidney volume, hypoxia index R2*, T1, perfusion, GFR, and magnetization transfer ratio (MTR) were measured to evaluate the FA-induced renal structural and functional changes during the development of chronic injury. In addition, we investigated the relationships between renal parameters. We hypothesized that multiparametric MRI could capture the longitudinal FA-induced changes in renal structure and function.
MATERIALS AND METHODS
Animals.
This study was approved by the Institutional Animal Care and Use Committee at the Mayo Clinic. To demonstrate the utility of the multiparametric MRI techniques of monitoring the FA-induced nephropathy, 8-wk-old male C57BL/6 mice (n = 15) (Jackson Laboratory) were used. Mice in the FA group (n = 10) underwent intraperitoneal injection of FA (Sigma-Aldrich, St. Louis, MO) dissolved in sodium bicarbonate solution (37.5 mg/ml per 0.3 mol/l) at a dose of 200 mg/kg. Five control mice were injected with the vehicle sodium bicarbonate solution. Two days after treatment, BUN level was measured from 200 μl blood collected from cheek bleeding to verify the development of AKI (23).
MRI study.
MRI studies were performed on a vertical 16.4 T scanner (Bruker, Billerica, MA) equipped with a 38-mm inner diameter birdcage coil. All 10 mice in the FA group underwent MRI at 2 wk posttreatment, after which half were euthanized for histological assessment of renal injury, and the other half underwent a repeat MRI study, followed by histology at 4 wk. All control mice underwent MRI and ex vivo studies at 2 wk after treatment. During MRI, mice were anesthetized with 2% isoflurane in a mouse chamber and maintained with 1% to 2% isoflurane in an animal holder in supine position. Warm air was blown on the mice to maintain the body temperature at ~36°C. Respiration and body temperature were monitored by a physiological monitoring system (SA Instruments, Stony Brook, NY). Multiparametric MRI was used to measure kidney volume, R2*, MTR, perfusion, T1, and GFR.
Kidney volume was measured using a 3D fast imaging with steady precession sequence with the following parameters: repetition time (TR) of 14 ms, echo time (TE) of 2.7 ms, flip angle of 20°, field of view of 5.12 × 2.56 × 1.28 cm3, matrix size of 256 × 128 × 64, and number of averages of 2. Images were acquired in the coronal plane. All other MRI scans were performed in axial slices with a field of view of 2.56 × 2.56 cm2.
Renal oxygenation was assessed by BOLD-MRI using a respiration-gated 3D multiecho gradient echo sequence to eliminate external field inhomogeneity and susceptibility artifacts (5) with the following imaging parameters: TR of 200 ms, TE of 3.5~24.5 ms, echo number of 8, flip angle of 25°, 3D slab thickness of 1 mm, matrix size of 128 × 128 × 8, and number of averages of 2.
In vivo renal fibrosis was assessed by MTI using an MT-prepared fast low angle shot (FLASH) sequence (11). FLASH images without MT (M0) were acquired with the following parameters: TR of 400 ms, TE of 2.9 ms, flip angle of 20°, slice thickness of 1 mm, slice number of 5, matrix size of 256 × 256, and number of averages of 8. Then MT-weighed images (Mt) were acquired by adding Gaussian MT pulses before FLASH acquisition. The MT pulse parameters were as follows: offset frequency of 1,500 Hz, pulse peak amplitude of 10 μT, pulse length of 9.13 ms, pulse bandwidth of 300 Hz, flip angle of 585°, and pulse number of 2.
Renal perfusion and T1 were measured by arterial spin labeling using a flow-sensitive alternating inversion recovery sequence with rapid acquisition with relaxation enhancement (RARE) with the following parameters: TR of 18,000 ms, TE of 5 ms, slice number of 1, slice thickness of 2 mm, inversion slab thickness of 6 mm, matrix size of 128 × 72, RARE factor of 72, and number of averages of 2. A total of 22 images were acquired with inversion delays from 40 to 18,000 ms (11).
GFR was measured using our previously developed DCE-MRI technique (13). A saturation recovery T1 measurement method with snapshot-FLASH readout was used to trace gadolinium (Gd) dynamics with a temporal resolution of 1 s/scan. Following acquisition of the proton density and 10 baseline T1-weighted images, 20 μl of 37.5 mM gadodiamide were injected through the tail vein within 2 s, after which images were acquired repetitively. Other imaging parameters included: recovery time for 2 slices of 0.25 and 0.57 s, encoding scheme centric, TR of 4.92 ms, TE of 0.8 ms, flip angle of 15°, slice thickness of 2 mm, matrix size of 128 × 128, and number of repetitions of 150. All multiparametric MRI parameters are summarized in Table 1.
Table 1.
