Abstract
Study Design:
Retrospective, multicenter review of adult scoliosis patients with minimum 2-year follow-up.
Objective:
Because the fractional curve (FC) of adult scoliosis can cause radiculopathy, we evaluated patients treated with either circumferential minimally invasive surgery (cMIS) or open surgery.
Methods:
A multicenter retrospective adult deformity review was performed. Patients included: age >18 years with FC >10°, ≥3 levels of instrumentation, 2-year follow-up, and one of the following: coronal Cobb angle (CCA) > 20°, pelvic incidence and lumbar lordosis (PI-LL) > 10°, pelvic tilt (PT) > 20°, and sagittal vertical axis (SVA) > 5 cm.
Results:
The FC was treated in 118 patients, 79 open and 39 cMIS. The FCs had similar coronal Cobb angles preoperative (17° cMIS, 19.6° open) and postoperative (7° cMIS, 8.1° open), but open had more levels treated (12.1 vs 5.7). cMIS patients had greater reduction in VAS leg (6.4 to 1.8) than open (4.3 to 2.5). With propensity matching 40 patients for levels treated (cMIS: 6.6 levels, N = 20; open: 7.3 levels, N = 20), both groups had similar FC correction (18° in both preoperative, 6.9° in cMIS and 8.5° postoperative). Open had more posterior decompressions (80% vs 22.2%, P < .001). Both groups had similar preoperative (Visual Analogue Scale [VAS] leg 6.1 cMIS and 5.4 open) and postoperative (VAS leg 1.6 cMIS and 3.1 open) leg pain. All cMIS patients had interbody grafts; 35% of open did. There was no difference in change of primary CCA, PI-LL, LL, Oswestry Disability Index, or VAS Back.
Conclusion:
Patients’ FCs treated with cMIS had comparable reduction of leg pain compared with those treated with open surgery, despite significantly fewer cMIS patients undergoing direct decompression.
Keywords: deformity, fractional curve, laminectomy, lumbar, lumbar interbody fusion, minimally invasive, MIS, radiculopathy, scoliosis
Introduction
In adult scoliosis patients, the decision to undergo surgery is typically driven by disability and pain. Many times, in well-balanced patients, the radiculopathy is the primary cause of significant disability and change in their functional status. Although stenosis in the concavity of the major curve of the scoliosis can be a cause of radiculopathy, the fractional curve (usually from L4 to S1) below the major curve is often the primary driver for the patient to pursue surgery. In addition to curve progression causing cephalocaudal foraminal (“up-down”) stenosis on the concavity of the fractional curve, the degeneration of the lower lumbar spine from L4 to S1 secondary to disc desiccation, collapse, and listhesis causes further loss of foraminal height. This loss of foraminal height eventually compresses the exiting nerve roots at the dorsal root ganglion, which is much less tolerant of compression than other parts of the nerve roots. Thus, the radiculopathy from the fractional curve may be refractory to conservative care because of loss of structural integrity of the spine to hold the neural foramen open.
Treatment of the fractional curve can be critical in alleviating patients’ pain, which may require extension of the fusion to either L5 or S1. Although indirect decompression is often performed in open scoliosis surgery, concomitant central and lateral recess stenosis may coexist, necessitating a direct decompressive laminectomy since the indirect decompression may be insufficient to properly alleviate the central stenosis. Traditionally, adult scoliosis has been treated in an open fashion, allowing for the direct decompression of the nerves in the fractional curve in addition to any realignment of the spine. However, minimally invasive surgery (MIS) has been increasingly used in adult deformity surgery, with potential benefits of reduction in morbidity and impact to the patient. A circumferential MIS (cMIS) surgery not only involves MIS or mini-open anterior or lateral interbody fusion but also percutaneous screw fixation without muscle stripping of the posterior spine. Such circumferential surgery relies on indirect decompression of the foramen, lateral recesses, and central canal through interbody distraction; it generally does not include direct decompressive laminectomy. Although prior studies have shown that indirect decompression improves foraminal height and central canal diameter, outcomes measures specifically related to the fractional curve of adult scoliosis have not been formally reported to our knowledge.1,2 The purpose of this study is to evaluate the outcomes of treatment of the fractional curve in adult scoliosis via cMIS techniques with interbody distraction versus traditional, open posterior surgical treatment.
