Abstract
While sodium-glucose cotransporter-2 (SGLT2) inhibitors have been used for the routine management of type 2 diabetes for several years, it is perhaps their natriuretic effects that are most important clinically. This natriuresis activates tubuloglomerular feedback, resulting in reduced glomerular hypertension and proteinuria, leading to renal protective effects in the EMPA-REG OUTCOME and CANVAS Program trials. In the cardiovascular system, it is likely that plasma volume contraction due to natriuresis in response to SGLT2 inhibition is at least in part responsible for the reduction in the risk of heart failure observed in these trials. We compare this mechanism of action with other antidiabetics. Importantly, other diuretic classes, including thiazide and loop diuretics, have not resulted in such robust clinical benefits in patients with type 2 diabetes, possibly because these older agents do not influence intraglomerular pressure directly. In contrast, SGLT2 inhibitors do have important physiological similarities with carbonic anhydrase inhibitors, which also act proximally, and have been shown to activate tubuloglomerular feedback.
Keywords: albuminuria, anhidrasa carbonic, diabetic kidney disease, natriuresis, sodium glucose type 2 inhibitor
INTRODUCTION
Diabetic kidney disease (DKD) is a common microvascular complication of diabetes mellitus (DM) and is an important, independent risk factor for the development of cardiovascular disease (14). Current clinical markers of DKD progression include worsening albuminuria and/or progressive glomerular filtration rate (GFR) decline (7). Approximately 20–30% of patients with DM (type 1 and 2) develop DKD (43), despite current therapeutic strategies (70) including glycemic, lipid, and blood pressure control and blockade of the renin-angiotensin-aldosterone system (RAAS) (31, 42, 70). As a consequence, DKD remains a leading cause of end-stage renal disease (ESRD) and renal replacement therapy (RRT) in developed countries (7, 36). The identification of novel therapeutic strategies that reduce the risk of DKD initiation and progression is therefore of a research priority.
Beyond older approaches outlined above, more recent mechanistic studies and clinical trial data have highlighted the role for novel antihyperglycemic agents to reduce the risk of DKD and cardiovascular complications (48, 79). Importantly, even though these agents, including sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP1-RAs), are approved as glucose-lowering therapies, the mechanisms associated with decreased cardiorenal risk are likely related to natriuretic rather than glucosuric effects. Accordingly, in this review, we focus on the potential role of natriuresis in the pathophysiology of DKD and the relationship between natriuresis and renal protection with newer antihyperglycemic agents.
THE PATHOGENESIS OF DKD
The pathogenesis of DKD is complex, multifactorial, and still incompletely understood (4, 14, 36). DKD is traditionally thought to predominantly impact the glomerulus (75), through a variety of mechanisms including intraglomerular hypertension, as described below, which promotes glomerulosclerosis and inflammation by increasing glomerular wall tension and shear stress, especially in the context of systemic hypertension. The diabetic milieu is, however, also associated with tubular injury and inflammation. Consequently, the concept of “diabetic tubulopathy” has emerged as a significant manifestation of DKD (86). Proteinuria that is typical of DKD can therefore either be due to damage to the glomerular barrier and its subsequent albuminuria and/or loss of the capacity of tubular protein absorption on the basis of tubular injury. Furthermore, the increase in glomerular protein filtration may cause direct tubular toxicity, resulting in tubular proteinuria and perpetuation of the injury process, although this remains controversial (86).
Activation of inflammatory pathways in the setting of diabetes is likely to be primarily related to ambient hyperglycemia, although other factors such as glomerular hemodynamic dysfunction and albuminuria may be involved. This inflammatory activation is achieved by several mechanisms: accumulation of advanced glycation end products and activation of oxidative stress, nuclear transcription factor-κB (NF-κB), protein kinase C-β, and other putative injury pathways (14). The final common result is extracellular matrix and mesangial expansion, thickening of the glomerular basement membrane and tubulointerstitial injury.
SODIUM AND DKD
From a renal mechanistic perspective, sodium (Na+) plays an important role in the pathogenesis of DKD for two main reasons. First, increased dietary Na+ intake and volume expansion are risk factors for hypertension, which promotes the initiation and progression of DKD (1). Second, the proximal renal tubular handling of Na+ likely plays an important role in the pathogenesis of renal hyperfiltration, which is generally defined as either a GFR ≥2 SD above the mean or GFR of ≥135 ml·min−1·1.73 m−2 (50). Renal hyperfiltration is thought to reflect underlying glomerular hypertension, which contributes to the initiation and progression of clinical DKD, including elevated albumin excretion and progressive renal function decline (71).
There are several theories that explain the onset of renal hyperfiltration in DM, which have been reviewed in detail elsewhere (61, 71). One of these theories, the tubular theory, suggests that increased Na+ reabsorption in DM at the proximal tubule via the SGLT1 and SGLT2 in the setting of hyperglycemia (21) leads to reduced Na+ delivery at the macula densa with consequent tubuloglomerular feedback (TGF) inhibition (8, 61). TGF suppression leads to afferent arteriolar vasodilation, increased glomerular pressure and renal hyperfiltration (71). The other major renal mechanism that contributes to hyperfiltration is the vascular theory, whereby RAAS activation in DM results in efferent arteriolar vasoconstriction via angiotensin II activity at the angiotensin II type 1 receptor, leading to glomerular hypertension and hyperfiltration (26, 35).
EFFECT OF THE TRADITIONAL TREATMENTS IN DKD
To date, the treatments to reduce the risk of DKD progression are inadequate and include glycemic and lipid control, management of hypertension, and reducing dietary salt intake (47). RAAS inhibitors are also used to reduce the risk of DKD progression (36, 68, 84, 85). For the impact of intensive glycemic control in the setting of type 2 DM, in the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR controlled evaluation (ADVANCE) study, this strategy decreased the onset and worsening nephropathy by 33% (P = 0.005) and the risk of new onset of microalbuminuria by 25% (P < 0.001) (89). Moreover, patients who received intensive glucose lowering in ADVANCE were less likely to develop ESRD than patients who received standard care [hazard ratio (HR): 0.35; P = 0.02] (89), and this benefit persisted in the follow up trial, ADVANCE-ON (HR,: 0.54; P < 0.01) (83). Likewise, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (24), the risk of albuminuria was clearly reduced at the end of the study in the intensive glycemic control group (HR: 0.72; P < 0.0001) (24).
In smaller, short-term trials with antihyperglycemic agents, the thiazolidinedione rosiglitazone reduced albuminuria in patients with type 2 DM (36), and similar effects have been shown with pioglitazone (36). In the case of dipeptidyl peptidase-4 (DPP4) inhibitors, although most of the available studies do not show significant renoprotective effects for this class of drugs (77), definitive conclusions cannot be drawn until the results of large cardiovascular trials (CVOT) in patients with DKD are complete. For example, the Multicenter, International, Randomized, Parallel Group Double-blind, Placebo-controlled, Cardiovascular Safety and Renal Microvascular Outcome Study with Linagliptin (CARMELINA study; NCT01897532) (60) should be able to further clarify the renoprotective effect of linagliptin, and this issue will be further explored in the Cardiovascular Outcome Study of Linagliptin vs. Glimepiride in Patients cith Type 2 Diabetes (CAROLINA trial; NCT01243424), which is using an active sulfonylurea comparator (41).
