Abstract
Recent developments have demonstrated that immunotherapies are capable of achieving durable antitumor responses in patients with metastatic cancer. One modality that has been able to induce durable complete regressions in patients with melanoma has been adoptive cell therapy (ACT). This has slowly been expanded to other cancer types using new approaches such as genetically engineered T-cells and other methods of antigen targeting. It now appears that immune targeting of mutated “neoantigens” plays a major role in successful ACT, as well as in other immunotherapies such as checkpoint inhibitors. This realization presents not only new challenges to ACT but also new opportunities in that all tumors now may have potential antigens to attack that can be revealed by tumor genomic sequencing. There are a variety of exciting approaches to translate these new findings into clinical trials applying ACT to the majority of cancer types.
Only recently has the goal of immunologically rejecting a metastatic cancer in a patient been realized in a consistent fashion. The concept has been advanced for over a century, but was only seen sporadically or anecdotally. Two major developments that have led to this success have been the demonstration that many cancer patients have a T-cell repertoire capable of recognizing their cancer and the realization that the tumor microenvironment is inhibitory to the function of this repertoire. A detailed molecular understanding of these phenomena has not only led to immunotherapies that consistently lead to tumor rejection but also point the way to achieving it in many different types of human cancer. Other chapters in this volume will detail the mechanisms and successes of checkpoint blockade, particularly in patients with melanoma, lung cancer, and other cancers, while this chapter will focus on the T-cell repertoire and methods of augmenting it to better achieve complete and lasting tumor eradication.
1. IDENTIFYING TUMOR-ASSOCIATED ANTIGENS RECOGNIZED BY T-CELLS
Prior to a molecular understanding of tumor rejection antigens, there was evidence that some T-cells from patients with cancer could immuno-logically recognize their cancer. The clearest and most consistent examples were from patients with melanoma, where tumor infiltrating lymphocytes (TIL) or peripheral blood lymphocytes (PBL) stimulated in vitro with tumor was shown to lyse their autologous tumor line in an major histocompatability complex (MHC)-specific fashion (Herin et al., 1987; Knuth, Danowski, Oettgen, & Old, 1984; Muul, Spiess, Director, & Rosenberg, 1987). One major advantage of studying melanoma was that patient-specific tumor lines could be readily established from surgical specimens in many patients and were available for molecular studies and immunological assays. In addition, this autologous tumor recognition by T-cells appeared to be much more prevalent in melanoma patients for reasons not understood at the time. When TIL, simply expanded in recombinant interleukin-2 (rIL-2), were cocultured with their autologous tumor (either cryopreserved fresh tumor, enzymatically digested single cell suspensions, or in vitro-maintained tumor cell lines) cytolysis in short-term chromium release assay or cytokine release could be demonstrated for most melanoma patients studied (Topalian, Solomon, & Rosenberg, 1989). These tumor-reactive T-cells were then concurrently investigated both clinically and immunologically.
