Abstract
Purpose:
This study compared the effectiveness of endothelial/Descemet’s membrane complex thickness obtained using high-definition anterior segment optical coherence tomography with endothelial cell density obtained using confocal microscopy as diagnostic tools in predicting corneal transplant rejection.
Methods:
This observational, prospective, cross-sectional study evaluated penetrating keratoplasty grafts. Slit lamp examination organized the grafts into healthy or rejecting grafts. Grafts were scanned using both high-definition anterior segment optical coherence tomography and confocal microscopy. Central corneal thickness, endothelial/Descemet’s membrane complex thickness, endothelial cell density, and coefficient of variation were each compared with the clinical status. Descemet’s rejection index, defined by endothelial/Descemet’s membrane complex thickness divided by central corneal thickness multiplied by 33, further compared endothelial/Descemet’s membrane complex thickness with central corneal thickness.
Results:
Endothelial/Descemet’s membrane complex thickness, central corneal thickness, and Descemet’s rejection index were all able to differentiate between clear and rejected corneal grafts (p < 0.0001, p = 0.001, and p = 0.012, respectively). Endothelial cell density and coefficient of variation did not correlate with the clinical status (p = 0.054 and p = 0.102, respectively). Endothelial/Descemet’s membrane complex thickness had the largest area under the curve using receiver operating characteristic curves (p < 0.0001). Endothelial/Descemet’s membrane complex thickness had a sensitivity of 86% and specificity of 81% with a cutoff value of >16.0 µm (p < 0.0001). The sensitivity and specificity of endothelial cell density were both 71% with a cutoff value of ⩽897 cells/mm2 (p = 0.053). There was a high correlation between endothelial/Descemet’s membrane complex thickness and both Descemet’s rejection index and central corneal thickness (p < 0.0001).
Conclusion:
Endothelial/Descemet’s membrane complex thickness measured by high-definition anterior segment optical coherence tomography is a useful parameter for the diagnosis of corneal graft rejection. The diagnostic performance of endothelial/Descemet’s membrane complex thickness was significantly better than that of endothelial cell density and central corneal thickness. Endothelial cell density and the coefficient of variation were unable to diagnose corneal graft rejection in our cross-sectional study.
Keywords: corneal graft rejection, Descemet’s membrane, endothelial cell density, optical coherence tomography
Introduction
The current standard of care for determining the status of penetrating keratoplasty (PK) grafts is by slit lamp examination.1 Using only the slit lamp, the clinician might fail to see early changes of rejection and it introduces subjectivity. The Cornea Donor Study defined corneal rejection by finding endothelial rejection line, stromal infiltrate, keratic precipitates, cells in the anterior chamber, or ciliary injection in a graft that was previous clear.2 Currently, there are no other imaging or objective parameters to define rejection in PKs.
To assist with corneal failure, ophthalmologists have used specular and confocal microscopy for the evaluation of corneal grafts at a cellular level.2,3 Parameters evaluated to attempt to predict corneal failure include endothelial cell density (ECD), central corneal thickness (CCT), coefficient of variation of cell area (polymegathism), and variation of cell shape (pleomorphism).2,4 Among PKs, Lass and colleagues5 found the 6-month postoperative ECD was predictive of late graft failure. This study did not evaluate correlation with rejection. It is thought that a lower ECD is a risk factor for late graft failure. However, many grafts with a low ECD <1000 cells/mm2, even as low as <500 cells/mm2, remained clear years after transplantation.5,6 CCT has also been correlated with graft failure, however, not rejection.7
The histopathology review of solid organ transplantation shows a common pattern of thickening of the basement membrane.8–12 Our group has shown that endothelial/Descemet’s membrane complex thickness (En/DMT) will mimic the changes seen in the basement membrane from solid organs with transplant rejection.13 We concluded that this thickening as seen in En/DMT is diagnostic of graft rejection. In addition, evaluation of the cornea is much less invasive when using high-definition optical coherence tomography (HD-OCT) as no biopsy is necessary. HD-OCT has the ability to image and determine the thickness of the En/DMT in vivo in a noninvasive fashion.14
This is the first study to compare En/DMT with ECD and coefficient of variation of cell area in the diagnosis of corneal graft rejection. It is our hope to give clinicians an objective tool to assist in predicting the viability of a PK graft.