Until recently, trials with antihyperglycemic agents have shown neutral cardiovascular effects and positive effects in the kidney but mainly on surrogate markers such as albuminuria. This paradigm has changed in the last 3 yr, due to results from GLP1-RA trials such as Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results–A Long-Term Evaluation (LEADER) (39) and Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) (38), as well as positive SGLT2 inhibitor cardiovascular outcomes such as Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) (88) and the CANVAS Program (48). For example, in the EMPA-REG OUTCOME and CANVAS Program trials, empagliflozin and canagliflozin, respectively, reduced the risk of the primary three-point major adverse cardiac event end point and also decreased the risk of reaching secondary, composite renal end points (48, 79). Furthermore, both agents reduced the risk of hospitalization for heart failure by >30% (13). These important clinical effects, especially related to DKD and heart failure risk have led to suggestions (22) that SGLT2 inhibitors act primarily as natriuretic agents. See Tables 1, 2, and 3 to see the summary of the results of renal outcomes in trials with antihyperglycemic agents.
Table 1.
Renal outcomes with SGLT2 inhibitors
| Drug | Trial | Number of Patients | Median Follow-Up Time, yr | Renal Outcome | Comment |
|---|---|---|---|---|---|
| Empagliflozin | EMPA-REG OUTCOME | 7,020 | 3.1 | 44% RR reduction of doubling of SCr (1.5 vs. 2.6%); P < 0.001 | |
| 38% RR reduction of progression to MA (11.2 vs. 16.2%); P < 0.001 | |||||
| 55% RR reduction of initiation of RRT (0.3 vs. 0.6%); P = 0.04 | |||||
| Slowing GFR decline (annual decrease 0.19 ± 0.11 vs. 1.67 ± 0.13 ml·min−1·1.73 m−2; P < 0.001) | |||||
| Canagliflozin | CANVAS PROGRAM | 10,142 | 4 | Mean eGFR (ml·min−1·1.73 m−2) difference from placebo (2.0 ml/min; 95% CI: 1.5–2.6) | |
| Mean ratio of UACR compared with placebo, eGFR categories (ml·min−1·1.73 m−2) (P heterogeneity = 0.01) | |||||
| eGFR >90: −17% | |||||
| eGFR 60–90: −17% | |||||
| eGFR 45–60: −26% | |||||
| eGFR <45: −13% | |||||
| Doubling of Scr, the need for RRT, or death from renal causes. (HR: 0.53; 95% CI: 0.33–0.84 for all subgroups eGFR; P heterogeneity = 0.21 and >0.50, respectively) | |||||
| CREDENCE (NCT02065791) | 4,461 | 5.5 | NA | Expected completion date: June of 2019 | |
| Dapagliflozin | DECLARE (NCT01730534)DAPA-CKD (NCT03036150) | 17,1504,000 | 4–54 | NANA | Expected completion date: April 2019 |
| Expected completion date: November of 2020 | |||||
| Ertugliflozin | VERTIS (NCT01986881) | 8,000 | 5–7 | NA | Expected completion date: June 2020 |
Studies are as follows: CANVAS PROGRAM (48): CANagliflozin cardio Vascular Assessment Study; Cardiovascular and Renal Outcomes with Canagliflozin According to Baseline Kidney Function: Data from the CANVAS Program (49); Canagliflozin and Renal Outcomes In Type 2 Diabetes: Results From The CANVAS Program Randomized Clinical Trials (53); CREDENCE (25): Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (NCT02065791); DAPA-CKD: a Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with CKD (NCT03036150); DECLARE-TIMI 58: Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (NCT01730534); EMPA-REG OUTCOME (88): Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes (79); VERTIS: Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study To Assess Cardiovascular Outcomes Following Treatment with Ertugliflozin (MK-8835/PF-04971729) in Subjects with Type 2 Diabetes Mellitus and Established Vascular Disease (NCT01986881). SGLT1, sodium glucose cotransporter 2; UACR, urinary albumin-to-creatinine ratio; RRT, renal replacement therapy; SCr, serum creatinine; MA, macroalbuminuria; HR, hazard ratio; RR, relative risk; eGFR, estimated glomerular filtration rate; CI, confidence interval; NA, not available.
Table 2.
Renal Outcomes with DPP4 inhibitors
| Drug | Trial | Number of Patients | Median Follow-Up Time, yr | Renal Outcome Change from Baseline Placebo/Intervention | Comment |
|---|---|---|---|---|---|
| Saxagliptin | SAVOR-TIMI 53 | 16,492 | 2.1 | Change in UACR (mg/g) Sstudy end: −34.3. P < 0.004 | |
| Change in UACR category Sstudy end: lowered by saxagliptin, eGFR (ml·min−1·1.73 m−2) | |||||
| eGFR >50 (n = 10,621). | |||||
| IMPROVED (10.4% SAXA vs. 8.5% PBO). WORSENED (12.7% SAXA vs. 15.1% PBO). P < 0.0001 | |||||
| eGFR ≤50 (n = 1739). | |||||
| IMPROVED (12.5% SAXA vs. 9.8% PBO). WORSENED (50.9% SAXA vs. 49.1% PBO). P = 0.041 | |||||
| Doubling of Scr HR 1.1 (95% CI: 0.89–1.36) | |||||
| Initiation of RRT HR 0.90 (95% CI: 0.61–1.32) | |||||
| Alogliptin | EXAMINE | 5,380 | 1.6 | Change in eGFR (ml·min−1·1.73 m−2), Δ from baseline: | |
| eGFR >90: −4.5 PBO/−6.7 ALOG | |||||
| eGFR 60–90: 1.0 PBO/0.6 ALOG | |||||
| eGFR 30–60: 2.1 PBO /1.1 ALOG | |||||
| eGFR <30: 1.6 PBO/0.2 ALOG | |||||
| Initiation of RRT n (%): 22(0.8) PBO vs. 24(0.9) ALOG; P = 0.88 | |||||
| Sitagliptin | TECOS | 14,671 | 4 | Mean difference between-group treatment difference in eGFR (ml·min−1·1.73 m−2) (95% CI) year 4: −1.34 (−1.76 to −0.91), P < 0.001 | |
| Mean difference in UACR (mg/g) (95% CI) year 4: −0.18 (−0.35 to −0.02), P = 0.031 | |||||
| Linagliptin | CARMELINA (NCT01897532) | 8,300 | 4 | NA | Expected completion date: January 2018 |
| Linagliptin (vs. glimepiride) | CAROLINA (NCT01243424) | 6,000 | 7.6 | NA | Expected completion date: September 2018 |
Data are modified from Muskiet et al. (46). Studies were as follows: CARMELINA (60): Cardiovascular and Renal Microvascular Outcome Study with Linagliptin in Patients with Type 2 Diabetes Mellitus (NCT01897532); CAROLINA (41): Cardiovascular Outcome Study of Linagliptin vs. Glimepiride in Patients with Type 2 Diabetes (NCT01243424); EXAMINE (80): Examination of Cardiovascular Outcomes with Alogliptin vs. Standard of Care; SAVOR-TIMI 53 (63): the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI) 53 Trial; Effect of Saxagliptin on Renal Outcomes in the SAVOR-TIMI 53 Trial (45); TECOS (18): Trial Evaluating Cardiovascular Outcomes with Sitagliptin; Effect of Sitagliptin on Kidney Function and Respective Cardiovascular Outcomes in Type 2 Diabetes: Outcomes From TECOS (10). DPP4, dipeptidyl peptidase-4; UACR, urinary albumin-to-creatinine ratio; RRT, renal replacement therapy; SCr, serum creatinine; MA, macroalbuminuria; HR, hazard ratio; NA, not available; PBO, placebo; SAXA, saxagliptin; ALOG, alogliptin.