Multiparametric MRI sequences and parameters
| Kidney Index | Sequence | TR, ms | TE, ms | Slice/Slab Thickness, mm | Slice/Slab Number | FOV, cm2 | Matrix Size | FA, ° | NA | Other Parameters |
|---|---|---|---|---|---|---|---|---|---|---|
| Volume | 3D-FISP | 14 | 2.7 | 12.8 | 1 | 5.12 × 2.56 | 256 × 128 × 64 | 20 | 2 | — |
| R2* | 3D-MGE | 200 | 3.5–24.5 | 1 | 1 | 2.56 × 2.56 | 128 × 128 × 8 | 25 | 2 | Echo number, 8 |
| MTR | MT-prepared FLASH | 400 | 2.9 | 1 | 5 | 2.56 × 2.56 | 256 × 256 | 20 | 8 | — |
| Perfusion & T1 | FAIR-RARE | 18,000 | 45 | 1 | 1 | 2.56 × 2.56 | 128 × 72 | 90 | 1 | RARE factor, 72 |
| Inversion slab thickness, 6 mm | ||||||||||
| Number of image pairs, 22 | ||||||||||
| Inversion time, 40 to 18,000 ms | ||||||||||
| GFR | Saturation recovery snapshot-FLASH | 4.92 | 0.8 | 2 | 2 | 2.56 × 2.56 | 128 × 128 | 15 | 1 | Saturation time, 0.25 and 0.57 s |
| Number of repetitions, 150 |
3D-FISP, three-dimensional fast imaging with steady precession; 3D-MGE, three-dimensional multiecho gradient echo; FA, flip angle; NA, number of averages; FAIR-RARE, flow-sensitive alternating inversion recovery sequence with rapid acquisition with relaxation enhancement; FLASH, fast low angle shot; FOV, field of view; GFR, glomerular filtration rate; MT, magnetization transfer; MTR, magnetization transfer ratio; TE, echo time; TR, repetition time.
Image analysis.
Kidney volume was quantified from the 3D fast imaging with steady precession images using Analyze (version 12.0, Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). Renal regions of interest (ROIs) were manually traced on all frames where the kidney was observed, from which total renal volume was calculated. All other MR images were analyzed using in-house-developed software modules developed in Matlab (MathWorks, Natick, MA).
For BOLD-MRI analysis, 8 images were reconstructed after zero-filling the k-space data to 256 × 256. The magnitude of all eight images was added to generate a single 1-mm two-dimensional image. T2* was quantified by pixel-wise monoexponential curve fitting of signal intensity over echo times, after which R2* was calculated as 1/T2* and used as an index of the blood oxygenation level.
Renal perfusion and T1 were quantified from the flow-sensitive alternating inversion recovery sequence with RARE images. The magnitude images were used to generate the global and slice-selective T1 maps by pixel-wise monoexponential fitting with two unknown parameters (inversion efficiency and T1). The perfusion coefficient was quantified as described previously (11). The global T1 maps were used to reflect tissue water content in this study.
The MTR was calculated pixel-wise as (M0−Mt)/M0 and used as an index of renal fibrosis (11, 12). To calculate the averaged MTR in different regions of the kidney, cortical and medullary ROIs were traced on the Mt images, showing the best contrast between cortex and medulla with reference to the M0 image and MTR map. Fluid-filled spaces, like large vessels and the pelvis, were excluded to avoid bias. Average MTR was calculated as the mean value from all ROIs of different slices. The same ROIs of the central slice were subsequently propagated and applied for quantification of R2*, renal perfusion, and T1.
The method for DCE-MRI analysis has been described previously (13). Briefly, kidney T1 values were measured using a simple two-point monoexponential fitting, and R1 was calculated as 1/T1. The averaged baseline T1 value was used for the calculation of change in R1 postinjection. ROIs were selected on the abdominal aorta (4–9 pixels, depending on its size), renal parenchyma, and inner medullary papilla for the measurement of arterial input function, contrast perfusion and filtration, and outflow, respectively. A modified two-compartmental model was used for the fitting of Gd dynamics, from which normalized GFR (nGFR) was quantified. Then single-kidney GFR was calculated as the product of nGFR and kidney volume.
Histology.
Mice were euthanized at 2 (n = 5) or 4 (n = 5) weeks after treatment to harvest and fix kidneys. Trichrome and hematoxylin-eosin staining was performed on 5-μm axial kidney slices to assess FA-induced tissue injuries. Fibrosis was quantified as the fraction of fibrotic area over the total cross-sectional area of the tissue using AxioVision (Carl Zeiss SMT, Oberkochen, Germany). Tissue injury was assessed by signs of tubular dilation or atrophy and the integrity of renal structure.
Statistical analysis.
Statistical analysis was performed using JMP 10.0 (SAS Institute, Cary, NC). Renal parameters were compared between the control and FA-treated mice using one-way analysis of variance followed by Tukey’s post hoc analysis. Pearson correlations were conducted on all available data at all time points to investigate the relationships between different renal parameters. Results were expressed as means ± standard deviations. P < 0.05 was considered statistically significant.
RESULTS
Longitudinal progression of renal structure and function.
Two days after injection, BUN level more than tripled in the FA mice compared with controls (78.7 ± 9.3 vs. 23.1 ± 2.7 mg/dl, P < 0.001), confirming FA-induced acute renal injury. Representative images of control and FA mouse kidneys at 2 and 4 wk after treatment are shown in Fig. 1. Whereas no change in kidney size was observed at 2 wk, the FA mice showed significantly smaller kidneys at 4 wk, as compared with the control mice (151.3 ± 31.1 vs. 184.9 ± 6.8 μl, P = 0.009).
Fig. 1.
