Abstract
The ability of IL-2 to expand T cells with maintenance of functional activity has been translated into the first reproducible effective human cancer immunotherapies. The administration of IL-2 can lead to durable, complete, and apparently curative regressions in patients with metastatic melanoma and renal cancer. The growth of large numbers of tumor-infiltrating lymphocytes with in vitro anti-cancer activity in IL-2 has led to the development of cell transfer therapies that are highly effective in patients with melanoma. The genetic modification of T cells with genes encoding αβ TCRs or chimeric Ag receptors and the administration of these cells after expansion in IL-2 have extended effective cell transfer therapy to other cancer types.
In November 1984, a 33-year-old woman with metastatic melanoma who had progressed through multiple prior treatments received the aggressive infusion of rIL-2. Within one month after treatment, biopsy of one of her tumors showed extensive necrosis, after two months, all tumor deposits were shrinking, and a few months later, all evidence of cancer was gone. This patient has remained disease-free for the past 29 years. She was the first cancer patient to respond to the administration of IL-2 and, thus, the first to demonstrate that a purely immunologic maneuver that stimulated T lymphocytes could mediate complete destruction of large, invasive, vascularized cancers in humans. This patient and thousands that subsequently received IL-2 played a major role in the introduction of immunotherapy into the mainstream of cancer treatment.
Several seminal events in the development of modern cellular immunology set the stage for the translation of immunologic concepts into effective immunotherapies for patients with cancer. The origins of cellular immunology are recent. The study of Abs dominated studies of immunology until the middle of the 20th century when it became apparent that the cellular arm of the immune system played a major role in immunologic reactions. The word “lymphocyte” was not listed in the index of the 1958 issue of The Journal of Immunology, and the ability of lymphocytes to directly identify and interact with Ags and components on the surface of other cells was not appreciated. The study of the direct role of lymphocytes in delayed hypersensitivity reactions and the importance of cellular immune reactions as mediators of tissue rejection brought the lymphocyte into the forefront of studies of immunology(1). These studies, however, were severely hampered by the inability to sustain the survival and growth of lymphocytes ex vivo until the identification in 1976 of a T cell growth factor (now known as IL-2), produced by lymphocytes, that was capable of growing T lymphocytes in vitro (2). The availability of this growth factor propelled studies of cellular immunology forward at a rapid pace.
IL-2 is a 15.5-kDa cytokine secreted predominately by Ag-simulated CD4+ T cells, but it can also be produced by CD8+ cells, NK cells, and activated dendritic cells. IL-2 can stimulate cells that express either a trimeric high-affinity IL-2 receptor containing the α-, β-, and γ-chains or a low-affinity dimeric receptor consisting of only the β- and γ-chains. In CD8 cells, IL-2 can simulate cell growth, as well as differentiation into memory and more terminally differentiated lymphocytes. IL-2 is the predominant factor responsible for the maintenance of CD4+ regulatory T cells and plays a role in the differentiation of CD4 T cells into a variety of subsets with different T cell functions. Translation of information derived from in vitro and murine tumor models led to the administration of this nonspecific T cell growth factor to patients with cancer and ultimately to the growth and adoptive cell transfer (ACT) of natural or genetically modified autologous human antitumor T cells expanded in vitro in IL-2 to treat a variety of cancer types (3). These findings have had a profound impact on the ability to manipulate the cellular immune system to successfully treat patients with cancer, and they represented the first reproducible demonstrations that manipulations of the immune system could mediate the regression of large human cancers.
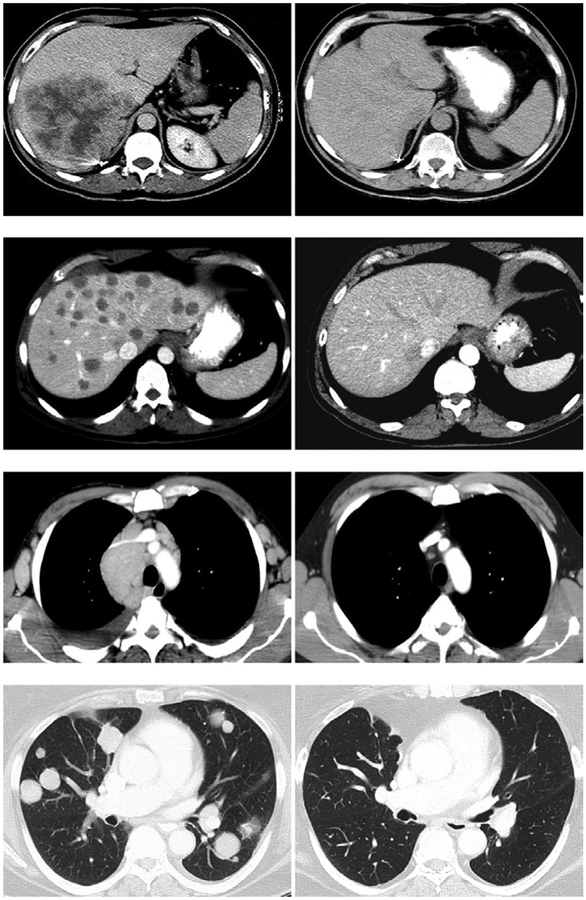
Examples of durable cancer regressions in patients with meta-static cancer resulting from IL-2–based therapies are shown in Fig. 1, including a patient with kidney cancer treated with IL-2, a patient with melanoma treated with autologous tumor-infiltrating lymphocytes (TILs), and patients with lymphoma or synovial cell sarcoma treated with autologous lymphocytes genetically engineered with chimeric Ag receptors (CARs) or TCRs that recognize the patient’s cancer.
FIGURE 1.
Upper panel, Fifty-six–year-old male with metastatic renal cell cancer to the liver and subcarinal lymph nodes was treated with high-dose bolus IL-2 in January 1994. Patient underwent a complete regression of all disease and remains disease-free 20 y later. Upper middle panel, Fifty-four–year-old male with metastatic melanoma to the lungs and liver was treated with autologous TILs plus IL-2 following a lymphodepleting regimen in December 2003. The patient underwent a complete regression of all disease and remains disease-free >10 y later. Lower middle panel, Fifty-year-old male with follicular non-Hodgkin’s lymphoma at multiple sites in the abdomen, mediastinum, and axillary lymph nodes treated with genetically engineered autologous peripheral lymphocytes expressing a gene encoding an anti-CD19 chimeric Ag receptor in May 2009. The patient underwent a dramatic regression of all disease following two cycles of treatment and is progression-free >4 y later. Lower panel, Sixty-seven–year-old female with metastatic synovial sarcoma to the lung and right pelvis treated with genetically engineered autologous peripheral lymphocytes expressing a gene encoding a TCR reactive with the NY-ESO-1 cancer testes Ag in August 2010. The patient was treated in August 2010 and has undergone a dramatic partial regression now ongoing >3 y later.