Methods
A retrospective review of 2 multicenter adult spinal deformity (ASD) databases with similar inclusion criteria was conducted. Institutional review board approval was obtained (#14-13 558, University of California, San Francisco). The first database included ASD patients from 11 institutions in the United States who underwent traditional open spine surgery and were enrolled into a prospective registry. The second database is a retrospective registry of patients from 10 institutions in the United States who underwent minimally invasive spine surgery that includes cMIS, posterior-only MIS (pMIS), stand-alone lateral interbody fusions, and hybrid techniques such as lateral interbody fusions with open posterior surgery. The OPEN database was prospectively collected but retrospectively reviewed. The cMIS is a retrospective database. Data was collected from October 2009 to September 2013. Inclusion criteria for both databases were age >18 years old and at least one of the following radiographic measurements: sagittal vertical axis (SVA) ≥5 cm, pelvic tilt (PT) ≥20°, lumbar scoliosis Cobb angle ≥20°, or a pelvic incidence and lumbar lordosis (PI-LL) mismatch of ≥10°. The patients were both revision and first-time surgeries. We did not exclude patients who had prior surgery. Patients with minimum 3 levels fused, had a minimum of 2-year follow-up, and fractional curves >10° were included for analysis in this study. In an attempt to create a more homogenous study population, only the cMIS patients were included and were propensity-matched by levels fused to the open cohort; hybrid patients (those with open posterior surgery) were excluded. Matching was done by assigning a propensity score using linear regression. Scores were then ranked, and 2 groups were created with similar propensity scores. Random sampling of the larger group was used to create an equal sample size in both groups. Chi-square tests and Mann-Whitney U tests were used to assess significant differences between the 2 groups, and significance was set at P < .05. All statistical analyses were done using IBM SPSS Statistics version 23 (Armonk, NY).
Results
One hundred sixty-five patients in the databases had complete 2-year data. One hundred and eighteen patients had their fractional curves treated: 79 open and 39 cMIS. The fractional curves were similar preoperative (17° cMIS, 19.6° open) and postoperative (7° cMIS, 8.1° open), but open had more levels treated (12.1 vs 5.7). cMIS had greater reduction in Visual Analogue Scale (VAS) leg (6.4 to 1.8) than open (4.3 to 2.5). When propensity-matched for levels treated (6.6 cMIS and 7.3 open), 40 patients had their fractional curves treated with either cMIS (n = 20) or with open (n = 20) surgery. Table 1 lists outcome data. Both groups had similar magnitude of fractional curve correction (6.9° in cMIS and 8.5° in open postoperative). Although cMIS patients had a lower estimated blood loss (809 cc vs 2299 cc), open patients had a higher SVA change (−19.6 mm vs +13.2 mm) and more pelvic fixation (55% vs 15%). When looking at the entire cohort, 84.8% of open patients underwent direct decompression, whereas 29.7% of cMIS did (P < .001). Operation room time was 468.1 minutes for cMIS and 402.7 minutes for Open (P = .233). The cMIS decompression was achieved during MIS transforaminal lumbar interbody fusion (TLIF), but there was no open decompressive laminectomy in the cMIS group other than the corridor created for the TLIF. When propensity-matched for levels instrumented, 80% of open patients underwent direct decompression, and 22.2% of cMIS did (P < .001). Of the entire cohort, 49.4% of the open group underwent interbody grafting (41.8% anterior lumbar interbody fusion [ALIF], 7.6% TLIF); when propensity-matched for levels fused, 35% of the open group underwent interbody grafting (30% ALIF, 5% TLIF). All patients in the cMIS groups underwent interbody grafting. Both groups had similar preoperative VAS leg (6.1 cMIS and 5.4 open) and postoperative VAS leg (1.6 cMIS and 3.1 open). There was no significant difference in magnitude of leg pain improvement between groups (VAS leg −4.4 cMIS vs −2.2 open, P = .055). There was no significant difference in preoperative and postoperative change of coronal Cobb angle of the major curve, PI-LL, LL, Oswestry Disability Index, or VAS back (see Table 1).
Table 1.