Methods
Study population
This study was submitted to and approved by the Saint Louis University Institutional Review Board, ID# 23109. Written informed consent, which was reviewed by the Saint Louis University Institutional Review Board, was obtained from each participant. Prior to testing, each participant was informed about the goals and protocol of the procedure.
Inclusion criteria for participation included full thickness corneal transplant and surgery performed greater than 1 month prior. This was a consecutive series of PK surgeries. All surgeries were uncomplicated. Exclusion criteria included corneal grafts with corneal infection. Slit lamp examination was performed on each eye by a masked cornea trained specialist (either SE or MC) in order to assign the examined corneal grafts into either a clear or rejected category. A graft was considered rejected if corneal edema was present in two consecutive visits with a documented history of rejection episodes (keratic precipitates, anterior chamber cells, new graft edema, and a Khodadoust line). Rejected grafts were diagnosed by detecting corneal graft edema with a history of causative rejection episode. All failed grafts included in this study had failed secondary to rejection. Furthermore, each participant received anterior segment HD-OCT and confocal microscopy imaging.
Image acquisition
Using an HD-OCT device (Cirrus HD-OCT; Carl Zeiss Meditec, Dublin, CA, USA), an image of the cornea was obtained for each study eye. The machine was placed in the 5-line anterior segment mode. With the participant sitting comfortably in the headrest, the focus was advanced and centered on the cornea. Images were acquired at the corneal vertex of each eye as the patient was asked to look at the fixation light.
The Confoscan 4 (Nidek Technologies, Inc., Fremont, CA, USA) was used to obtain confocal microscopy images. A drop of topical anesthetic (proparacaine hydrochloride ophthalmic solution 0.5%; Alcon Laboratories, Inc., Fort Worth, TX, USA) was placed in the corneal transplant eye. The 40× contact lens method was used to obtain images. Coupling saline gel was placed over the contact lens. The patient was placed comfortably in the headrest and told to fixate on a target. The lens was slowly advanced until the gel coupled with the central apex of the corneal transplant. Once the gel was in contact, the brightest reflex was obtained and images acquired as per Confoscan instruction. Images were uploaded and reviewed. The process was repeated until a clear image was obtained. No more than five attempts were made for the concern of patient discomfort.
Image analysis
HD-OCT image analysis was previously reported by our group.13–15 As previously reported by our group, optical coherence tomography (OCT) reflectivity identified two peaks in Fuch’s endothelial dystrophy patients. After Descemet’s stripping automated endothelial keratoplasty (DSAEK), the removed Descemet’s membrane thickness was measured by light microscopy. There was a significant correlation between OCT and histological measurement of Descemet’s thickness.15 In our current study, we located two hyper-reflective bands seen in HD-OCT images that represent the interfaces of the anterior and posterior sides of endothelial/Descemet’s membrane complex. As HD-OCT is unable to differentiate Descemet’s membrane from endothelial tissue or a retrocorneal membrane, we grouped all tissue posterior to the anterior hyper-reflective layer as endothelial/Descemet’s membrane complex. A caliper, provided by the HD-OCT image analysis software, was used to measure the En/DMT at the vertex of the cornea by MAS who was masked to the groups. Thickness was measured at the center of each image.
ECD was obtained from confocal microscopy images. By means of the Nidek Advanced Vision Information System (NAVIS; Nidek Technologies, Inc.), a variable frame technique was used to outline a clear polygonal area. All clear cells were outlined and included in the polygonal shape by an operator (CS). ECD was reported by the counted cells in the polygonal area. Variation of cell size, polymegathism, was determined by outlining the border of each cell.