IL-2 administration as a cancer treatment
The inability to sustain the survival and growth of T lymphocytes in vitro was a perplexing problem in the 1970s. Although responder T cells from mixed lymphocyte cultures could survive and grow for many months when cells were stimulated by allogeneic lymphocytes every 1–2 wk, the factors responsible for this sustained growth were unknown. An important step toward understanding this phenomenon was a report in 1976 that the culture of pools of lymphocytes from multiple donors in medium containing PHA gave rise to a supernatant factor that when continuously supplied could sustain the growth of T lymphocytes from normal human bone marrow for many months (2). The growing cells were identified as T cells based on their ability to form rosettes of sheep erythrocytes, the major marker of T cells at that time. These growing cells were distinguished from growing B cells by the absence of EBV, which could promote the long-term growth of B cells. These findings were quickly followed by studies, both in mice and humans, demonstrating that supernatants derived from lectin-stimulated normal lymphocytes could sustain the growth of both mouse and human lymphocytes in vitro with maintenance of Ag recognition (4–8). Purification of the super-natants showed that the growth-promoting properties were independent of the continued presence of the lectin in the supernatant (9–11). The ability of this T cell growth factor (shortly thereafter named IL-2) to mediate T cell survival and sustain function in vitro suggested that its administration could potentially stimulate functional T cells in vivo; however, these studies were severely hampered by the inability to obtain large amounts of purified IL-2.
A human Jurkat T cell tumor line was identified that secreted high levels of IL-2 following PHA stimulation, and three lots of purified preparations of IL-2 were produced for clinical testing by the E.I. DuPont Company (12) between July 1983 and February 1984. Sixteen patients with advanced cancer were treated with this purified natural IL-2 (13). IL-2 administration resulted in dose-related fever, chills, malaise, and mild reversible hepatic dysfunction, but no antitumor activity was noted in these patients.
Progress in the administration of IL-2, however, was stimulated by the cloning of the gene encoding IL-2 in 1983 (14) and the subsequent production and characterization in 1984 of the biological activity of rIL-2 produced in Escherichia coli (15, 16). PHA-stimulated human lymphocytes provided a yield of 0.7 μg/l supernatant, which was improved to 3 μg/l when PHA was used to stimulate the Jurkat T cell tumor line. In contrast, recombinant E. coli could produce 100 mg IL-2/l. The production of rIL-2 finally made it possible to evaluate the impact of the in vivo administration of large amounts of IL-2 in cancer-bearing mice and humans.
Early experiments demonstrated that the administration of rIL-2 to tumor-bearing mice could mediate the regression of small established pulmonary metastases as well as s.c. tumors in animal models (17), although it was necessary to reach significant IL-2–related toxicity before antitumor effects were seen. The modest results seen using IL-2 in animal models, however, provided the impetus for the first administration of rIL-2 in humans. Twenty patients were reported in 1985 (23 treated) who received a wide variety of different regimens and doses of rIL-2 (18). The half-life of IL-2 in humans was ~7 min with a later delayed clearance consistent with a two-compartment model as IL-2 was released from extravascular space into the plasma compartment. Marked depletion of all lymphoid cells was seen almost immediately after IL-2 administration, which rebounded after IL-2 was discontinued. Significant toxicities became apparent in these early studies, including fever, chills, malaise, arthralgias, and unexpected capillary leak, which led to weight gain from marked fluid retention. Levels of IFN and other cytokines were found in the serum, and although these interesting immunologic changes were seen, there was no evidence of tumor regression in any of these cancer patients treated with IL-2 alone.
It was early noted that the exposure of normal mouse splenocytes or human PBMCs to supernatants containing IL-2 could generate cells, later called lymphokine-activated killer (LAK) cells, that without further stimulation could recognize and kill cultured tumor cell lines and fresh human cancer cells in vitro (19–22). LAK cell precursors were not T cells and appeared related to the NK lineage. Multiple studies of the adoptive transfer of these LAK cells grown in vitro and administered to tumor-bearing mice showed in vivo antitumor activity, but only in models in which tumors were treated before they became vascularized (23–26). These studies stimulated a clinical trial in 30 patients with advanced cancer, first using i.v. administration of LAK cells generated with natural IL-2 and later LAK cells generated with rIL-2 (27). No anti-tumor responses were seen in any of these patients. Murine models indicated that the administration of IL-2 could increase the in vivo activity of LAK cells, and this work stimulated more aggressive attempts to administer IL-2 at the maximum tolerated doses in conjunction with LAK cells in humans.
These higher IL-2 doses finally led to the first demonstration that IL-2 administration was capable of mediating tumor regression in humans, and the results of this study were published in December 1985 (28). Twenty-five patients with metastatic cancer, in whom standard therapy had failed, were treated in the Surgery Branch at the National Cancer Institute with increasing doses of IL-2 until toxicity precluded further dose escalation. Although most patients treated in the early phase of this study received 60,000 IU/kg every 8 h, patients subsequently treated received 180,000 or 600,000 IU/kg. An every 8 h schedule of the bolus infusion of IL-2 was established based on the in vivo half-life of IL-2 and assured that serum levels were continuously maintained at concentrations necessary to stimulate high-affinity IL-2 receptors during the course of IL-2 administration. In this first series of 25 patients, including the first patient mentioned at the beginning of this review, 4 of 7 patients with metastatic melanoma and 3 of 3 patients with metastatic renal cancer exhibited regression of metastatic cancer, and thus these cancer types were the targets in many subsequent studies evaluating the clinical effectiveness of IL-2. A generalized capillary leak syndrome was induced by IL-2 in vivo that resulted in interstitial pulmonary infiltrates and substantial weight gain in patients. Similarly, serum creatinine and bilirubin levels were elevated in about half of the patients. The side effects were transient and returned to baseline following treatment.
