Abstract
Alcohol relapse plays a major role in alcohol dependence and is an important focus for the treatment of alcoholism. The alcohol deprivation effect (ADE) is a widely used paradigm in rodents to model the relapse episodes that occur in human alcoholics. Mesyl Salvinorin B (MSB) is a potent and selective kappa opioid receptor (KOP-r) full agonist, with fewer side effects (e.g., sedation or anhedonia) than classic KOP-r full agonists and a longer duration of action in mice than the structurally similar salvinorin A. We have recently found that MSB prevents cocaine seeking in a rat self-administration model and reduces excessive alcohol drinking in a mouse escalation model via a KOP-r-mediated mechanism. Here, we further investigated whether MSB alone (0.3-3 mg/kg) or in combination with naltrexone (mu-opioid receptor antagonist at 1 mg/kg) altered alcohol “relapse” drinking using a mouse ADE paradigm. Both male and female mice, exposed to 3-week intermittent access alcohol drinking in a two-bottle choice paradigm with 24-h access every other day, developed excessive alcohol intake and then displayed pronounced ADE after 1-week abstinence. Acute administration of MSB prevented the ADE at 3 mg/kg in both male and female mice. Upon investigation of potential synergistic effects between naltrexone and MSB, we found that acute administration of a combination of MSB (0.3 mg/kg) and naltrexone (1 mg/kg) reduced the ADE at doses lower than those individual effective doses, with no sex difference. Our study suggests that the KOP-r full agonist MSB both alone and in combination with naltrexone shows potential in alcohol “relapse” treatment models.
Keywords: alcohol deprivation effect, combined therapy, KOP-r, Mesyl Salvinorin B, naltrexone, relapse
INTRODUCTION
Activation of the kappa-opioid receptor (KOP-r) by the natural product salvinorin A (Sal A) has anti-addictive effects (including cocaine and amphetamine) in preclinical models of drug addiction [1, 2]. However, Sal A has a very short half-life, which limits its potential for clinical use. Mesyl Salvinorin B (MSB), an analogue of Sal A, is a potent KOP-r full agonist and has improved pharmacokinetic properties with fewer side effects (sedation in the locomotor activity test or anhedonia in the sucrose preference test) compared to the natural product Sal A or other “classic” KOP-r agonists [3, 4]. Recently, it has been found that acute administration of MSB significantly attenuates cocaine seeking in a rat self-administration model [3] and reduces alcohol drinking in a mouse escalation model in a dose-dependent manner [4]. This suggests that MSB may have potential utility in treating drug abuse. To date, no study has investigated the effects of MSB in alcohol “relapse” in rodent models.
After a period of imposed abstinence, the phenomenon of a transient increase in alcohol consumption observed in both humans and rodents has been termed the alcohol deprivation effect (ADE) [5, 6]. ADE has been demonstrated as an appropriate animal model for studying alcohol “relapse” drinking included in this study. While the ADE is widely studied in rats [7, 8], studies on the ADE in C57BL/6J mice have not been established after excessive alcohol drinking. Based on the above rat models, we recently developed a simple behavioral protocol that rapidly and reliably induced ADE in C57BL/6 mice [9]. In this model, after mice have access to intermittent access alcohol drinking for 3 weeks, both male and female mice display excessive alcohol consumption (15-25 g/kg/day) [4, 10]. After they experience 1 week of imposed abstinence, mice show a pronounced ADE when alcohol is presented again (a significant increase in alcohol intake after 4 hours of alcohol access), modeling relapse drinking that occur in human alcoholics. Based on our recent findings as mentioned above, we hypothesized that MSB would prevent alcohol “relapse” drinking in the mouse ADE model. In this study, therefore, we determined the pharmacological effects of MSB on ADE in both male and female mice, to explore its potential for development as an anti-relapse agent for alcoholism.