To further evaluate if the En/DMT is in proportion to the total corneal thickening of the graft from edema resulting from the rejection, a value was calculated as the Descemet’s rejection index (DRI). This value is the En/DMT divided by the CCT multiplied by a constant. We chose 33 to be the constant as this makes normal DRI values to be approximately equal to 1.13
Statistical analysis
Statistical analyses with SPSS software, version 22.0 (SPSS, Chicago, IL, USA), were performed to calculate descriptive statistics for all eyes. Means of En/DMT, ECD, CCT, polymegathism, and DRI were evaluated. The medians of these values were also reported to test for normality. The predictive accuracy, sensitivity and specificity, of all parameters was determined by generating a receiver operating characteristic (ROC) curve. Coefficient of correlation (R value) was used to compare correlation between all parameters above. Two-sided p values less than 0.05 were considered statistically significant. Values are presented as means ± standard deviation.
Results
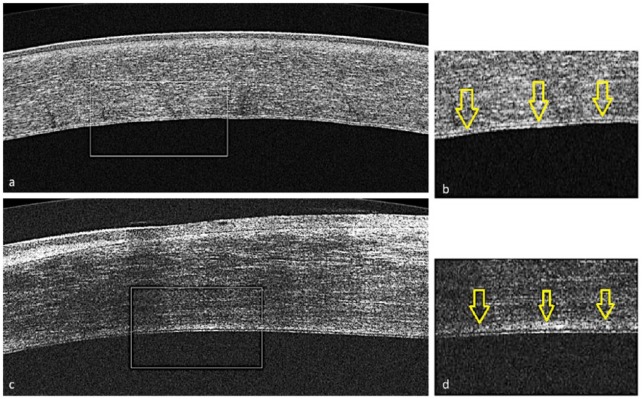
There were 31 participants and 37 eyes enrolled in this study, 18 women and 13 men. It was determined by a masked observer (either SE or MC) that 24 of the eyes were in the clear cornea group. Likewise, 13 eyes were qualified as rejected. Further demographics are displayed in Table 1. There was no significant difference between the mean age or years after corneal transplantation of the clear and rejected groups. A readable image was obtained by HD-OCT for measurable analysis in all of the 24 clear grafts and all 13 rejection grafts. The quality of all the obtained HD-OCT images was good enough to differentiate En/DMT from the rest of the cornea. An example comparing a clear graft HD-OCT image with a rejection graft image is shown in Figure 1. Confocal microscopy was able to obtain a readable image with all 24 clear grafts; however, only 7 of the 13 rejection graft images were readable enough to allow ECD measurement.
Table 1.
Characteristics of corneal grafts and mean values for ECD, En/DMT, CCT, polymegathism, and DRI.
| Clear group | Rejection group | p value (Mann–Whitney U test) | |
|---|---|---|---|
| Number of participants | 18 | 13 | |
| Number of eyes | 24 | 13 | |
| Age (years) | 67 ± 19 (27–92) | 53 ± 19 (31–80) | p = 0.080 |
| Gender | |||
| Men | 13 | 5 | |
| Women | 5 | 8 | |
| Postoperative time (years) | 7 ± 7 (1–22) | 6.4 ± 11 (1–36) | p = 0.88 |
| ECD (cells/mm2) | 1411 ± 714 (264–2069) | 870 ± 524 (212–1068) | p = 0.054 |
| En/DMT (µm) | 15.0 ± 2.9 (12.0–20.0) | 43.0 ± 45.7 (16.0–151.6) | p < 0.0001 |
| CCT (µm) | 526 ± 67 (606–315) | 775 ± 243 (471–1117) | p = 0.001 |
| Polymegathism (%) | 37.5 ± 12 (66.1–22.3) | 29.5 ± 7.5 (16.3–41.2) | p = 0.102 |
| DRI | 0.95 ± 0.19 (0.65–1.25) | 1.66 ± 1.37 (0.76–4.77) | p = 0.012 |
Values presented as means ± standard deviation (range).