Importantly, however, this was the first demonstration that a purely immunologic maneuver could lead to tumor regression in humans and stimulated substantial activity exploring the administration of IL-2 to patients (29). Tumors do not express IL-2 receptors and thus the antitumor activity was the result of IL-2 stimulation of immune cells. In 1987, the Surgery Branch at the National Cancer Institute reported the results of the first 157 consecutive patients with advanced cancer treated with high-dose IL-2, either alone or in conjunction with LAK cells (30). A maximum dose of 600,000–720,000 IU/kg every 8 h was established as the maximum tolerated dose. Thirty of the 157 patients showed objective cancer regressions, including 13 of 57 patients with renal cell cancer (23%) and 12 of 42 patients with melanoma (29%). Cancer regressions were durable and seven of the nine complete responses remained in remission at the time of publication and continued for years thereafter. A randomized trial of 181 patients with metastatic melanoma or renal cancer comparing treatment with IL-2 alone or in conjunction with LAK cells showed that the antitumor effects were due to IL-2 alone, and thus LAK cell administration was omitted in future studies (31).
These results then led to an explosion of studies utilizing IL-2 in patients with metastatic cancer using either a high-dose bolus regimen or a continuous infusion of IL-2 (32–34). Patients with metastatic melanoma or metastatic renal cell cancer were uniquely responsive to high-dose IL-2 administration, and except for patients with advanced non-Hodgkin’s lymphomas(35) only rare responses were seen in patients with other tumor types. Complete durable regressions of metastatic disease were a hallmark of IL-2 therapy in melanoma and renal cancer. In 1994 the Surgery Branch at the National Cancer Institute reported on 283 consecutive patients treated with high-dose bolus IL-2 with metastatic melanoma or renal cancer(32), which was updated to 409 consecutive patients 4 y later. This later study revealed a 15% incidence of objective regressions in 182 patients with metastatic melanoma (7% were complete) and a 19% overall response rate in 227 patients with metastatic renal cancer (9% were complete) (33). Twenty-seven of the 33 completely responding patients (82%) remained in ongoing continuous complete response from 39 to >148 mo from the onset of treatment and appeared to be cured. Tumor regressions were seen at all organ sites. Multi-institutional studies confirmed these single institution results. The continuous infusion of high-dose IL-2 was evaluated by the National Biotherapy Study Group in multiple trials using the continuous infusion of IL-2, often in combination with the administration of cells or other cytokines, and similar anti-tumor responses were seen (36). Two hundred fifty-five consecutive patients with metastatic kidney cancer from multiple institutions were entered into seven phase 2 clinical trials of high-dose bolus IL-2 sponsored by the Chiron Corporation resulting in an overall objective response rate of 14% with 5% achieving complete responses (37). The median duration of partial responses was 19 mo and the median response duration of complete responses was not reached because 8 of the 12 complete responders were ongoing, including many longer than 2 y. A recent analysis of 259 consecutive patients with metastatic kidney cancer treated in the Surgery Branch at the National Cancer Institute between 1986 and 2006 with high-dose IL-2 revealed that 23 (9%) patients experienced a complete response and only 4 of 23 developed disease recurrence(38). Thirty patients (11%) achieved a partial response for an overall objective response rate of 20%. Based on the results of the durability of responses in both single institution as well as multi-institutional studies, the U.S. Food and Drug Administration approved high-dose bolus IL-2 for the treatment of patients with metastatic renal cancer in 1992, thus becoming the first immunotherapy approved for the treatment of patients with cancer.
As these studies were being conducted, similar results were being achieved in multi-institutional studies of IL-2 for the treatment of patients with metastatic melanoma. Two hundred seventy patients were entered into clinical trials conducted at 22 different institutions using the high-dose bolus IL-2 regimen(39). The overall objective response rate was 16% with 17 complete responders (6%) and 26 partial responders (10%). The median duration in patients who achieved a complete response had not been reached with 10 of the 17 complete responders ongoing at 24–106 mo. Based on the durability of these responses, IL-2 was approved by the U.S. Food and Drug Administration for the treatment of patients with meta-static melanoma in 1998. Higher response rates were seen in patients with s.c. or cutaneous metastases, although the great majority of patients treated had visceral disease.
The early toxicities seen accompanying the administration of high-dose IL-2 were unexpected, but were a harbinger of toxicities seen with many immunologic maneuvers that followed. The underlying toxicity of IL-2 results from a capillary leak that leads to fluid extravasation into visceral organs that can compromise their function. Although serious biochemical abnormalities can be seen in the liver and kidney, these all returned to normal following the completion of treatment. An unusual aspect of the administration of IL-2 was the continuation of dosing until grade 3 or 4 toxicity was reached. There was a significant learning curve in the administration of IL-2 before it was realized that many of these toxicities were completely reversible (40). In the first 157 patients reported from the Surgery Branch at the National Cancer Institute there were 4 treatment-related deaths (28). The neutrophil chemotactic defects induced by IL-2 were probably associated with the high incidence of central line sepsis that accompanied IL-2 administration (41), although a prospective randomized study demonstrated that this complication could be virtually eliminated by the use of prophylactic oxacillin (42). As information was learned about the tolerance to IL-2, the administered doses in the first cycle of therapy decreased from an initial median of 13 doses to 7 doses without any decrease in the ongoing objective or complete response rates (43). A detailed study of 1241consecutive metastatic cancer patients treated with high-dose i.v. bolus IL-2 in the Surgery Branch at the National Cancer Institute showed a substantial decrease in toxicities in patients when IL-2 administration experience increased (43). Although treatment-related deaths were initially in the 2 to 4% range, mortalities dropped consistently, and in the last 809 consecutive patients reported and treated in the Surgery Branch at the National Cancer Institute in 1998 there were no deaths related to treatment with IL-2. Thus, with appropriate management and experience high-dose IL-2 can be safely administered to patients with metastatic cancer with treatment-related mortalities <1%.
The role of IL-2 in ACT of cancer
The use of IL-2 to grow T cells in vitro that can be used for adoptive cell therapy, as well as the administration of IL-2 to support the growth and survival of antitumor cells infused into patients, has been of major importance in translating basic studies of IL-2 to the clinic.