Renal volume by three-dimensional fast imaging with steady precession. Representative kidney images (A) and single-kidney volume (B) of control (n = 5) and folic acid (FA) mice at 2 (n = 10) and 4 (n = 5) wk after treatment. Kidney volume of FA mice showed no change at 2 wk but a significant decrease at 4 wk, indicating renal atrophy in the chronic phase. Statistical comparison was performed using one-way analysis of variance followed by Tukey’s post hoc analysis.
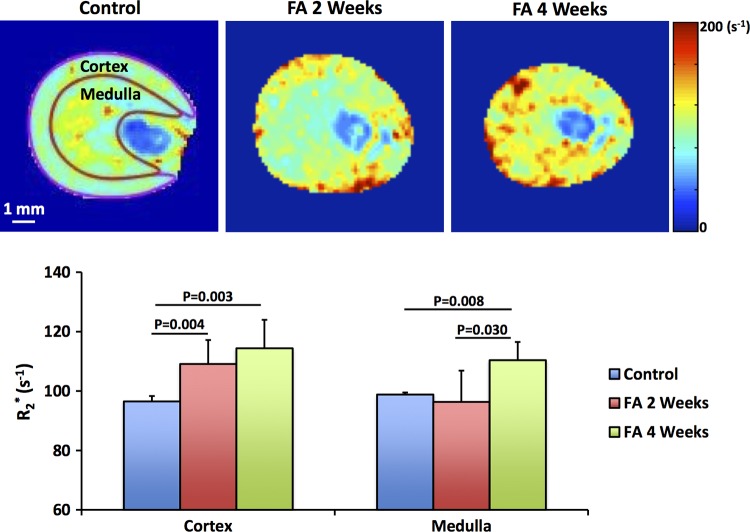
Representative R2* maps of the control and FA mouse kidneys are shown in Fig. 2. Patchy enhancement in R2* appeared in the renal cortex (109.1 ± 8.0 vs. 96.5 ± 1.8 s−1, P = 0.004) of FA mice at 2 wk, with no change in the medulla. At 4 wk, both the renal cortex (114.4 ± 9.6 s−1, P = 0.003) and medulla (110.4 ± 6.1 vs. 98.8 ± 0.6 s−1, P = 0.008) of FA mice showed elevated R2* as compared with the control kidneys (Fig. 2). Medullary R2* at 4 wk was also higher than at 2 wk (96.4 ± 10.5 s−1, P = 0.030).
Fig. 2.
Renal oxygenation by blood-oxygenation-dependent (BOLD-MRI). Representative R2* maps and measured cortical and medullary R2* of control (n = 5) and folic acid (FA) mice at 2 (n = 10) and 4 (n = 5) wk after treatment. Increasing levels of hypoxia are indicated by intensifying yellow and red colors. Patchy hypoxia was observed in the renal cortex at 2 wk, which evolved to global hypoxia at 4 wk. Statistical comparison was performed using one-way analysis of variance followed by Tukey’s post hoc analysis.
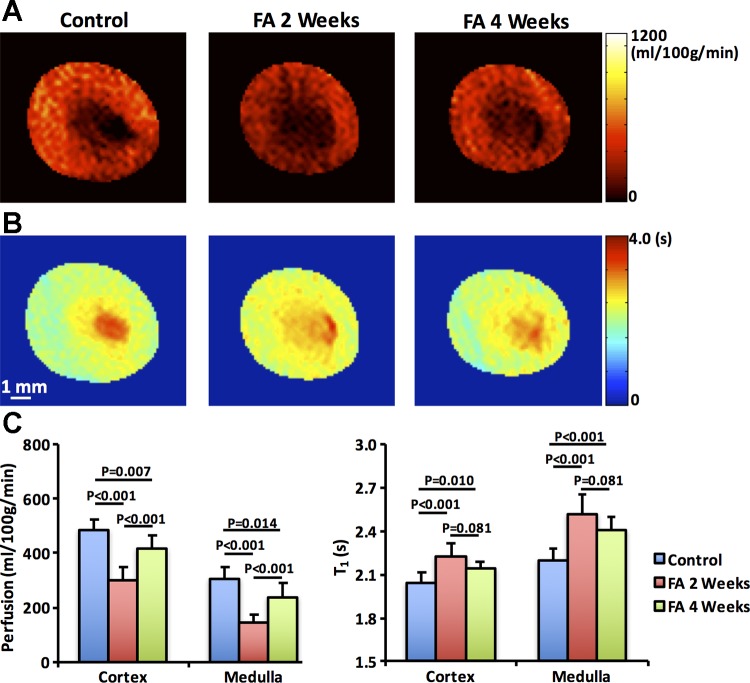
Figure 3, A and B shows the representative renal perfusion and T1 maps of control and FA mice, respectively. Renal perfusion and T1 showed a biphasic and reciprocal change after FA treatment. Compared with control kidneys (cortex, 485.5 ± 39.0 and medulla, 302.8 ± 43.5 ml·100 g−1·min−1), cortical (297.4 ± 51.1, P < 0.001) and medullary (145.4 ± 29.2, P < 0.001) perfusion in FA mice dropped markedly at 2 wk (Fig. 3C) but showed some recovery by 4 wk (cortex, 418.8 ± 48.4, P < 0.001 and medulla, 236.8 ± 52.3, both P < 0.001), although they remained lower than in control kidneys. Contrarily, cortical and medullary T1 rose at 2 wk compared with baseline by 8.8% and 14.6%, respectively. Both cortical and medullary T1 tended to decrease at 4 wk (Fig. 3C), although this drop has not reached statistical significance (P = 0.081 vs. 2 wk) and remained higher than in controls.