CCT, central corneal thickness; DRI, En/DMT rejection index, defined as En/DMT divided by CCT multiplied by 33; ECD, endothelial cell density; En/DMT, Descemet’s membrane complex thickness.
Figure 1.
The four images (a–d) represent a comparison of anterior segment high-definition optical coherence tomography images between clear (a and b) and rejection corneal grafts (c and d).Presets (b and d) show magnified images of the posterior part of the corresponding cornea (a and c), respectively. Descemet’s membrane complex is represented by the area posterior to the hyper-reflective line. This is delineated by arrows seen in b and d.
There was significant increase in En/DMT, DRI, and CCT in rejected corneal grafts as compared with clear grafts group (43 ± 45.7 versus 15 ± 2.9 µm, p < 0.0001; 1.66 ± 1.37 versus 0.95 ± 0.19; p = .012; and 775 ± 243 versus 526 ± 67 µm, p = 0.001, respectively). On the other hand, there was no significant difference between ECD and coefficient of variation in rejected corneal grafts and clear grafts (870 ± 524 versus 1411 ± 714 cells/mm2, p = 0.054, and 29.5 ± 7.5 and 37.5 ± 12 percent variation, p = .102, respectively). In total, 21% of the clear grafts measured an ECD <700 cells/mm2. These data are summarized in Tables 1 and 2.
Table 2.
Median values for ECD, En/DMT, CCT, polymegathism, and DRI.
| Clear group | Rejection group | |
|---|---|---|
| ECD (cells/mm2) | 597 | 437 |
| En/DMT (µm) | 16.0 | 23.9 |
| CCT (µm) | 539 | 814 |
| DRI | 0.95 | 1.13 |
Values presented as median values.
CCT, central corneal thickness; DRI, En/DMT rejection index, defined as En/DMT divided by CCT multiplied by 33; ECD, endothelial cell density; En/DMT, Descemet’s membrane complex thickness.
CCT as a measurement of corneal graft status and severity of rejection had a significant correlation with En/DMT (R = 0.585, p < 0.0001). DRI correlated with En/DMT as well (R = 0.705, p < 0.0001). This study did not find any correlation between DRI and CCT (p = 0.987). There was no significant correlation between ECD and polymegathism with all other parameters. These results are summarized in Table 3.
Table 3.
Nonparametric correlation between ECD, En/DMT, CCT, and polymegathism (R value).
| En/DMT | CCT | Polymegathism | DRI | |
|---|---|---|---|---|
| ECD | −0.293 (p = 0.110) | −0.294 (p = 0.109) | −0.182 (p = 0.336) | −0.128 (p = 0.492) |
| En/DMT | x | 0.585 (p < 0.0001) | −0.007 (p = 0.971) | 0.705 (p < 0.0001) |
| CCT | x | x | 0.023 (p = 0.905) | 0.003 (p = 0.987) |
| Polymegathism | x | X | x | 0.012 (p = 0.948) |
Values presented as correlation coefficient (R value). x represents that the value is not applicable.
CCT, central corneal thickness. DRI, En/DMT rejection index; ECD, endothelial cell density. En/DMT, Descemet’s membrane complex thickness.
In order to compare the diagnostic performance of En/DMT, CCT, ECD, polymegathism, and DRI in the diagnosis of graft rejection, we created ROC curves. These data are presented in Table 4. En/DMT was 86% sensitive and 81% specific when the thickness of the graft was greater than 16 µm (optimal cutoff value, p < 0.0001). ECD did not prove as accurate of a test showing 71% for both sensitivity and specificity (p = 0.053). The area under the curve was highest for En/DMT followed by CCT, ECD, and polymegathism, respectively (Figure 2).
Table 4.
