Abstract
Estrogens, acting synergistically with androgens, are known from animal experiments to be important in lower urinary tract symptoms (LUTS) and benign prostate enlargement. Human exposure to environmental estrogens occurs throughout the life span, but the urologic health risks in men are largely unknown. Bisphenol A (BPA) is an endocrine disruptor implicated in male urogenital malformations. Given the role of estrogens in male LUTS, we studied the effects of BPA administered in combination with testosterone (T) on the urinary voiding behavior of adult male mice. Adult male mice underwent subcutaneous implantation with slow-release pellets of 25 mg BPA or 2.5 mg estradiol-17β (E2), plus 25 mg T, and were compared with untreated (UNT) mice that underwent sham surgery. We studied urinary voiding behavior noninvasively for 1 mo before treatment and for 4 mo after treatment. After euthanasia, we evaluated bladder volume and mass. Mice treated with T+BPA had increased bladder volume (P < 0.05) and mass (P < 0.01) compared with UNT mice. After 4 mo of treatment with T+BPA, three of five mice developed voiding dysfunction in the form of droplet voiding or an intermediate pattern of voiding different from both UNT and T+E2-treated mice. Treatment of male mice with BPA or estradiol induces voiding dysfunction that manifests at later time points, implicating the endocrine disruptor, BPA, as a contributor to male LUTS.
Keywords: developmental origins of health and disease, LUTD, prostate, urinary dysfunction
INTRODUCTION
Bisphenol A (BPA) is a monomer in polycarbonate plastics that can leach from containers used to store food and beverages, dental implants, and epoxy resins in container linings. Although toxicokinetic studies indicate that BPA is rapidly metabolized by the liver after oral exposure, human biomonitoring data support that exposure to BPA is ubiquitous and found in over 90% of individuals in the United States (5, 42). Although the assumption has been that the majority of BPA exposure occurs via ingestion from food packaging (35), substantial nonfood exposure routes are also common in humans, including inhalation and transdermal routes (14, 39, 46). The estrogenic activity of BPA was first reported in 1936 (11). It is often stated that BPA is a weak estrogen compared with estradiol, because the binding affinity of BPA for classical nuclear estrogen receptors is lower than that of estradiol. It is well recognized that potency is impacted by tissue-specific coregulatory proteins and does not simply reflect affinity for nuclear receptors. Additionally, nonnuclear estrogen receptors associated with rapid-response enzyme cascades show equal potency for BPA and estradiol (48). BPA is now well accepted as an endocrine disruptor (45, 49). Although precise mechanisms in addition to its estrogenic activity remain unclear, BPA has been implicated in a wide range of health problems in humans, including peripheral vascular disease, insulin resistance, and obesity (6, 16, 23, 37, 38). Occupational exposure of adult men in China to BPA is associated with alterations in serum hormone levels and male sexual dysfunction (19, 20). US BPA occupational studies indicate that workers in plants that use BPA have higher serum levels of BPA than workers in China (13). In experimental animals, BPA exposure during development has been linked to male reproductive system abnormalities involving the testes, the epididymis, the seminal vesicles, and the prostate (44, 47). BPA exposure has also been linked to changes in gene expression within the prostate and the lower urinary tract of rodents (41).
Lower urinary tract symptoms (LUTS) affect at least 30% of men over the age of 50, and the prevalence increases with age (15). Multiple factors, including age, prostate enlargement, comorbid medical conditions, and bladder dysfunction, contribute to male LUTS (8). The standard of care for the better half of the 20th century consisted of surgical intervention to manage complications due to LUTS associated with benign prostatic hyperplasia (BPH). In the 1990s, noninvasive urodynamic testing, clinical history, and examinations were recommended before medical or surgical intervention (17, 22). Noninvasive testing for patients with LUTS includes uroflow, which is used to measure the flow rate during urination (9). The goal of this test is to improve clinical decisions, therefore reducing the number of men undergoing surgery for management of complications due to LUTS. Although the molecular mechanisms of BPH-related LUTS are unclear, the altered hormone environment in aging men has been explored (3, 4, 28). As men age, serum estrogens increase or remain constant, whereas testicular androgens decline (3). Although precise mechanisms remain unknown, estrogens have been implicated in benign prostatic enlargement and LUTS (27). Specifically, several studies have suggested a link between serum estradiol and LUTS (33), prostate size/volume (40), and risk of BPH (40). Despite the well-characterized estrogenic activity of BPA, the possibility that BPA exposure in adults could contribute to male LUTS has not been previously evaluated. The rationale for this study is that estrogen receptor ligands are involved in the development of lower urinary tract dysfunction (LUTD); however, little is known about the role of endocrine disruptors such as BPA in the development of LUTD. Therefore, the objective of this project was to use uroflow to determine the effects of adult BPA exposure on urinary voiding behavior in male mice.
MATERIALS AND METHODS
Steroid implantation.
C57BL/6 male mice (6–8 wk of age) were obtained from Charles River Laboratories (Wilmington, MA) and maintained under standard laboratory conditions using microisolator caging (12:12 light-dark cycle) with free access to food (Laboratory Autoclavable Rodent Diet 5010) and reverse osmosis water from Hydropac. All animal experiments and procedures were conducted under a protocol approved by the University of Rochester’s University Committee on Animal Resources. BPA (99% pure), estradiol-17β (E2), and testosterone (T) were obtained from Sigma Chemical (St. Louis, MO). Mice underwent surgical implantation of compressed hormone pellets containing 25 mg T and either 25 mg BPA or 2.5 mg E2 as described previously (30, 31). Control mice underwent the surgical procedure, but no pellet was implanted. For bladder measurements, untreated (UNT), n = 7; T+E2, n = 7; T+BPA, n = 10. Uroflow studies were done on a subset of mice (UNT, n = 2; T+E2, n = 5; T+BPA, n = 5). At the completion of the experiment, mice were euthanized with CO2, and the body mass was measured. Laparotomy was then performed, and bladder volume and mass were determined as previously described (25).