In human alcoholics and rodent models, pharmacological studies provide consistent evidence that the mu-opioid receptor (MOP-r) antagonist naltrexone (NTN) decreases alcohol relapse episodes [11], and relapse-like drinking in an ADE model [8]. In the present study, therefore, we used the well-known NTN as a reference compound to compare its effects on mouse ADE with those of MSB. Another particularly interesting question is whether the proper combination of these two drugs could be more effective in reducing alcohol “relapse” drinking than either drug alone, given that the two compounds have different mechanisms of actions (KOP-r agonism for MSB and MOP-r antagonism for NTN). Therefore, we specifically tested combinations of MSB and NTN using doses of each drug that, when given alone, had no effect on ADE.
MATERIAL AND METHDODS
Animals.
Male and female adult C57BL/6J mice (8 weeks of age) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in a temperature-controlled room (21 °C). Mice were placed on a 12-hour reverse light-dark cycle (lights off at 7:00 am) upon arrival, and acclimated for a week prior to testing. Mice were individually housed in ventilated cages fitted with steel lids and filter tops and given ad libitum access to food and water. Animal care and experimental procedures were conducted according to Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences 1996), and were approved by the Institutional Animal Care and Use Committee of the Rockefeller University.
Materials.
Mesyl Salvinorin B (MSB) was synthesized from Sal A as described previously [12], and dissolved in 1% DMSO. Ethanol solutions were prepared from 190 proof absolute ethyl alcohol (Pharmco-AAPER, Brookfield, CT, USA) and dissolved in tap water. Naltrexone hydrochloride was purchased from Sigma-Aldrich and dissolved in physiological saline.
Procedures.
1. Chronic intermittent access alcohol drinking.
In C57BL/6J mice, this model of excessive alcohol drinking is widely used by many laboratories [e.g., 9, 10], During alcohol drinking in their home cages for 3 weeks, mice had access to food and water at all times. This chronic intermittent access model (two-bottle choice paradigm with chronic alcohol exposure every other day) was described in detail in earlier reports (Table S1). Starting at 10:00 am (3 hours after lights off), both the alcohol (15%) solution and water sipper tubes were placed on their home cages. The position of the tubes on left or right side of the cage was randomly set to avoid the development of side preference. The alcohol tubes were filled with fresh alcohol solution, and placed for 24 hours before being replaced with the water tubes. Alcohol and water intake values were recorded after 4, 8 and 24 hours of alcohol access in the drinking days, and these data were used to calculate consumed alcohol intake (g/kg) and relative preference for alcohol (alcohol intake/total fluid intake).
2. Alcohol deprivation effect (ADE) after 3-week intermittent access alcohol drinking.
This model of alcohol “relapse” drinking in C57BL/6J mice has recently been developed by our laboratory [9], based on the rat models by others [7, 8], Briefly, mice experienced chronic intermittent access alcohol drinking (see the above Section 1) for 3 weeks (Table S1). In the baseline session on day 21 in week 3, 30% (but not 15%) alcohol and water intake values were recorded at 4, 8 and 24 hours. Then, alcohol tubes were taken away for 7 days. After 1-week abstinence, alcohol (30%) tubes were presented to the mice again at 10:00am (3 hours after the dark cycle) on day 28 (week 5) and the alcohol and water intakes were recorded at 4, 8 and 24 hours in the ADE session.
3. Acute administration MSB, NTN or their combination in the ADE model (Table S1).
Mice were randomly assigned as the vehicle- and drug-treated groups in each sex with similar alcohol intake in the baseline session. An experimenter, blinded to the treatments given to the experimental groups, injected the drug and vehicle. The mice in control groups received one vehicle injection before the ADE test on day 28; and the mice in test groups received one drug injection (MSB or NTN) or two drug injections (MSB followed by NTN) before the ADE test on day 28. Then, the alcohol tube was presented after the drug or vehicle injection, and alcohol and water intakes were recorded after alcohol access.