ROC curve data which represent the diagnostic performance of En/DMT, CCT, ECD, and polymegathism.
| Clear versus rejection group | ||
|---|---|---|
| En/DMT | p value | <0.0001 |
| AUC | 0.909 | |
| Sensitivity | 86% | |
| Specificity | 81% | |
| Cutoff | >16.0 µm | |
| CCT | p value | 0.0029 |
| AUC | 0.794 | |
| Sensitivity | 73% | |
| Specificity | 71% | |
| Cutoff | >563 µm | |
| ECD | p value | 0.053 |
| AUC | 0.744 | |
| Sensitivity | 71% | |
| Specificity | 71% | |
| Cutoff | ⩽897 cells/mm2 | |
| Polymegathism | p value | 0.1 |
| AUC | 0.292 | |
| Sensitivity | 71% | |
| Specificity | 70% | |
| Cutoff | ⩽30% |
AUC, area under the curve; CCT, central corneal thickness; ECD, endothelial cell density; En/DMT, Descemet’s membrane complex thickness; ROC, receiver operating characteristic.
Figure 2.
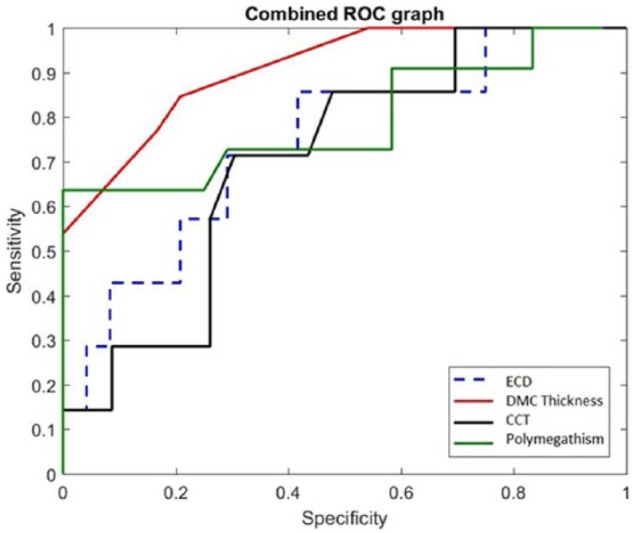
This graph represents the combined receiver operating characteristic curves of ECD, En/DMT, CCT, and polymegathism. Increased area under the curve represents a better sensitivity and specificity for the diagnostic test. En/DMT had the highest sensitivity and specificity compared with the other parameters. CCT, central corneal thickness; ECD, endothelial cell density; En/DMT, Descemet’s membrane complex thickness.
Discussion
The main goal of this study was to compare En/DMT obtained using HD-OCT with other diagnostic parameters to evaluate PK status. In this study, En/DMT, DRI, and CCT were able to predict whether a PK was clear or rejected as it correlated with the clinical exam. En/DMT was highly correlated with corneal rejection severity as measured by CCT. The CCT increase may be a combination of endothelial dysfunction during rejection and loss of ECD; this study is unable to differentiate the two. On the other hand, ECD and polymegathism obtained using confocal microscopy were unable to differentiate between clear and rejected grafts.
As there is no objective imaging to evaluate a snapshot evaluation of PK grafts, slit lamp is the current standard but introduces subjectivity. Our prior study was able to show the validity of En/DMT as a diagnostic tool.13 In total, 139 eyes with prior PK or DSAEK were separated clinically into rejecting or clear groups. Among actively rejecting grafts, En/DMT and DRI were significantly greater compared with clear grafts and controls. Diagnostic abilities of En/DMT and DRI were excellent when comparing actively rejecting grafts from clear grafts (100% and 96% sensitivity; 92.5% and 92.5% specificity).
Prior to this study, En/DMT has not been compared with ECD to evaluate rejection. Among grafts in rejection or failure, there is expected to be more ECD loss. Our results follow this trend; however, overall En/DMT growth was much more significant. This potentially may detect preclinical rejection; however, future longitudinal studies are necessary for newer keratoplasty techniques.