Fig. 3.
Renal perfusion and T1 by flow-sensitive alternating inversion recovery sequence with rapid acquisition with relaxation enhancement. Representative renal perfusion (A) and T1 (B) maps of control (n = 5) and folic acid (FA) mice at 2 (n = 10) and 4 (n = 5) wk after treatment. The measured renal perfusion and T1 (C) in renal cortex and medulla. Biphasic changes were observed in both renal perfusion and T1. A marked decrease in renal perfusion was observed at 2 wk, followed by a mild recovery at 4 wk. Renal T1 showed a dramatic increase at 2 wk but a tendency of decrease at 4 wk. The elevated T1 indicates increased fluid content because of tubular dilation in FA-treated mouse kidneys. Statistical comparison was performed using one-way analysis of variance followed by Tukey’s post hoc analysis.
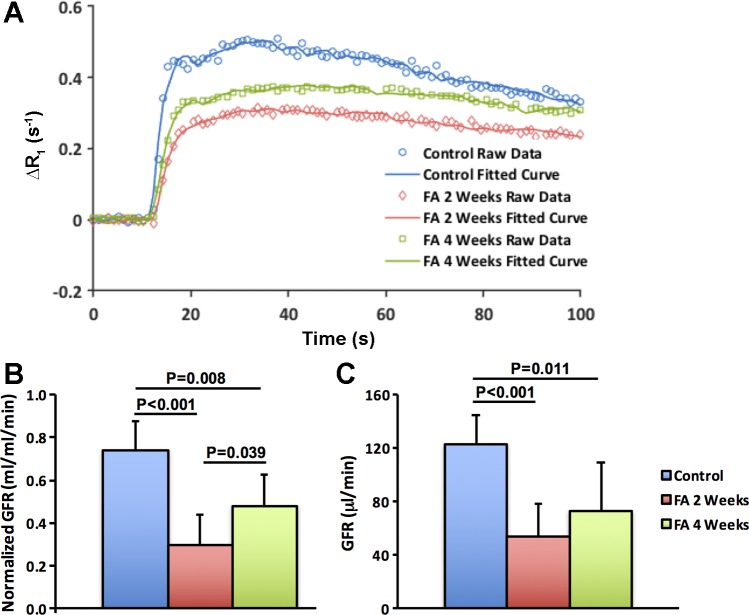
Representative renal DCE-MRI raw and model-fitted Gd dynamics in control and FA mice are shown in Fig. 4A. The two-compartmental model provided a robust fitting of experimentally acquired data in both control and FA mice. The FA mice showed particularly blunted Gd dynamics at 2 wk, followed by a slight recovery at 4 wk, which, nevertheless, remained lower than the control curve. Consequently, the model-fitted nGFR at 2 wk dropped significantly in FA compared with control mice (0.3 ± 0.12 vs. 0.72 ± 0.12 ml·ml−1·min−1, P < 0.001) and showed slight recovery from 2 to 4 wk (0.48 ± 0.12, P = 0.039, Fig. 4B). However, the calculated single-kidney GFR, as the product of nGFR and kidney volume, showed no increase from 2 to 4 wk (Fig. 4C), primarily because of kidney shrinkage at 4 wk (Fig. 1).
Fig. 4.
Glomerular filtration rate (GFR) by dynamic contrast-enhanced MRI. Representative experimentally acquired and model-fitted gadolinium dynamics (A) and measured normalized (B) and total (C) GFR in control (n = 5) and folic acid (FA) mice at 2 (n = 10) and 4 (n = 5) wk after treatment. The unit of normalized GFR is milliliters of filtrates per milliliter of tissue volume per minute (ml·ml tissue−1·min−1). The FA mice showed the lowest normalized GFR at 2 wk, followed by a slight recovery at 4 wk. However, because of renal atrophy at 4 wk, no change in total GFR was observed from 2 to 4 wk. Statistical comparison was performed using one-way analysis of variance followed by Tukey’s post hoc analysis. Δ, change in.