Laboratory studies of tumor-reactive T-cells led to a key discovery in our molecular understanding of the T-cell response to human cancer in 1991 when van der Bruggen et al. cloned the antigen recognized by a tumor-reactive T-cell clone from a patient with melanoma (van der Bruggen et al., 1991). Following this achievement, many antigens were cloned using T-cell clones often obtained from patients with melanoma (Boon, Cerottini, Van den Eynde, van der Bruggen, & Van, 1994; Kawakami et al., 1994). TIL were frequently the source of these clones and the initial antigens discovered were dominated by tissue differentiation antigens shared by melanomas and melanocytes (Rosenberg, Kawakami, Robbins, & Wang, 1996). These proteins are synthesized in huge amounts by cells in the melanocytic lineage, and the high frequency of their representation in tumor cDNA libraries may account for their early dominance in antigen identification. In addition, the attractiveness of targeting shared antigens which could potentially be attacked using “off-the-shelf” reagents focused attention on these types of targets. The other major class of shared target antigens was the tumor-germline antigens (initially termed tumor-testis antigens based on limited expression data). These were proteins, mostly encoded by genes on the X-chromosome, that were expressed during embryogenesis, but seldom on adult tissues other than germline or placental tissues. These were found to be immunogenic both by high-avidity humoral responses and by generating T-cell responses in patients with cancer (Jager et al., 1998; Scanlan et al., 2002). As more has been learned about the tumor-germline antigens, their main drawback seems to be a low prevalence of expression by the most common tumor histologic types, and inconsistent expression within individual patients and tumors (Park et al., 2016). Another class of shared antigens is normal proteins that are highly overexpressed by tumors, but expressed on some normal tissues. These included proteins such as CEA, mesothelin, or hTERT. Initial efforts to enhance T-cell repertoires against these self-antigens focused on vaccine strategies. Using dozens of approaches and enrolling thousands of patients, these vaccine strategies have only induced anecdotal objective responses in patients with measurable disease (Klebanoff, Acquavella, Yu, & Restifo, 2011). Clinical testing of vaccines has migrated more toward use in the adjuvant setting, but such studies require large randomized studies with years of follow-up, and have been largely negative as well. During this time, a large body of evidence has been developed which shows that there were many immunosuppressive influences in the tumor microenvironment that can suppress T-cell activation, expansion, or function. These included T-regulatory cells, inhibitory “checkpoint” receptors on T-cells, myeloid-derived suppressor cells (MDSC) and enzymes, and cytokines that impair T-cell function (Bronte & Mocellin, 2009; Gajewski et al., 2006). One approach to circumventing this local immunosuppression was to generate and expand a tumor-reactive T-cell repertoire ex vivo and adoptively transfer it to the patient along with other reagents that could support these cells or perhaps block local immunosuppressive factors.
2. CELL THERAPY WITH GENETICALLY ENGINEERED T-CELLS
A major advance that allowed this to be developed in concert with antigen discovery work was the ability to gene engineer mature human peripheral blood T-cells with high efficiency and safety (Morgan et al., 2006). Current techniques using gamma retroviruses or lentiviruses can introduce stable genetic changes with high frequency in human PBL without requiring selection and such cells have been administered to hundreds of patients without complications from gene modification after many years of follow-up. On cloning T-cell receptors (TCR) which recognized defined tumor-associated antigens, these TCR were genetically engineered into the PBL of HLA-appropriate patients and the expanded cells given in adoptive transfer protocols (Morgan et al., 2006). Another approach used a novel chimeric antigen receptor (CAR) where the ligand-binding domain was derived from the single chain variable fragment of a monoclonal antibody which bound a cell surface molecule on tumors (Eshhar, Waks, & Gross, 2014; Eshhar, Waks, Gross, & Schindler, 1993). This was covalently linked in tandem via a transmembrane domain to the CD3-zeta activating moiety from T-cells to trigger activation on ligation of the CAR in an MHC-independent fashion (often with interposed costimulatory domains to enhance activation or cell survival) (Brentjens et al., 2007). The design and use of CAR in gene-engineered T-cells is dealt with in greater detail elsewhere in this publication.