There was a statistically significant correlation found among CCT and En/DMT. This was expected because as the transplant begins to reject, the entire transplant thickens, increasing both CCT and En/DMT. Our group used the DRI, as explained above, to determine whether the En/DMT increases more in proportion to the entire CCT. We have previously shown the diagnostic ability of DRI in our prior study.13 The clear group had a DRI of 0.95 and a rejection group of 1.67. Therefore, the En/DMT thickened proportionally more than the CCT. This highlights that the thickening cannot be simply explained by corneal edema caused by endothelial decompensation accompanying graft rejection.
ECD and CCT in prior studies have been shown to predict late PK graft failure, however, not corneal rejection.5,7 Multiple possible etiologies for increased cell loss in PKs include surgical trauma, decreased nutrition from aqueous, decreased innervation, or subclinical inflammation.16 The Cornea Donor Study found at 6 months, 1 year, and at 5 years that ECD and CCT were associated with late graft failure.17 However, these studies do not comment on ECD or CCT with regard to PK rejection.
Clear grafts with low endothelial cell counts have been observed in multiple studies.6,18–21 Cornea Donor Study Investigator Group has, thus, recommended against using it for the follow-up of corneal grafts.22 Our study correlated with these findings, in that 5 of the 24 clear grafts had an ECD <700 cells/mm2. Solely relying on ECD may be cause for misinterpretation of graft status.
Another parameter to attempt to predict late graft failure, not rejection, is the coefficient of variation (polymegathism). Ing and colleagues17 did not find a statistically significant change from the 2-month postoperative examination and 5 years after surgery while looking at the coefficient of variation. Our study did not show a correlation with this parameter and rejection.
Acquiring clear images via confocal microscopy proved difficult among the corneal rejection grafts. In this study, only 54% of the rejection grafts produced a readable confocal image. Possible explanations for the difficulty may be due to light scattering from increased edema, poor ability of participant to keep eye on fixation target due to poor vision, or the confocal machine had difficulty focusing due to thickening of the tissue posterior to the endothelial layer. Meanwhile, HD-OCT produced clear readable images among all of the clear and rejection grafts without need of multiple repeat attempts. Logistically, HD-OCT imaging may be more practical for clinicians.
The mechanism of the En/DMT complex growth is unclear, and future histopathological and immunohistochemistry studies are warranted. There are multiple possible etiologies to explain the thickening of En/DMT. This observed thickening of the En/DMT may be secondary to the growth of basement membrane as seen in other organ transplants, that is, kidney and lung.8–12 Previously, our group has shown that the En/DMT grows 1.2 µm per decade in normal corneas and increases in thickness more so in a disease state like Fuchs corneal dystrophy.14 Another mechanism might be thickening secondary to immunological deposits or secondary to the development of a retrocorneal membrane.23–25 With the development of HD-OCT, it is now possible to image En/DMT in vivo and evaluate the growth of this layer.14,26 Our group has conducted an ex vivo histopathological study that included 54 corneal specimens.15 That study compared the thickness of Descemet’s membrane among rejected corneal graft specimens and clear corneas after enucleation secondary to melanoma. The Descemet’s membrane was significantly thicker in the rejected group, while only three had retrocorneal membranes and none of the rejected grafts had endothelium. Our ex vivo study, thus, suggested that the Descemet’s membrane was the source of the in vivo En/DMT thickening observed using the HD-OCT.
We acknowledge some limitations in this study. It was difficult to obtain clear images with confocal microscopy among the rejection patients. That led to missing confocal microscopy data in the corneal rejection group. However, this also highlights the disadvantage of relying on confocal microscopy. The surgical trauma is difficult to standardize for each graft. There were two surgeons and two different examiners who introduced observational bias. By slit lamp alone, it is difficult to differentiate between rejected grafts and secondary graft failure without inflammation, and these grafts are all included in the rejected/failure group. Our theory is that in each of these categories, En/DMT will increase. Also, this study was a cross-sectional prospective observational study. It would be valuable to compare age-matched controls and follow them over time to get more of a dynamic growth measurement of the En/DMT. Also, as it is a cross-sectional study, our study did not compare the difference in En/DMT growth and ECD loss with different preoperative corneal transplant indications (i.e. scarring, keratoconus). Further studies are warranted for this evaluation. Another limitation and future direction of this study is that the donor ECD was not available.