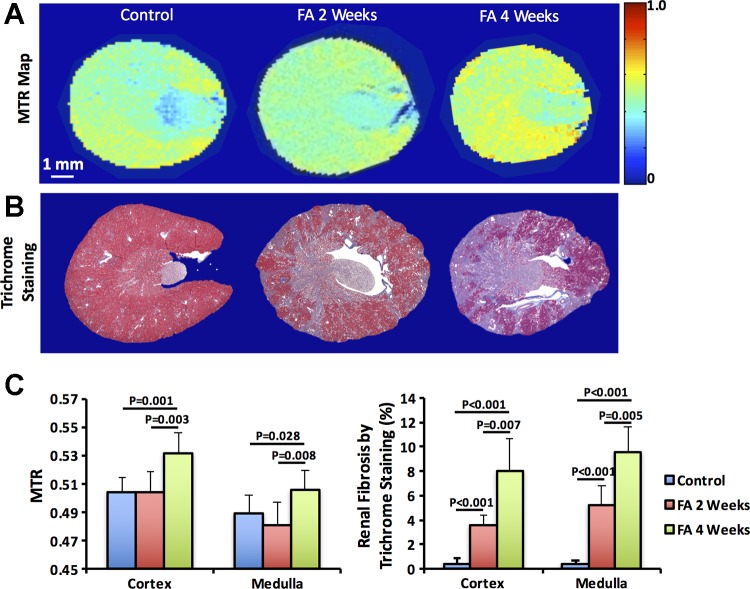
Representative MTR maps and trichrome-stained kidney sections are shown in Fig. 5, A and B, respectively. FA treatment induced patchy necrosis and a typical pattern of striated mild interstitial fibrosis at 2 wk, which exacerbated at 4 wk (Fig. 5B). The measured MTR and histological renal fibrosis are shown in Fig. 5C. Compared with control kidneys (cortex, 0.39 ± 0.45% and medulla, 0.40 ± 0.22%), histology showed significant renal fibrosis in FA mice at 2 wk (cortex, 3.54 ± 0.85%, P < 0.001 and medulla, 5.20 ± 1.57%, P < 0.001), which increased further by 4 wk (cortex, 8.03 ± 2.62%, P = 0.007 and medulla, 9.60 ± 2.04%, P = 0.005). In contrast, the measured MTR showed no change at 2 wk, yet it increased significantly in both the renal cortex (0.53 ± 0.01 vs. 0.50 ± 0.01, P = 0.001) and medulla (0.51 ± 0.01 vs. 0.49 ± 0.01, P = 0.028) at 4 wk.
Fig. 5.
Renal fibrosis by in vivo magnetization transfer imaging and ex vivo trichrome staining. Representative magnetization transfer ratio (MTR) maps (A) and trichrome-stained tissue sections (B) of control (n = 5) and folic acid (FA) mice at 2 (n = 10) and 4 (n = 5) weeks after treatment. Quantified cortical and medullary MTR and renal fibrosis (C). FA treatment induced patchy necrosis and mild interstitial fibrosis at 2 wk, which exacerbated at 4 wk. The measured MTR showed no change at 2 wk but increased significantly in both the renal cortex and medulla at 4 wk. Statistical comparison was performed using one-way analysis of variance followed by Tukey’s post hoc analysis.
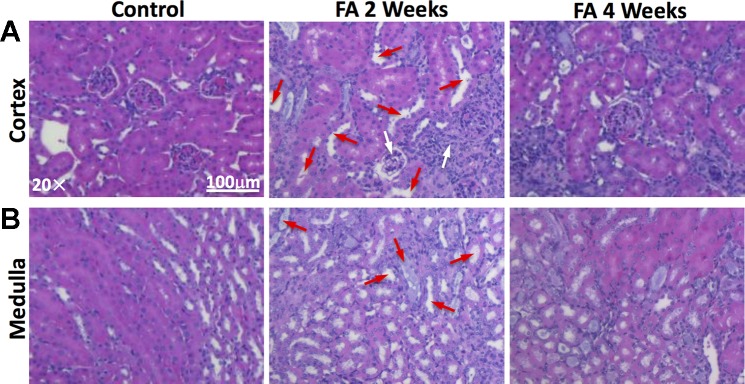
Representative hematoxylin-eosin-stained cortical and medullary sections are shown in Fig. 6. Significant tubular dilation, as marked by red arrows, occurred at 2 wk after FA treatment, possibly because of the obstruction caused by crystalized FA. Inflammatory infiltration, glomerular atrophy (marked by white arrows), and tubular necrosis were also observed. At 4 wk, FA-induced tissue injuries included primarily tubulointerstitial atrophy and fibrosis.
Fig. 6.
Folic acid (FA)-induced renal injuries by ex vivo hematoxylin-eosin (H&E) staining. Representative H&E-stained cortical (A) and medullary (B) tissue sections of control and FA mice at 2 and 4 wk after treatment. At 2 wk, significant tubular dilation (marked by red arrows), as well as inflammatory infiltration, glomerular atrophy (marked by white arrows), and tubular necrosis, were observed. At 4 wk, FA-induced tissue injuries included primarily tubulointerstitial atrophy and fibrosis.
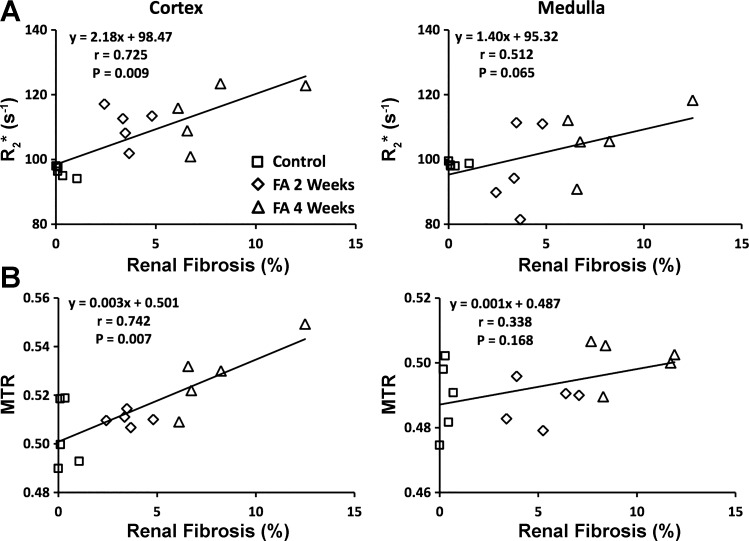
Correlation among renal indices.