Trials of such genetically retargeted T-cells showed that some could achieve tumor regression but also established the risks associated with antigen selection. Clinical trials of receptor-engineered T-cells targeting unmutated, shared tumor-associated antigens showed these antigens could divided into ones that: (1) were associated with unacceptable autoimmunity,(2) induced acceptable or tolerable autoimmunity, and (3) those that showed no apparent autoimmunity. As an example of the first outcome, T-cells transduced with TCRs against the melanoma/melanocyte differentiation antigens such as MART-1 or gp100 caused tumor regression but also attacked the skin, eyes, and inner ear due to the presence of melanocytes at those locations (Johnson et al., 2009). Rashes, vitiligo, and decreases in vision and hearing limited the utility of these reagents. On targeting CEA with a high-avidity TCR generated in an HLA-A2 transgenic mouse, some early evidence of antitumor activity was seen, but all patients treated developed severe, life-threatening colitis (Parkhurst et al., 2011). On the other hand, when the B-cell marker, CD19 (present on both benign and malignant T-cells) was targeted with CARs, impressive responses in patients with B-cell lymphomas and leukemias were seen (Goff et al., 2010; Kochenderfer et al., 2010; Maude et al., 2014). The accompanying B-cell aplasia that also resulted was manageable with surveillance for hypogammaglobulinemia and infection and appropriate supportive care. In view of the dramatic responses seen, the risk-benefit ratio of this anti-B-cell autoimmunity was considered acceptable. The best current example of a self-antigen effectively targeted with receptor-engineered T-cells with no apparent autoimmunity has been adoptive cell therapy (ACT) against the tumor-germline antigen, NY-ESO-1. Originally discovered by screening patient sera for antitumor IgG responses using the SEREX technique, this member of the tumor-germline family of antigens was found expressed by a patient’s esophageal cancer (Chen et al., 1997). It is also expressed by a small subset of melanomas and by 80% of synovial sarcomas due to a common translocation in this tumor. Autologous PBL from patients were transduced for clinical testing with an anti-NY-ESO-1 TCR that was HLA-A0201 restricted (Robbins et al., 2011). As is common with T-cell transfers, the recipient was immunosuppressed with a single cycle of nonmyeloablative chemotherapy prior to cell transfer. This transiently eliminates host T-regulatory cells and MDSC, induces the homeostatic cytokines IL-7 and IL-15, and depletes resident lymphocytes competing for these T-cell growth factors, all shown to enhance ACT or survival of T-cells after transfer in mouse models (Klebanoff, Khong, Antony, Palmer, & Restifo, 2005). When HLAA0201+ patients with measurable metastatic melanoma or synovial sarcoma were given anti-NY-ESO-1 receptor transduced T-cells (with a brief course of supportive systemic IL-2), objective response rates by RECIST criteria of 55% and 61%, respectively, were seen, with some complete and durable responses seen (Fig. 1; Robbins et al., 2015). No treatment-related autoimmunity was evident in these patients. A wide variety of other tumor-associated target antigens are being studied as targets for receptor-engineered T-cells, but have yet to be demonstrated to mediate major tumor regressions and to be safe.
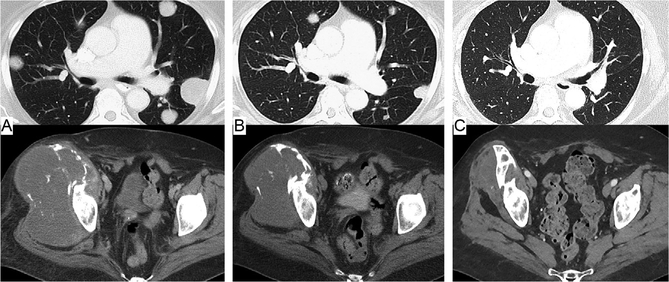
Figure 1.
Patient with an NY-ESO-1-expressing metastatic synovial cell sarcoma showing ongoing regression of lung metastases and a large pelvic primary tumor after a single adoptive transfer of autologous T-cells genetically engineered with a T-cell receptor recognizing NY-ESO-1. (A) Pretreatment scans, (B) 5 months posttreatment, and(C) 5 years posttreatment.