Demographic Data of Patients Undergoing cMIS Versus Open Treatment of the Fractional Curve in Adult Scoliosis Surgery.
| Unmatched Cohort | Matched Cohort | |||||
|---|---|---|---|---|---|---|
| cMIS | Open | P | cMIS | Open | P | |
| N | 39 | 79 | 20 | 20 | ||
| Age (years) | 62.7 | 59.9 | .162 | 61.9 | 60.5 | .82 |
| Female | 32 (82.1%) | 66 (83.5%) | .839 | 16 (50.0%) | 16 (50.0%) | .999 |
| Levels treated | 5.7 | 12.1 | <.001 | 6.6 | 7.3 | .678 |
| Staged | 24 (61.5%) | 14 (17.7%) | <.001 | 14 (70.0%) | 2 (10.0%) | <.001 |
| Transfusion | 16 (41.0%) | 72 (92.3%) | <.001 | 10 (50.0%) | 15 (75.0%) | .102 |
| Illiac fixation | 4 (10.3%) | 67 (84.8%) | <.001 | 3 (15.0%) | 11 (55.0%) | .008 |
| Preoperative PT | 23.3 | 24 | .476 | 22.4 | 25.6 | .166 |
| Preoperative PI | 52.7 | 54.6 | .487 | 49.3 | 53.9 | .283 |
| Preoperative PI-LL | 13 | 14.4 | .712 | 9.8 | 14.2 | .496 |
| Preoperative LL | 39.4 | 39.9 | .984 | 39 | 39.7 | .82 |
| Preoperative SVA | 34.6 | 56.3 | .111 | 27.2 | 50.3 | .134 |
| Preoperative max Cobb | 35.1 | 49.5 | <.001 | 36.2 | 42.8 | .149 |
| Preoperative fractional curve | 17 | 19.6 | .043 | 18 | 18 | .841 |
| Postoperative PT | 23.4 | 22.1 | .575 | 23.3 | 24.3 | .599 |
| Postoperative PI | 52.5 | 53.5 | .662 | 48.9 | 54.5 | .189 |
| Postoperative PI-LL | 10 | 1.6 | .006 | 8.8 | 4.8 | .48 |
| Postoperative LL | 43.2 | 51.9 | .002 | 41.4 | 49.8 | .08 |
| Postoperative SVA | 39 | 22.5 | .383 | 42.4 | 27.5 | .869 |
| Postoperative Cobb | 14 | 24.1 | .005 | 12.5 | 24.3 | .023 |
| Postoperative fractional curve | 7 | 8.1 | .56 | 6.9 | 8.5 | .351 |
| Preoperative VAS back | 6.8 | 7 | .52 | 6.4 | 6.9 | .496 |
| Preoperative VAS leg | 6.4 | 4.3 | .004 | 6.1 | 5.4 | .73 |
| Preoperative ODI | 50.6 | 42.7 | .029 | 49.2 | 42.8 | .221 |
| Postoperative VAS back | 3 | 2.9 | .645 | 3.1 | 3.5 | .874 |
| Postoperative VAS leg | 1.8 | 2.5 | .262 | 1.6 | 3.1 | .169 |
| Postoperative ODI | 28.3 | 26.3 | .535 | 27 | 22.2 | .443 |
| EBL | 672.4 | 2273.4 | <.001 | 808.8 | 2299.3 | .002 |
| ▵ PI-LL | −3.8 | −13.1 | .001 | −2.1 | −8.7 | .134 |
| ▵LL | 4.3 | 12.9 | .002 | 2.9 | 8.9 | .123 |
| ▵SVA | 2.4 | −36.2 | .002 | 13.2 | −19.6 | .036 |
| ▵ Max Cobb | −22.2 | −25.6 | .524 | −26.3 | −18.7 | .307 |
| ▵ Fractional curve | −10 | −11.8 | .255 | −11.1 | −9.7 | .478 |
| ▵ ODI | −22.4 | −15.6 | .06 | −22.2 | −19.1 | .593 |
| ▵ VAS back | −3.8 | −3.8 | .743 | −3.3 | −3.2 | .619 |
| ▵ VAS leg | −4.7 | −2 | <.001 | −4.4 | −2.2 | .055 |
| Decompression of nerve roots | 11 (29.7%) | 67 (84.8%) | <.001 | 4 (22.2%) | 16 (80.0%) | <.001 |
| Interbody | ALIF: 33 (41.8%) | ALIF: 6 (30.0%) | ||||
| Interbody | TLIF: 6 (7.6%) | TLIF: 2 (5.0%) | ||||
Abbreviations: cMIS, circumferential minimally invasive surgery; PT, pelvic tilt; PI, pelvic incidence; LL, lumbar lordosis; SVA, sagittal vertical axis; VAS, Visual Analogue Scale; ODI, Oswestry Disability Index; EBL, estimated blood loss; ALIF, anterior lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion.
