Abstract
Introduction:
Inflammation, or the prolonged resolution of inflammation, contributes to death from tuberculosis. Interest in inflammatory mechanisms and the prospect of beneficial immune modulation as an adjunct to antibacterial therapy has revived and the concept of host directed therapies has been advanced. Such renewed attention has however, overlooked the experience of such therapy with corticosteroids.
Areas covered:
The authors conducted literature searches and evaluated randomized clinical trials, systematic reviews and current guidelines and summarize these findings. They found evidence of benefit in meningeal and pericardial tuberculosis in HIV-1 uninfected persons, but less so in those HIV-1 co-infected and evidence of harm in the form of opportunist malignancy in those not prescribed antiretroviral therapy. Adjunctive corticosteroids are however of benefit in the treatment and prevention of paradoxical HIV-tuberculosis immune reconstitution inflammatory syndrome.
Expert Commentary:
Further high quality clinical trials and experimental medicine studies are warranted and analysis of materials arising from such studies could illuminate ways to improve corticosteroid efficacy or identify novel pathways for more specific intervention.
Keywords: Tuberculosis, corticosteroid, drug therapy, tuberculous meningitis, tuberculous pericarditis
1. Introduction: corticosteroids as a host-directed therapy in tuberculosis
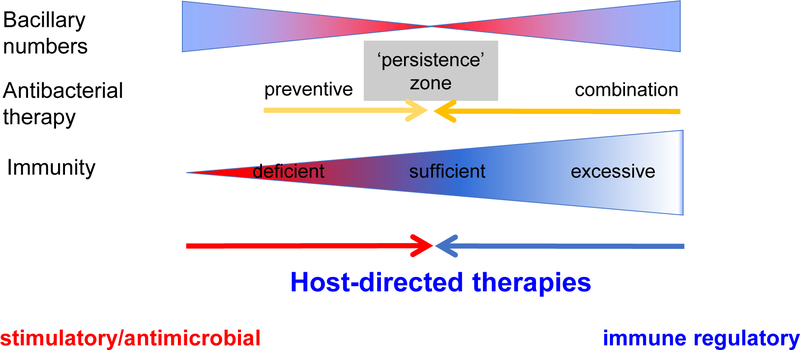
In 2016, 1.7 million people died from tuberculosis, including 0.4 million people co-infected with HIV-1 infection [1]. Delayed and poor diagnosis, social factors and limited access to care leading to late clinical presentation are contributors, as is antimicrobial drug resistant disease. However, the mechanism of death in the majority of cases remains surprisingly poorly understood. Pathological aberrant immunity to Mycobacterium tuberculosis is widely appreciated to contribute to mortality yet is under-researched. This has led to a recent increased interest in the prospect of host-directed therapies that seek either to augment immunity to facilitate bacillary clearance or as an adjunct to offset the inflammation that leads to tissue damage and which may impair antibacterial action (Figure 1 and [2]). Little of this increasing literature, however, acknowledges that by far the greatest clinical experience of such adjunctive anti-inflammatory therapy is with corticosteroids. Corticosteroids have potent anti-inflammatory and immunosuppressive effects and are among the most commonly used drugs to control inflammation in diverse conditions including infection and autoimmune diseases. They are commonly used in many respiratory conditions such as asthma, bronchiolitis, cystic fibrosis and tuberculosis. Over 50 clinical trials had been conducted to evaluate the effect of corticosteroids in pulmonary and extrapulmonary tuberculosis and a meta-analysis on 41 clinical trials that took place prior to September 2012 found that corticosteroids significantly reduced mortality in all forms of tuberculosis [3]. This review discusses such clinical evidence and reflects that the mechanisms remain largely uninvestigated in the context of human disease.
Figure 1.