Table 3.
Renal outcomes wit GLP1-RA
| Drug | Trial | Number of Patients | Median Follow-Up Time, yr | Renal Outcome (Change from Baseline Placebo/Intervention) | Comment |
| Lixisenatide | ELIXA | 6,068 | 2.1 | Change in UACR (%) month 24: 34/24; P = 0.004 | |
| Liraglutide | LEADER | 9,340 | 3.8 | Time to new onset of persistent MA; HR: 0.74 (0.60–0.91); P = 0.03 | |
| Persistent doubling of SCr; HR: 89 (0.67–1.19); P = 0.43 | |||||
| Need for continuous RRT; HR: 0.87 (0.61–1.24); P = 0.44 | |||||
| Death due to renal disease; HR: 1.59 (0.52–4.87); P = 0.41 | |||||
| Semaglutide | SUSTAIN 6 | 3,297 | 3 | New or worsening nephropathy; HR: 0.64 (0.46–0.88); P = 0.005 | |
| Persistent MA; HR: 0.54 (0.37–0.77) P = 0.001 | |||||
| Persistent doubling of Scr; HR: 1.28 (0.64–2.58) P = 0.48 | |||||
| Need for continuous RRT; HR: 0.91 (0.40–2.07) P = 0.83 | |||||
| Exenatide | EXSCEL | 14,000 | 3 | NA | |
| Dulaglutide | REWIND (NCT01394952) | 9,622 | 6.5 | NA | Expected completion date: April 2019 |
| Albiglutide | HARMONY Outcomes (NCT02465515) | 9,400 | 3–5 | NA | Expected completion date: May 2019 |
Data are modified from Muskiet et al. (46). Studies are as follows: ELIXA (56): Evaluation of Cardiovascular Outcomes in Patients with Type 2 Diabetes After Acute Coronary Syndrome during Treatment with AVE0010 (Lixisenatide); EXSCEL (23): Exenatide Study of Cardiovascular Event Lowering Trial; HARMONY Outcomes (NCT02465515): Effect of Albiglutide, When Added to Standard Blood Glucose Lowering Therapies, on Major Cardiovascular Events in Subjects with Type 2 Diabetes Mellitus; LEADER (39): Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results–A Long-Term Evaluation; Liraglutide and Renal Outcomes in Type 2 Diabetes (37); REWIND (NCT01394952): Researching Cardiovascular Events with a Weekly Incretin in Diabetes; SUSTAIN 6 (38): Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes. GLP1-RA, glucagon-like peptide-1 receptor agonist; UACR, urinary albumin-to-creatinine ratio; RRT, renal replacement therapy; SCr, serum creatinine; MA, macroalbuminuria; HR, hazard ratio; NA, not available.
Beyond improving glycemic control, SGLT2 inhibitors and GLP1-RA agents have the capacity to reduce blood pressure and albuminuria, which may contribute to nephroprotective effects (38, 39, 56). In the case of SGLT2 inhibitors, these agents reduce renal hyperfiltration through natriuresis, independent of glycemic control mechanisms. Empagliflozin and canagliflozin have demonstrated nephroprotective effects on the basis of a decrease in the slope of GFR decline and albuminuria progression (48, 88).
EFFECT OF THE NEW ANTIHYPERGLYCEMIC DRUGS IN DKD
SGLT2 Inhibitors
SGLT2 inhibitors are approved as antihyperglycemic agents in countries around the world and also reduce blood pressure and weight (21). From a renal perspective, beyond empirical evidence from CVOTs, SGLT2 inhibitors exert several salutary physiological effects, including induction of a natriuresis, reduction of hyperfiltration, and slowing of the progression of DKD (i.e., renal function loss and elevated albumin excretion) in animals and humans (21). In normal physiology, SGLT2 is responsible for the reabsorption of 5% of the total tubular Na+. During ambient hyperglycemia, the reabsorption capacity of tubular Na+ increases up to 14% due to the increased mRNA expression and/or protein activity of SGLT1 and SGLT2, leading to a decrease in Na+ delivery to the macula densa (21). This natriuresis also reduces tissue Na+ content, which may be important during states of volume expansion such as heart failure (81). For example, dapagliflozin treatment for 6 wk reduces the concentration of tissue Na+ measured by magnetic resonance imaging, which suggests that SGLT2 inhibitors decrease both the plasma volume and the amount of Na+ in the interstitial fluid compartment in humans (62).
Kimura (27) also suggested that SGLT2 inhibitors exert natriuretic effects that are comparable to loop diuretics, which act by inhibiting Cl− and Na+ reabsorption at the loop of Henle by blocking the Na+/K+/2Cl− cotransporter, thereby increasing distal electrolyte delivery. Similar to loop diuretics, SGLT2 inhibitors increase transit of Na+ and Cl− through the tubular system, which augments delivery of Na+ and Cl− to the macula densa distally. Increased distal Na+ and Cl− delivery with loop diuretics does not, however, activate TGF, as discussed below. In contrast, SGLT2 inhibitors also increase distal delivery of Na+ and Cl− to the macula densa, resulting in increased Na+ and Cl− reabsorption at this location and adenosine triphosphate catabolism to adenosine, which acts as a vasoconstrictor at the afferent arteriole via the adenosine 1 receptor (21, 59). SGLT2 inhibitor-mediated TGF promotes afferent vasoconstriction, reduces glomerular pressure, and causes a 30–50% decrease in albuminuria (21, 59). The reduction in glomerular hypertension is also postulated to account for the characteristic acute and reversible “dip” in GFR in response to SGLT2 inhibitors (3, 5, 21, 79).
Loop diuretics, which also cause an increase in distal delivery of Na+ to the macula densa, do not impact TGF pathways that are activated with SGLT2 inhibitors (44). Reabsorption of Na+ and Cl− at the macula densa depends on the Na+/K+/2Cl− cotransporter, which is inhibited by loop diuretics. Since loop diuretics block the same cotransporter at the loop of Henle leading to natriuresis and plasma volume contraction, TGF is unaffected in humans, likely as a consequence of the inability of Na+/Cl− to enter macula densa cells and trigger TGF (19, 51, 87). Importantly, other diuretic classes such as thiazides that act at the distal convoluted tubule, as well as agents acting at the cortical collecting duct (e.g., amiloride and mineralocorticoid antagonists), act too distally to impact TGF (22, 40, 76, 78, 87). The mechanism of action of SGLT2 inhibitors more closely resembles that of diuretics acting at the proximal tubule, such as acetazolamide (32).