3. CELL THERAPY WITH TIL
Even prior to conducting laboratory studies on antigen identification and clinical translation protocols using receptor-engineered T-cells, empirical trials using melanoma TIL were also undertaken. The availability of such autologous T-cells with consistent antitumor reactivity allowed the development of the principles and practices of adoptive lymphocyte transfer in patients. Over two-thirds of T-cells grown from resected melanoma metastases show autologous tumor recognition after being cultured in IL-2 (Goff et al., 2010). These can be readily expanded in vitro to large numbers, and used for adoptive T-cell transfer. This was initially done in patients with measurable metastatic melanoma with the patients receiving just TIL (grown in bulk from enzymatically dispersed tumor and administered without regard to in vitro reactivity) and systemic IL-2 (Rosenberg et al., 1988, 1994). Some patients also received a single dose of cyclophosphamide (at 25 mg/kg) prior to cell administration. Overall the objective response rate was 34% with neither differences between patients given or not given cyclophosphamide nor between patients who had or not had prior systemic IL-2 therapy alone. Despite giving a median of nearly 2×1011 cells per patient, many of these responses proved to be of short duration, with only 7% persisting for a year after treatment. Earlier studies of gene-marked TIL showed that in vivo survival after administration was very brief with most patients having no TIL detectable by RT-PCR a month later (Rosenberg et al., 1990). After this experience and with supportive data from murine models, transient host lymphodepletion using cyclophosphamide and fludarabine was introduced just prior to cell infusion. In addition, changes in the methods of growing and selecting TIL for administration were made. When TIL were grown from individual fragments of a resected metastasis, a high degree of heterogeneity was seen between the different fragment cultures. When fresh or cultured autologous tumor was available, these TIL fragment cultures could be shown to often differ in their tumor recognition. Therefore, for many patients, one was able to select subcultures with greater tumor recognition to expand and administer. In addition, the final expansion of these select fragment cultures was also made much faster and more consistent using a method incorporating anti-CD3 antibody and irradiated feeder cells in addition to IL-2 (Riddell & Greenberg, 1990). Ultimately, when all of these principles were employed in treating a group of patients with melanoma, an objective response rate of 54% was seen (using three different lymphodepleting regimens that did not confer significant differences in outcome) with 20% of these patients maintaining durable complete responses after 5–8 years of follow-up (Rosenberg et al., 2011; Table 1). The same high response rates were seen in patients previously unresponsive to IL-2 alone, demonstrating that the cell transfer was responsible for the regressions. High levels of persistent T-cell survival were also seen which were associated with a higher likelihood of response. More recently, this experience has been reproduced in another 101 patients with melanoma, where the overall and complete response rates were 53% and 24%, respectively (manuscript in preparation). These trials perhaps best illustrate the concept that adoptive T-cell transfer is capable of causing and sustaining complete regressions of disseminated cancer in patients.
Table 1.
Response Rate and Response Durations of 93 Patients with Measurable Metastatic Melanoma Treated with Preparative Lymphodepletion, TIL, and Interleukin-2 (Three Different Lymphodepleting Regimens Were Used but Showed No Significant Differences in Outcome so They Are Considered Together)
| PR | CR | ORR | ||
|---|---|---|---|---|
| Number of Patients (Duration in Months) | ||||
| 93 | 32 (34%) | 20 (22%) | 52 (56%) | |
| 84, 36, 29, 28, 21 | 137 +, 135 +, 134+, 124 + | |||
| 14, 14, 13, 12, 11 | 120+, 120+, 116+, 113 + | |||
| 9, 8, 7, 7, 7 | 104+, 101 +, 100+, 95 + | |||
| 6, 6, 6, 6, 6 | 94+, 94+, 94+,93 + | |||
| 5, 5, 4, 4, 4 | 82+, 64+, 63+, 19 | |||
| 3, 3, 3, 2, 2 | ||||
| 2, 2 | ||||
Most patients had progressed after prior IL-2 therapy and only two patients received more than one TIL transfer. The data are updated with a median follow-up of 9.8 years.