Discussion
The fractional curve of a scoliosis, which is defined as the curve below the major curve of an adult lumbar or thoracolumbar scoliosis, can oftentimes be the most symptomatic aspect of a patient’s disease pathology and will frequently be the principle reason a patient chooses surgery. This primarily occurs as result of narrowing of the neural foramen in the cephalad-caudad direction in the concavity of the fractional curve. This is often refractory to conservative care because there is compression via the pedicle against the disc or vertebral body of the level below. Because the etiology is bony compression versus ligamentous compression, the mechanical component of the pain can be much greater than the inflammatory component of the pain, and pain relief can be almost instantaneous on sitting down. In addition, the part of the nerve that is often compressed in the foramen secondary to up-down stenosis is the dorsal root ganglion (DRG). Because the cell bodies are within the DRG itself, compression of the DRG can be exquisitely painful and disabling, much more so than compression of the myelinated axon portion of the nerve root.3-5 Because of the bony compression of the DRG secondary to the fractional curve of scoliosis, it can often be critical to address the cephalad-caudad stenosis of the fractional curve in order to alleviate the patient’s leg pain. In our study, we used VAS leg pain scores as a reflection of treatment efficacy of the fractional curve. In addition, we used a criteria of fractional curve magnitude greater than 10° (L4 to S1) as a proxy for up-down foraminal stenosis on the concavity of the fractional curve causing leg pain.
Figure 1.
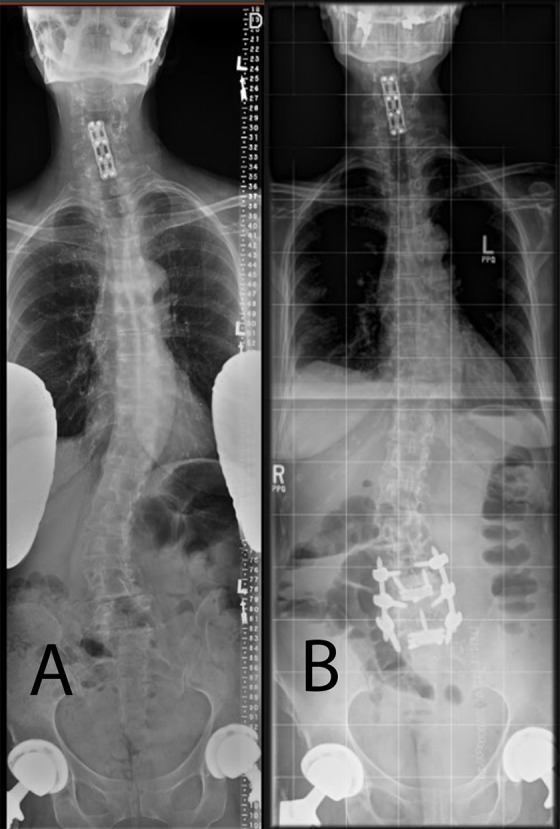
(A, B). Preoperative and postoperative images of a patient with adult scoliosis and radiculopathy being treated for the fractional curve only via anterior mini-open lumbar interbody fusion and percutaneous screw fixation without decompression.
Figure 2.
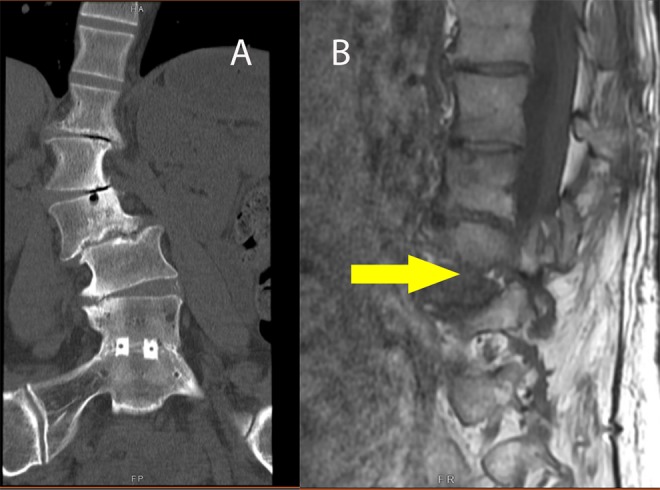
(A) Coronal computed tomography reconstruction showing coronal tilt of vertebra in the fractional curve resulting in “B,” foraminal, up-down stenosis (see arrow).
Figure 3.