The MSB doses were based on our recent publication [4]: the mice in test groups received one MSB injection (0.3, 1 or 3 mg/kg, i.p.), and the mice in control groups received one vehicle injection (1% DMSO). The NTN doses were also based on the above publication [4]: the mice in test groups received one NTN injection (0.5, 1 or 3 mg/kg, i.p.) and the mice in control groups received one saline injection. The MSB + NTN dose chosen was based on the above two experiments with each compound alone: the mice in test groups received the first i.p. injection of MSB (0.3 mg/kg) followed by the second i.p. injection of NTN (0.5 or 1 mg/kg) 20 min later; and the mice in control groups received one vehicle followed by saline.
Data analysis.
We performed power analyses to determine the number of animals required to provide statistical significances, based on the levels of differences seen previously [9], and predicted that these studies require 9-15 males and 9-10 females per group. As similar effects on the ADE with no significant sex differences were seen after the individual compounds and their combinations, data of each sex were analyzed and presented separately. Alcohol intake differences across the different groups were analyzed using two-way ANOVA for treatment (vehicle vs drug doses) and for sessions (baseline vs ADE) in each sex, with testing our a priori hypothesis that there were effects of ADE, NTN, MSB or their combinations, based on the published findings [7, 8] and our new hypothesis. This was followed by Newman-Keuls post-hoc tests. The accepted level of significance for all tests was p<0.05. All statistical analyses were performed using Statistica (version 5.5, StatSoft Inc, Tulsa, OK).
RESULTS
Effect of MSB on ADE in both male and female mice.
In this experiment, we tested the effect of MSB at 0.3, 1 or 3 mg/kg on alcohol intake. In the males at 4 hours (Figure 1A), two-way ANOVA revealed a significant effect of MSB treatment [F(1,84)=3.5, p<0.05], Session [F(1,84)=11, p<0.01], and a significant interaction between Session and Treatment [F(1,84)=2.8, p<0.05]. Post hoc analysis showed that: (1) the males had more intake in the ADE session on day 28 than the baseline on day 21 [p<0.05]; and (2) the 3 mg/kg MSB-treated males had less intake than the vehicle-treated ones in the ADE session [p<0.01]. In the females at 4 hours (Figure 1B), two-way ANOVA showed a significant effect of MSB treatment [F(1,72)=2.8, p<0.05], Session [F(1,72)=7.1, p<0.01], and a marginally significant interaction between Session and Treatment [F(1,72)=2.7, p=0.05]. Post hoc analysis further showed that: (1) the females had more intake in the ADE session than the baseline [p<0.05]; and (2) the 3 mg/kg MSB-treated females had less intake than the vehicle ones in the ADE session on day 28 [p<0.01]. However, MSB at either 0.3 or 1 mg/kg did not significantly reduce ADE in either males or females. For alcohol preference, there was no significant effect of ADE or acute MSB on preference ratio in either sex, and at 3 mg/kg dose, the results are shown in Table S2.
Figure 1.