4. CELL THERAPY TARGETING MUTATED “NEOANTIGENS”
An interesting observation during these trials with melanoma-derived TIL was that these TIL cultures often contained reactivity against melanoma/melanocyte associated differentiation antigens but infrequently induced vitiligo and rarely were associated with eye, ear, or any autoimmune toxicities. This suggested that the relevant target antigens might be something else. The (low)prevalence of reactivity against the known tumor-germline antigens also could not account for the high rates of response (Kvistborg et al., 2012). In some TIL cultures, clear autologous tumor recognition could be demonstrated but no recognition of HLA-matched melanomas was seen suggesting an antigen repertoire with private specificity was responsible. Several prior studies using expression cloning to identify tumor antigens had demonstrated that these mutated “neoantigens” were indeed targeted by some T-cells (Coulie et al., 1995; Robbins et al., 1996; Wolfel et al., 1995). The need to procure autologous tumor as a reagent and the difficulties of translating such reactivities into patient-specific therapeutics had blunted enthusiasm for this class of tumor antigen. Now, the advent of rapid and relatively inexpensive whole exomic sequencing (WES) greatly increased access to the genetic information needed to pursue this avenue of investigation. So studies were undertaken to explore tumor-specific mutated proteins as T-cell targets. A series of three melanomas with tumor-reactive TIL (of known HLA restriction) were fully sequenced and all nonsynonymous exomic mutations identified (Robbins et al., 2013). As most epitopes presented by MHC Class I are 9 or 10 amino acids in length, 19-amino acid sequences consisting of each mutant amino acid and its 9 flanking wild-type residues on either side were analyzed for every mutation and epitopes within them rank ordered using HLA-binding algorithms and the known HLA restriction element. Although there were thousands of potential “neoepitopes” for each tumor, when only the 40 candidate epitopes with the highest predicted affinity were synthesized and tested for recognition, multiple mutated epitopes were recognized by all three TIL. The three patient TIL recognized the 5th, 18th, 19th, and 38th best-binding peptides for patient #1; the 2nd, 17th, and 23rd for patient #2; and the 2nd, 4th, 24th, and 36th for patient #3. These experiments indicated that multiple high-avidity epitopes created by tumor-specific mutations in melanoma were frequently being processed and presented and were generating T-cell responses. Others also found that mutated antigens were being recognized by T-cell responses in patients responding to other immunotherapies such as checkpoint inhibitors.
Coincident with these laboratory discoveries, compelling circumstantial evidence was accumulating that immune recognition of mutated antigens was playing a significant role in other effective clinical immunotherapies. It has long been known that melanoma was a tumor histology that was often one of the most responsive to immunotherapies such as interleukin-2 and anti-CTLA4 and can rarely undergo some degree of spontaneous regression. When large numbers of human tumors were subjected to DNA sequencing, melanoma was found to be one of the most highly mutated of all human tumors, presumably due to UV mutagenesis (Lawrence et al., 2013). Interestingly, uveal and mucosal melanomas with no connection to UV irradiation were much less mutated and appeared much less responsive to the same immunotherapies (Krauthammer et al., 2012). As newer agents such as anti-PD1 or anti-PDL1 antibodies were tested, again melanoma was the most responsive histology (Topalian et al., 2012). Yet other tumors such as lung, bladder, and head and neck cancers with high rates of mutation (mostly related to cigarette carcinogens) also showed significant response rates (American Association for Cancer Research, 2015; Brahmer et al., 2015). Perhaps most compelling was the finding that most colon cancers, which had low rates of