The concept of host-directed therapies against tuberculosis
2. Corticosteroid efficacy in various disease forms of tuberculosis
2.1. Pulmonary tuberculosis
Globally pulmonary tuberculosis contributes an estimated 81% of all reported tuberculosis cases [1]. The proportion extrapulmonary varies by age, location, HIV-1 status and ascertainment (Table 1). The inflammatory immune response elicited by tuberculosis is responsible for much of the associated structural lung damage and subsequent functional impairment. Use of adjuvant anti-inflammatory agents such as corticosteroids for the treatment of pulmonary tuberculosis has been researched since the 1960s. Adverse pharmacokinetic interactions with tuberculosis treatment and corticosteroid-related side-effects have been areas of concern. Two systematic reviews have been conducted on adjunctive corticosteroid therapy in pulmonary tuberculosis, with contradictory conclusions. A systematic review published in 2003 included 11 RCTs and found that corticosteroids were safe and provide early and sustained benefit as determined by clinical and radiological features of pulmonary tuberculosis in selected patients with advanced disease. However, mortality was not assessed and conclusions were based on findings of small studies only two of which included rifampicin in treatment regimens [4]. A more recent Cochrane review updated the findings of this 2003 systematic review by inclusion of 18 trials (including the 11 trials from the 2003 review). The conclusion on this occasion was that there was no high-quality evidence of benefit in the context of contemporary antimicrobial treatment of tuberculosis, due to lack of large clinical trials using rifampicin in the treatment regimen [5].
Table 1:
| New and relapse | Pulmonary | Extra-pulmonary | Percentage | HIV-1-co-infected | Percentage | |
|---|---|---|---|---|---|---|
| Africa | 1,273,560 | 1,065,327 | 208,233 | 16% | 358,237 | 28% |
| The Americas | 221,008 | 186,940 | 34,068 | 15% | 20,528 | 9% |
| Eastern Mediterranean | 514,449 | 390,367 | 124,082 | 24% | 1,367 | 0% |
| Europe | 219,867 | 187,898 | 31,969 | 15% | 24,871 | 11% |
| South-East Asia | 2,707,879 | 2,291,793 | 416,086 | 15% | 60,245 | 2% |
| Western Pacific | 1,372,371 | 1,268,798 | 103,573 | 8% | 11,526 | 1% |
| GLOBAL | 6,309,134 | 5,391,123 | 918,011 | 15% | 476,774 | 8% |
Notifications of new and relapse tuberculosis cases (for which treatment history is unknown) for WHO regions in 2016 [1]
Only five trials evaluating adjunctive corticosteroid treatment for pulmonary tuberculosis have been conducted since the introduction of multi-drug, rifampicin-containing treatment regimens [6–10]. Only one included patients with HIV-1-associated tuberculosis [10]. Two trials included only patients with endobronchial tuberculosis [7,8], one of which was conducted in in children [7]. Four of these five studies reported no deaths. The only trial that did was that conducted in patients with HIV-1-associated pulmonary tuberculosis [10]. They used a high dose of prednisolone (2.75mg/kg daily for 4 weeks and then tapered over 4 weeks) and showed no survival benefit (18% deaths in prednisolone vs 15% deaths in placebo arms, p = 0.28). Three deaths of 17 in the prednisolone arm were attributed to this therapy, namely sickle cell crisis, hypertensive encephalopathy and meningitis. One retrospective observational study evaluating the role of steroids on 90-day mortality in patients with pulmonary tuberculosis admitted to an intensive care unit with acute respiratory failure showed decreased mortality after adjusting the analysis with the inverse probability of treatment weighted method [11].
In terms of microbiological outcomes, two of the five trials included sputum conversion as an endpoint. One study showed no difference in proportion of patients who were sputum negative at two months (92% vs 93% in those receiving adjuvant steroids compared to no steroids) [6]. A second study showed faster sputum clearance with a greater proportion of negative mycobacterial cultures at 1 month in the prednisolone arm compared to the placebo arm (62% vs 37%, p=0.001), but there were similar proportions of sputum culture negative patients at 2 months (86% vs 85%) [10].
With respect to treatment failure and relapse one study of the five reported treatment failure and showed similar proportions (1% vs 1%) in both arms. The same study showed similar proportions of relapse (patients developing recurrent tuberculosis within two years) with 8.6% in prednisolone group versus 11.7% with no prednisolone [10]. In another study, bacteriological relapse requiring treatment occurred in 3% of prednisolone treated versus 2% with no prednisolone [6]. A third study showed no relapse in either arm during 1–3 years of follow up [9].
In terms of clinical improvement, one study of five reported decreased time until patients became afebrile in the prednisolone treated arm versus the no prednisolone arm (13 days vs 37 days), with a mean decrease of 1.2°C within 72 hours in the prednisolone group versus an increase of 0.2°C in those without prednisolone (p=0.003) [9]. The same study was the only one to report change in weight, showing significantly increased weight in those receiving prednisolone compared to no prednisolone at 70 days after treatment (mean gain 7.2 kg vs 4.2 kg, p = 0.002) [9] and the only one to report duration of hospitalization, with shortened stay (53.4 days vs 71.3 days, p = 0.028) in the prednisolone arm [9].
