Abstract
Excessive vascular smooth muscle cell (SMC) proliferation, migration and extracellular matrix (ECM) synthesis are key events in the development of intimal hyperplasia, a pathophysiological response to acute or chronic sources of vascular damage that can lead to occlusive narrowing of the vessel lumen. Atherosclerosis, the primary cause of coronary artery disease, is characterised by chronic vascular inflammation and dyslipidemia, while revascularisation surgeries such as coronary stenting and bypass grafting represent acute forms of vascular injury. Gene knockouts of transforming growth factor-beta (TGFβ), its receptors and downstream signalling proteins have demonstrated the importance of this pleiotropic cytokine during vasculogenesis and in the maintenance of vascular homeostasis. Dysregulated TGFβ signalling is a hallmark of many vascular diseases, and has been associated with the induction of pathological vascular cell phenotypes, fibrosis and ECM remodelling. Here we present an overview of TGFβ signalling in SMCs, highlighting the ways in which this multifaceted cytokine regulates SMC behaviour and phenotype in cardiovascular diseases driven by intimal hyperplasia.
Keywords: Transforming growth factor-beta, Smads, Cardiovascular disease, Smooth muscle cells, Vascular cells, Revascularisation surgery
Highlights
-
•
Dysfunctional smooth muscle cells (SMCs) contribute to the development of intimal hyperplasia and coronary artery disease (CAD)
-
•
Transforming growth factor-beta (TGFβ) is a powerful regulator of SMC phenotype and function
-
•
The activities of TGFβ in CAD are context-dependent, with both atheroprotective and atherogenic roles in SMCs
-
•
TGFβ regulates SMC proliferation and ECM secretion after revascularisation surgery
-
•
The TGFβ signaling axis is an attractive target for therapies aimed at reducing SMC dysfunction and fibrosis in CAD
1. Introduction
Classic ultrastructural studies by Schwartz et al were the first to show the presence of morphologically identifiable vascular smooth muscle cells (SMCs) migrating though the internal elastic lamina following acute vascular injury in a rat model of balloon angioplasty [1]. Later, seminal work by Clowes et al using [3H]-thymidine labelling showed that over 40% of medial SMCs were actively proliferating 48 hours post-injury, indicating that a large proportion of SMCs within the vascular wall retain the capacity to re-enter the cell cycle and contribute to vascular remodelling and repair in adult animals [2]. This phenotypic plasticity of SMCs is now understood to play a significant role in the development of intimal hyperplasia, a pathological vascular remodelling process that occurs during the development of coronary artery disease following prolonged exposure to dyslipidaemia, hypertension and inflammation [[3], [4], [5]] or as a consequence of revascularisation surgery, such as coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) [6]. In the latter, a combination of ischemic-reperfusion injury, acute physical damage and increased longitudinal and circumferential shear stress results in endothelial cell (EC) activation, triggering the release of cytokines and growth factors, including transforming growth factor-beta (TGFβ) [7]. In concert, these growth factors and cytokines drive the de-differentiation of quiescent ‘contractile’ SMCs into an active ‘synthetic’ state, in which they display enhanced proliferation, migration and secretory capacity [6].
2. The TGFβ signalling pathway
TGFβ is the prototype of the highly-conserved TGFβ superfamily, members of which are potent regulators of SMC phenotype and function in vascular homeostasis and disease [8]. TGFβ superfamily share the same overall structure, consisting of two extended monomers held together by an intermolecular disulphide bond [9]. All TGFβ monomers incorporate a characteristic ‘cysteine knot’ structure, composed of three intramolecular disulphide bonds linking six conserved cysteine residues [10]. Three TGFβ isoforms are expressed in mammals (TGFβ 1-3) and are differentially localised in major blood vessels during development, with TGFβ1 highly localised to the tunica intima, TGFβ2 restricted to the tunica media and TGFβ3 expressed throughout the whole vessel wall [11,12]. In adults, TGFβ1 and TGFβ3 proteins are mainly localised to the arterial intima, with TGFβ1 present in around 50% of the intimal stellate-shaped SMC population [13]. TGFβ is secreted as part of a large latent complex (LLC), consisting of the C-terminal mature TGFβ peptide and N-terminal latency associated peptide (LAP) covalently bound to large latent TGFβ binding proteins (LTBP) [14]. LTBPs stabilise latent TGFβ complexes and facilitate their retention at the cell surface through direct interactions with fibrillin and other ECM proteins [15], while RGD sequences in the LAP target latent TGFβ to integrin receptors [16]. Activation of latent TGFβ at the cell surface is induced primarily by proteases such as furin and plasmin, which cleave the covalently-bound LAP-LTBP pair from the mature TGFβ molecule [17]. Proteolytic cleavage of LAP-LTBP yields short-lived, biologically active TGFβ homodimers which are able to interact with transmembrane TGFβ type III receptors such as betaglycan (also known as TβRIII) and endoglin [18]. Betaglycan is expressed in the majority of cell types, whereas endoglin is most abundantly expressed in vascular ECs, although recent studies have also shown localisation to SMCs in diseased vessels [[19], [20], [21], [22]]. Both betaglycan and endoglin are now thought to have important cellular functions beyond their actions as TGFβ co-receptors, which are reviewed at length elsewhere [23,24].
Binding to type III accessory receptors facilitates TGFβ signalling through presentation of ligand to signal transduction receptors at the cell surface. Active TGFβ homodimers signal via specific transmembrane heteromeric complexes comprised of two type I and two type II serine/threonine kinase receptors [25]. Five TGFβ superfamily type II receptors and seven type I receptors exist in mammals [26]. The type I and type II receptors are structurally similar with small cysteine-rich ECDs (100-140 amino acids), single TMDs (30-35 amino acids) and highly conserved intracellular serine/threonine kinase domains (S/TKD; 350-400 amino acids) [9]. Each member of the TGFβ superfamily binds to a characteristic combination of type I and type II receptors (Table 1). Analysis of the crystal structures of TGFβ ligand:receptor ternary complexes has revealed that the length and conformation of the ligand fingertips and receptor ligand binding loops are important determinants of ligand: receptor specificity [27]. These studies have illustrated that TGFβ ligands use their conserved Site IIa in their fingertip region to bind the β1 and β2 strands within the ECD of the TGFβ type II receptor (TβRII) [28]. Importantly, the β4-β5 region within the ECD of the TβRII contains a 5-8 amino acid insertion which ensures type II receptor specificity by blocking binding of TβRII to bone morphogenetic protein (BMP) ligands. Of the five mammalian type II receptors, TGFβ binds specifically to TβRII (also known as TGFBR2), which is highly expressed throughout the intima and media of adult vessels [13]. Early membrane crosslinking studies confirmed the expression of TβRII in SMCs, also showing binding of I125TGFβ1 to receptor complexes composed of type I, II and III TGFβ receptors [29]. TβRII ligand binding induces the assembly of type I and type II receptors into a heteromeric complex, within which constitutively active TβRII phosphorylates type I receptors at several serine and threonine residues within their conserved glycine-serine (GS) domains [8,30]. TGFβ ligands principally signal via activin receptor-like kinase 5 (ALK5, a type I receptor also known as TβRI) [14]. In addition to ALK5, TGFβ can also signal via another type I receptor called activin receptor-like kinase 1 (ALK1), via a distinct Smad-mediated signalling pathway to ALK5 [[31], [32], [33], [34], [35]]. While ALK5 is predominantly expressed in medial SMCs in vessels from healthy adult animals, ALK1 is chiefly localised to the endothelium, although it is upregulated in SMCs following acute vascular injury or during atherogenesis [[36], [37], [38]]. Following activation, type I TGFβ receptors propagate the signal inside the cell through activation of the canonical Smad signalling pathway, as well as other Smad-independent kinase pathways (Fig. 1; [25]). Readers are directed to a series of excellent reviews on TGFβ signalling via non-canonical kinase pathways [26,39,40].
Table 1.
- Ligands, receptors and R-Smads in the TGFβ superfamily
| Ligand | Type I receptor | Type II receptor | Type III receptor | R-Smad |
|---|---|---|---|---|
| TGFβ1 TGFβ2 TGFβ3 |
ALK1/5 | TβRII | Betaglycan Endoglin |
Smad1/5/8 Smad2/3 |
| BMP2 BMP4 |
ALK3/6 | BMPRII | RGM Betaglycan/Endoglin |
Smad1/5/8 |
| BMP5 BMP6 BMP7 |
ALK2/3/6 | BMPRII ActRIIA ActRIIB |
Betaglycan Endoglin |
Smad1/5/8 |
| BMP8A BMP8B |
ALK3/5 | BMPR2/ActRIIA ActRIIB/TβRII |
Not known | Smad1/5/8 Smad2/3 |
| BMP9 BMP10 |
ALK1/3/6 | BMPRII/ActRIIA | Endoglin | Smad1/5/8 |
| GDF7 GDF6 GDF5 |
ALK2/3/6 | BMPRII/ActRIIA/ ActRIIB |
Not known | Smad1/5/8 |
| AMH | ALK2/3/6 | AMHRII | Not known | Smad1/5/8 |
| Activin A/AB/B GDF8 GDF11 |
ALK4 | BMPRII ActRIIA ActRIIB |
Betaglycan Endoglin |
Smad2/3 |
| BMP16/Nodal | ALK7 | BMPRII/ActRIIA ActRIIB |
Not known | Smad2/3 |
TGFβ = transforming growth factor beta, BMP = bone morphogenetic protein, GDF = growth/differentiation factor, AMH = anti-Mϋllerian hormone, RGM = repulsive guidance molecule
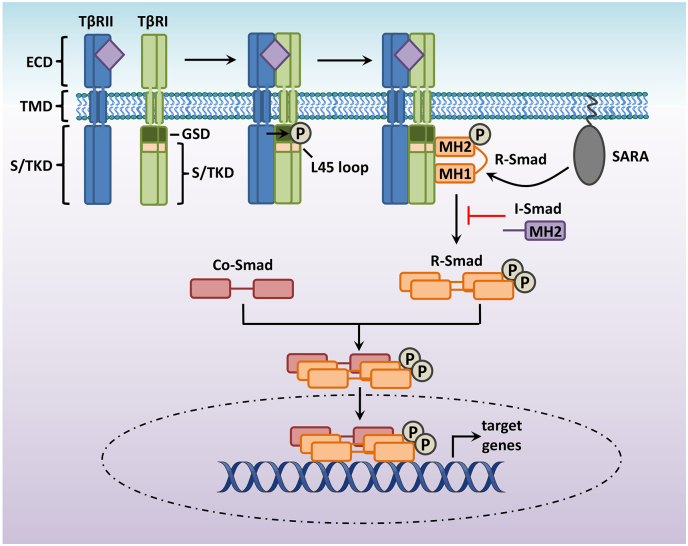
Fig. 1.
- Canonical TGFβ signalling pathway. Active TGFβ homodimers signal via binding to specific transmembrane receptor complexes comprised of two type I (TβRI) and two type II (TβRII) serine/threonine kinase receptors. TβRI and TβRII are structurally similar with small cysteine-rich extracellular domains (ECD), single transmembrane domains (TMD) and highly conserved intracellular serine/threonine domains (S/TKD). TGFβ binding to TβRII induces the assembly of TβRII and TβRI receptors into a heteromeric complex, within which constitutively active TβRII phosphorylates TβRI at several serine and threonine residues within its conserved glycine-serine domain (GSD). R-Smads become phosphorylated by the activated TβRI at their C-terminal SSXS motif. The L45 loop of TβRI and the L3 loop of the R-Smad MH2 domain determine R-Smad receptor specificity, with ALK5 specifically phosphorylating Smads 2 and 3. The adaptor protein Smad anchor for receptor activation (SARA) can also facilitate recognition of R-Smads by the receptors. I-Smads contain MH2 domains and can act to turn off Smad TGFβ signalling by interfering with Smad-receptor or Smad-Smad interactions. Phosphorylated R-Smads form a heteromeric complex with Co-Smad, accumulate in the nucleus and directly regulate the transcription of specific target genes.