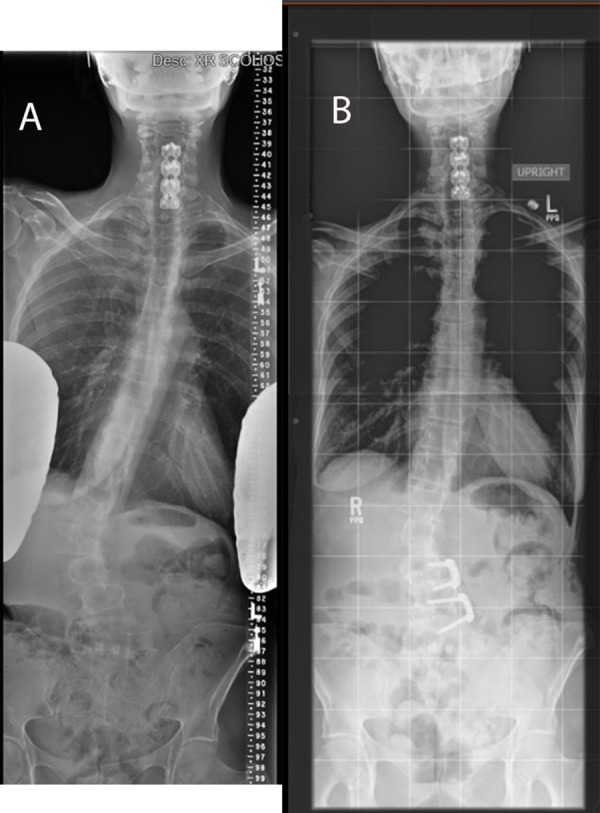
(A, B) Preoperative and postoperative images of the same patient in Figure 2, demonstrating anterior-only treatment of the fractional curve with minimally invasive lateral interbody fusion with anterior fixation.
Prior studies on adult scoliosis have shown that there is decreased blood loss and shorter hospital stays, and outcomes have been similar.6-19 Despite these advances, MIS techniques are still evolving, and open techniques remain the most common treatment for many deformities. In order to assess the efficacy of MIS adult deformity surgery, it is reasonable to compare MIS outcomes to open surgical outcomes.
In this study, when comparing cMIS treatment with open treatment of the fractional curve for ASD correction, we could show no significant difference in the VAS leg pain scores, even though the cMIS group had significantly fewer direct decompressive procedures than open surgery (22.2% vs 80%, P < .001). The other consideration is that because of the very nature of cMIS surgery, all the patients had interbody grafts; however, in the matched open cohort, only 35% underwent interbody grafting. The potential advantage of MIS surgery lies in the minimally invasive interbody techniques, which afford indirect decompression by not only increasing foraminal height but also by ligamentotaxis (tensioning the ligamentum flavum, which effectively pulls it away from the neural elements).20 Our results indicate that the MIS indirect decompression (with 22.2% also receiving some sort of direct decompression) reduced the VAS leg pain scores as much as open surgeries with 80% decompressive laminectomies, and 35% interbody grafts. This is potentially an important finding because indirect decompression does not have the long history as open laminectomy, and there are some who believe that direct decompression is still the best way to alleviate radiculopathy. This finding supports the validity of indirect decompression and the potential avoidance of risks faced by performing direct decompression, such as dural tears, epidural fibrosis, nerve injury, postoperative epidural hematoma, or inadvertent destabilization of the spine with aggressive bony removal adjacent to the end of the fusion.
Another consideration in this study is that the correction of the primary curve was similar between both groups, regardless of type of surgery. This is an important factor because much of the fractional curve symptoms stem from the up/down (cephalad/caudad) stenosis, which is usually secondary to the coronal angulation of the primary curve; that is, the greater the coronal Cobb of the major curve, the greater the coronal Cobb of the fractional curve to compensate. Thus, correction of the primary Cobb angle per se was not critical, but rather, the alleviation of the up/down stenosis from coronal Cobb of the fractional curve was. The coronal Cobb of the fractional curve itself may not have had a significant correction in the coronal plane but increasing the up-down dimensions of neural foramen improved outcomes. This could have been achieved with disc height restoration (while still keeping a coronal scoliosis) by the interbody device. The fact that both groups had similar correction of the primary coronal curve and concomitant similar decrease in VAS leg scores may reflect the mechanism of pain alleviation, regardless of direct decompression via laminectomy or not.
There has long been a debate as to whether to stop a long fusion at L5 or to extend the construct to the sacrum.21-23 One main reason for including L5-S1 is the obliquity of the lumbosacral segment. Such an obliquity contributes to the Cobb angle of the fractional curve. In our study, we included only patients in whom the fractional curve magnitude was greater than 10° and also had their fractional curves treated. Because of these inclusion criteria, patients whose distal construct stopped at L5 were not included in this study.