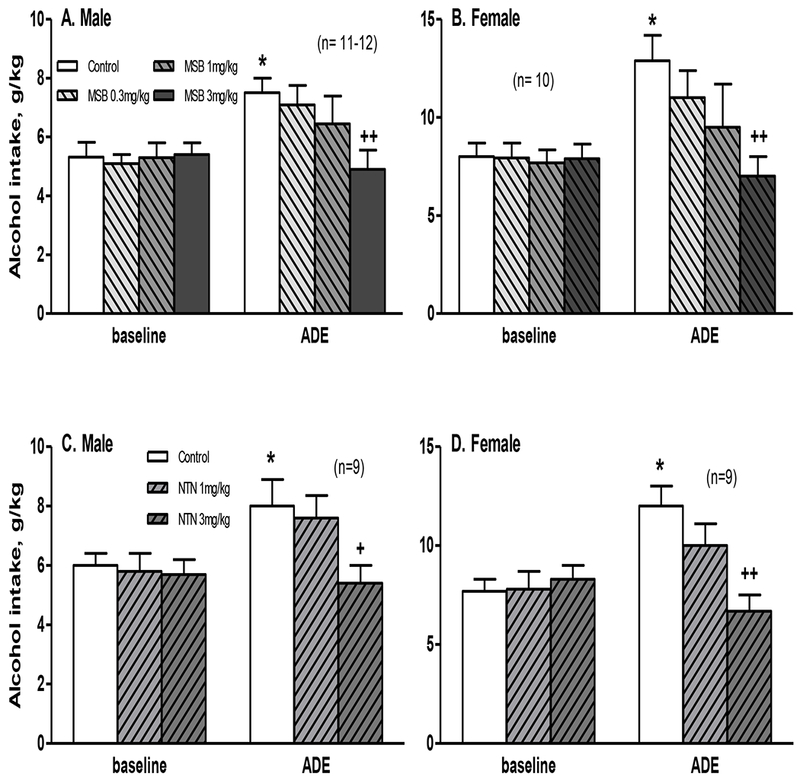
Effects of acute administration of Mesyl Sal B (MSB, 0.3-3 mg/kg) (A, B) or naltrexone (NTN, 1-3 mg/kg) (C, D) on alcohol intake in an alcohol deprivation effect (ADE) model at 4 hours in male and female mice after 1 week of abstinence from 3-week chronic intermittent access alcohol drinking. * p<0.05 vs. control baseline, and + p<0.05 or ++ p<0.01 vs. control ADE.
After 8 or 24 hours, there were no significant effects of acute MSB at 3 mg/kg on either alcohol intake (Table S3) or preference ratio (data not shown) in either sex. Acute MSB at 3 mg/kg did not change water intake after 4, 8 or 24 hours (Table S4).
Effect of NTN on ADE in both male and female mice.
In this experiment, we tested the effect of NTN at 0.5, 1 or 3 mg/kg in both sexes. Acute administration of NTN at 0.5 mg/kg did not show any effect on ADE in either males or females (data not shown).
At 1 and 3 mg/kg doses, the results on alcohol intake are shown in Figure 1. In the males at 4 hours (Figure 1C), two-way ANOVA revealed a significant effect of Session [F(1,48)=4.5, p<0.05] and a marginally significant effect of NTN treatment [F(1,48)=2.8, p=0.06]. To test our a priori hypothesis that NTN would reduce ADE [8], we included the post-hoc results showing that: (1) the males had more intake in the ADE session than the baseline [p<0.05]; and (2) the 3 mg/kg NTN-treated males had less intake than the vehicle-treated ones in the ADE session [p<0.05], though 2-way ANOVA did not show a significant effect of NTN. In the females at 4 hours (Figure 1D), two-way ANOVA revealed a significant effect of NTN treatment [F (1,48) = 4.0, p<0.05], Session [F(1,48)=6.3, p<0.05], and a significant interaction between Session and Treatment [F(1,48)=6.3, p<0.01]. Post hoc analysis showed that: (1) the females had more intake in the ADE session than the baseline [p<0.05]; and (2) the 3 mg/kg NTN-treated females had less intake than the vehicle ones in the ADE session [p<0.01]. For alcohol preference, there was no significant effect of ADE or acute NTN at 3 mg/kg on preference ratio in either sex (data not shown).
After 8 or 24 hours, there were no significant effects of acute NTN at 3 mg/kg on either alcohol intake or preference ratio in either sex (data not shown).
Effect of MSB combined with NTN on ADE in both male and female mice.
Finally, we tested the effect of 0.3 mg/kg MSB combined with 2 doses of NTN (0.5 and 1 mg/kg), and the MSB + NTN doses were chosen based on the above two experiments with each drug alone. Acute administration of MSB at 0.3 mg/kg combined with NTN at 0.5 mg/kg did not reduce ADE in either sex (data not shown).