3. Canonical Smad TGFβ signalling
Smad proteins are the principal intracellular mediators of TGFβ superfamily signalling. Of the eight Smad proteins expressed in mammals (Smads 1-8), Smads 2 and 3 are the primary receptor-regulated Smads (or R-Smads) activated by receptors for the three TGFβ ligands [25,41]. Smad4, also known as Co-Smad, serves as a common partner for all R-Smads. Smad6 and Smad7 are inhibitory Smads (I-Smads) which act to turn off Smad TGFβ signalling by interfering with Smad-receptor or Smad-Smad interactions [25]. In general, all Smads are widely expressed throughout development and in adult animals [42]. The R-Smads and Co-Smad share homologous N- and C-terminal regions, called the Mad-homology 1 (MH1) and MH2 domains respectively, separated by a divergent proline-rich linker region [43]. I-Smads contain conserved MH2 domains but do not possess MH1 domains [8]. With the exception of Smad2, the MH1 domains of Smads exhibit sequence specific DNA binding activity, whereas MH2 domains mediate Smad oligomerisation and Smad-receptor interactions [25,44]. The linker region of R-Smad contains multiple phosphorylation sites which allow specific crosstalk with other signalling pathways including mitogen-activated protein kinases (MAPKs) and cyclin-dependent kinases, and a PY motif which mediates specific interactions with the Smurf ubiquitin ligases [25].
In non-stimulated cells, Smads undergo a constant process of nucleocytoplasmic shuttling, with the rate of nuclear export being higher than the rate of import, such that the R-Smads are predominantly localised to the cytoplasm [45]. In contrast, I-Smads tend to be localised within the nucleus in non-stimulated cells and Smad4 is distributed equally between both compartments [46]. Upon ligand stimulation, R-Smads become phosphorylated by the activated type I receptor at their C-terminal SSXS motif, which increases their affinity for Smad4 [25]. The L45 loop of the type I receptor (located adjacent to its GS region) and the L3 loop of the R-Smad C-terminal domain determine R-Smad receptor specificity. The primary TGFβ type I receptor in SMCs, ALK5, specifically phosphorylates Smads 2 and 3 [47,48]. Receptor recognition of R-Smads can be facilitated by auxiliary proteins, such as the adaptor protein, Smad anchor for receptor activation (SARA). SARA contains a phospholipid binding FYVE domain which targets Smads 2 and 3 to the plasma membrane and early endosomes, where it facilitates their interaction with the activated TβRI [49]. Phosphorylated R-Smads form a heteromeric complex with Smad4 and accumulate in the nucleus following importin-mediated nuclear translocation [25].
Nuclear R-Smad/Smad4 complexes bind directly to Smad-binding elements (SBE) in the promoters of TGFβ target genes via a highly conserved β-hairpin loop within their MH1 domain [50]. Although many Smad-responsive promoter regions contain one or more SBEs [50], oligonucleotide binding assays have shown that Smad complexes can also recognise and bind GC-rich promoter sequences, demonstrating a relaxed DNA-binding specificity of the Smad MH1 domain [41]. As the affinity of Smad binding to a single SBE is insufficient to support sustained binding to DNA in the absence of co-operating transcriptional partners [50,51], they exert the majority of their effects on gene expression in co-operation with DNA binding co-factors, co-activators and co-repressors [41]. For example, the transcription factor δEF1 (also known as ZEB-1) is selectively expressed in SMCs and transactivates the promoters of SMC differentiation markers following TGFβ1 stimulation of SMCs, by directly binding Smad3 and serum response factor (SRF) [52]. Similarly, the transcriptional coactivator myocardin physically associates with Smad3 in SMCs, co-ordinately transactivating the promoters of the SM22α, smooth muscle myosin heavy chain (SMMHC) and smooth muscle α-actin genes (ACTA2; [53]). Thus, while Smad proteins are ubiquitously expressed, the expression of Smad transcriptional partners is generally restricted to certain cell types, thereby providing a mechanism for cell lineage-specific gene responses [41]. Readers are directed to two excellent recent reviews on the contextual control of gene transcription elicited by Smad proteins [54,55].
4. TGFβ in coronary artery disease
Coronary artery disease (CAD) is primarily caused by atherosclerosis, which leads to the formation of occlusive, lipid-rich plaques in affected vessels (Fig. 2A) [56]. Prolonged exposure to cardiovascular risk factors such as dyslipidemia, hypertension and inflammation promotes endothelial dysfunction, which precedes atherosclerotic lesion formation [[3], [4], [5]]. The increased vascular permeability of dysfunctional, activated endothelial cells (ECs) promotes the entry of low density lipoproteins (LDLs) from the circulation into the vascular intima. Proteoglycans in the arterial wall (such as versican, biglycan and decorin) bind and retain LDLs, which become oxidised (oxLDL; [[57], [58], [59]]). OxLDL induces the secretion of chemokines and the expression of leukocyte adhesion molecules, which together promote monocyte infiltration into the sub-endothelial space [60]. Within the intima, SMC- and EC-derived cytokines induce monocytes to differentiate into macrophages that engulf oxLDL, forming foam cells. In turn, inflammatory cells within the early lesion secrete cytokines and growth factors which promote the development of intimal hyperplasia. Resident SMCs are key drivers of intimal hyperplasia in the initiation and early progression of atherosclerosis, which is characterised by SMC dedifferentiation, proliferation and migration [61]. Secretory SMCs synthesise an abundant array of ECM components, which form a fibrous cap over the plaque, further encroaching on the vessel lumen [62]. Increased synthesis of proteoglycans by secretory SMCs also promotes lipoprotein retention in the growing lesions, while dedifferentiated SMCs acquire phenotypic characteristics of the osteoblast, adipocyte and macrophage lineages [63]. Advanced, rupture-prone plaques are characterised by lipid-rich necrotic cores (composed of apoptotic foam cells and cellular debris) thin fibrotic caps (a consequence of matrix metalloproteinase secretion), vascular calcification and neoangiogenesis.
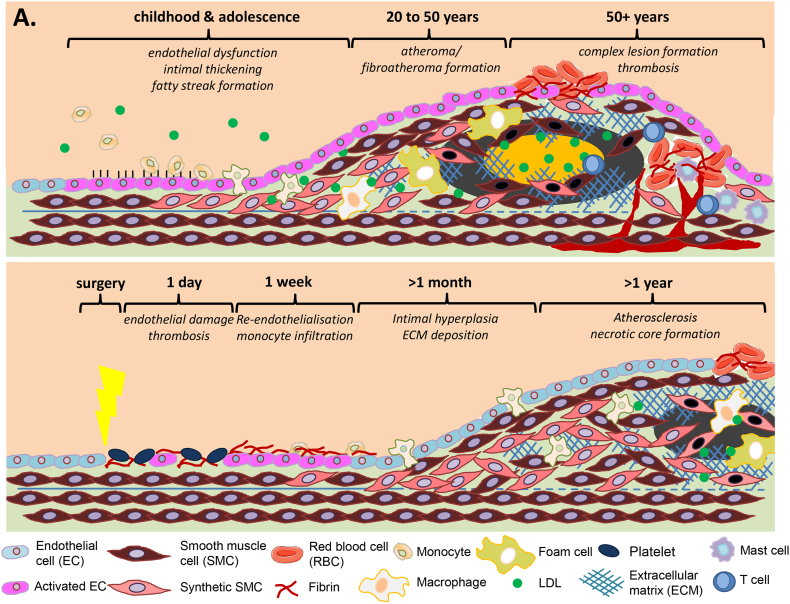
Fig. 2.
– Vascular remodelling during atherosclerosis (A) and after revascularisation surgery (B) (A) Atherosclerosis is initiated by the activation of the endothelium in response to oxidative, haemodynamic or biochemical stimuli. Activated endothelial cells (ECs) upregulate surface adhesion molecules and secrete growth factors and cytokines, promoting rolling adhesion of circulating leukocytes as well as activation of the underlying smooth muscle cells (SMCs). Activated SMCs dedifferentiate and start proliferating and migrating, contributing to the growing neointima. Leukocytes adhering to the endothelium migrate into the intima through diapedesis, maturing into macrophages and phagocytosing low density lipoproteins to become foam cells, characteristic of the ‘fatty streak’ lesions that can be observed from adolescence onwards. Fibroatheromas form from areas of intimal thickening, which consist of foam cells, remnants of apoptotic SMC and a lipid rich ECM pool. Early fibroatheromas are characterised by an acellular necrotic core and a thick fibrous cap, composed of collagen fibrils interspersed with SMCs. Advancing fibroatheromas contain cholesterol crystals, neovessels and lymphocytes, and have thin fibrous caps due to proteolytic ECM degradation, making these lesions particularly susceptible to rupture and thrombosis. Rupture and thrombosis frequently occurs at the shoulder regions of plaques, where mast cells accumulate and secrete pro-angiogenic factors and enzymes to further promote microvessel formation. (B) Vein graft implantation or coronary stent deployment induces endothelial damage and denudation. Within hours, platelets and red blood cells adhere to the endothelial layer, initiating a coagulation cascade that results in the deposition of fibrin-rich layers. In the weeks following surgery, circulating leukocytes attach and infiltrate the vascular endothelium, while SMCs in the media are activated and start migrating into the growing neointima. Growth factors and cytokines released by cells in the vessel wall induce SMC proliferation and ECM deposition, resulting in further intimal thickening and inward vascular remodelling. Intimal thickening can act as a substrate for superimposed atherosclerosis or neoatherosclerosis, which is frequently observed between 2-5 years following revascularisation surgery. The pathogenesis of superimposed atherosclerosis/neoatherosclerosis bears many similarities with native coronary artery atherosclerosis (A), albeit within a much shorter timeframe.
Several genome-wide association studies (GWAS) have identified an association between CAD and single nucleotide polymorphisms (SNPs) in genes encoding TGFβ signalling pathway components. For example, functional polymorphisms in the promoter, signal peptide sequence and coding sequence of the TGFβ1 gene are associated with increased risk of myocardial infarction [64,65]) and stroke [66]; meta-analyses have also shown an association between these polymorphisms and CAD [[67], [68], [69], [70]]. In addition, a joint analysis of two GWAS on CAD patients identified an association with an intronic SNP in the SMAD3 gene [71] which was later shown to reduce enhancer activity and attenuate Smad3 expression [72,73]. Clinical studies have illustrated that plasma levels of active TGFβ1 are markedly reduced in patients with advanced atherosclerosis compared with healthy controls [74,75]. In contrast, other groups have reported an increase in active TGFβ1 levels in the plasma of CAD patients, where patients with triple vessel disease had twice the level of circulating active TGFβ1 compared with those with no or mild CAD [76]. These differences may be due to discrepancies between sample preparation methods, which can affect the level of TGFβ1 protein detected in plasma [77]. Nevertheless, immunolocalisation studies of human atherosclerotic lesions strongly support a role for TGFβ in the pathogenesis of CAD, showing high levels of TGFβ1 and TGFβ3 in SMCs and macrophage-derived foam cells in early fatty streak lesions, co-localising with TβRII and ALK5 [13]. >50% of SMCs in early lesions also stain positive for phospho-Smad2, indicative of TGFβ signalling pathway activation in these cells [78]. Advanced fibrous lesions also express significant amounts of TGFβ1, whereas TGFβ3, TβRII and ALK5 are expressed at more reduced levels in the fibrous plaque and the associated media [13].
Studies in experimental models of atherosclerosis indicate that TGFβ can be both atheroprotective and atherogenic. Early animal studies using global TGFβ inhibition or genetic knockout approaches demonstrated that reduced availability of TGFβ was pro-atherogenic and associated with the development of macrophage-rich, pro-inflammatory plaques which were prone to rupture [79]. Mice heterozygous for the deletion of tgfb1 on a cholesterol-enriched diet had reduced SMC differentiation (determined by levels of αSMA and SMMHC, two mature SMC marker proteins), accelerated lipid lesion formation and increased vascular inflammation compared with wild-type littermate controls [80]. Similarly, administration of a neutralising anti-TGFβ1 antibody [81], or systemic infusion of a dominant negative TβRII in apolipoprotein E (ApoE)-deficient mice [82] significantly enhanced lipid infiltration in the vascular wall, decreased collagen type I and III secretion by SMCs and was associated with frequent intraplaque haemorrhages. Corroborating these TGFβ knockdown studies, overexpression of an activated TGFβ1 expression construct via viral gene transfer markedly reduced atherosclerotic lesion formation in fat-fed LDL receptor knockout mice [83]. In these animals, medial and intimal SMCs showed reduced expression of the oxidative stress marker nitrotyrosine, with CD68+ macrophage infiltration also substantially attenuated as a result of diminished SMC-derived M-CSF secretion [83]. Similarly, overexpression of active TGFβ1 in the hearts of ApoE-/- mice reduced aortic root plaque formation by decreasing inflammatory cell infiltration and increasing SMC collagen secretion to form more stable atherosclerotic lesions [84]. Interestingly, pre-incubation of rat SMCs with atorvastatin enhanced the TGFβ1-mediated activation of Smad2/3; similar results were observed in ApoE-/- mice treated with a moderate dose of statin, accompanied by increased collagen and αSMA staining in plaques [85]. Together, these studies support the ‘protective cytokine’ theory of atherosclerosis [86], indicating that TGFβ can protect against the development of unstable plaque lesions by promoting the expression of contractile SMC proteins, supressing leukocyte recruitment, and reinforcing the fibrous cap by enhancing ECM production by resident SMCs.