Uroflow.
Before hormone implantation (1 mo), we began assessment of urinary voiding behavior (2–3 sessions/wk) and continued until 4 mo after steroid hormone implantation (18, 51). Details of our uroflow testing apparatus have been previously described (18, 25). Briefly, mice were placed in individual metabolic cages suspended over milligram-resolution balances without fecal separation screens and offered a preferred solution to drink. Perturbations in the balance were monitored 10 times per second, and the character stream was received by our custom program (LabVIEW; National Instruments, Austin, TX) to generate displays of void mass, duration, and uroflow, which were verified by synchronized videos of voiding events.1
Statistical analysis.
For each individual mouse uroflow testing session, the median uroflow (g/s), peak uroflow in the first 2 s of the void (g/s), and median voided mass (g) were determined. During each uroflow testing session, individual uroflow curves for each mouse were examined by a single observer blinded to treatment condition, counted, and classified as either a sustained void (≥3-s duration) or droplet void (<3-s duration). For all uroflow parameters, the median of each measure was reported for the 1-mo period before treatment and 1, 2, 3, and 4 mo after treatment. Data analysis, using GraphPad Prism 7 (La Jolla, CA) and SAS (9.4), for comparisons of continuous variables consisted of one-way ANOVA where appropriate and repeated-measures two-way ANOVA with Bonferroni post hoc comparisons for uroflow measures that continued over time. Data were evaluated for homoscedasticity with Bartlett’s unequal variance test, and when necessary, rank transformation was performed. For body mass and bladder measurements, graphs show means ± SE of nontransformed data, although the bladder data were subjected to analysis of covariance, with body weight as the covariate. For uroflow parameters, graphs show medians for 1-mo time period. In all analyses, P < 0.05 was considered statistically significant.
RESULTS
Effects of BPA on mouse body mass and bladders.
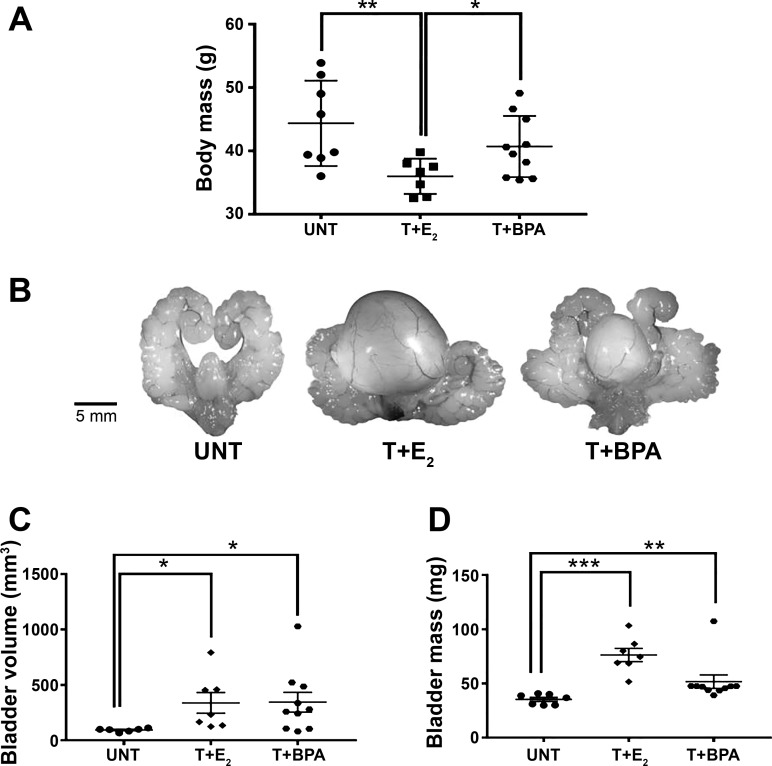
To assess effects of BPA in the mouse, we evaluated body mass and associated bladder changes. Because of unequal variances among the groups for bladder volume (Bartlett’s statistic, 30.44; P < 0.0001) and bladder mass (Bartlett’s statistic, 9.132; P = 0.0104), data were transformed using reciprocals to satisfy assumptions of ANOVA. As shown in Fig. 1A, there was a significant decrease in body mass in the T+E2 group relative to the UNT and T+BPA groups (overall ANOVA, P < 0.05; UNT vs. T+E2, P < 0.01; T+E2 vs. T+BPA, P < 0.05). The urogenital tracts from UNT, T+E2-treated, and T+BPA-treated mice for 4 mo are shown in Fig. 1B. Bladder volume was significantly altered by treatment (P < 0.01) and was significantly larger in mice treated with T+E2 (P < 0.05) and T+BPA (P < 0.05) compared with UNT (body weight did not account for a significant component of the variance, P = 0.52); in addition, when T+E2 and T+BPA groups were compared, there was no significant difference (Fig. 1C). Bladder mass was significantly altered by treatment (P < 0.0001, Fig. 1D), and body mass was not a significant covariate (P = 0.86). Specifically, bladder mass was increased relative to UNT among mice treated with T+E2 (P < 0.001) and T+BPA (P < 0.01). However, the average bladder mass was significantly higher among the mice treated with T+E2 compared with T+BPA (P < 0.01), indicating that BPA-induced bladder hypertrophy was less severe than the T+E2 treatment.
Fig. 1.