Despite some of the advantages of MIS, there are disadvantages of the MIS approach that must also be considered. First, because there is no direct decompression, if the patient remains symptomatic with leg pain postoperatively and there remains residual stenosis, the patient may require reoperation or further treatment of the residual stenosis. Second, the lack of exposure of the posterior elements prevents using the posterior bony surfaces for arthrodesis, both as graft material and fusion surface. Third, the placement of the percutaneous fixation relies completely on some type of imaging modality—either c-arm or navigation—whereas open screw fixation can be placed free-hand with standard anatomic landmarks. This may create additional time to the operation or increase radiation exposure to both the surgeon and the patient.
With regard to propensity matching the cohorts by levels fused, this was in order to try to compare comparable groups. That is, we felt that a comparison between a patient who was treated with an L4-S1, 2-level fusion fractional curve only to a patient who was treated T10 to the pelvis—which included the fractional curve—was not a similar comparison because of the difference of the magnitude of surgeries. Without matching, one could argue that a patient with a T10 or T3 to the pelvis operation did either better or worse than an L4-S1 fusion because of fusion of the entire Cobb angle and not just the fractional curve, better sagittal realignment, or other causes. Indeed, we found that the unmatched cohorts actually had a significantly higher rate of pain alleviation in the leg with the MIS cohort compared to the open cohort, possibly indicating a higher incidence of radiculitis or nerve damage or nerve stretching when fusing more levels. Thus, we felt that matching was important so that the 2 groups were similar for the comparison.
One could argue that the interbody is simply not necessary in open surgery because a wide decompression, stabilization, and correction of the curve would be sufficient to alleviate the stenosis; however, this is only possible in open surgery. We wished to see if a comparable outcome could be achieved with interbody fusion and minimally invasive surgery without open treatment. Because we found that the interbody graft without direct decompression was an effective treatment method for fractional curve pain, it shows that minimally invasive treatment of the fractional curve in scoliosis is a viable option if an interbody graft is used. When comparing the minimally invasive treatments—without direct decompression—to open treatments with decompression and finding similar outcomes, it provides evidence that minimally invasive may be a viable option in patients who have radiculopathy from the fractional curve.
There are limits to this study. First, it is a retrospective study. Unfortunately, because of the method of data collection in the cMIS group, only retrospective data is available. Second, the numbers are small, and the study may be underpowered to detect a significant difference. Because cMIS surgery for adult deformity is relatively new, the numbers are limited and hopefully with larger numbers more conclusive statements can be made. However, the main comparison factors are that the cMIS group had a significantly lower number of direct decompressions than the open group, yet the same reduction of leg pain.
Another limitation of this study is the small number of patients despite the relatively large numbers of patients within the databases. The number of patients with more than 4 levels of fusion in both cohorts was fairly large (426 patients); however, when radiographically excluding for patients who had fractional curves greater than 10°, the number of patients was reduced significantly. This is because many patients were sagittal deformities or patients with fractional curves that did not meet the criteria of greater than 10°. By further reducing to minimum 2-year follow-up, 165 patients who had 2-year follow-up were included. In addition, we also wanted to include patients who had leg pain from the fractional curve, not simply the radiographic finding. This narrowed the numbers down even more to 118 patients. Also, once matched for levels fused between open and MIS patients, we then narrowed the numbers down to 40 patients in order to create 2 homogeneous groups in terms of number of instrumented levels.
Another limitation to this study is why patients with higher VAS leg scores were more frequently treated with cMIS techniques. Since they were 2 different cohorts, the question itself may be difficult to answer. That is, since the cMIS cohort was treated by surgeons who felt very comfortable with both open and MIS techniques, the reason for selecting cMIS may be difficult to identify, and there may be a selection bias. However, the open cohort was treated by surgeons who primarily or exclusively perform open surgery only with minimal to no use of MIS techniques. Thus, unfortunately, because there are 2 cohorts by 2 different groups of surgeons, it will be difficult to answer the question about why there is a higher preoperative VAS score in the cMIS group.