Prior to the advent of IL-2, attempts to adoptively transfer T cells with antitumor activity as a therapeutic modality in animal tumor models were dependent on the ability to generate cells with antitumor activity by in vivo immunization (reviewed in Ref. 44). Although adoptive transfer of these T cells could mediate modest antitumor activity, the inability to grow these cells ex vivo severely limited progress, and the lack of human cells with specific antitumor reactivity was a major obstacle to the application of this approach in humans.
An important aspect of early studies of IL-2 in vitro was the demonstration that lymphocytes expanded long-term in IL-2 could retain specific Ag reactivity (4–8). Clonal T cell lines from mouse and human lymphocytes could be generated by limiting dilution and growth in IL-2 (45–47). Early mouse models of adoptive cell therapy using T cells resulting from immunization of mice with the Friend virus–induced leukemia, FBL-3, showed that the adoptive transfer of T cells i.p. to mice bearing i.p. and nodal FBL-3 exhibited in vivo antitumor effects (48). It was not at all clear, however, that large blastic-activated T cells grown in vitro in IL-2 could mediate systemic antitumor effects because early studies showed a dramatic accumulation of these large cells in the lungs for 1–2 d following i.v. administration (49, 50). An early test of the impact of the i.v. administration of cells grown in IL-2 demonstrated that the rejection of skin grafts in mice could be accelerated by the i.v. adoptive transfer of syngeneic cells with alloreactivity expanded in IL-2 (51). Similarly, in an extension of prior work performed using the FBL-3 leukemia (8), a palpable local tumor in the footpad as well as disseminated metastases could be cured by the i.v. administration of specific immune lymphocytes expanded in IL-2 (52). This provided a clear demonstration that i.v. injection of cells grown in IL-2 could circulate and manifest immune effector functions against palpable vascularized tumors in vivo.
Although T cells could be generated against the viral-induced FBL-3 lymphoma model, a major obstacle to the application of this cell transfer approach was the inability to generate T cell reactivity against transplantable solid tumors in mice or against naturally growing human cancers. Lymphocytes infiltrating into the stroma of solid tumors unexpectedly provided a source of such antitumor T cells. The growth of single-cell suspensions of a variety of murine and human solid tumors in IL-2 resulted in pure cultures of TILs free of contaminating tumor cells (19). The early growth of LAK cells in these cultures was limited, and as cells continued to grow the resulting lymphocytes often exhibited specific tumor reactivity against the tumor of origin and not other tumors.
The first report of the adoptive immunotherapy of murine tumors using syngeneic TILs plus IL-2 showed that large established, vascularized pulmonary, as well as hepatic metastases could be eliminated by this approach (53). Lymphodepletion prior to administration of specific TILs could cure most mice bearing visceral metastastic deposits from the MC38 colon adenocarcinoma, as well as mice bearing large methylcholanthrene-induced sarcomas. An important step in the development of cell transfer therapy for the treatment of human cancers was the demonstration that TILs obtained from resected metastatic melanoma deposits and grown in IL-2 developed specific cytolytic immune responses against the autologous human tumor (54). In vitro studies demonstrating the tumor specificity of human TILs stimulated the development of methods to grow human TILs in large numbers (55, 56) and then led to the first report of the ability of adoptive cell transfer to mediate the regression of large established cancers in patients with metastatic melanoma (57). In a paper reported in 1988 from the Surgery Branch at the National Cancer Institute, 20 patients with metastatic melanoma were treated with in vitro–expanded autologous TILs in conjunction with IL-2. Objective cancer regression was seen in 9 of 15 patients not previously treated with IL-2 and in 2 of 5 patients who had progressed through prior treatment with IL-2. Regression of cancers was seen in the lungs, liver, bones, skin, and s.c. sites. Studies of the adoptive transfer of [111In]-labeled TILs into patients with metastatic melanoma showed accumulation in the lungs for ~24 h and subsequent accumulation of administered TILs in tumor deposits (50, 58). From May 1987 through December 1992, 86 patients with metastatic melanoma were treated with 145 courses of autologous TILs plus high-dose bolus i.v. IL-2 at 720,000 IU/kg every 8 h (59). Because of evidence from animal models that prior lymphodepletion could improve the antitumor impact of cell administration, 57 of the 86 patients received a single i.v. dose of 25 mg/kg cyclophosphamide ~36 h prior to the TIL infusion. The overall objective response rate in these patients was 34% and was similar whether patients received prior cyclophosphamide or had previously been treated with high-dose IL-2. The rate of response was greater in patients treated with TILs from younger cultures, TILs with shorter doubling times, and TILs with higher lytic capacity against autologous tumor targets (60). Many of these responses, however, were of short duration with minimal apparent persistence of the transferred cells. In studies using retroviral insertion of a neomycin phophotransferase gene to mark TILs, barely0.01% of the administered cells could be detected in the circulation days after cell transfer (61). Attempts to improve the antitumor effects of TIL transfer in humans using highly selected tumor-reactive CD8+ clones reactive with melanocyte differentiation Ags did not result in objective tumor regression in patients with melanoma, suggesting that the polyclonal nature of tumor reactivity in TILs and possibly the presence of CD4+ cells were necessary to induce tumor rejection (62, 63).