A small study that included only patients with endobronchial tuberculosis reported no difference in forced vital capacity and forced expiratory capacity at 2 months between the steroid treated group and those without steroids (9.2% vs 10.4% and 13.1% vs 9.4%, respectively) [8].
2.2. Tuberculous meningitis
Tuberculous meningitis (TBM) is the most lethal form of tuberculosis, causing death or disability in almost half of those affected [12]. Since the advent of antitubercular treatment in the 1940s, there has been widespread recognition frequent paradoxical worsening of signs and symptoms upon initiation of treatment. This is particularly acute in TBM where the encased environment of the central nervous system is particularly vulnerable to the response of the host immune system to dead or dying bacteria. Particularly in the context of this paradoxical worsening of symptoms with antitubercular treatment, corticosteroids are the most widely used and researched host-directed therapy in TBM.
In terms of mortality, the first randomized controlled trial (RCT) of corticosteroids in TBM was reported in 1969 and demonstrated a non-significant reduction in mortality with treatment (RR 0.53, 95% CI 0.39 to 1.37; n = 23) [13]. Since then a further six published RCTs have investigated efficacy of corticosteroids in reducing mortality [14–19]. A recent Cochrane review found that in the pooled analysis of these seven trials alongside data from two unpublished trials there were 25% fewer deaths with corticosteroids (RR 0.75, 95% CI 0.65 to 0.87; 1337 participants) [20]. The largest of these studies was a randomized, double-blind placebo-controlled trial conducted in Vietnam in 545 patients with TBM [18]. In this trial treatment with dexamethasone was associated with a reduction in risk of death (RR 0.69, 95% CI 0.52 to 0.92; p = 0.01). Of the nine RCTs conducted since 1969, this is the only study for which long-term outcome data is available. These data demonstrated that at two years there was a non-significant trend towards survival in the dexamethasone group (RR 0.63 versus 0.55; p = 0.07); however at five years’ survival rates were similar (RR 0.54 versus 0.51; p = 0.51) [21]. In a subgroup analysis accounting for grade of TBM, the benefit of dexamethasone tended to persist over time in those with less severe (Grade 1) TBM, but not for more severe (Grade 2 or 3) TBM (5-year survival probability 0.69 versus 0.55, p = 0.07). However, the test of interaction between disease severity and effect size was not statistically significant (p = 0.36).
Of the nine studies included in the Cochrane review, eight reported on neurological disability at 2 to 24 months’ post diagnosis [20]. Pooled analysis of these data demonstrated no effect of corticosteroids (RR 0.92, 95% CI 0.71 to 1.20; 1314 participants). In the aforementioned Vietnamese study, there was also no significant reduction in the proportion of severely disabled patients (34 of 187 (18.2%) among survivors in the dexamethasone group vs 22 of 159 patients (13.8%) in the placebo group, p=0.27); or in the combined outcome of death and severe disability at 9 months (OR 0.81, 95% CI 0.58 to 1.13; p = 0.22) [21]. At five years there was no significant association between dexamethasone treatment and disability status (p=0.32) [18,21]. This trial, as well as the analysis from the recent Cochrane review highlight the lack of evidence to support the efficacy of corticosteroids to prevent disability in TBM.
Where TBM occurs in the context of HIV co-infection, there is a paucity of efficacy data to inform the use of corticosteroids. Of the nine RCTs taking place since 1969, only the 2004 Vietnamese study enrolled patients with HIV-1 co-infection (98 of the 545 patients were co-infected) [18]. Although not powered to test the efficacy of dexamethasone in HIV-1-associated TBM, there was no significant effect of dexamethasone on the combined endpoint of death and disability or on death alone (stratified relative risk of death 0.78; 95% CI 0.59 to 1.04; p = 0.08).
As mentioned earlier, corticosteroids have also been used to treat paradoxical worsening of symptoms upon use of effective antitubercular therapy. Of particular importance in patients with HIV-1 co-infection, is that initiation of antiretroviral therapy (ART) may lead to further severe paradoxical worsening named the immune reconstitution inflammatory syndrome (IRIS). Although in non-CNS IRIS use of prednisolone has been associated with more rapid symptom improvement [22], there is poor evidence of efficacy in TBM-IRIS. In a study that described the pathogenesis of TBM-IRIS, the use of corticosteroids failed to prevent very frequent (close to 50% incidence) IRIS in HIV-1 infected patients with TBM [23].