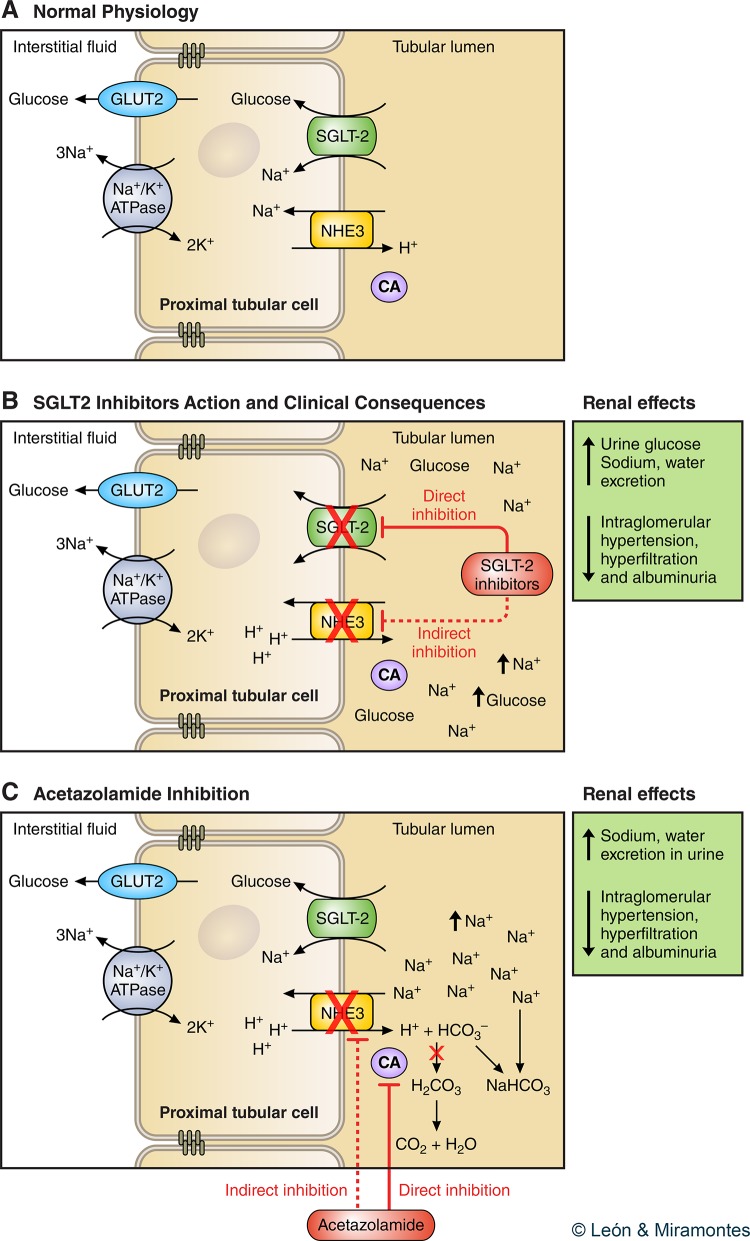
What is the mechanism by which SGLT2 inhibitors have clinical benefits? Approximately 30% of filtered Na+ is reabsorbed at the proximal convoluted tubule by NHE3, which is located in the apical membrane of the tubular epithelium (28). NHE3 is directly involved in Na+ reabsorption and indirectly in bicarbonate ions () reabsorption, sharing the same domain as SGLT2 in the epithelial membrane of the proximal convoluted tubule (Fig. 1A) (16, 55). It has previously been shown in animals that phlorizin and dapagliflozin reduce NHE3 activity, thereby limiting sodium bicarbonate (NaHCO3) reabsorption with resultant natriuresis. This action is likely independent of effects on glucose reabsorption (55). Empagliflozin also reduces the activity of NHE3 by means of phosphorylation (15, 28). Thus SGLT2 inhibitor-related natriuresis would result from the sum of inhibition of Na+ reabsorption by SGLT2 and NHE3 (Fig. 1B). Carbonic anhydrase is an enzyme expressed in the luminal membrane of the proximal convoluted tubules that leads to the formation of carbonic acid (H2CO3) and subsequent conversion to H2O and CO2. Carbonic anhydrase activates NHE3 and thereby favors tubular excretion of H+ and reabsorption of Na+ (28).
Fig. 1.
A: glucose reabsorption in the renal proximal tubule. Normal physiology. The basolateral sodium potassium adenosine triphosphate active transporter (Na+-K+ ATPase) pumps Na+ out and K+ into the cell to establish an inward Na+ gradient. This gradient is used for Na+ and glucose cotransport across the luminal brush border of the early proximal tubule through sodium glucose cotransporter 2 (SGLT2), and the glucose is passively returned via facilitative glucose transporter (GLUT2) to the interstitium/bloodstream. Na is reabsorbed via Na/H exchanger (NHE3). B: the SGLT2 inhibitor blocks Na/glucose reabsorption via SGLT2 and potentially inhibits NHE3. C: action of acetazolamide on carbonic anhydrase (CA) with its effect similar to that of SGLT2 inhibition. CA is an enzyme expressed in the luminal membrane of the proximal tubular cells, leading to the formation of carbonic acid (H2CO3) and subsequent conversion into H2O and CO2. CA activates NHE3 favoring mobilization of its substrates, allowing tubular excretion of H+ and reabsorption of Na+. Inhibition of this enzyme involves retention of bicarbonate (NaHCO3) in the renal tubule, which further increases diuresis.
Acetazolamide is a diuretic that acts at the proximal convoluted tubule by inhibiting carbonic anhydrase, thereby suppressing NHE3 activity. The inhibition of carbonic anhydrase causes H+ to accumulate in the epithelial cell, thereby decreasing tubular Na+ exchange, which results in natriuresis (Fig. 1C). In clinical practice, however, acetazolamide use is limited to specific scenarios such as altitude sickness (12). In animals, acetazolamide-induced increases in distal tubular Na+ and Cl− are associated with activation of TGF and expected renal hemodynamic effects including afferent arteriolar vasoconstriction and GFR decline, intrarenal hemodynamic changes that are not observed with furosemide administration (54, 73). In fact, in humans without diabetes, acetazolamide reduces GFR by 10–20% and also reduces renal hyperfiltration in obese nondiabetic individuals, intrarenal hemodynamic changes that are again not evident with equipotent doses of furosemide (57, 66, 87). Interestingly, in the context of acute altitude sickness, which can be associated with albuminuria, acetazolamide also reduces urine albumin excretion (6, 82). Perhaps most importantly in relation to DM, in patients with type 1 DM, acetazolamide increases proximal natriuresis assessed using renal lithium clearance techniques, while at the same time reducing GFR and albumin excretion (64, 65). SGLT2 inhibitors therefore appear to most closely resemble acetazolamide, rather than more distally acting traditional diuretic agents.