There is, however, an important caveat to these observations; while the induction of contractile marker proteins (such as αSMA and SM22α) by TGFβ can be viewed as atheroprotective in SMCs, very recent studies investigating the origin of αSMA+ cells within atherosclerotic lesions have demonstrated that activation of this transcriptional programme by TGFβ in endothelial cells (ECs) can instead promote the induction of atherosclerosis. Using endothelial lineage tracing mice on an ApoE-/- background (SclCreERT2; R26RstopYFP;ApoE−/−), Evrard et al found that TGFβ could induce endothelial-to-mesenchymal transition (EndMT) during atherogenesis, enhancing expression of αSMA and fibrotic markers in ECs without affecting collagen expression (87). Of note, immunohistological evaluation of human atherosclerotic lesions revealed a higher proportion of cells co-expressing endothelial and fibroblast markers in type VI plaques (complicated lesions with unstable features) compared to type V plaques (stable fibrocalcific lesions/fibroatheromas) supporting a role for TGFβ-induced EndMT in the clinical context [87]. There is also accumulating evidence that TGFβ can elicit atherogenic effects through its actions on SMCs in early plaque lesions. For example, while the promotion of contractile protein expression in SMCs is an important part of TGFβ’s anti-atherogenic repertoire during the later stages of plaque development, increased vascular resistance and SMC hypercontractility is also associated with the induction of atherosclerosis [88]. Additionally, TGFβ is now known to be a potent inducer of proteoglycan (PG) synthesis by SMCs, enhancing the gene expression and glycosaminoglycan (GAG) sidechain elongation of PGs such as biglycan [89,90] and versican [91]. PGs directly contribute to the initiation of atherosclerosis through their electrostatic interactions with lipoproteins, promoting the retention of lipoproteins in the sub-endothelial space (reviewed in [92]). Accordingly, treatment of atheroprone LDLr-/- mice with the TGFβ neutralising antibody 1D11 substantially repressed biglycan expression, reducing biglycan colocalisation with apoB lipoproteins and attenuating atherosclerotic lesion formation [93]. Finally, it has recently been recognised that TGFβ can drive the transdifferentiation of SMCs into proliferative, αSMA-positive migratory myofibroblasts, thereby contributing to the early development of atherosclerotic plaques, whilst on the other hand promoting stability of more advanced lesions through fibrotic cap formation [94,95]. Thus, while TGFβ generally acts as a potent pro-fibrotic and anti-inflammatory mediator in CAD, the pathophysiological outcome of these actions is highly context-dependent, varying according to the specific cell type, stage of atherosclerosis (early/advanced) and type of lesion (stable/unstable).
5. TGFβ in acute vascular injury: vein graft failure and restenosis
Revascularisation surgeries such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) are frequently prescribed for advanced or acute presentations of CAD, aiming to widen occluded coronary arteries. However, the long-term patency of such procedures is hampered by the development of intimal hyperplasia within the vessel, resulting in re-occlusion and the need for repeat intervention. Similar to the intimal hyperplasia (IH) that forms a fertile ‘soil’ for atherosclerosis in CAD, IH following revascularisation surgery is initiated by activation of the endothelium. Stent deployment (PCI) or exposure of venous bypass conduits to increased arterial shear stress (CABG) induces acute endothelial injury, leading to adhesion of circulating platelets and monocytes to the endothelium. Pro-inflammatory growth factors and cytokines released by activated endothelial cells, platelets and leukocytes drive SMC dedifferentiation, proliferation and migration, cellular processes that are critical to the development of IH. Medial SMC proliferation is rapidly induced following vascular injury and peaks around 7 days post-injury (10 - 20 % medial SMC proliferation; [96] [97]). Proliferating medial SMCs also migrate and accumulate in the intima, contributing to the overall lesion cell mass [98,99]. The resulting hyperplastic neointima can act as a substrate for accelerated atherosclerotic plaque formation, which further contributes to the occlusion of the vessel. Compared to native CAD, which takes decades to develop, vein graft atherosclerosis develops over a period of months to a few years. Lesions are also more concentric and diffuse than native atherosclerotic lesions and are more susceptible to thrombosis and rupture ([100,101]; reviewed in [102]). There is growing awareness that in-stent atherosclerosis (most frequently termed ‘neoatherosclerosis’) is one of the primary causes of late stent failure, with several studies showing that the development of neoatherosclerosis is accelerated in drug-eluting stents (DES) compared to first-generation bare-metal stents (BMS) [[103], [104], [105], [106]]. Although the mechanisms causing neoatherosclerosis have yet to be fully characterised, histopathological and intravascular imaging studies indicate that stent-induced shear stress, chronic inflammation and endothelial dysfunction may be key contributing factors (Fig. 2B) [102].
Clinical studies of restenotic arteries following balloon angioplasty were among the first to suggest that TGFβ plays a prominent role in the development of IH following revascularisation surgery. These studies showed that the mRNA expression of TGFβ1 was significantly elevated in restenotic lesions compared with both primary atherosclerotic lesions and control non-atherosclerotic tissues [107]. IHC co-staining for TGFβ1 and αSMA within sections taken adjacent to those studied by in situ hybridisation demonstrated that TGFβ1 was localised to the intimal and medial SMC layers in restenotic lesions [107]. Two further groups demonstrated that the majority of human restenotic lesions showed positive staining for TGFβ1 throughout the vascular media and intima, identifying SMCs as a key source of TGFβ1 in restenotic vessels [108,109]. Pre-clinical studies support this observation, showing that TGFβ1 is upregulated in SMCs at sites of acute vascular injury in rodents. TGFβ1 mRNA levels were significantly increased in rat carotid arteries 6 hours post-injury and remained significantly elevated for at least 2 weeks [110,111]. Increased TGFβ1 expression (both at the mRNA and protein level) 2 weeks after wounding was associated with a parallel increase in fibronectin, collagen I and collagen III mRNA expression, three pro-fibrotic genes known to be regulated by TGFβ [111]. In a porcine coronary angioplasty model, levels of active TGFβ1 were significantly elevated between 2 hours and 7 days following angioplasty, with immunohistochemical studies showing strong localisation to both SMCs and ECs [112]. TGFβ1 mRNA and protein levels have also been shown to be chronically upregulated 6 months post-grafting in a rabbit CABG model, accompanied by increases in connective tissue growth factor (CTGF), a well-defined TGFβ1 responsive gene [113].
Subsequently, numerous in vivo interventional studies have convincingly shown that TGFβ promotes IH in vein grafts and models of PCI (Table 2). Overexpression of TGFβ1 in porcine arteries using an expression plasmid resulted in increased procollagen, collagen and proteoglycan synthesis by neointimal SMCs and was accompanied by marked intimal and medial hyperplasia [114]. Furthermore, adenoviral overexpression of active TGFβ1 in uninjured rat arteries resulted in a hyperplasic neointima [96] or a larger collagen-rich neointima in grafted rat arteries compared with control [115]. Similarly, infusion of purified recombinant TGFβ1 into rats 2 weeks after carotid artery injury increased [3H]-thymidine-labelled SMC nuclei within the neointima, indicating that TGFβ1 stimulates SMC proliferation as well as collagen synthesis in this model of vascular injury [111]. Substantiating these TGFβ1 overexpression studies, inhibition of TGFβ by antisense treatment or by intravenous infusion of a soluble TβRII reduced IH and adventitial fibrosis in balloon-injured rat carotid arteries [116,117]. Interestingly, adenovirus-mediated overexpression of TGFβ3 (but not TGFβ1) in pig coronary arteries inhibited constrictive remodelling and reduced lumen loss after coronary angioplasty [118]. Similarly, direct infusion of TGFβ3 to goat carotid arteries after anastomosis reduced vessel wall thickness by 30%, in part by reducing collagen type VII content 3 months post-surgery [119]. In contrast to the increased intimal hyperplasia observed in interventional studies using TGFβ1, these groups also showed reduced SMC proliferation in TGFβ3-treated animals, which suggests that there may be differences in how SMCs respond to specific TGFβ isoforms in vivo. The intracellular signalling mechanisms that drive IH in response to TGFβ have yet to be fully characterised, however the majority of studies to date have identified an important role for the canonical Smad2/3 pathway. Kundi et al showed that carotid artery injury in rats leads to significant induction of Smad3 in medial SMCs, while overexpression of Smad3 via gene transfer resulted in increased collagen accumulation [120] and SMC proliferation via a p27-dependent mechanism [97]. Furthermore, adenoviral overexpression of Smad7 in rat balloon-injured arteries reduced intimal thickening, lumen area loss and collagen synthesis 14 days post-injury [121], with in vitro studies indicating that these effects were due to direct effects on resident SMCs [122,123]. Interestingly, oral dosing of an ALK5/ALK4 small molecule inhibitor following balloon injury decreased intimal collagen production but had no effect on intimal SMC proliferation [124], suggesting that other TGFβ receptor signalling pathways may be responsible for TGFβ-induced SMC proliferation.
Table 2.
In vivo studies employing different approaches to target TGFβ activity after vascular injury
|
Therapy |
Animal model | Outcome compared to control | Reference |
|---|---|---|---|
| Soluble TβRII | Rat carotid artery balloon injury | Reduced intimal thickening, constrictive remodelling, lumen area loss and collagen type I/III mRNA expression | Smith et al, 1999 [116] |
| ALK4/5/7 inhibitor (SB431542) | Rat carotid artery balloon injury | Reduced intimal thickening, neointimal SMC proliferation, reduced recruitment of MSCs | Zhao et al, 2016 [182] |
| ALK4/5 kinase inhibitor (SM16) | Rat carotid artery balloon injury | Reduced intimal thickening, inhibition of adventitial myofibroblast formation, collagen deposition | Fu et al, 2008 [124] |
| Anti-TGFβ1 ribozyme oligonucleotides | Rat carotid artery balloon injury | Reduced intimal thickening, TGFβ1 mRNA expression, collagen type I/III expression and synthesis | Yamamoto et al, 2000 [117] |
| Anti-TGFβ1 phosphorothioate oligonucleotides | Rabbit carotid artery balloon injury | Reduced intimal thickening, proteoglycan synthesis and TGFβ1 mRNA expression | Merrilees et al, 2000 [183] |
| Tranilast | Rat carotid artery balloon injury | Reduced SMC migration, TGFβ1 mRNA expression, TβRI/TβRII mRNA expression and αVβ3 mRNA expression | Ward et al, 1998 [184] |
| TGFβ1 antisense mRNA (adenoviral overexpression) | Rat femoral artery vein grafting | Reduced intimal thickening, reduced collagen and TIMP mRNA expression | Wolff et al, 2006 [115] |
| Recombinant TGFβ3 | Pig coronary artery balloon injury | Reduced constrictive remodelling, lumen area loss and increased collagen synthesis | Kingston et al, 2003 [118] |
| Smad7 (adenoviral overexpression) | Rat carotid artery balloon injury | Reduced intimal thickening, lumen area loss, collagen synthesis and adventitial fibroblast migration | Maallawaarachchi et al, 2005 |
| p38 MAPK inhibitors | Rat carotid artery balloon injury | Reduced intimal thickening and SMC proliferation | Ohashi et al, 2000 [185] |
| Pyrrole-imidazole polyamide targeting the TGFβ1 promoter | Rat carotid artery balloon injury | Reduced intimal thickening, TGFβ1, collagen and fibronectin mRNA expression and accelerated re-endothelialisation | Yao et al, 2009 [186] |
TGFβ = transforming growth factor beta, BMP = bone morphogenetic protein, GDF = growth/differentiation factor, AMH = anti-Mϋllerian hormone, RGM = repulsive guidance molecule
6. TGFβ signalling and SMC function
As stated in the introduction, studies have conclusively shown that TGFβ is a potent regulator of SMC phenotype and function. The atheroprotective effects of TGFβ are in part attributed to its capacity for stimulating SMC differentiation by inducing the expression of a large set of mature SMC genes (including αSMA, SM22α and SMMHC [125]) via Smad2 and/or Smad3, which interact with the SMC-specific promoters at putative SBEs [126,127]. TGFβ also induces serum response factor (SRF) protein expression and enhances its binding activity to CArG elements within the promoters of SMC marker genes [128]. Interestingly, Qiu et al have shown that Smad3 is the primary mediator for TGFβ1-induced SM22α expression, while Smad6 and Smad7 repress its activation [129]. Furthermore, the authors illustrated that Smad3 can bind to a SBE in the first exon of SM22α and directly associate with the SRF complex in response to TGFβ1 treatment [129]. TGFβ is also a potent inducer of the synthetic SMC phenotype, stimulating the production and secretion of collagen and proteoglycans by SMCs via direct and indirect interactions with the promoters of these genes [[130], [131], [132]]. However, the effects of TGFβ on SMC behaviour are more variable, with studies showing that TGFβ can both inhibit and stimulate SMC proliferation and migration. This may be due to the heterogeneous nature of SMCs, as evidenced by the varying gene expression patterns of human SMCs derived from primary atherosclerotic plaques, in-stent stenoses or healthy arteries [133]. At the molecular level, these differences have been attributed to varying levels of receptor expression, membrane localisation of receptors, availability of intracellular signalling mediators and presence of transcriptional co-regulators within the nucleus (reviewed in [54,134]). In the next section, we will highlight key findings on the regulation of SMC proliferation and migration by TGFβ in the context of intimal hyperplasia and CAD.