The pair-wise Pearson correlation coefficients between kidney volume, nGFR, and other cortical or medullary parameters are summarized in Tables 2 and 3. Renal R2* and MTR showed a strong association with renal fibrosis in the cortex (P = 0.009 and 0.007, respectively) but not in the medulla (Fig. 7, A and B). The nGFR showed a significant correlation with renal perfusion in both the cortex (P < 0.001) and medulla (P < 0.001), which also correlated significantly and inversely with their respective T1 values (cortex, P < 0.001 and medulla, P < 0.001). Similarly, nGFR showed a strong inverse correlation with renal T1 in both the cortex (P < 0.001) and medulla (P < 0.001).
Table 2.
Pair-wise Pearson correlation coefficients between kidney volume, nGFR, and cortical parameters
| Volume | R2* | Perfusion | nGFR | T1 | MTR | Fibrosis | |
|---|---|---|---|---|---|---|---|
| Volume | 1 | −0.448 | 0.135 | 0.390 | −0.441 | −0.487* | −0.467 |
| R2* | 1 | −0.342 | −0.450* | 0.486* | 0.581* | 0.725* | |
| Perfusion | 1 | 0.830* | −0.757* | 0.125 | −0.351 | ||
| nGFR | 1 | −0.843* | −0.069 | −0.405 | |||
| T1 | 1 | 0.096 | 0.421 | ||||
| MTR | 1 | 0.742* | |||||
| Fibrosis | 1 |
P < 0.05.
MTR, magnetization transfer ratio; nGFR, normalized glomerular filtration rate.
Table 3.
Pair-wise Pearson correlation coefficients between kidney volume, nGFR and medullary parameters
| Volume | R2* | Perfusion | nGFR | T1 | MTR | Fibrosis | |
|---|---|---|---|---|---|---|---|
| Volume | 1 | −0.416 | 0.042 | 0.390 | −0.414 | −0.347 | −0.508 |
| R2* | 1 | −0.275 | −0.348 | 0.498* | 0.378 | 0.512 | |
| Perfusion | 1 | 0.766* | −0.716* | 0.176 | −0.464 | ||
| nGFR | 1 | −0.833* | 0.044 | −0.553* | |||
| T1 | 1 | 0.159 | 0.469 | ||||
| MTR | 1 | 0.338 | |||||
| Fibrosis | 1 |
P < 0.05.
nGFR, normalized glomerular filtration rate; MTR, magnetization transfer ratio.
Fig. 7.
Correlations between ex vivo renal fibrosis and in vivo MRI parameters. Renal fibrosis by ex vivo trichrome staining showed significant correlations with in vivo R2* (A) and magnetization transfer ratio (MTR) (B) in the cortex but not medulla. FA, folic acid.
DISCUSSION
In this study, multiparametric MRI was used to longitudinally evaluate renal structural and functional changes in mice 2 and 4 wk after FA-induced AKI. While the kidney size, oxygenation, and fibrosis showed progressive deterioration, a transient impairment in renal perfusion, nGFR, and fluid content (T1) slightly improved by 4 wk, indicating partial functional recovery after the acute injury. We also observed direct correlations between renal fibrosis with cortical and medullary hypoxia and with cortical MTR and inverse correlations of renal perfusion and nGFR with T1. These observations suggest that multiparametric MRI allows for monitoring of the adaptive process within the kidney following kidney injury.
The FA-induced AKI animal model mimics important aspects of human AKI, including tubular epithelial cell injury, apoptosis, and inflammatory cell infiltration (25). Previous studies utilizing this model for investigation of AKI pathophysiology and treatment strategies evaluated renal function mostly by serum creatinine and BUN, which reflect limited aspects of the complex adaptive changes in single-kidney structure and function following acute insults. In comparison, MRI offers the opportunity for a noninvasive and comprehensive assessment of renal structure and function in vivo, which may provide greater insight into kidney injury mechanisms. Therefore, we used multiparametric MRI to longitudinally evaluate FA-induced kidney insults, including atrophy, hypoxia, increased fluid content, impaired hemodynamics, and fibrosis.
A cascade of renal injuries was observed at 2 wk after FA-induced AKI. Despite the unaltered kidney size, patchy tissue hypoxia, detected as R2* using BOLD-MRI, was observed in the renal cortex. This is in line with the previously reported in situ cortical hypoxia found ex vivo in regenerating tubules of murine kidneys 2 wk after FA treatment (27), confirming the usefulness of BOLD-MRI in noninvasive measurement of renal hypoxia. Surprisingly, no medullary hypoxia was detected at 2 wk. This might have been masked by the increased fluid content because of tubular dilation, as indicated by the elevated T1 (3). A similar increase in kidney water content has been reported in FA-induced nephropathy (21) as well as other kidney diseases, including renal artery stenosis (11, 14) and ischemia-reperfusion injury (10, 24). The increased fluid content in the kidney might also have hampered the ability of MTI in detecting mild renal fibrosis, especially in the medulla. Yet, the pathological changes in the kidney overall compromised renal perfusion and filtration function remarkably.