mutation, did not respond to these agents, but the subset with DNA mismatch repair defects (and much higher rates of mutation) was highly responsive to anti-PD1 as were a set of noncolorectal tumors with mismatch repair defects (Table 2; Le et al., 2015). Yet validation of the hypothesis that mutation-driven immune responses were involved in tumor rejection required that specific mutation-reactive T-cells be shown to cause tumor rejection when adoptively transferred to a patient. In 2014, Tran et al. studied a patient with chemotherapy refractory metastatic cholangiocarcinoma (Tran et al., 2014). A lung metastasis was resected, WES performed and multiple TIL cultures grown from individual fragments from the tumor. Initially, the patient was treated with Cy-Flu followed by 4×1010 bulk TIL from multiple fragments (without reactivity testing as there was no autologous tumor available) along with four doses of IL-2. The patient had a minor response but relapsed after 7 months. Her tumor was found to harbor 26 nonsynonymous mutations and minigenes encoding each mutated amino acids and the 12 wild-type residues on either side (to encompass not only short Class I presented epitopes, but all possible Class II presented epitopes) were synthesized and concatenated into three tandem minigenes. Autologous dendritic cells were transfected with RNA from these minigenes and each TIL fragment culture independently tested for recognition of any of the products of the tandem minigenes. One was reactive with one of the tandem minigenes and the individual minigene within it that conferred TIL recognition proved to encode ERBB2 interacting protein (ERBB2IP) containing an E805G mutation which was within the epitope presented by the Class II molecule HLA-DQB1*0601. One TIL culture was 95% pure for a VB22+ T-cell clone reactive with this peptide and it was grown to large numbers for retreatment. The patient then received another TIL infusion of 12×1010 cells, 95% of which were this CD4+ clone (retrospective analysis showed the first infusion contained 1/12th the amount of this clone), again with Cy-Flu and four doses of IL-2. Nearly 2 years later, she has had a near complete regression of her tumors which ongoing shrinkage (Fig. 2). Since then, investigation of multiple other tumors of various types has confirmed that mutation-reactive TIL can be found in most gastrointestinal cancers (Tran et al., 2014) as well as lung cancer, ovarian cancer, breast cancer, and pancreatic cancer. Current efforts are directed at determining if these T-cells can be effective in adoptive immunotherapy and optimizing their activity using combinations of other immunotherapeutic agents. The presence of these “nonself” antigens resulting from somatic mutations in human tumors and the potential for having high-affinity TCRs in the T-cell repertoire against them that have not been edited by central thymic deletion opens the door to finding endogenous antitumor responses to be exploited against most cancers types. Such endogenous reactivity against neoantigens may in fact be the common pathway responsible for the efficacy of currently approved immunotherapies such as interleukin-2 and checkpoint inhibitors (Van Allen et al., 2015).
Table 2.
Impact of Mismatch Repair Deficiency on Mutation Frequency and Response to Pembrolizumab
| Mismatch Repair- Deficient CRC | Mismatch Repair Proficient CRC | Mismatch Repair- Deficient Non-CRC | |
|---|---|---|---|
| Pts treated | 10 | 18 | 7 |
| Complete responses |
0 | 0 | 1 |
| Partial responses | 4 | 0 | 4 |
| Response rate | 40% | 0% | 71% |
| Pt tumors sequenced | 7 | 6 | 2 |
| Mean # somatic mutations | 1875 | 73 | 1455 |
CRC, colorectal cancer.
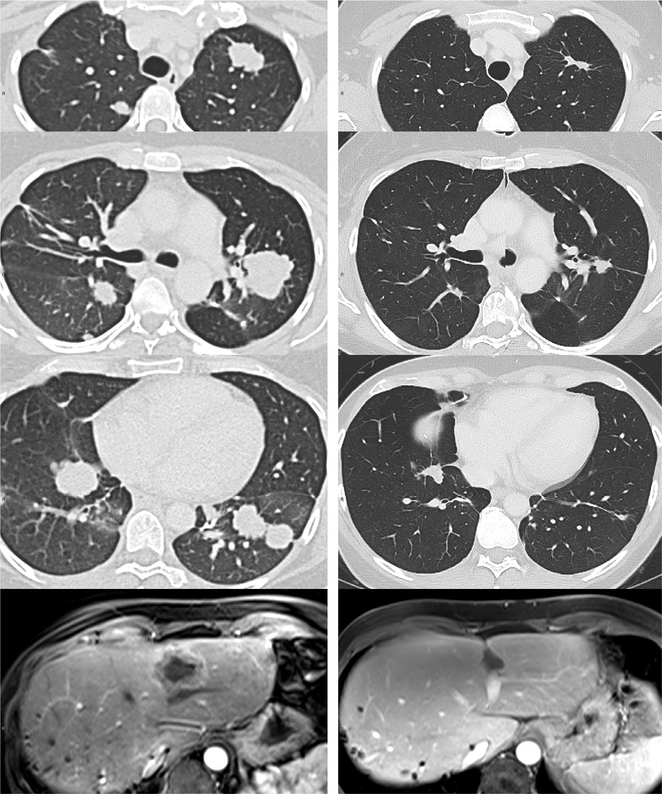
Figure 2.
Patient with metastatic cholangiocarcinoma after adoptive T-cell transfer of tumor infiltrating lymphocytes highly selected (95% clonal) for a CD4+ clone reactive with a mutated epitope in ERBB2 interactive protein, expressed by her cancer. Pre-treatment scans of lung and liver (left) and matching scans 20 months later (right) are shown.