7. TGFβ-regulated SMC proliferation
SMC responses to TGFβ in vitro are influenced by factors such as type of SMC (aortic, venous etc.), cellular density and concentration of TGFβ [134]. For example, Majack et al found that TGFβ1 inhibited proliferation of rat aortic SMCs at sub-confluent densities but potentiated SMC growth at high seeding densities [135]. Furthermore, treatment of cultured porcine coronary artery SMCs with low concentrations of TGFβ1 (0.025ng/mL) stimulated SMC proliferation, but attenuated SMC growth at concentrations of greater than 0.1 ng/mL [136]. The presence of other growth factors also appears to influence the effects of TGFβ on SMC proliferation. For instance, treatment of rat aortic SMCs with TGFβ1 had no significant effect on cell number in quiescent SMC cultures maintained in 1 % FBS, but markedly inhibited SMC proliferation in response to 5 % FBS or PDGF-BB in a dose-dependent manner [135,137]. Other studies, however, have shown that TGFβ potentiates the mitogenicity of FBS, PDGF-BB and bFGF, but only in confluent SMC cultures [138,139].
TGFβ-induced inhibition of SMC proliferation in vitro has been associated with G0/G1 cell cycle arrest through downregulation of the cell cycle regulator, cyclin-dependent kinase 1 (CDK1) [140]. Treatment of mouse aortic SMCs with TGFβ1 for 24 hours substantially reduced the percentage of cells in S phase and G2/M phase and increased the number of cells in G0/G1 [141]. Pharmacological inhibition of the p38 MAPK pathway (using 10μM SB203580) resulted in complete attenuation of TGFβ-dependent growth inhibition in the absence of any inhibitory effect on Smad2/3 signalling, as analysed by phosphorylation, nuclear translocation and reporter gene expression (141), indicating that p38 MAPK may mediate growth inhibition induced by TGFβ in SMCs. More recently, TGFβ has been shown to inhibit PDGF-induced SMC proliferation through downregulation of Cyclin D1 [142], a key regulator of cell cycle transition from G1 to S phase [143]. Here the authors demonstrated that treatment of human aortic SMCs with TGFβ1 significantly inhibited PDGF-BB-induced Cyclin D1 mRNA and protein expression after 24 hours. Interestingly, inhibition of ALK5 using 10 μM SB431542 or siRNA-mediated knockdown of Smad4 completely abolished the inhibitory effect of TGFβ on PDGF-induced Cyclin D1 expression and restored SMC proliferation in response to PDGF, suggesting that this occurs through a Smad-dependent mechanism [142].
In contrast, certain studies have shown that TGFβ1 can indirectly promote SMC proliferation in confluent cultures by inducing PDGF-A gene expression and autocrine production of PDGF-AA [138,144]. Both these studies found that TGFβ-induced rat aortic SMC proliferation was mimicked by treatment with exogenous PDGF-AA (> 5 ng/ml) and partially inhibited by neutralising antibodies to PDGF-AA [138,144]. However, a later study showed that while TGFβ induced an 8-fold increase in PDGF concentration after 24 hours, application of this conditioned medium (containing ~ 1 ng/mL PDGF-AA) to aortic SMCs did not increase mitogenic activity, indicating that induction of PDGF-AA production by TGFβ cannot fully account for the effects of TGFβ on the proliferation of rat aortic SMCs under all in vitro culture conditions [145]. Indeed, TGFβ has also been shown to directly stimulate SMC proliferation through a Smad-dependent mechanism. For instance, Mao et al demonstrated that aortic SMCs from smooth muscle-specific Smad4 knockout mice display a 62 % reduction in proliferation in vitro (as determined by BrdU labelling), compared with SMCs from wild-type mice [146]. Furthermore, shRNA-mediated knockdown of Smad2 and Smad3 within wild-type SMCs significantly reduced SMC proliferation in response to 20 % FBS and the expression of SMC-specific marker genes [146].
Despite the contrasting in vitro data for the effects of TGFβ on SMC proliferation, the majority of in vivo evidence indicates that TGFβ is a potent stimulator of arterial SMC proliferation [96,97,111,147]. For instance, infusion of recombinant TGFβ1 into rats after carotid artery balloon injury resulted in a significant increase in the number of [3H]-thymidine labelled SMC nuclei within the neointima, compared with untreated rat coronary arteries [111]. Similarly, Schulick et al noted that localised adenoviral over-expression of TGFβ1 in the endothelium of uninjured rat carotid arteries resulted in substantial intimal thickening after 4 weeks with marked cellular proliferation (measured by BrdU incorporation) when compared with control arteries [96]. TGFβ-induced SMC proliferation in vivo has been shown to be mediated via a Smad3-dependent mechanism, involving the phosphorylation and nuclear export of the cyclin-dependent kinase inhibitor p27 [97]. Adenoviral overexpression of Smad3 within balloon-injured rat carotid arteries significantly enhanced intimal thickening after 14 days and was associated with increased PCNA expression within intimal SMCs [97] and increased pERK MAPK expression within whole arteries and isolated SMCs [147]. Conflicting studies performed using a more damaging, inflammatory model of femoral artery wire injury showed enhanced neointimal hyperplasia and increased SMC proliferation in Smad3 knockout mice, indicating that the role of TGFβ in the arterial response to injury can vary as a function of the inflammatory microenvironment [148]. Thus, TGFβ/Smad3 can directly enhance SMC proliferation in vivo through transactivation of the ERK MAPK signalling pathway; other indirect mechanisms may account for the enhanced or repressed proliferative responses observed, including modulation of the inflammatory microenvironment or release of sequestered mitotic growth factors following ECM degradation.
8. TGFβ-regulated SMC migration
Similar to SMC proliferation, TGFβ has been shown to variably stimulate and inhibit SMC migration. Early in vitro studies performed in venous and arterial-derived SMCs showed that PDGF-BB, b-FGF or serum-induced migration is inhibited by TGFβ1 in a concentration-dependent manner and this effect is independent of cellular density [149,150]. TGFβ1 can suppress PDGF-BB-induced up-regulation of MMP-2 within rat arterial SMCs, suggesting that the indirect effects of TGFβ1 on SMC migration may partly be due to the inhibition of downstream pro-migratory genes [151]. Conversely, studies also show that TGFβ can directly stimulate SMC migration. For instance, aortic SMCs from smooth muscle-specific Smad4 knockout mice displayed significantly reduced migration in response to serum or PDGF-BB in vitro, compared with SMCs from wild-type mice [146]. Furthermore, inhibition of ALK5 using the kinase inhibitor SB431542 or shRNA-mediated knockdown of Smad2 or Smad3 significantly attenuated SMC migration in response to serum stimulation [146]. In vitro studies performed on aortic SMCs have shown that TGFβ can also regulate SMC migration via indirect mechanisms involving the up-regulation of avβ3 mRNA expression, an integrin which is highly expressed following vascular injury and is important in driving SMC migration [[152], [153], [154], [155]]. Pre-treatment of human aortic SMCs with TGFβ1 was associated with enhanced migration in response to vitronectin, a serum glycoprotein which promotes cell spreading and attachment through integrin receptor binding [154]. Furthermore, treatment of injured rat carotid artery SMCs with a TGFβ1 neutralising antibody completely abrogated TGFβ1-induced integrin β3 mRNA up-regulation [156]. Interestingly, treatment of rats with genistein (a tyrosine kinase inhibitor) following carotid artery injury markedly inhibited injury-induced up-regulation of TGFβ1, TGFβ3, integrin av and β3 mRNA expression, compared with vehicle-treated arteries, suggesting that induction of TGFβ following vascular injury is broadly reliant on tyrosine kinases [156].
9. Therapeutic targeting of TGFβ in CAD: challenges and opportunities
As documented above, TGFβ plays a fundamental role in the regulation of vascular function by affecting SMC proliferation, migration, differentiation and ECM production in CAD. Mutations in genes encoding TGFβ ligands and receptors are also associated with several developmental disorders and vascular diseases, including Marfan syndrome type 2, Loeys-Dietz syndrome, and other vasculopathies with clinical presentations that include thoracic aortic aneurysms and dissections [[157], [158], [159]]. Hence, components of the TGFβ signalling pathway are important therapeutic targets for a wide range of vascular pathologies.
Numerous pre-clinical studies have employed different approaches to inhibit TGFβ signalling after vascular injury, which have been shown to reduce intimal thickening compared with controls. However these approaches have yet to translate to significant clinical gain in the cardiovascular disease arena, with no TGFβ therapeutics currently on the market. Promisingly, small-scale clinical trials demonstrated that oral administration of 600 mg/day tranilast (N-(3,4-dimethoxycinnamoyl) anthranilic acid), a non-specific inhibitor of TGFβ biosynthesis, was associated with a significantly reduced risk of restenosis following PCI, compared with placebo (17.6% vs. 39.4% at 3 months) [160,161]. Originally developed as a treatment for allergic disorders such as chronic rhinitis and bronchial asthma, tranilast has also successfully been used (both orally and topically) as an anti-fibrotic agent in the treatment of hypertrophic scars or keloids [[162], [163], [164]]. However, the large-scale randomised double-bind clinical trial PRESTO (Prevention of REStenosis with Tranilast and its Outcomes) examining the effects of tranilast treatment in 11,484 patients after PCI failed to show improved clinical outcome (death, MI or repeat revascularisation) compared with placebo [165]. Worryingly, this trial highlighted some potential adverse effects of tranilast, including hyperbilirubinemia, increased serum creatinine and alanine transaminases, indicative of liver abnormalities. Fortunately these adverse effects were reversed upon cessation of treatment, however the lack of primary and secondary endpoint efficacy in this large-scale trial highlights the complexity of targeting TGFβ using systemic approaches in multimorbid, highly diverse groups of patients.
Nevertheless, TGFβ therapeutics are advancing in clinical trials for other indications, particularly fibrosis and oncology, and results appear to be positive [166,167]. Indeed, Pirfenidone (5-methyl-1-phenyl-2-[1H]-pyridone), which inhibits TGFβ production and activity, was approved by the FDA in October 2014 for treatment of idiopathic pulmonary fibrosis (IPF). IPF is a devastating progressive lung disease, with a median survival from time of diagnosis of 3 years; Pirfenidone was approved on the basis of phase III clinical trials showing a reduction in forced vital capacity decline (a measure of lung function) and improved progression-free survival compared to placebo (ASCEND study [168]). In the oncology field, Galunisertib (LY2157299 monohydrate) a small molecule inhibitor of the ALK5 kinase, has been evaluated in >10 clinical trials (alone or in combination with e.g alkylating agents) for different types of cancer [169]. The most advanced trial currently in progress is a phase II/III randomised, placebo-controlled trial that has enrolled ~140 patients with myelodysplastic syndrome (MDS; NCT02008318); interim data from this trial shows good tolerance of the drug and haematological improvement in 26% of patients enrolled. Of note, recent trials investigating the use of Galunisertib have utilised an adapted, intermittent dosing regimen (14 days on, 14 days off) due to preclinical studies showing proliferative, inflammatory changes in the heart valves and aortae of rats when continuously dosed with Galunisertib [170]. Although no medically significant cardiotoxicities were observed in a first-in-human dose study administering Galunisertib to glioma patients [171], the potential for serious adverse events with high-dose, systemic TGFβ agonists or antagonists should not be underestimated. Ultimately, localised and pathway-specific targeting of TGFβ signalling will be required in order to achieve optimal therapeutic efficacy whilst avoiding undesired off-target effects.