Combined with a higher dose of 1 mg/kg NTN, the results on alcohol intake are shown in Figure 2. In the males at 4 hours (Figure 2A), two-way ANOVA revealed a significant effect of Session [F(1,44)=5.9, p<0.05] and a marginally significant effect of MSB + NTN treatment [F(1,44)=3.7, p=0.05], and a marginally significant interaction between Session and Treatment [F(1,44)=3.0, p=0.08]. To test our a priori hypothesis that there was an effect of MSB + NTN, we included the post-hoc results showing that: (1) the males had more intake in the ADE session than the baseline [p<0.01]; and (2) the MSB + NTN-treated males had less intake than the vehicle ones in the ADE session [p<0.05], though 2-way ANOVA did not show a significant effect of MSB + NTN. In the females at 4 hours (Figure 2B), two-way ANOVA revealed a significant effect of Session [F(1,32)=15.6, p<0.005], a marginally significant effect of MSB + NTN treatment [F(1,32)=3.4, p=0.06], and a marginally significant interaction between Session and Treatment [F(1,32)=3.3, p=0.07]. To test our a priori hypothesis that there was an effect of MSB + NTN, we included the post-hoc results showing that: (1) the females had more intake in the ADE session than the baseline [p<0.01]; and (2) the MSB + NTN-treated females had less intake than the vehicle ones in the ADE session [p<0.05], though 2-way ANOVA did not show a significant effect of MSB + NTN. For alcohol preference, there was no significant effect of ADE or MSB + NTN on preference ratio in either sex (data not shown).
Figure 2.
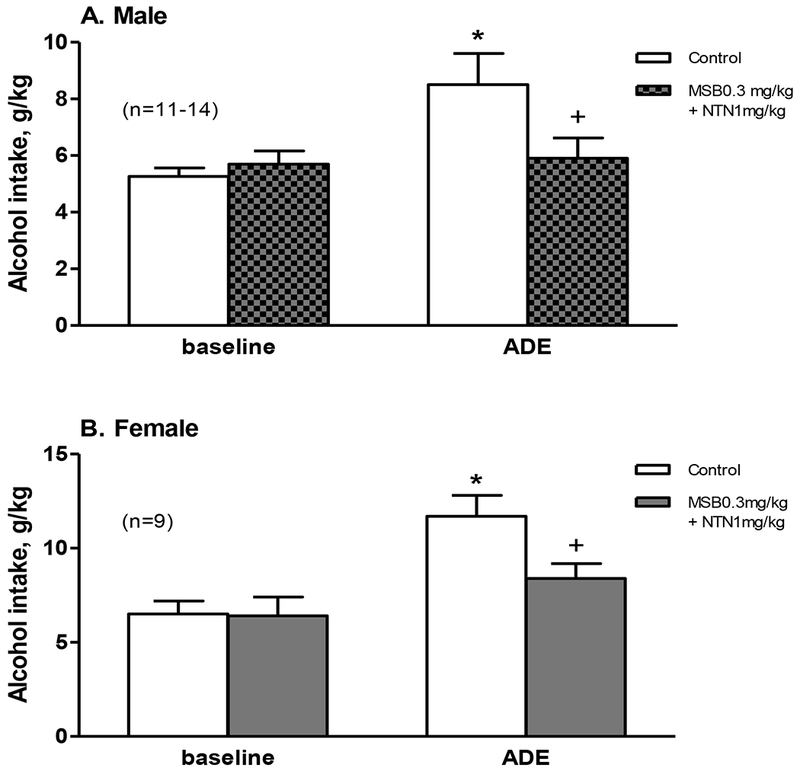
Effects of acute administration of Mesyl Sal B (MSB, 0.3 mg/kg) combined with naltrexone (NTN, 1 mg/kg) on alcohol intake in an alcohol deprivation effect (ADE) model at 4 hours in male (A, n=11-14) and female (B, n=9) mice after 1 week of abstinence from 3-week chronic intermittent access alcohol drinking. * p<0.05 vs. control baseline, and + p<0.05 vs. control ADE.