Other Beneficial Effects of SGLT2 Inhibitors in DKD
SGLT2 inhibition is associated with other beneficial renal effects beyond influences on glycemic control or low blood pressure, including suppression of fibrotic, inflammatory, and renal hypoxia pathways. Due to blockade of SGLT2 at the proximal tubule, glucose does not enter renal tubular cells thereby decreasing the generation of free radicals and inflammatory markers (NK-κB, interleukin 6, and monocyte protective protein-1) and fibrosis (fibronectin and transforming growth factor-β). The inflammatory and fibrotic effects at the renal level have been studied in experimental studies; for an example, studies using human proximal tubular cells (HK2) showed that SGLT2 inhibition decreased the production of inflammatory and fibrotic markers induced by high glucose (52). These in vitro findings suggest that SGLT2 inhibitors may provide renoprotection in diabetes by averting glucose from entering proximal tubule cells (74). In humans with type 2 DM, dapagliflozin also reduced levels of interleukin-6 and markers of proximal tubular cell injury (11).
In addition to reducing levels of inflammatory mediators, SGLT2 inhibition may prevent renal hypoxia states characteristic of DM that occur on the basis of augmented glucose cotransport leading to increased energy consumption (29). As a consequence of greater ATP-dependent solute reabsorption, more energy is consumed to reabsorb the increased load of filtered glucose, leading to reduced renal oxygen tension. Accordingly, inhibition of SGLT2 can reduce the state of renal hypoxia that is characteristic of DKD, thereby exerting a protective “β-blocker”-like effect in the kidney (17).
GLP1-RAs and DPP4 Inhibitors
Other antihyperglycemic agents, GLP1-RA and DPP4 inhibitors, are approved based on glucose-lowering activity but also have natriuretic properties. Similar to SGLT2 inhibitors, GLP1-RAs also exert weight loss and blood pressure lowering and albuminuria-lowering effects in addition to inducing natriuresis (70). Albumin excretion-related benefits have been reported in large cardiovascular safety studies with GLP1-RA such as Evaluation of Cardiovascular Outcomes in Patients with Type 2 Diabetes After Acute Coronary Syndrome during Treatment with AVE0010 (ELIXA) (56), LEADER (39), and SUSTAIN-6 (38). For example, in the ELIXA study (56) the median urinary albumin-to-creatinine ratio increased by 34% in the patients treated with placebo but only 24% in patients treated with lixisenatide (difference between treatments; P = 0.004). In the LEADER study using liraglutide, the onset of albuminuria was reduced by 26% [HR: 0.74 (0.60–0.91)] and the urinary albumin-to-creatinine ratio was reduced by 19% (confidence interval: 0.14–0.24%) (39). Finally, in SUSTAIN-6 (38), a reduction in the occurrence of albuminuria was observed in the group receiving semaglutide compared with placebo (semaglutide vs placebo, 2.5 vs. 4.9%). In cardiovascular safety trials, the DPP4 inhibitors such as saxagliptin and sitagliptin exert either neutral or modest reductions in albuminuria in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction 53 trial (SAVOR-TIMI 53) (63) and Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) (18) trials, respectively. In contrast with SGLT2 inhibitors and GLP1RAs, DPP4 inhibitors have not been shown to reduce blood pressure or body weight.
In comparison with TGF-related mechanisms leading to reductions in albumin excretion with SGLT2 inhibitors, the mechanisms whereby GLP-1-RA and DPP4 provide nephroprotection remain incompletely understood. GLP-1 promotes natriuresis through inhibition of NHE3 and stimulation of atrial natriuretic peptide, although this latter mechanism does not appear to be relevant in humans (34). The natriuresis should promote TGF-mediated afferent arteriolar vasoconstriction and decreased glomerular pressure and albuminuria (26). Despite the natriuresis, which is uniformly seen with GLP1-RAs in humans (2, 20, 34, 67, 72), and in contrast with SGLT2 inhibitors, GLP1-RA does not impact GFR, possibly due to direct endothelial vasorelaxation by GLP1 leading to overall neutral effect on afferent arteriolar tone and renal function (58).
Whereas SGLT2 inhibitors and GLP1RAs induce a proximal natriuresis, DPP4 inhibitor-associated natriuresis is independent of GLP1 and NHE3 in experimental models and instead depends on the activity of a chemokine called stromal cell-derived factor-1α (SDF-1α) (69). In the Akita mouse model, DPP4 inhibition induced natriuresis with linagliptin is dependent on intact SDF-1α activity and is abolished with pharmacological inhibition of SDF-1α with AMD3100 (69). In patients with type 2 diabetes, DPP4 inhibition with sitagliptin enhances natriuresis without impacting renal or systemic hemodynamic parameters (35) and also increased SDF-1α levels. In this cohort, total fractional Na+ excretion increased without changes in lithium-derived measures of proximal Na+ handling, strongly implicating distal tubular Na+ handling pathways. Therefore, while the specific tubular transport mechanisms are not yet defined, existing preclinical and clinical data suggest that pathways downstream from the macula densa are responsible for natriuresis with DPP4 inhibition (35). Since GLP1-RA and DPP4 inhibitors appear not to impact intrarenal hemodynamic function, albumin excretion-lowering effects are unlikely to be a consequence of changes in glomerular pressure and instead were due to suppression of anti-inflammatory pathways (30, 33, 35).
For the effects on neurohormones, and perhaps as expected based on the mild contraction of plasma volume, SGLT2 inhibitors agents are associated with modest increases in circulating and urinary levels of RAAS mediators in patients with diabetes (8, 9). This does not have any known clinical implications, since patients in the EMPA-REG OUTCOME and CANVAS Program trials benefitted from SGLT2i regardless of the use of background RAAS inhibitors therapies. Further work is, however, required to determine if there are synergistic effects in specific patient subgroups, such as in those with heart failure, in larger dedicated heart failure trials such as EMPEROR-preserved, EMPEROR-reduced, and DAPA-HF.
CONCLUSION
Experimental and human data suggest that proximal tubular Na+ avidity leads to maladaptive glomerular hypertension and hyperfiltration, thereby promoting DKD. Drugs that restore distal Na+ delivery to the macula densa, such as SGLT2 inhibitors and acetazolamide, attenuate glomerular hypertension and hyperfiltration and also reduce albumin excretion. Although other antihyperglycemic agents aside from SGLT2 inhibitors also promote natriuretic effects and reduce albumin excretion, these physiological effects are unlikely to be connected to their clinical benefits. Further investigation is required to determine how to optimally combine antihyperglycemic agents, such as SGLT2 inhibitors and GLP1RAs, that have complementary physiological effects around natriuresis, as well as weight loss, glucose, and blood pressure lowering, and cardiorenal protection.