While there are acknowledged challenges associated with using global approaches for targeting TGFβ in multimorbid CAD patients, new avenues with the potential for more focused targeting of TGFβ in SMCs have recently opened up. In the last decade, next-generation sequencing studies have identified non-coding RNA (ncRNA) sequences residing in intergenic regions of the genome. These non-coding transcripts are now known to have multiple functions, regulating the transcription and translation of proximal and distant protein-coding genes in a context-specific manner (reviewed in [172]). Recent studies have begun to elucidate the interactions between them and TGFβ pathway components, identifying novel potential therapeutic targets for CAD. Early studies showed that TGFβ could alter the expression of numerous microRNAs (miRs) in various human tissues and cells, the effects of which appear to be cell-type specific [173]. Microarray analysis in human carotid artery SMCs revealed a number of differentially expressed miRs following TGFβ1 treatment, including miR-143/145, which was significantly up-regulated by TGFβ1 in a concentration- and time-dependent manner [174]. Treatment of SMC with a specific inhibitor of p38MAPK completely blocked TGFβ1-induced miR-143/145 expression and attenuated the expression of SMC contractile genes (including CNN1, TAGLN and ACTA2) in response to TGFβ1 stimulation [174], identifying an additional mechanism through which TGFβ1 can promote SMC differentiation. Interestingly the miR-143/145 cluster, which is highly enriched in SMCs, has been shown to be significantly decreased following acute arterial injury [175] and in mouse atherosclerotic lesions [175]. Genetic knockout of miR-143/145 led to a reduction in the number of contractile arterial SMCs and a corresponding increase in synthetic SMCs, as determined by electron microscopy [176]. Neointimal lesions were also frequently observed in the femoral arteries of aged miR-143/145-/- mice, with no lesions observed in wild-type animals [176]. TGFβ has also been shown to regulate the expression of miR-21 through promoting the processing of pri-miR-21 into pre-miR-21 by the Drosha complex [177]. Importantly, miR-21 is over-expressed in murine and porcine models of vein grafting and is highly expressed within αSMA+ SMCs of failed human vein grafts [178]. Genetic ablation or antisense oligonucleotide-mediated knockdown of miR-21 significantly attenuated injury-induced neointima formation by inhibiting SMC proliferation and migration and inducing SMC apoptosis, highlighting the potential therapeutic benefit of miR-21 inhibition [178,179]. Together, these studies indicate that TGFβ-regulated miRNAs play a critical role in controlling SMC phenotype transitions and the response of the vascular wall to injury, underlining their potential as therapeutic targets. Targeting SMC-enriched, disease-dysregulated miRs downstream of TGFβ may be a more rational approach for achieving therapeutic efficacy whilst avoiding undesired side-effects.
10. Concluding remarks
TGFβ was initially identified in the early 1980’s, when Anita Roberts and Michael Sporn purified a ‘transformation factor’ that could render healthy cells malignant [180]. The first observation that this Janus-like cytokine could have multifunctional effects was made shortly thereafter, in studies showing that TGFβ could synergise with PDGF to stimulate fibroblast colony formation (CF) whilst inhibiting epidermal growth factor-induced CF [181]. From these early beginnings, the field of TGFβ research – and indeed the TGFβ superfamily - has expanded exponentially, with papers on TGFβ now numbering in the tens of thousands. Nevertheless, important questions have yet to be fully answered, and our understanding of the many TGFβ paradoxes remains incomplete. The advent of next-generation sequencing (NGS) has provided some clarification, identifying hitherto unknown genetic and phenotypic overlaps between patients who develop cardiovascular disease and those with inherited vascular conditions caused by mutations in TGFβ genes. Alongside, investigations following on from the Human Genome Project have started unravelling the complexity of the transcriptome, identifying non-coding RNA sequences that both regulate and are regulated by TGFβ signalling. These and other studies have greatly enhanced our mechanistic understanding of TGFβ, and the many levels at which this pleiotropic cytokine is controlled. From early experiments showing that TGFβ enhances the secretion of ECM proteins, we are now beginning to grasp how the cellular microenvironment in turn influences the actions of TGFβ; this is of particular relevance to coronary artery disease and intimal hyperplasia, during which extensive vascular remodelling occurs. Elucidation of these and other questions regarding the actions and interactions of TGFβ will, we hope, lead to the development of localised and pathway-specific therapies that effectively and selectively target the pathological actions of TGFβ.
Sources of funding
Dr. Low is supported by a British Heart Foundation PhD Studentship (FS/12/66/30003), Prof. Baker is supported by the British Heart Foundation Chair of Translational Cardiovascular Sciences (CH/11/2/28733) and Dr. Bradshaw is supported by a Personal Research Fellowship from the Royal Society of Edinburgh (RSE/33457).
References
- 1.Schwartz S.M., Stemerman M.B., Benditt E.P. The aortic intima. II. Repair of the aortic lining after mechanical denudation. Am. J. Pathol. 1975;81(1):15–42. [PMC free article] [PubMed] [Google Scholar]
- 2.Clowes A.W., Reidy M.A., Clowes M.M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab. Investig. 1983;49(3):327–333. [PubMed] [Google Scholar]
- 3.Vita J.A., Treasure C.B., Nabel E.G., McLenachan J.M., Fish R.D., Yeung A.C. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81(2):491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 4.John S., Schlaich M., Langenfeld M., Weihprecht H., Schmitz G., Weidinger G. Increased bioavailability of nitric oxide after lipid-lowering therapy in hypercholesterolemic patients: a randomized, placebo-controlled, double-blind study. Circulation. 1998;98(3):211–216. doi: 10.1161/01.cir.98.3.211. [DOI] [PubMed] [Google Scholar]
- 5.Schachinger V., Britten M.B., Elsner M., Walter D.H., Scharrer I., Zeiher A.M. A positive family history of premature coronary artery disease is associated with impaired endothelium-dependent coronary blood flow regulation. Circulation. 1999;100(14):1502–1508. doi: 10.1161/01.cir.100.14.1502. [DOI] [PubMed] [Google Scholar]
- 6.Rzucidlo E.M., Martin K.A., Powell R.J. Regulation of vascular smooth muscle cell differentiation. J. Vasc. Surg. 2007;45 doi: 10.1016/j.jvs.2007.03.001. Suppl A:A25-32. [DOI] [PubMed] [Google Scholar]
- 7.de Vries M.R., Simons K.H., Jukema J.W., Braun J., Quax P.H. Vein graft failure: from pathophysiology to clinical outcomes. Nat. Rev. Cardiol. 2016;13(8):451–470. doi: 10.1038/nrcardio.2016.76. [DOI] [PubMed] [Google Scholar]
- 8.ten Dijke P., Hill C.S. New insights into TGF-beta-Smad signalling. Trends Biochem. Sci. 2004;29(5):265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Hinck A.P. Structural studies of the TGF-betas and their receptors - insights into evolution of the TGF-beta superfamily. FEBS Lett. 2012;586(14):1860–1870. doi: 10.1016/j.febslet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Hinck A.P., Huang T. TGF-beta antagonists: same knot, but different hold. Structure. 2013;21(8):1269–1270. doi: 10.1016/j.str.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhurst R.J., Lehnert S.A., Faissner A., Duffie E. TGF beta in murine morphogenetic processes: the early embryo and cardiogenesis. Development. 1990;108(4):645–656. doi: 10.1242/dev.108.4.645. [DOI] [PubMed] [Google Scholar]
- 12.Millan F.A., Denhez F., Kondaiah P., Akhurst R.J. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991;111(1):131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- 13.Bobik A., Agrotis A., Kanellakis P., Dilley R., Krushinsky A., Smirnov V. Distinct patterns of transforming growth factor-beta isoform and receptor expression in human atherosclerotic lesions - Colocalization implicates TGF-beta in fibrofatty lesion development. Circulation. 1999;99(22):2883–2891. doi: 10.1161/01.cir.99.22.2883. [DOI] [PubMed] [Google Scholar]
- 14.ten Dijke P., Arthur H.M. Extracellular control of TGFbeta signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 2007;8(11):857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 15.Rifkin D.B. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J. Biol. Chem. 2005;280(9):7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 16.Munger J.S., Harpel J.G., Giancotti F.G., Rifkin D.B. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol. Biol. Cell. 1998;9(9):2627–2638. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saharinen J., Hyytiainen M., Taipale J., Keski-Oja J. Latent transforming growth factor-beta binding proteins (LTBPs) - structural extracellular matrix proteins for targeting TGF-beta action. Cytokine Growth Factor Rev. 1999;10(2):99–117. doi: 10.1016/s1359-6101(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 18.Piek E., Heldin C.H., Ten Dijke P. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 1999;13(15):2105–2124. [PubMed] [Google Scholar]
- 19.Cheifetz S., Bellon T., Cales C., Vera S., Bernabeu C., Massague J. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem. 1992;267(27):19027–19030. [PubMed] [Google Scholar]
- 20.Lopez-Casillas F., Wrana J.L., Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73(7):1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 21.Piao M., Tokunaga O. Significant expression of endoglin (CD105), TGFbeta-1 and TGFbeta R-2 in the atherosclerotic aorta: an immunohistological study. J. Atheroscler. Thromb. 2006;13(2):82–89. doi: 10.5551/jat.13.82. [DOI] [PubMed] [Google Scholar]
- 22.Gore B., Izikki M., Mercier O., Dewachter L., Fadel E., Humbert M. Key role of the endothelial TGF-beta/ALK1/endoglin signaling pathway in humans and rodents pulmonary hypertension. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilandzic M., Stenvers K.L. Reprint of: Betaglycan: a multifunctional accessory. Mol. Cell. Endocrinol. 2012;359(1-2):13–22. doi: 10.1016/j.mce.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Kapur N.K., Morine K.J., Letarte M. Endoglin: a critical mediator of cardiovascular health. Vasc. Health Risk Manag. 2013;9:195–206. doi: 10.2147/VHRM.S29144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 26.Massague J. TGFbeta signalling in context. Nat. Rev. Mol. Cell Biol. 2012;13(10):616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller T.D., Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012;586(14):1846–1859. doi: 10.1016/j.febslet.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 28.Radaev S., Zou Z., Huang T., Lafer E.M., Hinck A.P., Sun P.D. Ternary complex of transforming growth factor-beta1 reveals isoform-specific ligand recognition and receptor recruitment in the superfamily. J. Biol. Chem. 2010;285(19):14806–14814. doi: 10.1074/jbc.M109.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCaffrey T.A., Consigli S., Du B., Falcone D.J., Sanborn T.A., Spokojny A.M. Decreased type II/type I TGF-beta receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-beta1. J. Clin. Invest. 1995;96(6):2667–2675. doi: 10.1172/JCI118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmierer B., Hill C.S. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007;8(12):970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 31.Goumans M.J., Valdimarsdottir G., Itoh S., Rosendahl A., Sideras P., ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21(7):1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goumans M.J., Valdimarsdottir G., Itoh S., Lebrin F., Larsson J., Mummery C. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol. Cell. 2003;12(4):817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 33.Blaney Davidson E.N., Remst D.F., Vitters E.L., van Beuningen H.M., Blom A.B., Goumans M.J. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 2009;182(12):7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 34.Curado F., Spuul P., Egana I., Rottiers P., Daubon T., Veillat V. ALK5 and ALK1 play antagonistic roles in transforming growth factor beta-induced podosome formation in aortic endothelial cells. Mol. Cell. Biol. 2014;34(24):4389–4403. doi: 10.1128/MCB.01026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finnson K.W., Parker W.L., ten Dijke P., Thorikay M., Philip A. ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J. Bone Miner. Res. 2008;23(6):896–906. doi: 10.1359/jbmr.080209. [DOI] [PubMed] [Google Scholar]