After 8 or 24 hours, there were no significant effects of the MSB + NTN on either alcohol intake or preference ratio in either sex (data not shown).
DISCUSSION
Our main objective in the present study was to investigate the potential of MSB in reducing relapse-like drinking in mice after 1 week of abstinence from chronic 3-week excessive alcohol drinking. A transient increase in alcohol consumption after a period of imposed abstinence has been labeled as the ADE, which is regarded as an animal model of “relapse” drinking behavior and craving with good predictive validity [6]. In the present study, mice of both sexes displayed the ADE with increased alcohol intake after 1 week of abstinence (Figures 1 and 2). We used systemically active MSB to study these effects because of its resistance to metabolism and thereby longer-lasting effects relative to its parent compound Sal A [4]. We found that acute administration of MSB significantly reduced ADE in both males and females at 3 mg/kg (Figure 1). These results are consistent with previous reports of MSB’s effects on cocaine seeking and alcohol drinking behaviors which were shown to be KOP-r mediated, as the selective KOP-r antagonist nor-BNI blocked these effects [3, 4]. Our new results clearly demonstrate that MSB reduced alcohol “relapse” in a mouse model, which would constitute an interesting extension to the anti-relapse properties of MSB observed in drug-prime induced cocaine seeking behavior [3].
Many studies have demonstrated that classic KOP-r full agonists increase alcohol drinking [13, 14], induce alcohol-seeking behavior in a rat reinstatement model [15] and promote alcohol “relapse” drinking in rat ADE models [14] (see an updated review [16]). Therefore, our new data that the KOP-r full agonist MSB reduced, rather than triggered, “relapse” drinking, presents a scenario that seems to contradict the results of classic KOP-r agonists. After chronic excessive alcohol consumption, however, the endogenous dynorphin (a G-protein and beta-arrestin dependent agonist [17, 18])/KOP-r systems are activated in several neuronal structures. Either the increased release of dynorphin [19] or enhanced KOP-r activity [13] produces sedation, dysphoria, anxiety- and depression-like behaviors that may drive excessive and “relapse” drinking. In support of this concept, dynorphin levels (both mRNA and peptide) and KOP-r activity are found to be increased in the rat central amygdala after chronic alcohol exposure [20–22]. Pharmacologically, selective blockade of KOP-r attenuates alcohol drinking and stress-induced alcohol-seeking in mice [4] and rats [23, 24], though one study did not find any effect of nor-BNI on “relapse” drinking in rats during either early withdrawal or chronic abstinence [14]. Unlike dynorphin, however, MSB does not induce sedation [the locomotor activity test] or anhedonic signs of depression-like behavior [the sucrose preference test] in rats or mice [3, 4], and could act as a G-protein dependent agonist, which was suggested by our recent report [3] but needs further study. In a pilot study, MSB did not cause aversion (the conditioned place aversion test) or anxiety-like behavior (the elevated plus maze test) in rats, but with pro-depressive effects in the forced swim test, suggesting that the compound may have less severe side effects. MSB could possibly compete with excessive dynorphin to bind the KOP-r, thereby reducing beta-arrestin signaling. This could be responsible, at least in part, for the observed reduction of excessive alcohol intake in the ADE mice, as MSB may reverse the dynorphin-enhanced dysphoria and/or anxiety-like behavior presented during alcohol abstinence. Together, these data support the notion that MSB may exhibit different cellular and behavioral properties than dynorphin or classic KOP-r full agonists with drug-seeking effects (such as U50,488H and 1169,593). Therefore, the development of new KOP-r agonists with reduced side effects may have the potential to yield useful compounds for the treatment of drug addiction. Our studies are in line with growing research into the identification of functionally selective KOP-r ligands for the development of anti-addictive compounds [25–27].