GRANTS
D. Z. Cherney is supported by the Canadian Institutes of Health Research, as well as Diabetes Action Canada, a Strategy for Patient-Oriented Research initiative supported by the Canadian Institutes for Health Research. D. Z. Cherney also receives support from the Department of Medicine and Heart and Stroke Richard Lewar Centre of Excellence in Cardiovascular Research, University of Toronto and the Banting and Best Diabetes Centre, University of Toronto. D. Z. Cherney is the recipient of a University of Toronto, Department of Medicine Merit Award. P. Bjornstad receives salary and research support from National Institute of Diabetes and Digestive and Kidney Diseases Grants T32-DK-063687 and K23-DK-116720. P. Bjornstad is also a recipient of a Research Fellowship from Juvenile Diabetes Research Foundation and International Society of Pediatric and Adolescent Diabetes, and receives research support from Juvenile Diabetes Research Foundation, Thrasher Foundation, Childhood Diabetes Foundation, and Colorado Clinical and Translational Sciences Institute and Center for Women’s Health Research at University of Colorado.
DISCLOSURES
D. L. Jiménez has received speaking honoraria from Boehringer Ingelheim, Janssen, AstraZeneca, Sanofi, Novo Nordisk, Eli Lilly, and Esteve. J. P. González has received consulting fees or speaking honoraria from Boehringer Ingelheim, Janssen, AstraZeneca, Merck, Sanofi, and Novo Nordisk, Bayer. D. Z. Cherney receives operating funding from Boehringer Ingelheim, Janssen, AstraZeneca, and Merck. D. Z. Cherney has received consulting fees or speaking honoraria from Boehringer Ingelheim, Janssen, AstraZeneca, Merck, Mitsubishi-Tanabe, Sanofi, and Abbvie. P. Bjornstad has received travel support from Boehringer Ingelheim and speaking honoraria from Horizon Pharma.
AUTHOR CONTRIBUTIONS
D.L.J., D.Z.I.C., P.B., and J.P.M.G. conceived and designed research; D.L.J. and J.P.M.G. prepared figures; D.L.J., D.Z.I.C., P.B., L.C.G., and J.P.M.G. drafted manuscript; D.L.J., D.Z.I.C., P.B., L.C.G., and J.P.M.G. edited and revised manuscript; D.L.J., D.Z.I.C., P.B., L.C.G., and J.P.M.G. approved final version of manuscript.
REFERENCES
- 1.Allen TJ, Cooper ME, O’Brien RC, Bach LA, Jackson B, Jerums G. Glomerular filtration rate in streptozocin-induced diabetic rats. Role of exchangeable sodium, vasoactive hormones, and insulin therapy Diabetes 39: 1182–1190, 1990. doi: 10.2337/diab.39.10.1182. [DOI] [PubMed] [Google Scholar]
- 2.Barker P, Creasey PE, Dhatariya K, Levy N, Lipp A, Nathanson MH, Penfold N, Watson B, Woodcock T; Membership of the Working Party . Peri-operative management of the surgical patient with diabetes 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 70: 1427–1440, 2015. doi: 10.1111/anae.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC; EMPA-REG RENAL trial investigators . Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2: 369–384, 2014. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 4.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes 21: 279–286, 2014. doi: 10.1097/MED.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjornstad P, Laffel L, Tamborlane WV, Simons G, Hantel S, von Eynatten M, George J, Marquard J, Cherney DZ. Acute effect of empagliflozin on fractional excretion of sodium and eGFR in youth with type 2 diabetes. Diabetes Care 41: e129–e130, 2018. doi: 10.2337/dc18-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradwell AR, Delamere JP. The effect of acetazolamide on the proteinuria of altitude. Aviat Space Environ Med 53: 40–43, 1982. [PubMed] [Google Scholar]
- 7.Cherney D, Lund SS, Perkins BA, Groop P-H, Cooper ME, Kaspers S, Pfarr E, Woerle HJ, von Eynatten M. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 59: 1860–1870, 2016. doi: 10.1007/s00125-016-4008-2. [DOI] [PubMed] [Google Scholar]
- 8.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 9.Cherney DZ, Perkins BA, Soleymanlou N, Xiao F, Zimpelmann J, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M, Burns KD. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int 86: 1057–1058, 2014. doi: 10.1038/ki.2014.246. [DOI] [PubMed] [Google Scholar]
- 10.Cornel JH, Bakris GL, Stevens SR, Alvarsson M, Bax WA, Chuang L-M, Engel SS, Lopes RD, McGuire DK, Riefflin A, Rodbard HW, Sinay I, Tankova T, Wainstein J, Peterson ED, Holman RR; TECOS Study Group . Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care 39: 2304–2310, 2016. doi: 10.2337/dc16-1415. [DOI] [PubMed] [Google Scholar]
- 11.Dekkers CC, Petrykiv S, Laverman GD, Cherney DZ, Gansevoort RT, Heerspink HJ. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab 20: 1988–1993, 2018. doi: 10.1111/dom.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippi L, Bagnoli F, Margollicci M, Zammarchi E, Tronchin M, Rubaltelli FF. Pathogenic mechanism, prophylaxis, and therapy of symptomatic acidosis induced by acetazolamide. J Investig Med 50: 125–132, 2002. doi: 10.2310/6650.2002.31297. [DOI] [PubMed] [Google Scholar]
- 13.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME® trial investigators . Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J 37: 1526–1534, 2016. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 93: 137–188, 2013. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y, Gerasimova M, Mayoux E, Masuda T, Vallon V. SGLT2 inhibitor empagliflozin increases renal NHE3 phosphorylation in diabetic akita mice: possible implications for the prevention of glomerular hyperfiltration. Diabetologia 57: 1–564, 2014. [Google Scholar]
- 16.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 12: 78–89, 2015. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert RE. SGLT2 inhibitors: β blockers for the kidney? Lancet Diabetes Endocrinol 4: 814, 2016. doi: 10.1016/S2213-8587(16)30237-6. [DOI] [PubMed] [Google Scholar]
- 18.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 373: 232–242, 2015. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 19.Guidi E, Colussi G, Rombolà G, Airaghi C, Minetti E, Malberti F. Evaluation of the tubuloglomerular feedback system in human subjects Exp Nephrol 3: 61–64, 1995. www.ncbi.nlm.nih.gov/pubmed/7712144. [PubMed] [Google Scholar]