Mice treated with testosterone + bisphenol A (T+BPA) displayed enlarged bladder mass and volume. A: postnecropsy, the body mass was determined. There was a significant decrease in body mass in the testosterone + estradiol-17β (T+E2)-treated mice relative to the untreated (UNT, P < 0.01) and T+BPA (P < 0.05)-treated mice. B: urogenital tracts from UNT, T+E2-treated, and T+BPA-treated mice for 4 mo. C: at the time of necropsy, bladders were measured in situ with a precision caliper in three dimensions, and the volume was estimated as an ellipsoid. There was a significant increase in bladder volume in the T+E2 (P < 0.05) and T+BPA (P < 0.05) groups relative to UNT. Body weight did not account for a significant component of the variance (P = 0.52). D: bladders were carefully dissected, emptied of urine, and blotted dry, and the mass was determined. There was a significant increase in bladder mass in the T+E2 (P < 0.0001) and T+BPA (P < 0.01) groups relative to UNT. Body mass was not a significant covariate (P = 0.86). *P < 0.05, **P < 0.01, ***P < 0.001 in post hoc testing.
Uroflow.
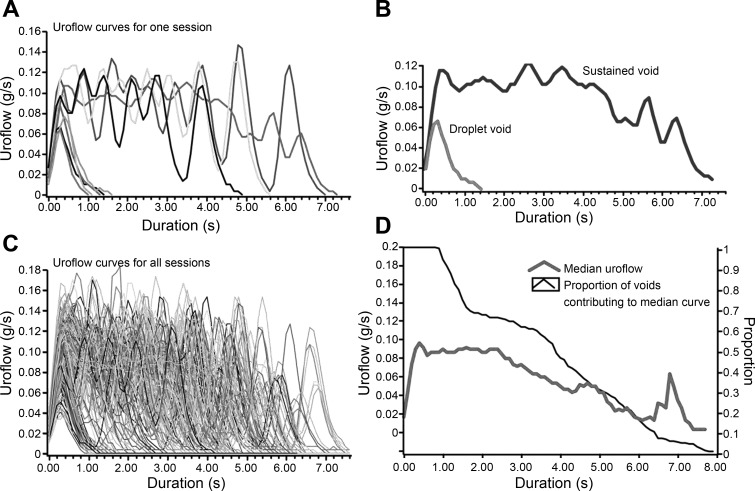
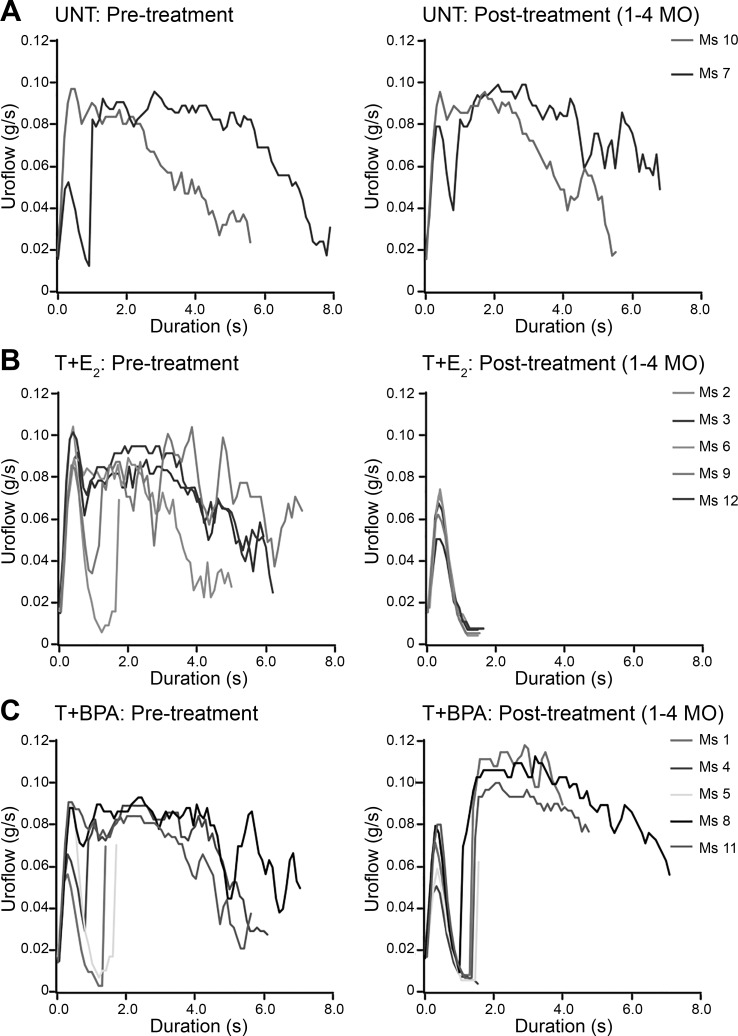
To assess urinary dysfunction, we compared urinary voiding in individual mice utilizing modified urinary metabolic cages. We used the uroflow curves (Fig. 2, A and B) for all sessions in a time window of interest (Fig. 2C) to create median uroflow curves for each mouse (Fig. 2D). We compared median uroflow curves that reflect the 1-mo time period before hormone implantation with median uroflow curves reflecting 1–4 mo after treatment. The two UNT mice exhibited median uroflow curves consistent with sustained voiding pattern before sham surgery that was similar to median uroflow curves following surgery. One of two UNT mice [mouse 7 (Ms 7)] did display a minor droplet-voiding component to the median uroflow curve that was present both before surgery and postsurgery for subcutaneous pellet implantation (Fig. 3A). Among mice treated with T+E2, median uroflow curves before hormone pellet implantation were similar to those of UNT mice (Fig. 3, A and B). In the 4 mo following hormone implantation, five of five mice treated with T+E2 displayed median uroflow curves consistent with a pattern of exclusively droplet voiding. Among mice treated with T+BPA, two mice (Ms 1 and Ms 5) displayed predominantly droplet voiding before treatment, whereas the remaining three mice had median uroflow curves consistent with sustained voiding similar to the UNT mice. Posttreatment, all mice treated with T+BPA displayed a mixture of median uroflow curves consistent with droplet voiding (Ms 4) or an intermediate pattern of voiding (Ms 8 and Ms 11) different from both UNT and T+E2-treated mice (Fig. 3C).