In conclusion, we see that En/DMT has the potential of being an important diagnostic tool to assist clinicians in the diagnosis of PK rejection. Slit lamp exam continues to be the most accurate and reliable method to diagnose corneal graft rejection. We propose in this study that En/DMT may be another objective way to help clinicians manipulate management in the postoperative period.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an NEI K23 award (K23EY026118), NEI core center grant to the University of Miami (P30 EY014801), Research to Prevent Blindness (RPB), and the American Society of Cataract and Refractive Surgery (ASCRS) Foundation. The funding organization had no role in the design or conduct of this research.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: United States Non-Provisional Patents (Application Nos 8992023 and 61809518), and PCT/US2018/013409. Patents and PCT are owned by University of Miami and licensed to Resolve Ophthalmics, LLC. Mohamed Abou Shousha is an equity holder and sits on the Board of Directors for Resolve Ophthalmics, LLC.
Synopsis: In this observational study, endothelial/Descemet’s membrane complex thickness was a more accurate diagnostic test compared with endothelial cell density at predicting whether a corneal transplant was in rejection or not.
ORCID iD: Mohamed Abou Shousha  https://orcid.org/0000-0002-4508-0492
https://orcid.org/0000-0002-4508-0492
Contributor Information
Christopher Smith, Saint Louis University Eye Institute, St. Louis, MO, USA.
Daniel Kaitis, Saint Louis University Eye Institute, St. Louis, MO, USA.
Jordan Winegar, Saint Louis University Eye Institute, St. Louis, MO, USA.
Sean Edelstein, Saint Louis University Eye Institute, St. Louis, MO, USA.
Matthew Council, Saint Louis University Eye Institute, St. Louis, MO, USA.
George Kontadakis, Institute of Vision & Optics, University of Crete, Crete, Greece.
Rocio Bentivegna, Saint Louis University Eye Institute, St. Louis, MO, USA.
Mohamed Abou Shousha, Department of Ophthalmology, Bascom Palmer Eye Institute, 900 NW 17th Street, Miami, FL 33136, USA.
References
- 1. Tan DTH, Dart JKG, Holland EJ, et al. Corneal transplantation. Lancet 2012; 379: 1749–1761. [DOI] [PubMed] [Google Scholar]
- 2. Dunn SP Gal RL Kollman C et al.;. Writing Committee for the Cornea Donor Study Research Group. Corneal graft rejection 10 years after penetrating keratoplasty in the cornea donor study. Cornea 2014; 33: 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bourne WM. Corneal endothelium – past, present, and future. Eye Contact Lens 2010; 36: 310–314. [DOI] [PubMed] [Google Scholar]
- 4. McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea 2008; 27: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lass JH, Sugar A, Benetz BA, et al. ; Cornea Donor Study Investigator Group. Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch Ophthalmol 2010; 128: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Visby E, Hjortdal J, Nielsen K. Evaluation of grafted patients with donor corneas that today are more than 100 years old. Acta Ophthalmol 2014; 92: 478–481. [DOI] [PubMed] [Google Scholar]
- 7. Verdier DD, Sugar A, Baratz K, et al. ; Cornea Donor Study Investigator Group. Corneal thickness as a predictor of corneal transplant outcome. Cornea 2013; 32: 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taddesse-Heath L, Kovi J. Electron microscopic findings in hepatic allograft rejection. J Natl Med Assoc 1994; 86: 779–782. [PMC free article] [PubMed] [Google Scholar]