- 20.Gutzwiller J-P, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 89: 3055–3061, 2004. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 21.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 22.Heise T, Jordan J, Wanner C, Heer M, Macha S, Mattheus M, Lund SS, Woerle HJ, Broedl UC. Acute pharmacodynamic effects of empagliflozin with and without diuretic agents in patients with type 2 diabetes mellitus. Clin Ther 38: 2248–2264.e5, 2016. doi: 10.1016/j.clinthera.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 377: 1228–1239, 2017. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I; ACCORD trial group . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 376: 419–430, 2010. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, Bull S, Cannon CP, Charytan DM, de Zeeuw D, Edwards R, Greene T, Heerspink HJ, Levin A, Pollock C, Wheeler DC, Xie J, Zhang H, Zinman B, Desai M, Perkovic V; CREDENCE study investigators . The canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 46: 462–472, 2017. doi: 10.1159/000484633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalra S, Singh V, Nagrale D. Sodium-glucose cotransporter-2 inhibition and the glomerulus: a review. Adv Ther 33: 1502–1518, 2016. doi: 10.1007/s12325-016-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura G. Diuretic action of sodium-glucose cotransporter 2 inhibitors and its importance in the management of heart failure. Circ J 80: 2277–2281, 2016. doi: 10.1253/circj.CJ-16-0780. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan D, Liu L, Wiebe SA, Casey JR, Cordat E, Alexander RT. Carbonic anhydrase II binds to and increases the activity of the epithelial sodium-proton exchanger, NHE3. Am J Physiol Renal Physiol 309: F383–F392, 2015. doi: 10.1152/ajprenal.00464.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol 314: F969–F984, 2018. doi: 10.1152/ajprenal.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y-S, Jun H-S. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm 2016: 3094642, 2016. doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.León Jiménez D, Castilla Guerra L, López Chozas JM, Miramontes González JP. Update concept of the dual blocking of the renin-angiotensin-aldosteron system. A new therapeutic option? Med Clin (Barc) 150: 33–38, 2018. doi: 10.1016/j.medcli.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 32.León Jiménez D, Gómez Huelgas R, Miramontes González JP. The mechanism of action of sodium-glucose co-transporter 2 inhibitors is similar to carbonic anhydrase inhibitors. Eur J Heart Fail 20: 409, 2018. doi: 10.1002/ejhf.1068. [DOI] [PubMed] [Google Scholar]
- 33.Lovshin JA. Glucagon-like peptide-1 receptor agonists: a class update for treating type 2 diabetes. Can J Diabetes 41: 524–535, 2017. doi: 10.1016/j.jcjd.2017.08.242. [DOI] [PubMed] [Google Scholar]
- 34.Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 38: 132–139, 2015. doi: 10.2337/dc14-1958. [DOI] [PubMed] [Google Scholar]
- 35.Lovshin JA, Rajasekeran H, Lytvyn Y, Lovblom LE, Khan S, Alemu R, Locke A, Lai V, He H, Hittle L, Wang W, Drucker DJ, Cherney DZI. Dipeptidyl peptidase 4 inhibition stimulates distal tubular natriuresis and increases in circulating SDF-1α1-67 in patients with type 2 diabetes. Diabetes Care 40: 1073–1081, 2017. [Correction in Diabetes Care 40: 1420–1421, 2017.] 10.2337/dc17-0061. [DOI] [PubMed] [Google Scholar]
- 36.Lytvyn Y, Bjornstad P, Pun N, Cherney DZ. New and old agents in the management of diabetic nephropathy. Curr Opin Nephrol Hypertens 25: 232–239, 2016. doi: 10.1097/MNH.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB; LEADER Steering Committee and Investigators . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 377: 839–848, 2017. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 38.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375: 1834–1844, 2016. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 39.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375: 311–322, 2016. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Maldonado M, Gely R, Tapia E, Benabe JE. Role of macula densa in diuretics-induced renin release. Hypertension 16: 261–268, 1990. doi: 10.1161/01.HYP.16.3.261. [DOI] [PubMed] [Google Scholar]
- 41.Marx N, Rosenstock J, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, Espeland MA, Bluhmki E, Mattheus M, Ryckaert B, Patel S, Johansen OE, Woerle HJ. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA®). Diab Vasc Dis Res 12: 164–174, 2015. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McFarlane P, Cherney D, Gilbert RE, Senior P; Diabetes Canada Clinical Practice Guidelines Expert Committee . Chronic kidney disease in diabetes. Can J Diabetes 42, Suppl 1: S201–S209, 2018. doi: 10.1016/j.jcjd.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving H-H, Steffes MW; American Diabetes Association . Nephropathy in diabetes. Diabetes Care 27, Suppl 1: S79–S83, 2004. doi: 10.2337/diacare.27.2007.S79. [DOI] [PubMed] [Google Scholar]
- 44.Mordi NA, Mordi IR, Singh JS, Baig F, Choy AM, McCrimmon RJ, Struthers AD, Lang CC. Renal and Cardiovascular Effects of sodium-glucose cotransporter 2 (SGLT2) inhibition in combination with loop Diuretics in diabetic patients with Chronic Heart Failure (RECEDE-CHF): protocol for a randomised controlled double-blind cross-over trial. BMJ Open 7: e018097, 2017. doi: 10.1136/bmjopen-2017-018097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, Im K, Rozenberg A, Yanuv I, Stahre C, Ray KK, Iqbal N, Braunwald E, Scirica BM, Raz I. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 Trial. Diabetes Care 40: 69–76, 2017. doi: 10.2337/dc16-0621. [DOI] [PubMed] [Google Scholar]
- 46.Muskiet MH, Tonneijck L, Smits MM, van Baar MJ, Kramer MH, Hoorn EJ, Joles JA, van Raalte DH. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 13: 605–628, 2017. doi: 10.1038/nrneph.2017.123. [DOI] [PubMed] [Google Scholar]
- 47.Muskiet MH, Tonneijck L, Smits MM, Kramer MH, Heerspink HJ, van Raalte DH. Pleiotropic effects of type 2 diabetes management strategies on renal risk factors. Lancet Diabetes Endocrinol 3: 367–381, 2015. doi: 10.1016/S2213-8587(15)00030-3. [DOI] [PubMed] [Google Scholar]
- 48.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 49.Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, Fulcher G, Desai M, Li Q, Deng H, Rosenthal N, Jardine MJ, Bakris G, Perkovic V. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function: data from the CANVAS program. Circulation CIRCULATIONAHA.118.035901, 2018. doi: 10.1161/CIRCULATIONAHA.118.035901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Nicola L, Gabbai FB, Liberti ME, Sagliocca A, Conte G, Minutolo R. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am J Kidney Dis 64: 16–24, 2014. doi: 10.1053/j.ajkd.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Odlind B, Beermann B, Selén G, Persson AE. Renal tubular secretion of piretanide and its effects on electrolyte reabsorption and tubuloglomerular feedback mechanism J Pharmacol Exp Ther 225: 742–746, 1983. https://www.ncbi.nlm.nih.gov/pubmed/6864530. [PubMed] [Google Scholar]
- 52.Panchapakesan U, Pegg K, Gross S, Komala MG, Mudaliar H, Forbes J, Pollock C, Mather A. Effects of SGLT2 inhibition in human kidney proximal tubular cells–renoprotection in diabetic nephropathy? PLoS One 8: e54442, 2013. doi: 10.1371/journal.pone.0054442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Matthews DR, Neal B. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 6: 691–704, 2018. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 54.Persson AE, Wright FS. Evidence for feedback mediated reduction of glomerular filtration rate during infusion of acetazolamide. Acta Physiol Scand 114: 1–7, 1982. doi: 10.1111/j.1748-1716.1982.tb06945.x. [DOI] [PubMed] [Google Scholar]
- 55.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 25: 2028–2039, 2014. doi: 10.1681/ASN.2013060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 373: 2247–2257, 2015. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 57.Puschett JB, Goldberg M. The relationship between the renal handling of phosphate and bicarbonate in man J Lab Clin Med 73: 956–969, 1969. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC371858/. [PubMed] [Google Scholar]