By 4 wk, renal function partly recovered, despite deterioration in renal structure. The 3D volumetric MRI revealed a significant decrease in kidney volume at 4 wk, indicating renal atrophy and remodeling in the chronic phase of FA-induced renal damage. Nevertheless, tubular dilation tended to regress, which might be partly associated with the detection of hypoxia in the renal medulla. The negative correlation between renal hemodynamics and T1 suggests that the recovery in renal perfusion and filtration at 4 wk may also be associated with resolution of the acute injury phase. However, this resolution was accompanied by progressive fibrosis and tissue atrophy, which may represent chronic kidney remodeling. These findings also highlight the recovery capacity of renal function. Despite the loss of functioning tissue, renal perfusion and normalized GFR partly recovered by 4 wk, possibly by increasing blood flow and filtration in relatively preserved areas. As a result, the detection of residual renal damage after acute insults may be difficult to appreciate by assessment of global filtration function alone.
Given the resolved fluid accumulation and elevated renal fibrosis, MTR in both the renal cortex and medulla showed measurable increases at 4 wk. Overall, the correlation between MTR and renal fibrosis by histology was found significant in the cortex but not in the medulla. These findings underscore the limits of MTR as an in vivo measure of renal fibrosis, for which a concurrent measure of tissue T1 and perfusion may be useful to evaluate tissue fluid content.
There are limitations in our study. First, control mice underwent MRI only at 2 but not 4 wk after treatment. However, we have previously found stable renal parameters, including volume, R2*, perfusion, and MTR, in normal mice from 2 to 4 wk (11). Second, because we focused on the development of fibrosis, FA-induced renal changes at time points earlier than 2 wk were not investigated. Gupta et al. (8) showed marked renal hypertrophy from 12 to 36 h after FA treatment, which may have reflected tubular swelling. An investigation of the renal function at such earlier time points is warranted in future studies. Third, only male mice were used in this study. Future studies are needed to validate the findings in female mice. Furthermore, the causal relationship of residual renal damage with susceptibility to future second insults also needs to be established.
In conclusion, multiparametric MRI captured the longitudinal progression in kidney structure, hypoxia, fluid accumulation, and dysfunction in mice treated with FA. Whereas kidney structure and hypoxia showed progressive deterioration, renal perfusion and normalized GFR showed a mild recovery by 4 wk, accompanied by a slight regression of tubular dilation. Therefore, multiparametric MRI provides an important tool for noninvasive, integrated, and longitudinal assessment of FA-induced kidney injury and may be useful for evaluation of treatment strategies.
GRANTS
This study was partly supported by NIH Grants DK-104273 (to L. O. Lerman), DK-102325 (to L. O. Lerman), and HL-123160 (to L. O. Lerman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.J., H.T., S.I.M., and L.O.L. conceived and designed research; K.J. and P.K.M. performed experiments; K.J. and T.A.P. analyzed data; K.J. and L.O.L. interpreted results of experiments; K.J. and T.A.P. prepared figures; K.J. and L.O.L. drafted manuscripts; K.J., H.T., P.K.M., S.I.M., and L.O.L edited and revised manuscript; K.J., T.A.P., H.T., P.K.M., S.I.M., and L.O.L approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Mariam P. Alexander from the Department of Laboratory Medicine and Pathology and Aditya S. Pawar from the Division of Nephrology and Hypertension at Mayo Clinic (Rochester, MN) for comments and suggestions in renal histology.
REFERENCES
- 1.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 380: 756–766, 2012. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Cheng CW, Rifai A, Ka SM, Shui HA, Lin YF, Lee WH, Chen A. Calcium-binding proteins annexin A2 and S100A6 are sensors of tubular injury and recovery in acute renal failure. Kidney Int 68: 2694–2703, 2005. doi: 10.1111/j.1523-1755.2005.00740.x. [DOI] [PubMed] [Google Scholar]
- 3.de Miguel MH, Yeung HN, Goyal M, Noh JW, Aisen AM, Phan SH, Wiggins RC. Evaluation of quantitative magnetic resonance imaging as a noninvasive technique for measuring renal scarring in a rabbit model of antiglomerular basement membrane disease. J Am Soc Nephrol 4: 1861–1868, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Doi K, Okamoto K, Negishi K, Suzuki Y, Nakao A, Fujita T, Toda A, Yokomizo T, Kita Y, Kihara Y, Ishii S, Shimizu T, Noiri E. Attenuation of folic acid-induced renal inflammatory injury in platelet-activating factor receptor-deficient mice. Am J Pathol 168: 1413–1424, 2006. doi: 10.2353/ajpath.2006.050634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebrahimi B, Crane JA, Knudsen BE, Macura SI, Grande JP, Lerman LO. Evolution of cardiac and renal impairment detected by high-field cardiovascular magnetic resonance in mice with renal artery stenosis. J Cardiovasc Magn Reson 15: 98, 2013. doi: 10.1186/1532-429X-15-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang TC, Alison MR, Cook HT, Jeffery R, Wright NA, Poulsom R. Proliferation of bone marrow-derived cells contributes to regeneration after folic acid-induced acute tubular injury. J Am Soc Nephrol 16: 1723–1732, 2005. doi: 10.1681/ASN.2004121089. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Goodnough CL, Erokwu BO, Farr GW, Darrah R, Lu L, Dell KM, Yu X, Flask CA. Arterial spin labeling-fast imaging with steady-state free precession (ASL-FISP): a rapid and quantitative perfusion technique for high-field MRI. NMR Biomed 27: 996–1004, 2014. doi: 10.1002/nbm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Puri V, Sharma R, Puri S. Folic acid induces acute renal failure (ARF) by enhancing renal prooxidant state. Exp Toxicol Pathol 64: 225–232, 2012. doi: 10.1016/j.etp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 9.He S, Liu N, Bayliss G, Zhuang S. EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury. Am J Physiol Renal Physiol 304: F356–F366, 2013. doi: 10.1152/ajprenal.00553.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hueper K, Peperhove M, Rong S, Gerstenberg J, Mengel M, Meier M, Gutberlet M, Tewes S, Barrmeyer A, Chen R, Haller H, Wacker F, Hartung D, Gueler F. T1-mapping for assessment of ischemia-induced acute kidney injury and prediction of chronic kidney disease in mice. Eur Radiol 24: 2252–2260, 2014. doi: 10.1007/s00330-014-3250-6. [DOI] [PubMed] [Google Scholar]
- 11.Jiang K, Ferguson CM, Ebrahimi B, Tang H, Kline TL, Burningham TA, Mishra PK, Grande JP, Macura SI, Lerman LO. Noninvasive assessment of renal fibrosis with magnetization transfer MR imaging: validation and evaluation in murine renal artery stenosis. Radiology 283: 77–86, 2017. doi: 10.1148/radiol.2016160566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang K, Ferguson CM, Woollard JR, Zhu X, Lerman LO. Magnetization transfer magnetic resonance imaging noninvasively detects renal fibrosis in swine atherosclerotic renal artery stenosis at 3.0 T. Invest Radiol 52: 686–692, 2017. doi: 10.1097/RLI.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang K, Tang H, Mishra PK, Macura SI, Lerman LO. Measurement of murine single-kidney glomerular filtration rate using dynamic contrast-enhanced MRI. Magn Reson Med 79: 2935–2943, 2018. doi: 10.1002/mrm.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang K, Tang H, Mishra PK, Macura SI, Lerman LO. A rapid T1 mapping method for assessment of murine kidney viability using dynamic manganese-enhanced magnetic resonance imaging. Magn Reson Med 80: 190–199, 2018. doi: 10.1002/mrm.27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung JH, Choi JE, Song JH, Ahn SH. Human CD36 overexpression in renal tubules accelerates the progression of renal diseases in a mouse model of folic acid-induced acute kidney injury. Kidney Res Clin Pract 37: 30–40, 2018. doi: 10.23876/j.krcp.2018.37.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury: an increasing global concern. Lancet 382: 170–179, 2013. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, James MT. Acute Kidney Injury. Ann Intern Med 167: ITC66–ITC80, 2017. doi: 10.7326/AITC201711070. [DOI] [PubMed] [Google Scholar]
- 18.Li LP, Tan H, Thacker JM, Li W, Zhou Y, Kohn O, Sprague SM, Prasad PV. Evaluation of renal blood flow in chronic kidney disease using arterial spin labeling perfusion magnetic resonance imaging. Kidney Int Rep 2: 36–43, 2017. doi: 10.1016/j.ekir.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long DA, Woolf AS, Suda T, Yuan HT. Increased renal angiopoietin-1 expression in folic acid-induced nephrotoxicity in mice. J Am Soc Nephrol 12: 2721–2731, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Ortega A, Rámila D, Izquierdo A, González L, Barat A, Gazapo R, Bosch RJ, Esbrit P. Role of the renin-angiotensin system on the parathyroid hormone-related protein overexpression induced by nephrotoxic acute renal failure in the rat. J Am Soc Nephrol 16: 939–949, 2005. doi: 10.1681/ASN.2004040328. [DOI] [PubMed] [Google Scholar]
- 22.Prasad PV. Evaluation of intra-renal oxygenation by BOLD MRI. Nephron Clin Pract 103: c58–c65, 2006. doi: 10.1159/000090610. [DOI] [PubMed] [Google Scholar]
- 23.Street JM, Souza AC, Alvarez-Prats A, Horino T, Hu X, Yuen PS, Star RA. Automated quantification of renal fibrosis with Sirius Red and polarization contrast microscopy. Physiol Rep 2: e12088, 2014. doi: 10.14814/phy2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tewes S, Gueler F, Chen R, Gutberlet M, Jang MS, Meier M, Mengel M, Hartung D, Wacker F, Rong S, Hueper K. Functional MRI for characterization of renal perfusion impairment and edema formation due to acute kidney injury in different mouse strains. PLoS One 12: e0173248, 2017. doi: 10.1371/journal.pone.0173248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen X, Peng Z, Li Y, Wang H, Bishop JV, Chedwick LR, Singbartl K, Kellum JA. One dose of cyclosporine A is protective at initiation of folic acid-induced acute kidney injury in mice. Nephrol Dial Transplant 27: 3100–3109, 2012. doi: 10.1093/ndt/gfr766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang HC, Zuo Y, Fogo AB. Models of chronic kidney disease. Drug Discov Today Dis Models 7: 13–19, 2010. doi: 10.1016/j.ddmod.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol 163: 2289–2301, 2003. doi: 10.1016/S0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]