Based on multiple animal models demonstrating that lymphodepletion was necessary for the effectiveness of the transferred cells, a trial was conducted in the Surgery Branch at the National Cancer Institute that dramatically changed the field of ACT in humans (64). A vigorous nonmyeloablative conditioning regimen consisting of high-dose cyclophosphamide and fludarabine was given immediately before cell transfer, which resulted in complete elimination of normal lymphocytes from peripheral blood for ~8 d before reconstitution of endogenous lymphocytes occurred. Six of 13 patients in this trial exhibited an objective cancer response, and for the first time there was substantial persistence and sometimes a clonal repopulation of the transferred cells that could represent 75% of all circulating CD8+ cells at 6–12 mo after infusion (64). In a subsequent study of 43 patients, a 49% objective response rate was achieved, and substantial persistence of the transferred cells was seen in most responding patients (65, 66). Two additional pilot trials of ACT in 25 patients each were conducted in the Surgery Branch at the National Cancer Institute to evaluate the impact of increased lymphodepletion by adding either 2 or 12 Gy total body irradiation (TBI) to the preparative chemotherapy regimen prior to the adoptive transfer of cells (65, 66). Objective response rates by Response Evaluation Criteria in Solid Tumors criteria in these two trials were 52 and 72%, respectively. Five of 25 (20%) patients receiving 2 Gy TBI and 10 of 25 (40%) of patients receiving 12 Gy TBI achieved a complete response. Nineteen of the 20 complete responders in this total of 93 patients remained in complete regression at 70–114 mo following the adoptive transfer of cells. Seventeen of the 20 completely responding patients had visceral metastases and all but 2 of the complete responders had progressive disease following other prior systemic therapy, including IL-2, chemotherapy, and anti-CTLA4. These rates of overall response and durable complete responses in patients with metastatic melanoma exceed those of other treatments for patients with this disease. Similar results using the adoptive transfer of TILs following a lymphodepleting preparative regimen have been reported by groups at the Sheba Medical Center in Israel (67), as well as the M.D. Anderson Hospital (68) and the Moffit Cancer Center in the United States (69).
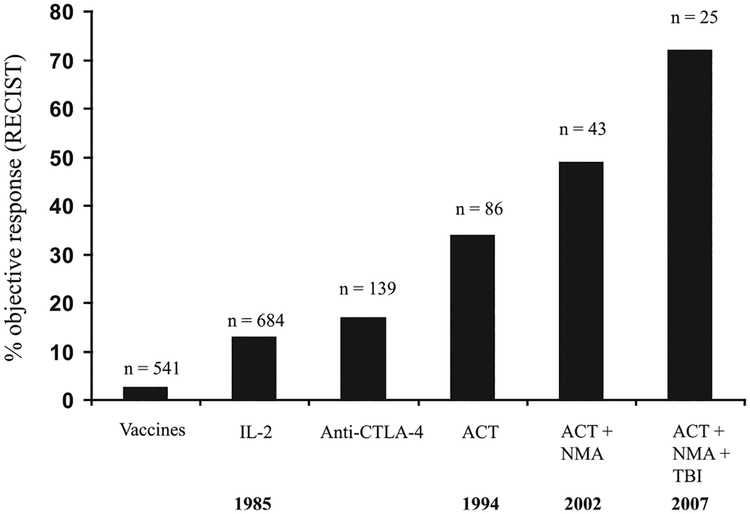
Thus, a continuous improvement in the treatment of patients with metastatic melanoma has been achieved utilizing IL-2–based immunotherapies (Fig. 2). Response rates in the Surgery Branch at the National Cancer Institute increased from 15% seen with IL-2 alone to 34% using ACT/TILs to 49% with ACT/TILs preceded by a preparative lymphodepleting chemotherapy regimen to 72% following maximum lympho-depletion, including TBI.
FIGURE 2.
Objective response rates (using Response Evaluation Criteria in Solid Tumors criteria) using various forms of cancer immunotherapy of patients with metastatic melanoma treated in the Surgery Branch at the National Cancer Institute. Only anecdotal responses have been seen utilizing cancer vaccine approaches. A response rate of 2.6% was seen using 541 different vaccines in 440 patients with metastatic cancer (84). Using an anti-CTLA Ab, a response rate of ~15% was seen but varied with the dose and schedule of administration (85). Utilizing IL-2 response rates of ~15% were seen that increased to 34% when IL-2 was given following ACT. The response rates increased to 49% when cell transfer was preceded by a nonmyeloablative chemotherapy (NMA) consisting of cyclophosphamide (60 mg/kg 2×) and fludarabine (25 mg/kg 5×) and was increased to 72% when cells were transferred following NMA plus 12 Gy TBI. The years of these reports are shown on the bottom line of the figure.
Studies in mouse models have helped to elucidate the important mechanisms that underlie successful ACT therapy (reviewed in Ref. 70). The lymphodepletion prior to cell transfer eliminates endogenous T regulatory cells and myeloid-derived suppressor cells as well as endogenous lymphocytes that compete with the transferred cells for growth-promoting cytokines such as IL-7 and IL-15. Less differentiated cells were more effective in mouse models than were terminally differentiated cells, and these murine studies were in accord with findings in humans that showed that cells with long telomeres and cells that expressed markers such as CD27 and CD28, indicative of less differentiated cells, were more effective in mediating cancer rejection (66). Persistence of the transferred cells in the circulation 1 mo after transfer correlated with clinical effectiveness of the cell transfer. Additionally, a role for T regulatory cells in humans was documented by showing that there was an inverse correlation between the rate of clinical response and a reappearance of CD4+Foxp3+ T regulatory cells in the circulation following the lymphodepletion (71).
The ability of TILs to mediate tumor rejection in humans led to multiple studies to define the Ags recognized by TILs responsible for tumor rejection. Early studies identified melanoma/melanocyte Ags such as MART-1 and gp100 that were recognized by many TILs (72, 73), although more recent evidence suggests that individual mutations expressed by autologous cancers represent the cancer rejection Ags targeted by TIL (74). The administration of lymphocytes genetically engineered to express high-affinity anti–MART-1 or anti-gp100 cells caused a low level of antitumor responses but often mediated significant toxicities against normal melanocytes in the eye and the ear (75, 76). In contrast, TILs mediated complete tumor rejection in the absence of melanocyte toxicity in most patients, suggesting that these shared melanocyte Ags were not the tumor rejection Ags (66). Recent studies using exomic sequencing to identify tumor mutations have further demonstrated the recognition of unique mutations by TILs(74). These findings also help explain why melanoma is uniquely responsive to IL-2 as well as TIL therapy. Melanomas contain an average of ~300 exomic mutations, and with the exception of smoking-induced lung cancers and cancers that arise from mismatch repair gene mutations, other solid tumors often contain a fifth or less the number of mutations found in melanomas (77). Although TILs can be grown from virtually any type of cancer, antitumor responses are rare when TILs from other tumor types are administered, likely due to the paucity of mutated Ags recognized. The larger the number of mutations, the more likely a processed peptide will exhibit the strong binding to an appropriate cell surface MHC molecule necessary for tumor recognition. Future ACT studies are likely to emphasize the use of lymphocytes reactive with unique mutations on a variety of tumor types.