- 58.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155: 1280–1290, 2014. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 59.Rajasekeran H, Lytvyn Y, Bozovic A, Lovshin J, Diamandis E, Cattran D, Husain M, Perkins BA, Advani A, Reich HN, Kulasingam V, Cherney D. Urinary adenosine excretion in Type 1 diabetes. Am J Physiol Renal Physiol 313: F184–F191, 2017. doi: 10.1152/ajprenal.00043.2017. [DOI] [PubMed] [Google Scholar]
- 60.Rosenstock J, Perkovic V, Alexander JH, Cooper ME, Marx N, Pencina MJ, Toto RD, Wanner C, Zinman B, Baanstra D, Pfarr E, Mattheus M, Broedl UC, Woerle HJ, George JT, von Eynatten M, McGuire DK; CARMELINA® investigators . Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA®): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol 17: 39, 2018. doi: 10.1186/s12933-018-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes 3: 1–6, 2012. doi: 10.4239/wjd.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmieder R, Ott C, Linz P, Jumar A, Friedrich S, Titze J, Hammon M, Uder M, Kistner I. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content. J Hypertens 34: e76, 2016. doi: 10.1097/01.hjh.0000500051.20830.98. 29301520 [DOI] [Google Scholar]
- 63.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 369: 1317–1326, 2013. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 64.Skøtt P, Hommel E, Arnold-Larsen S, Parving HH. Effect of carbonic anhydrase inhibitors on glomerular filtration rate in diabetic nephropathy Br Med J (Clin Res Ed) 294: 549, 1987. doi: 10.1136/bmj.294.6571.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skøtt P, Hommel E, Bruun NE, Arnold-Larsen S, Parving HH. Effects of acetazolamide on kidney function in type 1 (insulin-dependent) diabetic patients with diabetic nephropathy Diabetologia 31: 806–810, 1988. doi: 10.1007/BF00277481. [DOI] [PubMed] [Google Scholar]
- 66.Skøtt P, Hommel E, Bruun NE, Arnold-Larsen S, Parving HH. The acute effect of acetazolamide on glomerular filtration rate and proximal tubular reabsorption of sodium and water in normal man Scand J Clin Lab Invest 49: 583–587, 1989. doi: 10.3109/00365518909089139. [DOI] [PubMed] [Google Scholar]
- 67.Skov J, Dejgaard A, Frøkiær J, Holst JJ, Jonassen T, Rittig S, Christiansen JS. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab 98: E664–E671, 2013. doi: 10.1210/jc.2012-3855. [DOI] [PubMed] [Google Scholar]
- 68.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 17: 1703–1709, 2006. doi: 10.1681/ASN.2005080872. [DOI] [PubMed] [Google Scholar]
- 69.Takashima S, Fujita H, Fujishima H, Shimizu T, Sato T, Morii T, Tsukiyama K, Narita T, Takahashi T, Drucker DJ, Seino Y, Yamada Y. Stromal cell-derived factor-1 is upregulated bydipeptidyl peptidase-4 inhibition and hasprotective roles in progressive diabeticnephropathy. Kidney Int 90: 783–796, 2016. doi: 10.1016/j.kint.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 70.Thomas MC. The potential and pitfalls of GLP-1 receptor agonists for renal protection in type 2 diabetes. Diabetes Metab 43, Suppl 1: 2S20–2S27, 2017. doi: 10.1016/S1262-3636(17)30069-1. [DOI] [PubMed] [Google Scholar]
- 71.Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, Joles JA. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 28: 1023–1039, 2017. doi: 10.1681/ASN.2016060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser AH, Ter Wee PM, Diamant M, Joles JA, van Raalte DH. Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 39: 2042–2050, 2016. doi: 10.2337/dc16-1371. [DOI] [PubMed] [Google Scholar]
- 73.Tucker BJ, Steiner RW, Gushwa LC, Blantz RC. Studies on the tubulo-glomerular feedback system in the rat. The mechanism of reduction in filtration rate with benzolamide J Clin Invest 62: 993–1004, 1978. doi: 10.1172/JCI109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304: F156–F167, 2013. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 74: 351–375, 2012. doi: 10.1146/annurev-physiol-020911-153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WH, Mullens W. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur J Heart Fail 16: 133–142, 2014. doi: 10.1002/ejhf.35. [DOI] [PubMed] [Google Scholar]
- 77.de Vos LC, Hettige TS, Cooper ME. New glucose-lowering agents for diabetic kidney disease. Adv Chronic Kidney Dis 25: 149–157, 2018. doi: 10.1053/j.ackd.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Wang H, D’Ambrosio MA, Ren Y, Monu SR, Leung P, Kutskill K, Garvin JL, Janic B, Peterson EL, Carretero OA. Tubuloglomerular and connecting tubuloglomerular feedback during inhibition of various Na transporters in the nephron. Am J Physiol Renal Physiol 308: F1026–F1031, 2015. doi: 10.1152/ajprenal.00605.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 80.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 369: 1327–1335, 2013. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 81.Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc 7: e007046, 2018. doi: 10.1161/JAHA.117.007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winterborn MH, Bradwell AR, Chesner IM, Jones GT. The origin of proteinuria at high altitude Postgrad Med J 63: 179–181, 1987. doi: 10.1136/pgmj.63.737.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong MG, Perkovic V, Chalmers J, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, MacMahon S, Mancia G, Marre M, Matthews D, Neal B, Poulter N, Rodgers A, Williams B, Zoungas S; ADVANCE-ON Collaborative Group . Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care 39: 694–700, 2016. doi: 10.2337/dc15-2322. [DOI] [PubMed] [Google Scholar]
- 84.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest 77: 1925–1930, 1986. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zatz R, Meyer TW, Rennke HG, Brenner BM. Predominance of hemodynamic rather than metabolic factors in the pathogenesis of diabetic glomerulopathy Proc Natl Acad Sci USA 82: 5963–5967, 1985. doi: 10.1073/pnas.82.17.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeni L, Norden AGW, Cancarini G, Unwin RJ. A more tubulocentric view of diabetic kidney disease. J Nephrol 30: 701–717, 2017. doi: 10.1007/s40620-017-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zingerman B, Herman-Edelstein M, Erman A, Bar Sheshet Itach S, Ori Y, Rozen-Zvi B, Gafter U, Chagnac A. Effect of acetazolamide on obesity-induced glomerular hyperfiltration: a randomized controlled trial. PLoS One 10: e0137163, 2015. doi: 10.1371/journal.pone.0137163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 89.Zoungas S, de Galan BE, Ninomiya T, Grobbee D, Hamet P, Heller S, MacMahon S, Marre M, Neal B, Patel A, Woodward M, Chalmers J, Cass A, Glasziou P, Harrap S, Lisheng L, Mancia G, Pillai A, Poulter N, Perkovic V, Travert F; ADVANCE Collaborative Group . Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diabetes Care 32: 2068–2074, 2009. doi: 10.2337/dc09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]