Fig. 2.
Classification of uroflow patterns measured noninvasively. A: all uroflow curves for one session are plotted. B: voids are qualitatively classified by the shape of the uroflow curve into sustained void vs. droplet void (quantitative threshold for a droplet void defined as duration <3 s). C: uroflow curves of each void for all sessions show a mixed pattern of sustained and droplet voiding. D: median uroflow curve is generated from all curves in C. At shorter durations, more voids contribute to the median uroflow curve.
Fig. 3.
Testosterone + bisphenol A (T+BPA)-treated mice displayed voiding dysfunction in the form of droplet or an intermediate pattern of voiding after 4 mo. To compare individual mice before and after hormone treatment, uroflow curves generated by each urinary void were summarized into median uroflow curves. A: pretreatment and posttreatment uroflow curves were similar in two untreated (UNT) mice. B: whereas pretreatment uroflow curves were similar among the mice treated with testosterone + estradiol-17β (T+E2) and UNT, posttreatment, all 5 of the mice treated with T+E2 displayed predominantly droplet voiding. C: among mice treated with T+BPA, pretreatment uroflow curves were similar to those of UNT, whereas uroflow curves from all T+BPA-treated mice displayed a droplet void component and void components intermediate between those of the UNT and T+E2-treated mice. Ms 1–12, mice 1–12.
Urinary voiding patterns.
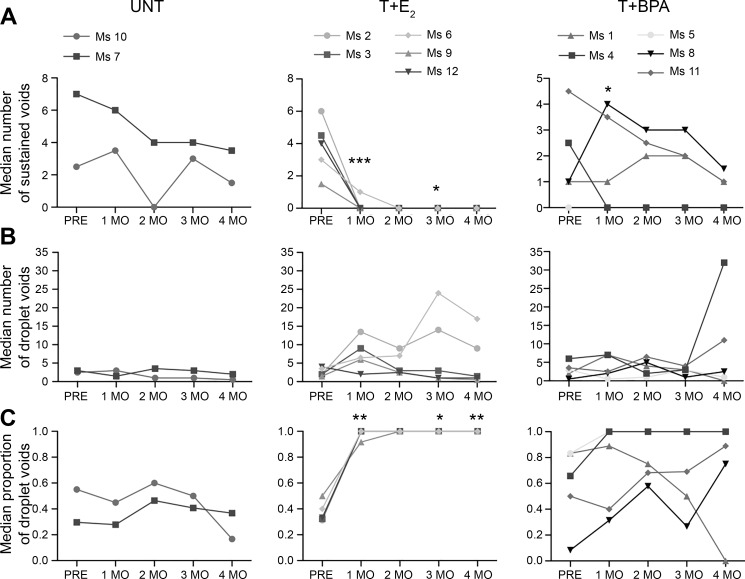
To determine whether T+BPA-treated mice developed LUTD similar to that of mice with LUTD by T+E2 treatment, we analyzed voiding patterns. To quantify differences in voiding patterns, we examined the frequency of sustained voids and droplet voids and the proportion of droplet voids (defined as the number of droplet voids divided by the total number of voids per session, including sustained and droplet). For each mouse on each testing day (Fig. 2A), every voiding event was classified as either a sustained void or a droplet void (Fig. 2B), and the median number of each type of void was computed for the 1-mo time period. Analysis of the number of sustained voids showed effects for interaction (P = 0.0010), time (P < 0.0001), and treatment (P = 0.0400). As shown in Fig. 4A, at all time points, UNT mice displayed sustained voids (range of median frequency, 0–7.5). Prior to treatment, T+E2 mice had a median frequency ranging from 1.5 to 6 sustained voids per session. In all 4 mo following treatment with T+E2, the median number of sustained voids decreased to zero, reaching statistical significance compared with UNT at 1 mo (P < 0.001) and 3 mo (P < 0.05) posttreatment. Among the T+BPA mice before treatment, there was a wide range of median sustained void counts, with one mouse (Ms 5) displaying a median number of zero sustained voids before treatment. Mice treated with T+BPA exhibited increased sustained voids relative to UNT mice at 1 mo posttreatment (P < 0.05), but other time points were not significantly different.
Fig. 4.
Testosterone + bisphenol A (T+BPA) caused voiding dysfunction in male mice over 4 mo of treatment. For each session, individual voids from each mouse were classified as sustained or droplet (defined as >3 s duration), expressed as frequency per session, or proportion of droplet voids, and summarized by median for each month. A: among untreated (UNT) mice, the median number of sustained voids did not change significantly over time, whereas there was a marked decrease in sustained voids among the mice treated with testosterone + estradiol-17β (T+E2) following hormone treatment. Among mice treated with T+BPA, there was an increase in the number of sustained voids at 1 mo but not at later time points [effects for interaction (P = 0.0010), time (P < 0.0001), and treatment (P = 0.0398)]. B: median number of droplet voids was consistent among all mice before treatment. Although there were not consistent differences in the frequency of droplet voids, 2 of 5 mice treated with T+E2 showed markedly increased frequency of droplet voiding at 3 and 4 mo, and 2 of 5 mice treated with T+BPA displayed substantially increased median droplet voids at the final month after treatment [effects for interaction (P = 0.6007), time (P = 0.8056), and treatment (P = 0.5082)]. C: median proportion of droplet voids was consistent among UNT mice over time, whereas it increased substantially among all mice treated with T+E2. Among mice treated with T+BPA, 4 of 5 mice displayed a proportion of droplet voids greater than that of UNT at 4 mo [effects for interaction (P = 0.0077), time (P = 0.0024), and treatment (P = 0.0228)]. Ms 1–12, mice 1–12; PRE, pretreatment. *P < 0.05, **P < 0.01, ***P < 0.001 in post hoc testing.