The ability to genetically modify human T-cells with high efficiency is not confined to only redirecting specificity. It also allows the function of T-cells to be altered to better effect tumor rejection. One recent clinical experience introducing IL-12 secretion into melanoma TIL to improve antigen processing and paracrine cytokine secretion in the tumor microenvironment resulted in a 63% objective response rate in a group of 16 patients given much smaller numbers of TIL than usual with just lymphodepletion but no IL-2 (Zhang et al., 2015). Although encouraging, the responses proved mostly short-lived and toxic IL-12 levels were seen in one patient. Other pharmacologic and methodological approaches applied during T-cell growth or in vivo are also being investigated to modify effector functions, and circumvent local immunosuppression or control the state of differentiation of transferred T-cells is being investigated based on preclinical data (Gattinoni et al., 2009; Hinrichs et al., 2008). Here again, the adoptive transfer of in vitro expanded T-cells represents the best opportunity to precisely “sculpt” the T-cell response using genetic and somatic modifications to achieve tumor rejection.
5. THE FUTURE OF ACT
To consistently achieve complete and durable tumor rejections with T-cell transfer, several problems need to be overcome. It appears that enhancement of the antitumor T-cell repertoire must be combined with measures to overcome the immunosuppressive local tumor microenvironment, so immunotherapeutic combinations will likely be necessary. Removing the tumor-reactive T-cells and growing them in vitro provides a potent way to rapidly increase their number and separates them from potentially detrimental measures applied to suppress the hostile tumor microenvironment. Logistically, the hurdle of growing very large numbers of T-cells on a patient-specific basis also needs to be addressed with either better engineering or more potent T-cells. Only a limited number of normal self-antigens have been safely and successfully targeted by adoptive T-cell therapy, but these are largely absent in the most common solid tumors. This exposes our lack of good shared target antigens and the paucity of off-the-shelf reagents that can be used against the most common human cancer types. The advent of efficient methods to genetically analyze individuals’ tumors has made the pursuit of tumor-specific mutated antigens feasible and attractive. Yet the obstacle of mutational heterogeneity within tumors must be addressed and new means of enriching for the correct T-cells are needed. Still, this concept of “engrafting” a patient with a greatly enhanced T-cell repertoire against their cancer through adoptive transfer may accomplish the first critical step leading to successful tumor rejection.
REFERENCES
- American Association for Cancer Research. (2015). Expanding the reach of anti-PD-1 therapy. Cancer Discovery, 5, 684–685. [DOI] [PubMed] [Google Scholar]
- Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, & Van PA (1994). Tumor antigens recognized by T lymphocytes. Annual Review of Immunology, 12, 337–365. [DOI] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. The New England Journal of Medicine, 373, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La PK, et al. (2007). Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clinical Cancer Research, 13, 5426–5435. [DOI] [PubMed] [Google Scholar]
- Bronte V, & Mocellin S (2009). Suppressive influences in the immune response to cancer.Journal of Immunotherapy, 32, 1–11. [DOI] [PubMed] [Google Scholar]
- Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, et al. (1997). A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proceedings of the National Academy of Sciences of the United States of America, 94, 1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulie PG, Lehmann F, Lethe B, Herman J, Lurquin C, Andrawiss M, et al. (1995). A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proceedings of the National Academy of Sciences of the United States of America, 92, 7976–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar Z, Waks T, & Gross G (2014). The emergence of T-bodies/CAR T cells. CancerJournal, 20, 123–126. [DOI] [PubMed] [Google Scholar]
- Eshhar Z, Waks T, Gross G, & Schindler DG (1993). Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences of the United States of America, 90, 720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. (2006). Immune resistance orchestrated by the tumor microenvironment. Immunological Reviews, 213, 131–145. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. (2009). Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nature Medicine, 15, 808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SL, Smith FO, Klapper JA, Sherry R, Wunderlich JR, Steinberg SM, et al. (2010). Tumor infiltrating lymphocyte therapy for metastatic melanoma: Analysis of tumors resected for TIL. Journal of Immunotherapy, 33, 840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin M, Lemoine C, Weynants P, Vessiere F, Van PA, Knuth A, et al. (1987). Production of stable cytolytic T-cell clones directed against autologous human melanoma. International Journal of Cancer, 39, 390–396. [DOI] [PubMed] [Google Scholar]
- Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, et al. (2008). IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood, 111, 5326–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jager D, et al. (1998). Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: Definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. The Journal of Experimental Medicine, 187, 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. (2009). Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood, 114, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, et al. (1994). Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proceedings of the National Academy of Sciences of the United States of America, 91, 3515–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Acquavella N, Yu Z, & Restifo NP (2011). Therapeutic cancer vaccines: Are we there yet? Immunological Reviews, 239, 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Khong HT, Antony PA, Palmer DC, & Restifo NP (2005). Sinks, suppressors and antigen presenters: How lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends in Immunology, 26, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuth A, Danowski B, Oettgen HF, & Old LJ (1984). T-cell-mediated cytotoxicity against autologous malignant melanoma: Analysis with interleukin 2-dependent T-cell cultures. Proceedings of the National Academy of Sciences of the United States of America, 81, 3511–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. (2010). Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood, 116, 4099–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. (2012). Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nature Genetics, 44, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvistborg P, Shu CJ, Heemskerk B, Fankhauser M, Thrue CA, Toebes M, et al. (2012). TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology, 1, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. (2013). Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature, 499, 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. (2015). PD-1 blockade in tumors with mismatch-repair deficiency. The New England Journal of Medicine, 372, 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England Journal of Medicine, 371, 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. (2006). Cancer regression in patients after transfer of genetically engineered lymphocytes. Science, 314, 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muul LM, Spiess PJ, Director EP, & Rosenberg SA (1987). Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. Journal of Immunology, 138, 989–995. [PubMed] [Google Scholar]
- Park TS, Groh EM, Patel K, Kerkar SP, Lee CC, & Rosenberg SA (2016). Expression of MAGE-A and NY-ESO-1 in primary and metastatic cancers. Journal of Immunotherapy, 39(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. (2011). T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Molecular Therapy, 19, 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell SR, & Greenberg PD (1990). The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. Journal of Immunological Methods, 128, 189–201. [DOI] [PubMed] [Google Scholar]
- Robbins PF, El-Gamil M, Li YF, Kawakami Y, Loftus D, Appella E, et al. (1996). A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. The Journal of Experimental Medicine, 183, 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, et al. (2015). A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: Long-term follow-up and correlates with response. Clinical Cancer Research, 21, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, et al. (2013). Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nature Medicine, 19, 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. (2011). Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of Clinical Oncology, 29, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, et al. (1990). Gene transfer into humans—Immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. The New England Journal of Medicine, 323, 570–578. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Kawakami Y, Robbins PF, & Wang R (1996). Identification of the genes encoding cancer antigens: Implications for cancer immunotherapy. Advances in Cancer Research, 70, 145–177. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. (1988). Use of tumor-infiltrating lymphocytes and interleukin-2 in the immuno-therapy of patients with metastatic melanoma. A preliminary report. The New England Journal of Medicine, 319, 1676–1680. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. (2011). Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clinical Cancer Research, 17, 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, et al. (1994). Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. Journal of the National Cancer Institute, 86, 1159–1166. [DOI] [PubMed] [Google Scholar]
- Scanlan MJ, Welt S, Gordon CM, Chen YT, Gure AO, Stockert E, et al. (2002). Cancer-related serological recognition of human colon cancer: Identification of potential diagnostic and immunotherapeutic targets. Cancer Research, 62, 4041–4047. [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine, 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Solomon D, & Rosenberg SA (1989). Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. Journal of Immunology, 142, 3714–3725. [PubMed] [Google Scholar]
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. (2014). Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science, 344, 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. (2015). Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science, 350, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De PE, Van den Eynde B, et al. (1991). A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science, 254, 1643–1647. [DOI] [PubMed] [Google Scholar]
- Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, et al. (1995). A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science, 269, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, et al. (2015). Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clinical Cancer Research, 21, 2278–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]