Because antitumor TILs can be reproducibly grown only from melanoma, efforts have turned to the genetic engineering of lymphocytes to express receptors capable of recognizing tumors. Transduction into normal autologous lymphocytes grown in IL-2 of genes encoding conventional αβ TCRs or CARs that rely on the Ag combining sites of an Ab fused to T cell intracellular molecules has significantly expanded the range of tumors that can be treated by adoptive cell therapy(3). The first example of the successful use of genetically engineered lymphocytes grown in IL-2 to mediate tumor rejection in humans was reported in 2006 using autologous lymphocytes genetically engineered with retroviruses encoding a high-affinity receptor against the MART-1 melanoma/melanocyte Ag and later against the gp100 Ag (75, 76). Although antitumor responses were seen, toxicity in the eye and ear due to melanocyte destruction in those organs precluded the further clinical use of these receptors. Shortly thereafter it was shown that transduction of normal lymphocytes with genes encoding TCRs against the cancer testes Ag, NY-ESO-1, could mediate tumor regression in patients with synovial cell sarcomas as well as melanomas (78). There are >100 different cancer testes Ags, and many are now being explored as potential tumor targets of ACT.
The exquisite specificity and sensitivity of the immune system in targeting Ags has resulted in substantial toxicity in normal tissues when target Ags are expressed even in low levels such as carcinoembryonic Ag or ERB-B2 (79, 80). However, ACT using genetically engineered cells to attack Ags expressed on tumors as well as on nonessential normal tissues has shown some success. The first report of ACT using a CAR targeting CD19 to mediate regression of CD19-expressing lymphomas in humans was published in 2010 (81). A substantial tumor regression was induced in this patient now going beyond 4 y. Normal B cells were also eliminated because they are the only other cells that express CD19, and Ig transfusions can partially overcome this B cell defect. Subsequent studies showed the effectiveness of this approach in the treatment of patients with CD19 expressing acute and chronic lymphocytic leukemias (reviewed in Ref. 82). In addition to adding antitumor receptors to T cells grown in IL-2, a wide variety of genetic alterations of lymphocytes can potentially be used to increase the antitumor activity of the transferred cells in ACT. The introduction of a gene encoding a single-chain IL-12 has been shown to increase the antitumor activity of transferred cells in mouse models, and clinical trials using TILs transduced to express single-chain IL-12 have begun (83).
Conclusions
The translation of basic findings concerning IL-2 had a profound impact on the development of cancer immunotherapy. The administration of IL-2 as well as the adoptive transfer of antitumor T cells grown in IL-2 represented the first effective immunotherapies for cancer in humans and have provided one of the first curative systemic therapies for any solid tumor. These studies played a major role in enabling immunotherapy to join the mainstream of cancer treatment.
Abbreviations used in this article:
- ACT
adoptive cell transfer
- CAR
chimeric Ag receptor
- LAK
lymphokine-activated killer
- TBI
total body irradiation
- TIL
tumor-infiltrating lymphocyte
Footnotes
Disclosures
The author has no financial conflicts of interest.
References
- 1.Mitchison NA 1955. Studies on the immunological response to foreign tumor transplants in the mouse. I. The role of lymph node cells in conferring immunity by adoptive transfer. J. Exp. Med 102: 157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan DA, Ruscetti FW, and Gallo RG. 1976. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 193: 1007–1008. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA 2011. Cell transfer immunotherapy for metastatic solid cancer: what clinicians need to know. Nat. Rev. Clin. Oncol 8: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillis S, and Smith KA. 1977. Long term culture of tumour-specific cytotoxic T cells. Nature 268: 154–156. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Schwarz S, and Spiess PJ. 1978. In vitro growth of murine T cells. II. Growth of in vitro sensitized cells cytotoxic for alloantigens. J. Immunol 121: 1951–1955. [PubMed] [Google Scholar]
- 6.Strausser JL, and Rosenberg SA. 1978. In vitro growth of cytotoxic human lymphocytes. I. Growth of cells sensitized in vitro to alloantigens. J. Immunol 121: 1491–1495. [PubMed] [Google Scholar]
- 7.Gillis S, Baker PE, Ruscetti FW, and Smith KA. 1978. Long-term culture of human antigen-specific cytotoxic T-cell lines. J. Exp. Med 148: 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheever MA, Greenberg PD, and Fefer A. 1981. Specific adoptive therapy of established leukemia with syngeneic lymphocytes sequentially immunized in vivo and in vitro and nonspecifically expanded by culture with interleukin 2. J. Immunol 126: 1318–1322. [PubMed] [Google Scholar]
- 9.Kurnick JT, Grönvik K-O, Kimura AK, Lindblom JB, Skoog VT,Sjöberg O, and Wigzell H. 1979. Long term growth in vitro of human T cell blasts with maintenance of specificity and function. J. Immunol 122: 1255–1260. [PubMed] [Google Scholar]
- 10.Rosenberg SA, Schwarz S, Spiess PJ, and Brown JM. 1980. In vitro growth of murine T cells. III. Method for separation of T cell growth factor (TCGF) from concanavalin A and biological activity of the resulting TCGF. J. Immunol. Methods 33: 337–350. [DOI] [PubMed] [Google Scholar]
- 11.Lotze MT, and Rosenberg SA. 1981. In vitro growth of cytotoxic human lymphocytes. III. The preparation of lectin-free T cell growth factor (TCGF) and an analysis of its activity. J. Immunol 126: 2215–2220. [PubMed] [Google Scholar]
- 12.Robb RJ, Kutny RM, and Chowdhry V. 1983. Purification and partial sequence analysis of human T-cell growth factor. Proc. Natl. Acad. Sci. USA 80: 5990–5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lotze MT, Frana LW, Sharrow SO, Robb RJ, and Rosenberg SA. 1985. In vivo administration of purified human interleukin 2. I. Half-life and immuno-logic effects of the Jurkat cell line-derived interleukin 2. J. Immunol 134: 157–166. [PubMed] [Google Scholar]
- 14.Taniguchi T, Matsui H, Fujita T, Takaoka C, Kashima N, Yoshimoto R, and Hamuro J. 1983. Structure and expression of a cloned cDNA for human inter-leukin-2. Nature 302: 305–310. [DOI] [PubMed] [Google Scholar]
- 15.Devos R, Plaetinck G, Cheroutre H, Simons G, Degrave W, Tavernier J,Remaut E, and Fiers W. 1983. Molecular cloning of human interleukin 2 cDNA and its expression in E. coli. Nucleic Acids Res. 11: 4307–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Grimm EA, McGrogan M, Doyle M, Kawasaki E, Koths K, and Mark DF. 1984. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science 223: 1412–1414. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Mulé JJ, Spiess PJ, Reichert CM, and Schwarz SL. 1985. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J. Exp. Med 161: 1169–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotze MT, Matory YL, Ettinghausen SE, Rayner AA, Sharrow SO,Seipp CA, Custer MC, and Rosenberg SA. 1985. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J. Immunol 135: 2865–2875. [PubMed] [Google Scholar]