Since droplet voids have previously been shown to be associated with LUTD, we compared the frequency between groups. For the frequency of droplet voids, there were no significant effects of treatment or time (P > 0.1). As shown in Fig. 4B, a low rate of droplet voiding was observed in UNT mice at all time points, with a range of 0.5–3.0 median number of droplet voids per session. Mice in all groups had a similar number of droplet voids pretreatment (T+E2 range, 1.5–4.0; T+BPA range, 0.5–6.0). However, among T+E2 mice posttreatment, there were two mice (Ms 2 and Ms 6) that exhibited an increase in the median number of droplet voids at all posttreatment time points, although the comparison of this treatment with the UNT group did not reach statistical significance. Among mice treated with T+BPA, there were two mice (Ms 4 and Ms 11) at 4 mo posttreatment that had an increased median number of droplet voids, but again, this treatment group did not differ significantly from UNT.
Because we observed differences in the voiding patterns among the groups, we determined the proportion of droplet voids in each session and compared the median of this proportion in 1-mo intervals (Fig. 4C). We found effects for interaction (P = 0.077), time (P = 0.0024), and treatment (P = 0.0228). As shown in Fig. 4C, UNT mice had a consistent proportion of droplet voids over time (range, 0.17–0.60). Prior to treatment, mice treated with T+E2 had a median proportion of droplet voids similar to that of UNT (range, 0.31–0.5); however, after treatment, mice treated with T+E2 had a significantly higher proportion of droplet voids compared with UNT (1 mo, P < 0.01; 3 mo, P < 0.05; 4 mo, P < 0.01). For the T+BPA mice before treatment, there was a large range of median proportion of droplet voids (0.08–0.83). Following treatment, two of five mice treated with T+BPA (Ms 4 and Ms 5) displayed exclusively droplet voids, whereas two others (Ms 11 and Ms 8) displayed a high proportion of droplet voids by 4 mo (0.89 and 0.75, respectively). The remaining mouse (Ms 1) displayed a high proportion of droplet voiding before treatment and following treatment, which decreased during the study period to display no droplet voids by 4 mo. Because of the individual variability of the voiding patterns displayed among the T+BPA mice, there were no statistically significant differences relative to UNT mice.
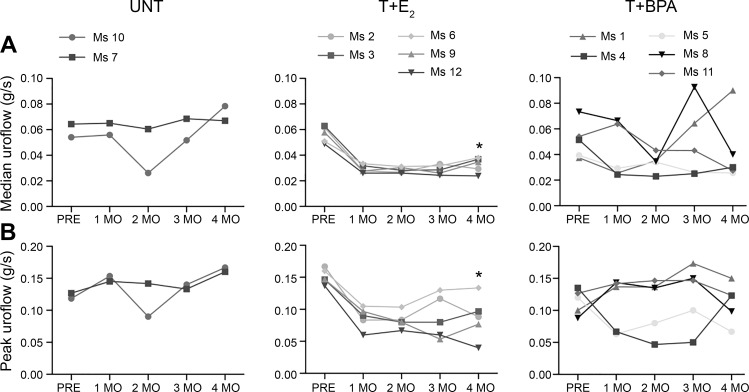
Another measurement of LUTD is median uroflow. As such, we assessed median uroflow before hormone treatment and at each time point following treatment. There were significant effects for time (P = 0.0252) and treatment (P = 0.0405) but not for interaction (P > 0.1). Even though the interaction effect was not significant, it appeared as though uroflow patterns differed over time within groups. As shown in Fig. 5A, median uroflow before treatment was similar among all groups, and the UNT mice did not change over time. However, among mice treated with T+E2, there was a decrease in median uroflow following hormone treatment that reached statistical significance at 4 mo (P < 0.05). Among mice treated with T+BPA, there were no consistent differences in median uroflow compared with UNT, although four of five mice treated with T+BPA (Ms 4, Ms 5, Ms 8, and Ms 11) displayed median uroflow at 4 mo that was similar to that of mice treated with T+E2. Because peak uroflow is a clinically relevant measure associated with bladder outlet obstruction in human male LUTS, we examined the changes over time in peak uroflow (Fig. 5B). We found effects for interaction (P = 0.0078) but not for time or treatment (P > 0.1). Peak uroflow was similar at all time points in UNT mice. Following hormone treatment, mice treated with T+E2 exhibited decreased peak uroflow, reaching statistical significance at 4 mo posttreatment (P < 0.05, Fig. 5B). Among mice treated with T+BPA, there were no consistent or statistically significant differences in peak uroflow compared with UNT.
Fig. 5.