Alternatively, MSB, as a KOP-r agonist, decreases dopamine concentrations in the striatum, and acute administration of alcohol leads to increased dopamine levels in the same regions, suggesting that MSB opposes the alcohol effect by modulating the dopamine system [28]. Also, KOP-r activation stimulates the hypothalamic-pituitary-adrenal (HPA) axis in humans and rodents, and thus the enhanced HPA function may reduce alcohol drinking and craving, as demonstrated in humans [29]. This latter possible mechanism is currently under investigation in our laboratories.
In our recent study, the combination of NTN and MSB together displayed a synergistic effect on reducing excessive alcohol intake in both male and female mice. The effect of this combination was alcohol-specific, as demonstrated by the lack of any effect on sucrose or saccharin consumption [4]. Here, we provide further experimental data showing that this MSB + NTN combination is more effective and potentially more beneficial in reducing alcohol “relapse” drinking than either drug alone, as the effects of these combined, low-dose administrations of two compounds on ADE were greater than those of either drug alone. The single-receptor targeted pharmacotherapies (NTN, acamprosate on NMDA receptors) have shown modest therapeutic value over placebo, suggesting a further need for greater efficacy. Our study in rodent ADE model suggests that the combination of MSB with NTN may be more efficacious in treating alcohol relapse than NTN alone. By targeting multiple neurotransmitter receptors implicated in different components of alcohol addiction, combination medications are likely to have enhanced efficacy over the traditional single-receptor approach.
Conclusion.
Consistent with our recent study on cocaine seeking in rats, our new finding here has provided further promising in vivo data showing that the KOP-r agonist MSB, in combination with NTN, may offer a novel strategy to treat alcohol relapse, possibly with less adverse effects.
Supplementary Material
Acknowledgement:
This work was supported by NIH AA021970 (YZ), DA018151 (TEP) and Miriam and Sheldon G. Adelson Medical Research Foundation (MJK).
Footnotes
Conflict of interest: All authors declare that they have no conflicts of interest.
REFERENCES
- 1.Negus SS, Mello NK, Portoghese PS, Lin CE, Effects of kappa opioids on cocaine self-administration by rhesus monkeys, J. Pharmacol. Exp. Ther 282 (1997) 44–55. [PubMed] [Google Scholar]
- 2.Morani AS, Kivell B, Prisinzano TE, Schenk S, Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats, Pharmacol. Biochem. Behav 94 (2009) 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonson B, Morani AS, Ewald AW, Walker L, Kumar N, Simpson D, Miller JH, Prisinzano TE, Kivell B, Pharmacology and anti-addiction effects of the novel κ opioid receptor agonist Mesyl Sal B, a potent and long-acting analogue of salvinorin A, Br. J. Pharmacol 172 (2015) 515–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Crowley RS, Ben K, Prisinzano TE, Kreek MJ, Synergistic blockade of alcohol escalation drinking in mice by a combination of novel kappa opioid receptor agonist Mesyl Salvinorin B and naltrexone, Brain Res. 1662 (2017a) 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burish TG, Maisto SA, Cooper AM, Sobell MB, Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students, J. Stud. Alcohol 42 (1981) 1013–1020. [DOI] [PubMed] [Google Scholar]
- 6.Vengeliene V, Bilbao A, Spanagel R, The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice, Alcohol 48 (2014) 313–320. [DOI] [PubMed] [Google Scholar]
- 7.Holter SM, Spanagel R, Effects of opiate antagonist treatment on the alcohol deprivation effect in long-term ethanol-experienced rats, Psychopharmacology 145 (1999) 360–369. [DOI] [PubMed] [Google Scholar]
- 8.Heyser CJ, Moc K, Koob GF, Effects of naltrexone alone and in combination with acamprosate on alcohol deprivation effect in rats, Neuropsychopharmacology 28 (2003) 1463–1471. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Schwartz B, Giza J, Gross S, Lee F, Kreek MJ, Blockade of alcohol escalation and “relapse” drinking by pharmacological FAAH inhibition in male and female C57BL/6J mice. Psychopharmacology 234 (2017b) 2955–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA, Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% alcohol, Alcohol. Clin. Exp. Res 35 (2011) 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Malley SS, Jaffe A, Chang G, Schottenfeld RS, Meyer RE, Rounsaville BJ, Naltrexone and coping skills therapy for alcohol dependence: a controlled study, Arch. Gen. Psychiatry 49 (1992) 881–887. [DOI] [PubMed] [Google Scholar]