- 19.Yron I, Wood TA Jr., Spiess PJ, and Rosenberg SA. 1980. In vitro growth of murine T cells. V. The isolation and growth of lymphoid cells infiltrating syngeneic solid tumors. J. Immunol 125: 238–245. [PubMed] [Google Scholar]
- 20.Grimm EA, Mazumder A, Zhang HZ, and Rosenberg SA. 1982. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes.J. Exp. Med 155: 1823–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm EA, Ramsey KM, Mazumder A, Wilson DJ, Djeu JY, and Rosenberg SA. 1983. Lymphokine-activated killer cell phenomenon. II. Precursor phenotype is serologically distinct from peripheral T lymphocytes, memory cytotoxic thymus-derived lymphocytes, and natural killer cells. J. Exp. Med 157: 884–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang JC, Mulé JJ, and Rosenberg SA. 1986. Murine lymphokine-activated killer (LAK) cells: phenotypic characterization of the precursor and effector cells. J. Immunol 137: 715–722. [PubMed] [Google Scholar]
- 23.Mule JJ, and Rosenberg SA. 1985. Successful adoptive immunotherapy of established metastases with lymphokine activated killer cells and recombinant interleukin-2 In Immune Responses to Metastases. Herberman RB, Wiltrout RH, and Gorelik E, eds. CRC, Boca Raton, FL, p. 69–94. [Google Scholar]
- 24.Rosenberg SA 1985. Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J. Natl. Cancer Inst 75: 595–603. [PubMed] [Google Scholar]
- 25.Ettinghausen SE, and Rosenberg SA. 1986. Immunotherapy of murine sarcomas using lymphokine activated killer cells: optimization of the schedule and route of administration of recombinant interleukin-2. Cancer Res. 46: 2784–2792. [PubMed] [Google Scholar]
- 26.Lafreniere R, and Rosenberg SA. 1985. Successful therapy of hepatic metastases from several murine tumors using lymphokine activated killer cells and recombinant interleukin-2. Surg. Forum 36: 392–394. [PubMed] [Google Scholar]
- 27.Lotze MT, Line BR, Mathisen DJ, and Rosenberg SA. 1980. The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implications for the adoptive immunotherapy of tumors.J. Immunol 125: 1487–1493. [PubMed] [Google Scholar]
- 28.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE,Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT, et al. 1985. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med 313: 1485–1492. [DOI] [PubMed] [Google Scholar]
- 29.Lotze MT, Chang AE, Seipp CA, Simpson C, Vetto JT, and Rosenberg SA. 1986. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA 256: 3117–3124. [PubMed] [Google Scholar]
- 30.Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S,Linehan WM, Robertson CN, Lee RE, Rubin JT, et al. 1987. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med 316: 889–897. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE,Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA, et al. 1993. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J. Natl. Cancer Inst 85: 622–632. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS,Parkinson DR, Seipp CA, Einhorn JH, and White DE. 1994. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271: 907–913. [PubMed] [Google Scholar]
- 33.Rosenberg SA, Yang JC, White DE, and Steinberg SM. 1998. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann. Surg 228: 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ,Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, et al. 2003. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J. Clin. Oncol 21: 3127–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber JS, Yang JC, Topalian SL, Schwartzentruber DJ, White DE, and Rosenberg SA. 1992. The use of interleukin-2 and lymphokine-activated killer cells for the treatment of patients with non-Hodgkin’s lymphoma. J. Clin. Oncol 10: 33–40. [DOI] [PubMed] [Google Scholar]
- 36.Dillman RO, Church C, Oldham RK, West WH, Schwartzberg L, and Birch R. 1993. Inpatient continuous-infusion interleukin-2 in 788 patients with cancer. The National Biotherapy Study Group experience. Cancer 71: 2358–2370. [DOI] [PubMed] [Google Scholar]
- 37.Fyfe GA, Fisher RI, Rosenberg SA, Sznol M, and Parkinson DR. 1996. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J. Clin. Oncol 14: 2410–2411. [DOI] [PubMed] [Google Scholar]
- 38.Klapper JA, Downey SG, Smith FO, Yang JC, Hughes MS,Kammula US, Sherry RM, Royal RE, Steinberg SM, and Rosenberg SA. 2008. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 113: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K,Abrams J, Sznol M, Parkinson D, Hawkins M, et al. 1999. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol 17: 2105–2116. [DOI] [PubMed] [Google Scholar]
- 40.Schwartzentruber DJ 1995. Biologic therapy with interleukin-2: clinical applications. Principles of administration and management of side effects In Biologic Therapy of Cancer, 2nd ed. DeVita VT Jr., Hellman S, and Rosenberg SA, eds. Lippincott Williams & Wilkins, Philadelphia, p. 235–249. [Google Scholar]
- 41.Klempner MS, Noring R, Mier JW, and Atkins MB. 1990. An acquired chemotactic defect in neutrophils from patients receiving interleukin-2 immuno-therapy. N. Engl. J. Med 322: 959–965. [DOI] [PubMed] [Google Scholar]
- 42.Bock SN, Lee RE, Fisher B, Rubin JT, Schwartzentruber DJ, Wei JP,Callender DPE, Yang JC, Lotze MT, Pizzo PA, and Rosenberg SA. 1990. A prospective randomized trial evaluating prophylactic antibiotics to prevent triple-lumen catheter-related sepsis in patients treated with immunotherapy. J. Clin. Oncol 8: 161–169. [DOI] [PubMed] [Google Scholar]
- 43.Kammula US, White DE, and Rosenberg SA. 1998. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer 83: 797–805. [PubMed] [Google Scholar]
- 44.Rosenberg SA, and Terry WD. 1977. Passive immunotherapy of cancer in animals and man. Adv. Cancer Res 25: 323–388. [DOI] [PubMed] [Google Scholar]
- 45.Baker PE, Gillis S, and Smith KA. 1979. Monoclonal cytolytic T-cell lines. J. Exp. Med 149: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg SA, Spiess PJ, and Schwarz S. 1980. In vitro growth of murine T cells. IV. Use of T-cell growth factor to clone lymphoid cells. Cell. Immunol 54: 293–306. [DOI] [PubMed] [Google Scholar]
- 47.Lotze MT, Strausser JL, and Rosenberg SA. 1980. In vitro growth of cytotoxic human lymphocytes. II. Use of T cell growth factor (TCGF) to clone human T cells. J. Immunol 124: 2972–2978. [PubMed] [Google Scholar]
- 48.Greenberg PD, Cheever MA, and Fefer A. 1981. Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1+2− lymphocytes. J. Exp. Med 154: 952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathisen DJ, and Rosenberg SA. 1980. Comparison of in vivo cell distribution following either intraperitoneal or intravenous injection of lymphoid cells. Transplantation 29: 347–349. [DOI] [PubMed] [Google Scholar]