Testosterone + bisphenol A (T+BPA) did not significantly alter median or peak uroflow in male mice. A: among untreated (UNT) mice, there were no changes over time in median uroflow, contrasted to mice treated with testosterone + estradiol-17β (T+E2), who had a significant decrease by 4 mo. Among mice treated with T+BPA, 4 of 5 displayed decreased uroflow, although the difference was not statistically significant in post hoc testing [effects for interaction (P = 0.2696), time (P = 0.0252), and treatment (P = 0.0405)]. B: peak uroflow (measured in the first 0–2 s of the urinary void) did not change over time among UNT mice but decreased at 4 mo among mice treated with T+E2. Peak uroflow among mice treated with T+BPA did not change over time [effects for interaction (P = 0.0078), time (P = 0.1703), and treatment (P = 0.1757)]. Ms 1–12, mice 1–12; PRE, pretreatment. *P < 0.05 in post hoc testing.
DISCUSSION
To our knowledge, we report for the first time that treatment of male mice with BPA, in an androgenic environment that models the human male hormonal milieu, induces bladder enlargement, hypertrophy, and urinary voiding dysfunction. This is consistent with bladder enlargement and hypertrophy in male mice treated with T+E2 (25). As expected, we found that droplet voiding was present in mice treated with T+E2 in the first month following hormone treatment, whereas urinary voiding dysfunction manifested at later time points in mice treated with T+BPA.
After 1 mo of hormone treatment, we observed consistent decreases in median and peak uroflow among positive control (T+E2-treated) mice. Whereas consistent differences in uroflow were not present in the entire group of mice treated with T+BPA, after 4 mo of treatment, male mice treated with T+BPA showed intermediate median uroflow values that were similar to those of the mice treated with T+E2 and substantially decreased relative to UNT. Decreases in median and peak uroflow that we observed in mice treated with T+E2 are consistent with the bladder outlet obstruction that is commonly observed in men with enlarged prostates. However, the late manifestations of voiding dysfunction we observed in mice treated with T+BPA are consistent with the relatively weaker estrogenic activity of BPA compared with E2.
At all time points, UNT mice exhibited, on average, <5 droplet voids per session, suggesting that some degree of droplet-voiding behavior is expected in male mice. This is consistent with the known importance of scent-marking behavior in male mice (1). It is known that when placed in a novel environment, dominant male mice have increased urine marks compared with subordinate animals (10). Although our study did not address the dominance status of individual mice, we speculate that the droplet voiding observed in the one T+BPA mouse in the baseline period, before subcutaneous pellet implantation, may be explained by the dominance status and territory marking inherent in some mice. The bladder abnormalities observed in male mice treated with T+E2 and T+BPA indicate that the droplet voiding observed in these groups following subcutaneous hormone pellet implantation is not explained by urine scent marking alone.
Given the increased variability in urinary voiding patterns among the mice treated with T+BPA, we conclude that our study was underpowered to detect differences in urinary voiding behavior compared with control mice. However, we feel that the urinary voiding dysfunction among mice treated with T+BPA observed at month 4, although not consistently or statistically different, may be a clinically relevant finding in terms of the urinary voiding function of a subset of T+BPA-treated animals that appeared to be affected by BPA. Individual differences in the response to BPA could reflect differences in sensitivity that are related to stressors or other factors during development, consistent with the developmental origins of health and disease hypothesis (50).
The idea that diseases present in adults may have originated very early in development is well accepted (42). Indeed, many studies suggest that humans and animals are most sensitive to endocrine disruptors, such as BPA, during development. However, our findings suggest that adult exposure to BPA, which occurs throughout the life span, is also of concern. Moreover, widespread, lifelong exposure of humans to BPA has only occurred in the “age of plastics” beginning in the 1970s. Therefore, men with cumulative lifetime exposure to BPA are now reaching middle age, when LUTS and other prostate pathologies are prominent (21, 29). Given that male LUTS is highly prevalent and a significant burden to the health care system today, the implication of BPA in LUTS pathophysiology is particularly troubling. There are numerous challenges inherent in modeling human exposures to BPA in experimental animals. BPA exposure in humans occurs continuously and throughout the life span. In humans, the majority of BPA exposure occurs via ingestion (4), dermal routes (14), and inhalation (34). Ingested BPA undergoes extensive first-pass metabolism in the liver. When BPA is administered subcutaneously, it does not undergo first-pass metabolism; therefore, this route of drug administration has limitations when trying to model human exposure based only on BPA in food or beverages. In fact, BPA and a number of its replacements have been detected at very high levels in thermal receipt paper (41a). The fact that there are numerous analogs of BPA that have estrogenic activity, as well as other estrogenic chemicals, must be considered in terms of cumulative exposure to environmental estrogens, given the finding that exposure to estradiol markedly impacts LUTS in male mice (24, 32).
In conclusion, the present findings implicate BPA exposure in adulthood in male lower urinary tract dysfunction. These findings are consistent with our prior research showing that exposure of adult male mice to E2 and T is associated with LUTS (25). Thus, BPA, which is a known estrogenic endocrine-disrupting chemical and was considered for use as an estrogenic drug in the 1930s, also has the capacity to cause LUTS in male mice. An important issue to consider is whether the dose of BPA and method of administration that we used result in serum BPA levels that are relevant to human levels. With regard to the route of drug administration, as discussed above, there is evidence that there are routes of exposure (inhalation and transdermal) that do not result in rapid first-pass metabolism of BPA, thus leading to much higher levels of bioactive (unconjugated) BPA in blood relative to levels found after intragastric gavage administration (46). This exposure leads to levels that were found in adult men and women as high as 7.2 ng/ml after transdermal exposure due to holding a cashier’s receipt that is coated with free BPA (14). Mean human blood levels of BPA have been reported to range from 0.3 to 4 ng/ml (43), but it is well recognized that there are substantial individual differences in the metabolism of estrogenic drugs and chemicals, and thus some individuals have blood levels of bioactive BPA well within the range we found in this study (14). In addition, our use of subcutaneous pellets to administer BPA would lead to continuous exposure rather than widely spaced exposure. Importantly, there is evidence from studies with human populations, including from the National Health and Nutrition Examination Survey conducted by the Centers for Disease Control and Prevention, that exposure to BPA in the US population is continuous (39). Additional research is necessary to determine the differences in endogenous sex steroid hormones that may result from BPA administration, since BPA decreases serum testosterone in men and rodents (2, 7, 36). It will also be important to investigate whether BPA causes these effects via classical or nonclassical estrogen receptor pathways and determine which estrogen receptors, as well as other receptors, are important in mediating the action of BPA in the male lower urinary tract.