- 36.Garrido-Martin E.M., Blanco F.J., Roque M., Novensa L., Tarocchi M., Lang U.E. Vascular injury triggers Kruppel-like factor 6 mobilization and cooperation with specificity protein 1 to promote endothelial activation through upregulation of the activin receptor-like kinase 1 gene. Circ. Res. 2013;112(1):113–127. doi: 10.1161/CIRCRESAHA.112.275586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Y., Bennett B.J., Wang X., Rosenfeld M.E., Giachelli C., Lusis A.J. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ. Res. 2010;107(4):485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seki T., Hong K.H., Oh S.P. Nonoverlapping expression patterns of ALK1 and ALK5 reveal distinct roles of each receptor in vascular development. Lab. Investig. 2006;86(2):116–129. doi: 10.1038/labinvest.3700376. [DOI] [PubMed] [Google Scholar]
- 39.Moustakas A., Heldin C.H. Non-Smad TGF-beta signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. Pt 16. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19(1):128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massague J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19(23):2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 42.Attisano L., Lee-Hoeflich S.T. The Smads. Genome Biol. 2001;2(8) doi: 10.1186/gb-2001-2-8-reviews3010. (REVIEWS3010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 44.Massague J., Chen Y.G. Controlling TGF-beta signaling. Genes Dev. 2000;14(6):627–644. [PubMed] [Google Scholar]
- 45.Massague J. How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 2000;1(3):169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 46.Schmierer B., Hill C.S. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor beta-dependent nuclear accumulation of Smads. Mol. Cell. Biol. 2005;25(22):9845–9858. doi: 10.1128/MCB.25.22.9845-9858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng X.H., Derynck R. A kinase subdomain of transforming growth factor-beta (TGF-beta) type I receptor determines the TGF-beta intracellular signaling specificity. EMBO J. 1997;16(13):3912–3923. doi: 10.1093/emboj/16.13.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y.G., Hata A., Lo R.S., Wotton D., Shi Y., Pavletich N. Determinants of specificity in TGF-beta signal transduction. Genes Dev. 1998;12(14):2144–2152. doi: 10.1101/gad.12.14.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsukazaki T., Chiang T.A., Davison A.F., Attisano L., Wrana J.L. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell. 1998;95(6):779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y., Wang Y.F., Jayaraman L., Yang H., Massague J., Pavletich N.P. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell. 1998;94(5):585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- 51.Zawel L., Dai J.L., Buckhaults P., Zhou S., Kinzler K.W., Vogelstein B. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol. Cell. 1998;1(4):611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura G., Manabe I., Tsushima K., Fujiu K., Oishi Y. Imai Y, et al. delta EF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Dev. Cell. 2006;11(1):93–104. doi: 10.1016/j.devcel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Qiu P., Ritchie R.P., Fu Z., Cao D., Cumming J., Miano J.M. Myocardin enhances Smad3-mediated transforming growth factor-beta1 signaling in a CArG box-independent manner: Smad-binding element is an important cis element for SM22alpha transcription in vivo. Circ. Res. 2005;97(10):983–991. doi: 10.1161/01.RES.0000190604.90049.71. [DOI] [PubMed] [Google Scholar]
- 54.David CJ, Massague J. Contextual determinants of TGF beta action in development, immunity and cancer (vol 19, pg 419, 2018). Nat. Rev. Mol. Cell Biol. 2018;19(7):479-. [DOI] [PubMed]
- 55.Hill C.S. Transcriptional Control by the SMADs. Csh Perspect Biol. 2016;8(10) doi: 10.1101/cshperspect.a022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Libby P. Changing concepts of atherogenesis. J. Intern. Med. 2000;247(3):349–358. doi: 10.1046/j.1365-2796.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 57.Williams K.J., Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995;15(5):551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams K.J., Tabas I. The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol. 1998;9(5):471–474. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Tabas I., Williams K.J., Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 60.Steinberg H.O., Bayazeed B., Hook G., Johnson A., Cronin J., Baron A.D. Endothelial dysfunction is associated with cholesterol levels in the high normal range in humans. Circulation. 1997;96(10):3287–3293. doi: 10.1161/01.cir.96.10.3287. [DOI] [PubMed] [Google Scholar]
- 61.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 62.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 63.Durham A.L., Speer M.Y., Scatena M., Giachelli C.M., Shanahan C.M. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018;114(4):590–600. doi: 10.1093/cvr/cvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cambien F., Ricard S., Troesch A., Mallet C., Generenaz L., Evans A. Polymorphisms of the transforming growth factor-beta 1 gene in relation to myocardial infarction and blood pressure. The Etude Cas-Temoin de l'Infarctus du Myocarde (ECTIM) Study. Hypertension. 1996;28(5):881–887. doi: 10.1161/01.hyp.28.5.881. [DOI] [PubMed] [Google Scholar]
- 65.Koch W., Hoppmann P., Mueller J.C., Schomig A., Kastrati A. Association of transforming growth factor-beta1 gene polymorphisms with myocardial infarction in patients with angiographically proven coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2006;26(5):1114–1119. doi: 10.1161/01.ATV.0000217747.66517.11. [DOI] [PubMed] [Google Scholar]
- 66.Sie M.P., Uitterlinden A.G., Bos M.J., Arp P.P., Breteler M.M., Koudstaal P.J. TGF-beta 1 polymorphisms and risk of myocardial infarction and stroke: the Rotterdam Study. Stroke. 2006;37(11):2667–2671. doi: 10.1161/01.STR.0000244779.30070.1a. [DOI] [PubMed] [Google Scholar]
- 67.Morris D.R., Moxon J.V., Biros E., Krishna S.M., Golledge J. Meta-analysis of the association between transforming growth factor-beta polymorphisms and complications of coronary heart disease. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu Y., Boer J.M., Barsova R.M., Favorova O., Goel A., Muller M. TGFB1 genetic polymorphisms and coronary heart disease risk: a meta-analysis. BMC Med. Genet. 2012;13:39. doi: 10.1186/1471-2350-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y.Y., Zhou Y.H., Gong G., Geng H.Y., Yang X.X. TGF-beta1 Gene -509C/T Polymorphism and Coronary Artery Disease: An Updated Meta-Analysis Involving 11,701 Subjects. Front. Physiol. 2017;8:108. doi: 10.3389/fphys.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verweij N., Eppinga R.N., Hagemeijer Y., van der Harst P. Identification of 15 novel risk loci for coronary artery disease and genetic risk of recurrent events, atrial fibrillation and heart failure. Sci. Rep. 2017;7(1):2761. doi: 10.1038/s41598-017-03062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coronary Artery Disease C., Samani N.J., Deloukas P., Erdmann J., Hengstenberg C., Kuulasmaa K. Large scale association analysis of novel genetic loci for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2009;29(5):774–780. doi: 10.1161/ATVBAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nikpay M., Goel A., Won H.H., Hall L.M., Willenborg C., Kanoni S. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015;47(10):1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turner A.W., Martinuk A., Silva A., Lau P., Nikpay M., Eriksson P. Functional Analysis of a Novel Genome-Wide Association Study Signal in SMAD3 That Confers Protection From Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2016;36(5):972–983. doi: 10.1161/ATVBAHA.116.307294. [DOI] [PubMed] [Google Scholar]
- 74.Grainger D.J., Kemp P.R., Metcalfe J.C., Liu A.C., Lawn R.M., Williams N.R. The serum concentration of active transforming growth factor-beta is severely depressed in advanced atherosclerosis. Nat. Med. 1995;1(1):74–79. doi: 10.1038/nm0195-74. [DOI] [PubMed] [Google Scholar]
- 75.Erren M., Reinecke H., Junker R., Fobker M., Schulte H., Schurek J.O. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler. Thromb. Vasc. Biol. 1999;19(10):2355–2363. doi: 10.1161/01.atv.19.10.2355. [DOI] [PubMed] [Google Scholar]
- 76.Wang X.L., Liu S.X., Wilcken D.E. Circulating transforming growth factor beta 1 and coronary artery disease. Cardiovasc. Res. 1997;34(2):404–410. doi: 10.1016/s0008-6363(97)00033-3. [DOI] [PubMed] [Google Scholar]
- 77.Grainger D.J., Mosedale D.E., Metcalfe J.C. TGF-beta in blood: a complex problem. Cytokine Growth Factor Rev. 2000;11(1-2):133–145. doi: 10.1016/s1359-6101(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 78.van Dijk R.A., Engels C.C., Schaapherder A.F., Mulder-Stapel A., Ten Dijke P., Hamming J.F. Visualizing TGF-beta and BMP signaling in human atherosclerosis: a histological evaluation based on Smad activation. Histol. Histopathol. 2012;27(3):387–396. doi: 10.14670/HH-27.387. [DOI] [PubMed] [Google Scholar]
- 79.Grainger D.J. Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler. Thromb. Vasc. Biol. 2004;24(3):399–404. doi: 10.1161/01.ATV.0000114567.76772.33. [DOI] [PubMed] [Google Scholar]
- 80.Grainger D.J., Mosedale D.E., Metcalfe J.C., Bottinger E.P. Dietary fat and reduced levels of TGFbeta1 act synergistically to promote activation of the vascular endothelium and formation of lipid lesions. J. Cell Sci. 2000;113:2355–2361. doi: 10.1242/jcs.113.13.2355. Pt 13. [DOI] [PubMed] [Google Scholar]
- 81.Mallat Z., Gojova A., Marchiol-Fournigault C., Esposito B., Kamate C., Merval R. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 2001;89(10):930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 82.Lutgens E., Gijbels M., Smook M., Heeringa P., Gotwals P., Koteliansky V.E. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler. Thromb. Vasc. Biol. 2002;22(6):975–982. doi: 10.1161/01.atv.0000019729.39500.2f. [DOI] [PubMed] [Google Scholar]
- 83.Li D., Liu Y., Chen J., Velchala N., Amani F., Nemarkommula A. Suppression of atherogenesis by delivery of TGFbeta1ACT using adeno-associated virus type 2 in LDLR knockout mice. Biochem. Biophys. Res. Commun. 2006;344(3):701–707. doi: 10.1016/j.bbrc.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Frutkin A.D., Otsuka G., Stempien-Otero A., Sesti C., Du L., Jaffe M. TGF-beta 1 Limits Plaque Growth, Stabilizes Plaque Structure, and Prevents Aortic Dilation in Apolipoprotein E-Null Mice. Arterioscler. Thromb. Vasc. Biol. 2009;29(9) doi: 10.1161/ATVBAHA.109.186593. (1251-U41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez-Vita J., Sanchez-Galan E., Santamaria B., Sanchez-Lopez E., Rodrigues-Diez R., Blanco-Colio L.M. Essential role of TGF-beta/Smad pathway on statin dependent vascular smooth muscle cell regulation. PLoS One. 2008;3(12) doi: 10.1371/journal.pone.0003959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grainger D.J. TGF-beta and atherosclerosis in man. Cardiovasc. Res. 2007;74(2):213–222. doi: 10.1016/j.cardiores.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 87.Evrard S.M., Lecce L., Michelis K.C., Nomura-Kitabayashi A., Pandey G., Purushothaman K.R. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 2016;7 doi: 10.1038/ncomms11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cavieres V., Valdes K., Moreno B., Moore-Carrasco R., Gonzalez D.R. Vascular hypercontractility and endothelial dysfunction before development of atherosclerosis in moderate dyslipidemia: role for nitric oxide and interleukin-6. Am J Cardiovasc Dis. 2014;4(3):114–122. [PMC free article] [PubMed] [Google Scholar]
- 89.Schonherr E., Jarvelainen H.T., Kinsella M.G., Sandell L.J., Wight T.N. Platelet-derived growth factor and transforming growth factor-beta 1 differentially affect the synthesis of biglycan and decorin by monkey arterial smooth muscle cells. Arterioscler. Thromb. 1993;13(7):1026–1036. doi: 10.1161/01.atv.13.7.1026. [DOI] [PubMed] [Google Scholar]