- 50.Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL,Yang JC, Yolles P, Larson SM, and Rosenberg SA. 1989. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J. Clin. Oncol 7: 250–261. [DOI] [PubMed] [Google Scholar]
- 51.Rosenstein M, Eberlein T, Kemeny MM, Sugarbaker PH, and Rosenberg SA. 1981. In vitro growth of murine T cells. VI. Accelerated skin graft rejection caused by adoptively transferred cells expanded in T cell growth factor. J. Immunol 127: 566–571. [PubMed] [Google Scholar]
- 52.Eberlein TJ, Rosenstein M, and Rosenberg SA. 1982. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J. Exp. Med 156: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg SA, Spiess P, and Lafreniere R. 1986. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233: 1318–1321. [DOI] [PubMed] [Google Scholar]
- 54.Muul LM, Spiess PJ, Director EP, and Rosenberg SA. 1987. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J. Immunol 138: 989–995. [PubMed] [Google Scholar]
- 55.Topalian SL, Muul LM, Solomon D, and Rosenberg SA. 1987. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J. Immunol. Methods 102: 127–141. [DOI] [PubMed] [Google Scholar]
- 56.Topalian SL, Solomon D, Avis FP, Chang AE, Freerksen DL,Linehan WM, Lotze MT, Robertson CN, Seipp CA, Simon P, et al. 1988. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J. Clin. Oncol 6: 839–853. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL,Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. 1988. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med 319: 1676–1680. [DOI] [PubMed] [Google Scholar]
- 58.Griffith KD, Read EJ, Carrasquillo JA, Carter CS, Yang JC, Fisher B,Aebersold P, Packard BS, Yu MY, and Rosenberg SA. 1989. In vivo distribution of adoptively transferred indium-111-labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma. J. Natl. Cancer Inst 81: 1709–1717. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ,Weber JS, Parkinson DR, Seipp CA, Einhorn JH, and White DE. 1994. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl. Cancer Inst 86: 1159–1166. [DOI] [PubMed] [Google Scholar]
- 60.Schwartzentruber DJ, Hom SS, Dadmarz R, White DE, Yannelli JR,Steinberg SM, Rosenberg SA, and Topalian SL. 1994. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J. Clin. Oncol 12: 1475–1483. [DOI] [PubMed] [Google Scholar]
- 61.Rosenberg SA, Aebersold PM, Cornetta K, Kasid A, Morgan RA, Moen R,Karson EM, Lotze MT, Yang JC, Topalian SL, et al. 1990. Gene transfer into humans: immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med 323: 570–578. [DOI] [PubMed] [Google Scholar]
- 62.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL,Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, et al. 2001. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J. Immunother 24: 363–373. [DOI] [PubMed] [Google Scholar]
- 63.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ,Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, et al. 2002. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J. Immunother 25: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P,Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298: 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U,Robbins PF, Huang J, Citrin DE, Leitman SF, et al. 2008. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol 26: 5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS,Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. 2011. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res 17: 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D,Kubi A, Shoshani N, Zikich D, Ohayon Y, et al. 2013. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin. Cancer Res 19: 4792–4800. [DOI] [PubMed] [Google Scholar]
- 68.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R,Lizee G, Mahoney S, Alvarado G, et al. 2012. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin. Cancer Res 18: 6758–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S,Kudchadkar R, Zager J, Gibney G, Sondak VK, et al. 2012. Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J. Immunother 35: 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gattinoni L, Powell DJ Jr., Rosenberg SA, and Restifo NP. 2006. Adoptive immunotherapy for cancer: building on success. Nat. Rev. Immunol 6: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao X, Ahmadzadeh M, Lu YC, Liewehr DJ, Dudley ME, Liu F,Schrump DS, Steinberg SM, Rosenberg SA, and Robbins PF. 2012. Levels of peripheral CD4+FoxP3+ regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood 119: 5688–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E,Yannelli JR, Adema GJ, Miki T, and Rosenberg SA. 1994. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. USA 91: 6458–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL,Miki T, and Rosenberg SA. 1994. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl. Acad. Sci. USA 91: 3515–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC,Teer JK, Cliften P, Tycksen E, et al. 2013. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med 19: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC,Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. 2006. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS,Kammula US, Royal RE, Sherry RM, Wunderlich JR, et al. 2009. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., and Kinzler KW. 2013. Cancer genome landscapes. Science 339: 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM,Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. 2011. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol 29: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA,Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, et al. 2011. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther 19: 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, and Rosenberg SA. 2010. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther 18: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ, et al. 2010. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116: 4099–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kochenderfer JN, and Rosenberg SA. 2013. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol 10: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z,Palmer DC, Reger RN, Borman ZA, Zhang L, et al. 2010. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lympho-depleted hosts. Cancer Res. 70: 6725–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosenberg SA, Yang JC, and Restifo NP. 2004. Cancer immunotherapy: moving beyond current vaccines. Nat. Med 10: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE,Levy CL, Rosenberg SA, and Phan GQ. 2012. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin. Cancer Res 18: 2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]