- 12.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE, Neoclerodane diterpenes as a novel scaffold for mu opioid receptor ligands, J. Med. Chem 48 (2005) 4765–4771. [DOI] [PubMed] [Google Scholar]
- 13.Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR, Supersensitive kappa opioid receptors promotes ethanol withdrawal-related behaviors and reduce dopamine signaling in the nucleus accumbens, Int. J. Neuropsychopharmacol 19 (2016) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holter SM, Henniger MS, Lipkowski AW, Spanagel R, Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats, Psychopharmacology 153 (2000) 93–102. [DOI] [PubMed] [Google Scholar]
- 15.Funk D, Coen K, Lê AD, The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav, 4 (2014) 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson RI, Becker HC, Role of the Dynorphin/Kappa Opioid Receptor System in the Motivational Effects of Ethanol, Alcohol. Clin. Exp. Res 41 (2017) 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maillet EL, Milon N, Heghinian MD, Fishback J, Schürer SC, Garamszegi N, Mash DC, Noribogaine is a G-protein biased κ-opioid receptor agonist, Neuropharmacology 99 (2015) 675–688. [DOI] [PubMed] [Google Scholar]
- 18.White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL, The G protein-biased κ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo, J. Pharmacol. Exp. Ther 352 (2015) 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C, A microdialysis profile of dynorphin A(1-8) release in the rat nucleus accumbens following alcohol administration, Alcohol. Clin. Exp. Res 30 (2006) 982–990. [DOI] [PubMed] [Google Scholar]
- 20.D’Addario C, Caputi FF, Rimondini R, Gandolfi O, Del Borrello E, Candeletti S, Romualdi P, Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain, Addict. Biol 13 (2013) 425–433. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Colombo G, Gessa GL, Kreek MJ, Effects of voluntary alcohol drinking on corticotropin-releasing factor and preprodynorphin mRNA levels in the central amygdala of Sardinian alcohol-preferring rats, Neurosci. Lett 554 (2013) 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM, The one-two punch of alcoholism: role of central amygdala dynorphin/kappa-opioid receptors, Biol. Psychiatry 75 (2014) 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker BM, Koob GF, Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence, Neuropsychopharmacology 33 (2008) 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deehan GA Jr., McKinzie DL, Carroll FI, McBride WJ, Rodd ZA, The long-lasting effects of JDTic, a kappa opioid receptor antagonist, on the expression of ethanol-seeking behavior and the relapse drinking of female alcohol-preferring (P) rats, Pharmacol. Biochem. Behav 101 (2012) 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aubé J, Jones SR, Martin TJ, Bohn LM, Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria, Sci. Signal 9 (2016) ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schattauer SS, Kuhar JR, Song A, Chavkin C, Nalfurafine is a G-protein biased agonist having significantly greater bias at the human than rodent form of the kappa opioid receptor, Cell. Signal 32 (2017) 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend EA, Naylor JE, Negus SS, Edwards SR, Qureshi HN, McLendon HW, McCurdy CR, Kapanda CN, do Carmo JM, da Silva FS, Hall JE, Sufka KJ, Freeman KB, Effects of nalfurafine on the reinforcing, thermal antinociceptive, and respiratory-depressant effects of oxycodone: modeling an abuse-deterrent opioid analgesic in rats. Psychopharmacology 234 (2017) 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spanagel R, Herz A, Shippenberg TS, The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study, J. Neurochem 55 (1990) 1734–1740. [DOI] [PubMed] [Google Scholar]
- 29.O’Malley S, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ, Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamic-pituitary-adrenocortical axis, Psychopharmacology 160 (2002) 19–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