Another limitation of this study is that even though both databases were retrospectively reviewed, the collection was prospective in one and retrospective in the other. The OPEN database was prospectively collected but retrospectively reviewed. The cMIS is a retrospective database. Even though the prospective cohort is followed in a prospective manner, the clinical data is retrospectively reviewed. Thus, the clinical scores are collected retrospectively in both groups. This was a limitation that we could not control; however, radiographic, surgical, and clinical parameters should not be affected. Radiographs were all measured centrally in the same way, data extracted from op notes and progress notes to collect surgical and outcomes data. Thus, the statistical methods should still be appropriate because it is comparing retrospective evaluation of data to retrospective evaluation data, even though one set was prospectively collected. In addition, by having these 2 different databases, different groups of surgeons enrolled into the open and into the MIS cohorts. Moreover, the MIS surgeons did not enroll their deformity patients, which were treated in an open fashion (only MIS patients), which could create another bias. This could produce selection biases in this study and it is an inherent limitation of this study. Future studies will have the MIS surgeons include both their open and MIS cohorts into enrollment, not just MIS patients.
Finally, another limitation is that the patient cohorts are not purely either decompression without interbody fusion or interbody fusion without decompression. We had tried to further match the cohorts into pure cohorts, but it would have resulted in too few patients in each group to make a meaningful statement. This raises concerns regarding the heterogeneity of the patient population, with the assertion that there is one group with more interbody fusion compared to another, and thus, the patient populations are different. However, after matching the cohorts, the 2 patient populations were not different with regard to their deformity and are actually are matched well preoperatively. When looking at the 2 different patient populations, it is inherently not necessary to match the cohorts on variables that are not statistically different. For example, the unmatched cohorts had statistically different major Cobb angles; however, after matching for levels instrumented fusion, that difference was no longer there. Thus, none of the preoperative radiographic parameters were statistically different after the match, so from a pretreatment standpoint, the treated deformities were actually homogeneous populations and not different from a deformity and radiographic standpoint. However, the treatment modalities were different, and that is part of our fundamental research question. In addition, we would propose that the uniqueness of this comparison, uniqueness of this pathology, minimum 2-year follow-up, and health-related quality-of-life data would make this study at the minimum “proof of concept” that MIS treatment without direct decompression of fractional curves is a viable option. In addition, many more patients in the cMIS group underwent staged surgeries. However, it is unclear if the staging of the surgeries improved the pain perception by the patients. This is a question that can be addressed in future studies.
Conclusions
In the treatment of the fractional curve of adult scoliosis, patients treated with cMIS achieved similar reduction in leg pain compared with those treated with traditional open surgery, even though significantly fewer cMIS patients underwent direct decompression of the fractional curve nerve roots. Although our study includes minimum 2-year follow-up, long-term data will be needed to truly determine if cMIS treatment of the fractional curve without laminectomy will ultimately be as effective as open, direct decompression.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Alimi M, Hofstetter CP, Tsiouris AJ, Elowitz E, Härtl R. Extreme lateral interbody fusion for unilateral symptomatic vertical foraminal stenosis. Eur Spine J. 2015;24(suppl 3):346–352. doi:10.1007/s00586-015-3940-z. [DOI] [PubMed] [Google Scholar]
- 2. Mundis GM, Akbarnia BA, Phillips FM. Adult deformity correction through minimally invasive lateral approach techniques. Spine (Phila Pa 1976). 2010;35(26 suppl):S312–S321. doi:10.1097/BRS.0b013e318202495f. [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression. Part 2: morphological and immunohistochemical changes of dorsal root ganglion. J Orthop Res. 2004;22:180–188. doi:10.1016/S0736-0266(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 4. Tan ZY, Donnelly DF, LaMotte RH. Effects of a chronic compression of the dorsal root ganglion on voltage-gated Na+ and K+ currents in cutaneous afferent neurons. J Neurophysiol. 2006;95:1115–1123. doi:10.1152/jn.00830.2005. [DOI] [PubMed] [Google Scholar]