In summary, BPA is well known as an endocrine disruptor that affects the developing and adult male urogenital system. To evaluate the role of adult BPA exposure in male LUTS, we treated male mice with BPA and supplemented with T to simulate the hormonal milieu of older men. Treatment of male mice with T+BPA induces bladder enlargement, hypertrophy, and, in some mice, urinary voiding dysfunction, implicating this endocrine disruptor as a contributor to male LUTS.
GRANTS
This work was supported by NIH Grants U54-DK-104310 (W. A. Ricke), ES-001332 (W. A. Ricke), RC2-ES-O18764 (F. S. vom Saal and W. A. Ricke), and UO1-ES-020952 (F. S. vom Saal and W. A. Ricke). T. M. Nicholson is a trainee in the Medical Scientist Training Program at the University of Rochester funded by NIH Grant T32-GM-07356 and Ruth L. Kirschstein National Research Service Award F30-DK-093173. J. L. Nguyen is a trainee in the Molecular and Environmental Toxicology program at the University of Wisconsin-Madison funded by NIH Grant T32-ES-007015 and Science and Medicine Graduate Research Scholars.
DISCLAIMERS
The content is the sole responsibility of the authors and does not represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.M.N. and W.A.R. conceived and designed research; T.M.N. and J.A.T. performed experiments; T.M.N., J.L.N., J.A.T., F.S.v.S., R.W.W., and W.A.R. analyzed data; T.M.N., J.L.N., G.E.L., J.A.T., F.S.v.S., R.W.W., and W.A.R. interpreted results of experiments; T.M.N. prepared figures; T.M.N. drafted manuscript; T.M.N., J.L.N., G.E.L., F.S.v.S., R.W.W., and W.A.R. edited and revised manuscript; T.M.N., J.L.N., and W.A.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mike Moses, Nikesha Haynes, Pam Weller, Emily Ricke, Kristen Uchtmann, and the Ricke laboratory for assistance with animal husbandry and feedback on this manuscript.
Footnotes
Supplemental Material for this article is available online at the American Journal of Physiology-Renal Physiology website.
REFERENCES
- 1.Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behav Brain Res 182: 73–79, 2007. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arase S, Ishii K, Igarashi K, Aisaki K, Yoshio Y, Matsushima A, Shimohigashi Y, Arima K, Kanno J, Sugimura Y. Endocrine disrupter bisphenol A increases in situ estrogen production in the mouse urogenital sinus. Biol Reprod 84: 734–742, 2011. doi: 10.1095/biolreprod.110.087502. [DOI] [PubMed] [Google Scholar]
- 3.Bélanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab 79: 1086–1090, 1994. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 4.Bernoulli J, Yatkin E, Konkol Y, Talvitie EM, Santti R, Streng T. Prostatic inflammation and obstructive voiding in the adult Noble rat: impact of the testosterone to estradiol ratio in serum. Prostate 68: 1296–1306, 2008. doi: 10.1002/pros.20791. [DOI] [PubMed] [Google Scholar]
- 5.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116: 39–44, 2008. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res 111: 825–830, 2011. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro B, Sánchez P, Torres JM, Preda O, del Moral RG, Ortega E. Bisphenol A exposure during adulthood alters expression of aromatase and 5α-reductase isozymes in rat prostate. PLoS One 8: e55905, 2013. doi: 10.1371/journal.pone.0055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol 49: 651–659, 2006. doi: 10.1016/j.eururo.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Clement KD, Burden H, Warren K, Lapitan MC, Omar MI, Drake MJ. Invasive urodynamic studies for the management of lower urinary tract symptoms (LUTS) in men with voiding dysfunction. Cochrane Database Syst Rev 4: CD011179, 2015. doi: 10.1002/14651858.CD011179.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939–941, 1973. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 11.Dodds EC, Lawson W. Synthetic oestrogenic agents without the phenanthrene nucleus. Nature 137: 996–996, 1936. doi: 10.1038/137996a0. [DOI] [Google Scholar]
- 13.Hines CJ, Jackson MV, Deddens JA, Clark JC, Ye X, Christianson AL, Meadows JW, Calafat AM. Urinary bisphenol A (BPA) concentrations among workers in industries that manufacture and use BPA in the USA. Ann Work Expo Health 61: 164–182, 2017. doi: 10.1093/annweh/wxw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hormann AM, Vom Saal FS, Nagel SC, Stahlhut RW, Moyer CL, Ellersieck MR, Welshons WV, Toutain PL, Taylor JA. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA). PLoS One 9: e110509, 2014. doi: 10.1371/journal.pone.0110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin DE, Milsom I, Kopp Z, Abrams P, Artibani W, Herschorn S. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: impact of overactive bladder. Eur Urol 56: 14–20, 2009. doi: 10.1016/j.eururo.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300: 1303–1310, 2008. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 17.Lepor H. Medical treatment of benign prostatic hyperplasia. Rev Urol 13: 20–33, 2011. doi: 10.3909/riu0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung YY, Schwarz EM, Silvers CR, Messing EM, Wood RW. Uroflow in murine urethritis. Urology 64: 378–382, 2004. doi: 10.1016/j.urology.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Zhou Z, Qing D, He Y, Wu T, Miao M, Wang J, Weng X, Ferber JR, Herrinton LJ, Zhu Q, Gao E, Checkoway H, Yuan W. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod 25: 519–527, 2010. doi: 10.1093/humrep/dep381. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Miao M, Zhou Z, Gao E, Chen J, Wang J, Sun F, Yuan W, Li DK. Exposure to bisphenol-A and reproductive hormones among male adults. Environ Toxicol Pharmacol 39: 934–941, 2015. doi: 10.1016/j.etap.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 8: e55387, 2013. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan SA, Penson DF, Ulchaker JC, Wei JT. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 185: 1793–1803, 2011. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 23.Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS One 5: e8673, 2010. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mesnage R, Phedonos A, Arno M, Balu S, Corton JC, Antoniou MN. Editor’s highlight: transcriptome profiling reveals bisphenol A alternatives activate estrogen receptor alpha in human breast cancer cells. Toxicol Sci 158: 431–443, 2017. doi: 10.1093/toxsci/kfx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW, Ricke WA. Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology 153: 5556–5565, 2012. doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation 82: 184–199, 2011. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev 81: 1535–1565, 2001. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 29.Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci 3: 10, 2009. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricke WA, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, Cunha GR. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer 118: 2123–2131, 2006. doi: 10.1002/ijc.21614. [DOI] [PubMed] [Google Scholar]
- 31.Ricke WA, Wang Y, Kurita T, Hayward SW, Cunha GR. Hormonal and stromal regulation of normal and neoplastic prostatic growth. Prog Mol Subcell Biol 40: 183–216, 2005. doi: 10.1007/3-540-27671-8_8. [DOI] [PubMed] [Google Scholar]
- 32.Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123: 643–650, 2015. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohrmann S, Nelson WG, Rifai N, Kanarek N, Basaria S, Tsilidis KK, Smit E, Giovannucci E, Platz EA. Serum sex steroid hormones and lower urinary tract symptoms in Third National Health and Nutrition Examination Survey (NHANES III). Urology 69: 708–713, 2007. doi: 10.1016/j.urology.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Rudel RA, Brody JG, Spengler JD, Vallarino J, Geno PW, Sun G, Yau A. Identification of selected hormonally active agents and animal mammary carcinogens in commercial and residential air and dust samples. J Air Waste Manag Assoc 51: 499–513, 2001. doi: 10.1080/10473289.2001.10464292. [DOI] [PubMed] [Google Scholar]
- 35.Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect 119: 914–920, 2011. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scinicariello F, Buser MC. Serum testosterone concentrations and urinary bisphenol A, benzophenone-3, triclosan, and paraben levels in male and female children and adolescents: NHANES 2011–2012. Environ Health Perspect 124: 1898–1904, 2016. doi: 10.1289/EHP150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect 120: 1297–1300, 2012. doi: 10.1289/ehp.1104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar A, Teppala S, Sabanayagam C. Urinary bisphenol A levels and measures of obesity: results from the National Health and Nutrition Examination Survey 2003–2008. ISRN Endocrinol 2012: 965243, 2012. doi: 10.5402/2012/965243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect 117: 784–789, 2009. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki K, Ito K, Ichinose Y, Kurokawa K, Suzuki T, Imai K, Yamanaka H, Honma S. Endocrine environment of benign prostatic hyperplasia: prostate size and volume are correlated with serum estrogen concentration. Scand J Urol Nephrol 29: 65–68, 1995. doi: 10.3109/00365599509180541. [DOI] [PubMed] [Google Scholar]
- 41.Taylor JA, Richter CA, Suzuki A, Watanabe H, Iguchi T, Coser KR, Shioda T, vom Saal FS. Dose-related estrogen effects on gene expression in fetal mouse prostate mesenchymal cells. PLoS One 7: e48311, 2012. doi: 10.1371/journal.pone.0048311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41a.U.S. Environmental Protection Agency Bisphenol A Alternatives in Thermal Paper. Washington, DC: U.S. EPA, 2014. [Google Scholar]
- 42.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet 17: 407–434, 2012. doi: 10.1590/S1413-81232012000200015. [DOI] [PubMed] [Google Scholar]
- 43.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118: 1055–1070, 2010. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health 14: 239–260, 1998. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 45.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 113: 926–933, 2005. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.vom Saal FS, Welshons WV. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Mol Cell Endocrinol 398: 101–113, 2014. doi: 10.1016/j.mce.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vrooman LA, Oatley JM, Griswold JE, Hassold TJ, Hunt PA. Estrogenic exposure alters the spermatogonial stem cells in the developing testis, permanently reducing crossover levels in the adult. PLoS Genet 11: e1004949, 2015. doi: 10.1371/journal.pgen.1004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson CS, Jeng YJ, Guptarak J. Endocrine disruption via estrogen receptors that participate in nongenomic signaling pathways. J Steroid Biochem Mol Biol 127: 44–50, 2011. doi: 10.1016/j.jsbmb.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 111: 994–1006, 2003. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winett L, Wallack L, Richardson D, Boone-Heinonen J, Messer L. A framework to address challenges in communicating the developmental origins of health and disease. Curr Environ Health Rep 3: 169–177, 2016. doi: 10.1007/s40572-016-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood RW, Baggs RB, Schwarz EM, Messing EM. Initial observations of reduced uroflow in transgenic adenocarcinoma of murine prostate. Urology 67: 1324–1328, 2006. doi: 10.1016/j.urology.2005.12.019. [DOI] [PubMed] [Google Scholar]