- 90.Little P.J., Tannock L., Olin K.L., Chait A., Wight T.N. Proteoglycans synthesized by arterial smooth muscle cells in the presence of transforming growth factor-beta1 exhibit increased binding to LDLs. Arterioscler. Thromb. Vasc. Biol. 2002;22(1):55–60. doi: 10.1161/hq0102.101100. [DOI] [PubMed] [Google Scholar]
- 91.Kahari V.M., Larjava H., Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J. Biol. Chem. 1991;266(16):10608–10615. [PubMed] [Google Scholar]
- 92.Wight T.N. A role for proteoglycans in vascular disease. Matrix Biol. 2018;71–72:396–420. doi: 10.1016/j.matbio.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang T., Wilson P.G., Thompson J.C., Nelson C., Yoder M.H., Tannock L.R. Prevention of TGFbeta induction attenuates angII-stimulated vascular biglycan and atherosclerosis in Ldlr-/- mice. J. Lipid Res. 2013;54(8):2255–2264. doi: 10.1194/jlr.P040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thannickal V.J., Lee D.Y., White E.S., Cui Z., Larios J.M., Chacon R. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003;278(14):12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 95.Gao P.J., Li Y., Sun A.J., Liu J.J., Ji K.D., Zhang Y.Z. Differentiation of vascular myofibroblasts induced by transforming growth factor-beta1 requires the involvement of protein kinase Calpha. J. Mol. Cell. Cardiol. 2003;35(9):1105–1112. doi: 10.1016/s0022-2828(03)00207-4. [DOI] [PubMed] [Google Scholar]
- 96.Schulick A.H., Taylor A.J., Zuo W., Qiu C.B., Dong G., Woodward R.N. Overexpression of transforming growth factor beta1 in arterial endothelium causes hyperplasia, apoptosis, and cartilaginous metaplasia. Proc. Natl. Acad. Sci. U. S. A. 1998;95(12):6983–6988. doi: 10.1073/pnas.95.12.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsai S., Hollenbeck S.T., Ryer E.J., Edlin R., Yamanouchi D., Kundi R. TGF-beta through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am. J. Physiol. Heart Circ. Physiol. 2009;297(2):H540–H549. doi: 10.1152/ajpheart.91478.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nemenoff R.A., Horita H., Ostriker A.C., Furgeson S.B., Simpson P.A., VanPutten V. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler. Thromb. Vasc. Biol. 2011;31(6):1300–1308. doi: 10.1161/ATVBAHA.111.223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chappell J., Harman J.L., Narasimhan V.M., Yu H., Foote K., Simons B.D. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ. Res. 2016;119(12):1313–1323. doi: 10.1161/CIRCRESAHA.116.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walts A.E., Fishbein M.C., Matloff J.M. Thrombosed, ruptured atheromatous plaques in saphenous vein coronary artery bypass grafts: ten years' experience. Am. Heart J. 1987;114(4 Pt 1):718–723. doi: 10.1016/0002-8703(87)90780-0. [DOI] [PubMed] [Google Scholar]
- 101.Safian R.D. Accelerated atherosclerosis in saphenous vein bypass grafts: a spectrum of diffuse plaque instability. Prog. Cardiovasc. Dis. 2002;44(6):437–448. doi: 10.1053/pcad.2002.123471. [DOI] [PubMed] [Google Scholar]
- 102.Yahagi K., Kolodgie F.D., Otsuka F., Finn A.V., Davis H.R., Joner M. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 2016;13(2):79–98. doi: 10.1038/nrcardio.2015.164. [DOI] [PubMed] [Google Scholar]
- 103.Higo T., Ueda Y., Oyabu J., Okada K., Nishio M., Hirata A. Atherosclerotic and thrombogenic neointima formed over sirolimus drug-eluting stent: an angioscopic study. JACC Cardiovasc. Imaging. 2009;2(5):616–624. doi: 10.1016/j.jcmg.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 104.Nakazawa G., Finn A.V., Vorpahl M., Ladich E.R., Kolodgie F.D., Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J. Am. Coll. Cardiol. 2011;57(4):390–398. doi: 10.1016/j.jacc.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 105.Otsuka F., Byrne R.A., Yahagi K., Mori H., Ladich E., Fowler D.R. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur. Heart J. 2015;36(32):2147–2159. doi: 10.1093/eurheartj/ehv205. [DOI] [PubMed] [Google Scholar]
- 106.Nakamura D., Attizzani G.F., Toma C., Sheth T., Wang W., Soud M. Failure Mechanisms and Neoatherosclerosis Patterns in Very Late Drug-Eluting and Bare-Metal Stent Thrombosis. Circ Cardiovasc Interv. 2016;9(9) doi: 10.1161/CIRCINTERVENTIONS.116.003785. [DOI] [PubMed] [Google Scholar]
- 107.Nikol S., Isner J.M., Pickering J.G., Kearney M., Leclerc G., Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J. Clin. Invest. 1992;90(4):1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yutani C., Imakita M., Ishibashi-Ueda H., Tsukamoto Y., Nishida N., Ikeda Y. Coronary atherosclerosis and interventions: pathological sequences and restenosis. Pathol. Int. 1999;49(4):273–290. doi: 10.1046/j.1440-1827.1999.00861.x. [DOI] [PubMed] [Google Scholar]
- 109.Chung I.M., Gold H.K., Schwartz S.M., Ikari Y., Reidy M.A., Wight T.N. Enhanced extracellular matrix accumulation in restenosis of coronary arteries after stent deployment. J. Am. Coll. Cardiol. 2002;40(12):2072–2081. doi: 10.1016/s0735-1097(02)02598-6. [DOI] [PubMed] [Google Scholar]
- 110.Madri J.A., Reidy M.A., Kocher O., Bell L. Endothelial cell behavior after denudation injury is modulated by transforming growth factor-beta1 and fibronectin. Lab. Investig. 1989;60(6):755–765. [PubMed] [Google Scholar]
- 111.Majesky M.W., Lindner V., Twardzik D.R., Schwartz S.M., Reidy M.A. Production of transforming growth factor beta 1 during repair of arterial injury. J. Clin. Invest. 1991;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chamberlain J., Gunn J., Francis S.E., Holt C.M., Arnold N.D., Cumberland D.C. TGF beta is active, and correlates with activators of TGF beta, following porcine coronary angioplasty. Cardiovasc. Res. 2001;50(1):125–136. doi: 10.1016/s0008-6363(01)00199-7. [DOI] [PubMed] [Google Scholar]
- 113.Jiang Z., Tao M., Omalley K.A., Wang D., Ozaki C.K., Berceli S.A. Established neointimal hyperplasia in vein grafts expands via TGF-beta-mediated progressive fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2009;297(4):H1200–H1207. doi: 10.1152/ajpheart.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nabel E.G., Shum L., Pompili V.J., Yang Z.Y., San H., Shu H.B. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc. Natl. Acad. Sci. U. S. A. 1993;90(22):10759–10763. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wolff R.A., Malinowski R.L., Heaton N.S., Hullett D.A., Hoch J.R. Transforming growth factor-beta1 antisense treatment of rat vein grafts reduces the accumulation of collagen and increases the accumulation of h-caldesmon. J. Vasc. Surg. 2006;43(5):1028–1036. doi: 10.1016/j.jvs.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 116.Smith J.D., Bryant S.R., Couper L.L., Vary C.P., Gotwals P.J., Koteliansky V.E. Soluble transforming growth factor-beta type II receptor inhibits negative remodeling, fibroblast transdifferentiation, and intimal lesion formation but not endothelial growth. Circ. Res. 1999;84(10):1212–1222. doi: 10.1161/01.res.84.10.1212. [DOI] [PubMed] [Google Scholar]
- 117.Yamamoto K., Morishita R., Tomita N., Shimozato T., Nakagami H., Kikuchi A. Ribozyme oligonucleotides against transforming growth factor-beta inhibited neointimal formation after vascular injury in rat model: potential application of ribozyme strategy to treat cardiovascular disease. Circulation. 2000;102(11):1308–1314. doi: 10.1161/01.cir.102.11.1308. [DOI] [PubMed] [Google Scholar]
- 118.Kingston P.A., Sinha S., Appleby C.E., David A., Verakis T., Castro M.G. Adenovirus-mediated gene transfer of transforming growth factor-beta3, but not transforming growth factor-beta1, inhibits constrictive remodeling and reduces luminal loss after coronary angioplasty. Circulation. 2003;108(22):2819–2825. doi: 10.1161/01.CIR.0000097068.49080.A0. [DOI] [PubMed] [Google Scholar]
- 119.Ghosh J., Baguneid M., Khwaja N., Murphy M.O., Turner N., Halka A. Reduction of myointimal hyperplasia after arterial anastomosis by local injection of transforming growth factor beta3. J. Vasc. Surg. 2006;43(1):142–149. doi: 10.1016/j.jvs.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 120.Kundi R., Hollenbeck S.T., Yamanouchi D., Herman B.C., Edlin R., Ryer E.J. Arterial gene transfer of the TGF-beta signalling protein Smad3 induces adaptive remodelling following angioplasty: a role for CTGF. Cardiovasc. Res. 2009;84(2):326–335. doi: 10.1093/cvr/cvp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mallawaarachchi C.M., Weissberg P.L., Siow R.C. Smad7 gene transfer attenuates adventitial cell migration and vascular remodeling after balloon injury. Arterioscler. Thromb. Vasc. Biol. 2005;25(7):1383–1387. doi: 10.1161/01.ATV.0000168415.33812.51. [DOI] [PubMed] [Google Scholar]
- 122.Rodriguez-Vita J., Sanchez-Lopez E., Esteban V., Ruperez M., Egido J., Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111(19):2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 123.Li J.H., Huang X.R., Zhu H.J., Johnson R., Lan H.Y. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003;63(6):2010–2019. doi: 10.1046/j.1523-1755.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- 124.Fu K., Corbley M.J., Sun L., Friedman J.E., Shan F., Papadatos J.L. SM16, an orally active TGF-beta type I receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arterioscler. Thromb. Vasc. Biol. 2008;28(4):665–671. doi: 10.1161/ATVBAHA.107.158030. [DOI] [PubMed] [Google Scholar]
- 125.Guo X., Chen S.Y. Transforming growth factor-beta and smooth muscle differentiation. World J. Biol. Chem. 2012;3(3):41–52. doi: 10.4331/wjbc.v3.i3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen S., Kulik M., Lechleider R.J. Smad proteins regulate transcriptional induction of the SM22alpha gene by TGF-beta. Nucleic Acids Res. 2003;31(4):1302–1310. doi: 10.1093/nar/gkg224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu B., Wu Z., Phan S.H. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am. J. Respir. Cell Mol. Biol. 2003;29(3):397–404. doi: 10.1165/rcmb.2003-0063OC. Pt 1. [DOI] [PubMed] [Google Scholar]
- 128.Hirschi K.K., Lai L., Belaguli N.S., Dean D.A., Schwartz R.J., Zimmer W.E. Transforming growth factor-beta induction of smooth muscle cell phenotpye requires transcriptional and post-transcriptional control of serum response factor. J. Biol. Chem. 2002;277(8):6287–6295. doi: 10.1074/jbc.M106649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiu P., Feng X.H., Li L. Interaction of Smad3 and SRF-associated complex mediates TGF-beta1 signals to regulate SM22 transcription during myofibroblast differentiation. J. Mol. Cell. Cardiol. 2003;35(12):1407–1420. doi: 10.1016/j.yjmcc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 130.Kubota K., Okazaki J., Louie O., Kent K.C., Liu B. TGF-beta stimulates collagen (I) in vascular smooth muscle cells via a short element in the proximal collagen promoter. J. Surg. Res. 2003;109(1):43–50. doi: 10.1016/s0022-4804(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 131.Verrecchia F., Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118(2):211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 132.Leask A., Abraham D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Q.J., Goddard M., Shanahan C., Shapiro L., Bennett M. Differential gene expression in vascular smooth muscle cells in primary atherosclerosis and in stent stenosis in humans. Arterioscler. Thromb. Vasc. Biol. 2002;22(12):2030–2036. doi: 10.1161/01.atv.0000042206.98651.15. [DOI] [PubMed] [Google Scholar]
- 134.Khan R., Agrotis A., Bobik A. Understanding the role of transforming growth factor-beta1 in intimal thickening after vascular injury. Cardiovasc. Res. 2007;74(2):223–234. doi: 10.1016/j.cardiores.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 135.Majack R.A. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. J. Cell Biol. 1987;105(1):465–471. doi: 10.1083/jcb.105.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hwang D.L., Latus L.J., Lev-Ran A. Effects of platelet-contained growth factors (PDGF, EGF, IGF-I, and TGF-beta) on DNA synthesis in porcine aortic smooth muscle cells in culture. Exp. Cell Res. 1992;200(2):358–360. doi: 10.1016/0014-4827(92)90183-9. [DOI] [PubMed] [Google Scholar]