- 9. Aita K, Yamaguchi Y, Horita S, et al. Thickening of the peritubular capillary basement membrane is a useful diagnostic marker of chronic rejection in renal allografts. Am J Transplant 2007; 7: 923–929. [DOI] [PubMed] [Google Scholar]
- 10. Siddiqui MT, Garrity ER, Martinez R, et al. Bronchiolar basement membrane changes associated with bronchiolitis obliterans in lung allografts: a retrospective study of serial transbronchial biopsies with immunohistochemistry. Mod Pathol 1996; 9: 320–328. [PubMed] [Google Scholar]
- 11. Shimizu T, Ishida H, Shirakawa H, et al. Clinicopathological analysis of transplant glomerulopathy cases. Clin Transplant 2009; 23(Suppl. 20): 39–43. [DOI] [PubMed] [Google Scholar]
- 12. Roufosse CA, Shore I, Moss J, et al. Peritubular capillary basement membrane multilayering on electron microscopy: a useful marker of early chronic antibody-mediated damage. Transplantation 2012; 94: 269–274. [DOI] [PubMed] [Google Scholar]
- 13. Abou Shousha M, Yoo SH, Sayed MS, et al. In vivo characteristics of corneal endothelium/Descemet’s membrane complex for the diagnosis of corneal graft rejection. Am J Ophthalmol 2017; 178: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shousha MA, Perez VL, Wang J, et al. Use of ultra-high-resolution optical coherence tomography to detect in vivo characteristics of Descemet’s membrane in Fuchs’ dystrophy. Ophthalmology 2010; 117: 1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. VanDenBerg R, Diakonis VF, Shousha MA, et al. Descemet’s membrane thickening as a sign for the diagnosis of corneal graft rejection: an ex vivo study. Cornea 2017; 36: 1535–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Investig Ophthalmol Vis Sci 1997; 38: 779–782. [PubMed] [Google Scholar]
- 17. Ing JJ, Ing HH, Nelson LR, et al. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology 1998; 105: 1855–1865. [DOI] [PubMed] [Google Scholar]
- 18. Sugar A, Gal RL, Kollman C, et al. ; Writing Committee for the Cornea Donor Study Research Group. Factors associated with corneal graft survival in the cornea donor study. JAMA Ophthalmol 2015; 133: 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen LB, Hjortdal J, Ehlers N. Longterm follow-up of penetrating keratoplasty for keratoconus. Acta Ophthalmol 2010; 88: 347–351. [DOI] [PubMed] [Google Scholar]
- 20. Kus MM, Seitz B, Langenbucher A, et al. Endothelium and pachymetry of clear corneal grafts 15 to 33 years after penetrating keratoplasty. Am J Ophthalmol 1999; 127: 600–602. [DOI] [PubMed] [Google Scholar]
- 21. Basak SK. Low endothelial cell count and clear corneal grafts. Indian J Ophthalmol 2004; 52: 151–153. [PubMed] [Google Scholar]
- 22. Lass JH, Sugar A, Benetz BA, et al. Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch Ophthalmol 2010; 128: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sherrard ES, Rycroft PV. Retrocorneal membranes. I. Their origin and structure. Br J Ophthalmol 1967; 51: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jakobiec FA, Bhat P. Retrocorneal membranes: a comparative immunohistochemical analysis of keratocytic, endothelial, and epithelial origins. Am J Ophthalmol 2010; 150: 230.e2–242.e2. [DOI] [PubMed] [Google Scholar]
- 25. Vargas LG, Vroman DT, Solomon KD, et al. Epithelial downgrowth after clear cornea phacoemulsification: report of two cases and review of the literature. Ophthalmology 2002; 109: 2331–2335. [DOI] [PubMed] [Google Scholar]
- 26. Tao A, Chen Z, Shao Y, et al. Phacoemulsification induced transient swelling of corneal Descemet’s endothelium complex imaged with ultra-high resolution optical coherence tomography. PLoS ONE 2013; 8: e80986. [DOI] [PMC free article] [PubMed] [Google Scholar]