- 5. Wang T, Hurwitz O, Shimada SG, et al. Chronic compression of the dorsal root ganglion enhances mechanically evoked pain behavior and the activity of cutaneous nociceptors in mice. PLoS One. 2015;10:e0137512 doi:10.1371/journal.pone.0137512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang MY, Mummaneni PV, Fu KM, et al. ; Minimally Invasive Surgery Section of the International Spine Study Group. Less invasive surgery for treating adult spinal deformities: ceiling effects for deformity correction with 3 different techniques. Neurosurg Focus. 2014;36:E12 doi:10.3171/2014.3.FOCUS1423. [DOI] [PubMed] [Google Scholar]
- 7. Wang MY, Mummaneni PV. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus. 2010;28:E9 doi:10.3171/2010.1.FOCUS09286. [DOI] [PubMed] [Google Scholar]
- 8. Wang MY. Improvement of sagittal balance and lumbar lordosis following less invasive adult spinal deformity surgery with expandable cages and percutaneous instrumentation. J Neurosurg Spine. 2013;18:4–12. doi:10.3171/2012.9.SPINE111081. [DOI] [PubMed] [Google Scholar]
- 9. Wang MY. Less invasive mini-open adult spinal deformity surgery. Neurosurg Focus. 2013;35(2 suppl):video 1. doi:10.3171/2013.V2.FOCUS13186. [DOI] [PubMed] [Google Scholar]
- 10. Uribe JS, Smith DA, Dakwar E, et al. Lordosis restoration after anterior longitudinal ligament release and placement of lateral hyperlordotic interbody cages during the minimally invasive lateral transpsoas approach: a radiographic study in cadavers. J Neurosurg Spine. 2012;17:476–485. doi:10.3171/2012.8.SPINE111121. [DOI] [PubMed] [Google Scholar]
- 11. Uribe JS, Deukmedjian AR, Mummaneni PV, et al. ; International Spine Study Group. Complications in adult spinal deformity surgery: an analysis of minimally invasive, hybrid, and open surgical techniques. Neurosurg Focus. 2014;36:E15 doi:10.3171/2014.3.FOCUS13534. [DOI] [PubMed] [Google Scholar]
- 12. Park P, Wang MY, Lafage V, et al. ; International Spine Study Group. Comparison of two minimally invasive surgery strategies to treat adult spinal deformity. J Neurosurg Spine. 2015;22:374–380. doi:10.3171/2014.9.SPINE131004. [DOI] [PubMed] [Google Scholar]
- 13. Mummaneni PV, Shaffrey CI, Lenke LG, et al. ; Minimally Invasive Surgery Section of the International Spine Study Group. The minimally invasive spinal deformity surgery algorithm: a reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg Focus. 2014;36:E6 doi:10.3171/2014.3.FOCUS1413. [DOI] [PubMed] [Google Scholar]
- 14. Dhall SS, Wang MY, Mummaneni PV. Clinical and radiographic comparison of mini-open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine. 2008;9:560–565. doi:10.3171/SPI.2008.9.08142. [DOI] [PubMed] [Google Scholar]
- 15. Deukmedjian AR, Dakwar E, Ahmadian A, Smith DA, Uribe JS. Early outcomes of minimally invasive anterior longitudinal ligament release for correction of sagittal imbalance in patients with adult spinal deformity. ScientificWorldJournal. 2012;2012:789698 doi:10.1100/2012/789698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anand N, Baron EM, Kahwaty S. Evidence basis/outcomes in minimally invasive spinal scoliosis surgery. Neurosurg Clin N Am. 2014;25:361–375. doi:10.1016/j.nec.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 17. Anand N, Baron EM, Khandehroo B, Kahwaty S. Long-term 2- to 5-year clinical and functional outcomes of minimally invasive surgery for adult scoliosis. Spine (Phila Pa 1976). 2013;38:1566–1575. doi:10.1097/BRS.0b013e31829cb67a. [DOI] [PubMed] [Google Scholar]
- 18. Anand N, Baron EM, Thaiyananthan G, Khalsa K, Goldstein TB. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech. 2008;21:459–467. doi:10.1097/BSD.0b013e318167b06b. [DOI] [PubMed] [Google Scholar]
- 19. Anand N, Rosemann R, Khalsa B, Baron EM. Mid-term to long-term clinical and functional outcomes of minimally invasive correction and fusion for adults with scoliosis. Neurosurg Focus. 2010;28:E6 doi:10.3171/2010.1.FOCUS09278. [DOI] [PubMed] [Google Scholar]
- 20. Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal. 2012;2012:456346 doi:10.1100/2012/456346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bridwell KH, Edwards CC, 2nd, Lenke LG. The pros and cons to saving the L5-S1 motion segment in a long scoliosis fusion construct. Spine (Phila Pa 1976). 2003;28:S234–S242. doi:10.1097/01.BRS.0000092462.45111.27. [DOI] [PubMed] [Google Scholar]
- 22. Cho KJ, Suk SI, Park SR, et al. Arthrodesis to L5 versus S1 in long instrumentation and fusion for degenerative lumbar scoliosis. Eur Spine J. 2009;18:531–537. doi:10.1007/s00586-009-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sardar ZM, Ouellet JA, Fischer DJ, Skelly AC. Outcomes in adult scoliosis patients who undergo spinal fusion stopping at L5 compared with extension to the sacrum. Evid Based Spine Care J. 2013;4:96–104. doi:10.1055/s-0033-1357360. [DOI] [PMC free article] [PubMed] [Google Scholar]