- 137.Owens G.K., Geisterfer A.A., Yang Y.W., Komoriya A. Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J. Cell Biol. 1988;107(2):771–780. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Majack RA, Majesky MW, Goodman LV. Role of PDGF-A expression in the control of vascular smooth muscle cell growth by transforming growth factor-beta. J. Cell Biol. 1990;111(1):239-47. [DOI] [PMC free article] [PubMed]
- 139.Little P.J., Allen T.J., Hashimura K., Nigro J., Farrelly C.A., Dilley R.J. High glucose potentiates mitogenic responses of cultured ovine coronary smooth muscle cells to platelet derived growth factor and transforming growth factor-beta1. Diabetes Res. Clin. Pract. 2003;59(2):93–101. doi: 10.1016/s0168-8227(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 140.Reddy K.B., Howe P.H. Transforming growth factor beta 1-mediated inhibition of smooth muscle cell proliferation is associated with a late G1 cell cycle arrest. J. Cell. Physiol. 1993;156(1):48–55. doi: 10.1002/jcp.1041560108. [DOI] [PubMed] [Google Scholar]
- 141.Seay U., Sedding D., Krick S., Hecker M., Seeger W., Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J. Pharmacol. Exp. Ther. 2005;315(3):1005–1012. doi: 10.1124/jpet.105.091249. [DOI] [PubMed] [Google Scholar]
- 142.Martin-Garrido A., Williams H.C., Lee M., Seidel-Rogol B., Ci X., Dong J.T. Transforming Growth Factor beta Inhibits Platelet Derived Growth Factor-Induced Vascular Smooth Muscle Cell Proliferation via Akt-Independent, Smad-Mediated Cyclin D1 Downregulation. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klein E.A., Assoian R.K. Transcriptional regulation of the cyclin D1 gene at a glance. J. Cell Sci. 2008;121(23):3853–3857. doi: 10.1242/jcs.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Battegay E.J., Raines E.W., Seifert R.A., Bowen-Pope D.F., Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63(3):515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- 145.Stouffer G.A., Owens G.K. Tgf-Beta Promotes Proliferation of Cultured Smc Via Both Pdgf-Aa-Dependent and Pdgf-Aa-Independent Mechanisms. J. Am. Coll. Cardiol. 1994;A162-A doi: 10.1172/JCI117199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mao X., DeBenedittis P., Sun Y., Chen J.F., Yuan K.Y., Jiao K. Vascular Smooth Muscle Cell Smad4 Gene Is Important for Mouse Vascular Development. Arterioscler. Thromb. Vasc. Biol. 2012;32(9):2171. doi: 10.1161/ATVBAHA.112.253872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Suwanabol P.A., Seedial S.M., Shi X., Zhang F., Yamanouchi D., Roenneburg D. Transforming growth factor-beta increases vascular smooth muscle cell proliferation through the Smad3 and extracellular signal-regulated kinase mitogen-activated protein kinases pathways. J. Vasc. Surg. 2012;56(2):446–454. doi: 10.1016/j.jvs.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kobayashi K., Yokote K., Fujimoto M., Yamashita K., Sakamoto A., Kitahara M. Targeted disruption of TGF-beta-Smad3 signaling leads to enhanced neointimal hyperplasia with diminished matrix deposition in response to vascular injury. Circ. Res. 2005;96(8):904–912. doi: 10.1161/01.RES.0000163980.55495.44. [DOI] [PubMed] [Google Scholar]
- 149.Mii S., Ware J.A., Kent K.C. Transforming growth factor-beta inhibits human vascular smooth muscle cell growth and migration. Surgery. 1993;114(2):464–470. [PubMed] [Google Scholar]
- 150.Engel L., Ryan U. TGF-beta 1 reverses PDGF-stimulated migration of human aortic smooth muscle cells in vitro. In Vitro Cell Dev Biol Anim. 1997;33(6):443–451. doi: 10.1007/s11626-997-0062-x. [DOI] [PubMed] [Google Scholar]
- 151.Risinger G.M., Jr., Updike D.L., Bullen E.C., Tomasek J.J., Howard E.W. TGF-beta suppresses the upregulation of MMP-2 by vascular smooth muscle cells in response to PDGF-BB. Am. J. Phys. Cell Phys. 2010;298(1):C191–C201. doi: 10.1152/ajpcell.00417.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Basson C.T., Kocher O., Basson M.D., Asis A., Madri J.A. Differential modulation of vascular cell integrin and extracellular matrix expression in vitro by TGF-beta 1 correlates with reciprocal effects on cell migration. J. Cell. Physiol. 1992;153(1):118–128. doi: 10.1002/jcp.1041530116. [DOI] [PubMed] [Google Scholar]
- 153.Janat M.F., Argraves W.S., Liau G. Regulation of vascular smooth muscle cell integrin expression by transforming growth factor beta1 and by platelet-derived growth factor-BB. J. Cell. Physiol. 1992;151(3):588–595. doi: 10.1002/jcp.1041510319. [DOI] [PubMed] [Google Scholar]
- 154.Brown S.L., Lundgren C.H., Nordt T., Fujii S. Stimulation of migration of human aortic smooth muscle cells by vitronectin: implications for atherosclerosis. Cardiovasc. Res. 1994;28(12):1815–1820. doi: 10.1093/cvr/28.12.1815. [DOI] [PubMed] [Google Scholar]
- 155.Srivatsa SS, Fitzpatrick LA, Tsao PW, Reilly TM, Holmes DR, Jr., Schwartz RS, et al. Selective alpha v beta 3 integrin blockade potently limits neointimal hyperplasia and lumen stenosis following deep coronary arterial stent injury: evidence for the functional importance of integrin alpha v beta 3 and osteopontin expression during neointima formation. Cardiovasc. Res. 1997;36(3):408-28. [DOI] [PubMed]
- 156.Ward M.R., Agrotis A., Kanellakis P., Dilley R., Jennings G., Bobik A. Inhibition of protein tyrosine kinases attenuates increases in expression of transforming growth factor-beta isoforms and their receptors following arterial injury. Arterioscler. Thromb. Vasc. Biol. 1997;17(11):2461–2470. doi: 10.1161/01.atv.17.11.2461. [DOI] [PubMed] [Google Scholar]
- 157.Bertoli-Avella A.M., Gillis E., Morisaki H., Verhagen J.M.A., de Graaf B.M., van de Beek G. Mutations in a TGF-beta Ligand, TGFB3, Cause Syndromic Aortic Aneurysms and Dissections. J. Am. Coll. Cardiol. 2015;65(13):1324–1336. doi: 10.1016/j.jacc.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pardali E., Goumans M.J., ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20(9):556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 159.Pardali E., Ten Dijke P. TGFbeta signaling and cardiovascular diseases. Int. J. Biol. Sci. 2012;8(2):195–213. doi: 10.7150/ijbs.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tamai H., Katoh O., Suzuki S., Fujii K., Aizawa T., Takase S. Impact of tranilast on restenosis after coronary angioplasty: tranilast restenosis following angioplasty trial (TREAT) Am. Heart J. 1999;138(5):968–975. doi: 10.1016/s0002-8703(99)70025-6. Pt 1. [DOI] [PubMed] [Google Scholar]
- 161.Tamai H., Katoh K., Yamaguchi T., Hayakawa H., Kanmatsuse K., Haze K. The impact of tranilast on restenosis after coronary angioplasty: the Second Tranilast Restenosis Following Angioplasty Trial (TREAT-2) Am. Heart J. 2002;143(3):506–513. doi: 10.1067/mhj.2002.120770. [DOI] [PubMed] [Google Scholar]
- 162.Azuma H., Banno K., Yoshimura T. Pharmacological properties of N-(3',4'-dimethoxycinnamoyl) anthranilic acid (N-5'), a new anti-atopic agent. Br. J. Pharmacol. 1976;58(4):483–488. doi: 10.1111/j.1476-5381.1976.tb08614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Suzuki H., Tanaka K., Kaneshige H., Nakagami K., Noguchi E. The effects of long term Tranilast administration on bronchial hypersensitivity in asthmatics. Panminerva Med. 1989;31(2):88–93. [PubMed] [Google Scholar]
- 164.Kohavi L., Sprecher E., Zur E., Artzi O. The Effect of Tranilast 8% Liposomal Gel Versus Placebo on Post-Cesarean Surgical Scars: A Prospective Double-Blind Split-Scar Study. Dermatol. Surg. 2017;43(9):1157–1163. doi: 10.1097/DSS.0000000000001140. [DOI] [PubMed] [Google Scholar]
- 165.Holmes D.R., Jr., Savage M., LaBlanche J.M., Grip L., Serruys P.W., Fitzgerald P. Results of Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2002;106(10):1243–1250. doi: 10.1161/01.cir.0000028335.31300.da. [DOI] [PubMed] [Google Scholar]
- 166.Akhurst R.J. Targeting TGF-beta Signaling for Therapeutic Gain. Cold Spring Harb. Perspect. Biol. 2017;9(10) doi: 10.1101/cshperspect.a022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Akhurst R.J., Hata A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.King T.E., Jr., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 169.Herbertz S., Sawyer J.S., Stauber A.J., Gueorguieva I., Driscoll K.E., Estrem S.T. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479–4499. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Anderton M.J., Mellor H.R., Bell A., Sadler C., Pass M., Powell S. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol. Pathol. 2011;39(6):916–924. doi: 10.1177/0192623311416259. [DOI] [PubMed] [Google Scholar]
- 171.Rodon J., Carducci M.A., Sepulveda-Sanchez J.M., Azaro A., Calvo E., Seoane J. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin. Cancer Res. 2015;21(3):553–560. doi: 10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ballantyne M.D., McDonald R.A., Baker A.H. lncRNA/MicroRNA interactions in the vasculature. Clin. Pharmacol. Ther. 2016;99(5):494–501. doi: 10.1002/cpt.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Butz H., Racz K., Hunyady L., Patocs A. Crosstalk between TGF-beta signaling and the microRNA machinery. Trends Pharmacol. Sci. 2012;33(7):382–393. doi: 10.1016/j.tips.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 174.Long X., Miano J.M. Transforming growth factor-beta1 (TGF-beta1) utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J. Biol. Chem. 2011;286(34):30119–30129. doi: 10.1074/jbc.M111.258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cordes K.R., Sheehy N.T., White M.P., Berry E.C., Morton S.U., Muth A.N. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256) doi: 10.1038/nature08195. (705-U80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Boettger T., Beetz N., Kostin S., Schneider J., Kruger M., Hein L. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J. Clin. Investig. 2009;119(9):2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Davis B.N., Hilyard A.C., Lagna G., Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McDonald R.A., White K.M., Wu J., Cooley B.C., Robertson K.E., Halliday C.A. miRNA-21 is dysregulated in response to vein grafting in multiple models and genetic ablation in mice attenuates neointima formation. Eur. Heart J. 2013;34(22):1636–1643. doi: 10.1093/eurheartj/eht105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ji R., Cheng Y., Yue J., Yang J., Liu X., Chen H. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 2007;100(11):1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 180.Roberts A.B., Anzano M.A., Lamb L.C., Smith J.M., Frolik C.A., Marquardt H. Isolation from murine sarcoma cells of novel transforming growth factors potentiated by EGF. Nature. 1982;295(5848):417–419. doi: 10.1038/295417a0. [DOI] [PubMed] [Google Scholar]
- 181.Roberts A.B., Anzano M.A., Wakefield L.M., Roche N.S., Stern D.F., Sporn M.B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc. Natl. Acad. Sci. U. S. A. 1985;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhao W., Wang C., Liu R., Wei C., Duan J., Liu K. Effect of TGF-beta1 on the Migration and Recruitment of Mesenchymal Stem Cells after Vascular Balloon Injury: Involvement of Matrix Metalloproteinase-14. Sci. Rep. 2016;6 doi: 10.1038/srep21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Merrilees M., Beaumont B., Scott L., Hermanutz V., Fennessy P. Effect of TGF-beta(1) antisense S-oligonucleotide on synthesis and accumulation of matrix proteoglycans in balloon catheter-injured neointima of rabbit carotid arteries. J. Vasc. Res. 2000;37(1):50–60. doi: 10.1159/000025713. [DOI] [PubMed] [Google Scholar]
- 184.Ward M.R., Sasahara T., Agrotis A., Dilley R.J., Jennings G.L., Bobik A. Inhibitory effects of tranilast on expression of transforming growth factor-beta isoforms and receptors in injured arteries. Atherosclerosis. 1998;137(2):267–275. doi: 10.1016/s0021-9150(97)00275-x. [DOI] [PubMed] [Google Scholar]
- 185.Ohashi N., Matsumori A., Furukawa Y., Ono K., Okada M., Iwasaki A. Role of p38 mitogen-activated protein kinase in neointimal hyperplasia after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2000;20(12):2521–2526. doi: 10.1161/01.atv.20.12.2521. [DOI] [PubMed] [Google Scholar]
- 186.Yao E.H., Fukuda N., Ueno T., Matsuda H., Nagase H., Matsumoto Y. A pyrrole-imidazole polyamide targeting transforming growth factor-beta1 inhibits restenosis and preserves endothelialization in the injured artery. Cardiovasc. Res. 2009;81(4):797–804. doi: 10.1093/cvr/cvn355. [DOI] [PubMed] [Google Scholar]