2.3. Pericardial tuberculosis
Tuberculous pericarditis presents with a broad spectrum of clinicopathological phenotypes. At one extreme, the bacillary load within the pericardium is relatively high, pericardial fluid Mycobacterium tuberculosis cultures become rapidly positive, PCR-based diagnostic techniques are frequently positive and the pericardial involvement often occurs as part of disseminated disease with multi-organ involvement [24,25]. Such multibacillary pericardial tuberculosis has been ascribed to impaired host immunity such as that encountered in people with advanced HIV-1 co-infection with CD4 counts well below 200/mm3. The morbidity and mortality in the multibacillary form may be directly related to the bacillary replication, in conjunction with immune mediated injury [24]. At the other clinicopathological extreme is a paucibacillary condition, in which pericardial fluid cultures are frequently negative despite long incubation, PCR-based techniques are seldom positive, and the aetiological diagnosis of tuberculous pericarditis is assumed, based on positive biomarkers and the absence of alternative diagnoses [26,27].
The paucibacillary form of the disease can manifest as:
accumulation and re-accumulation of exudative inflammatory fluid despite pericardiocentesis;
compromised cardiac function with cardiac tamponade; and
chronic pericardial damage presenting as constrictive pericarditis and death [28].
All of these complications are presumed to be due to the detrimental effects of an inflammatory response to tuberculous antigens within the confined potential pericardial space.
The mainstay of therapy in both forms of disease is antitubercular therapy together with antiretroviral therapy in those HIV-1 co-infected [29]. Where immune mediated injury is dominant, host-directed therapies aimed at attenuating the destructive inflammatory effects, such as glucocorticoids, have long been advocated, although strong evidence of benefit was lacking [30]. There have been four RCTs involving approximately 660 HIV-1 uninfected participants in total that tested whether corticosteroids added to antitubercular therapy reduce the mortality associated with tuberculous pericarditis [31–34]. All participants were either HIV seronegative or, in the studies conducted prior to the HIV pandemic, presumed uninfected. The pooled data from these studies suggests that adjunctive corticosteroids given either via direct intra-pericardial injection or taken orally, may reduce all-cause mortality by approximately 20%, risk ratio (RR) 0.80, 95% CI 0.59–1.09, with a significant reduction in pericarditis related death RR 0.39 95% CI 0.19–0.80 [35]. The cumulative data from the three trials that have evaluated the impact of steroids on mortality in HIV-1 co-infected participants (80% of whom were not on antiretroviral therapy) did not demonstrate any benefit [35].
The impact of corticosteroids on morbidity due to tuberculous pericarditis has been more difficult to evaluate from the available evidence, for two main reasons. First, there have been varying methods of, and time to, outcome assessments in the different studies. Second, there have also been differing rates of evacuation of pericardial fluid prior to the administration of steroids. However, in the largest (1400 patient) randomized placebo-controlled study to date, despite the lower than expected overall incidence of constrictive pericarditis (possibly attributable to the high proportion of HIV-1 co-infected participants), the rate of pericardial constriction was reduced by 44% (4.56/100 pt. years vs. 2.58/100 pt. years) in patients administered prednisolone. The authors cautioned in their study, that the rate of AIDS related malignancies amongst participants with advanced HIV-1 not prescribed combination ART was slightly but significantly increased compared to controls [34].
2.4. Other forms of tuberculosis
Other common sites of extrapulmonary tuberculosis include lymph nodes and pleura. The use of adjunctive corticosteroids in the management of these clinical syndromes is not routine. The available evidence around corticosteroid use in tuberculous lymphadenitis, pleurisy and peritonitis is presented below. Of note is that most of the available literature for these clinical syndromes is observational in nature, often conducted before contemporary antitubercular chemotherapy became standard. Studies are often limited by small sample size and by the use a variety of corticosteroid regimens making inter-study comparison difficult. The strength of evidence is therefore not great.
Controlled trials of corticosteroid use in tuberculous lymphadenitis exist, but these have been conducted in children with a focus on mediastinal lymphadenopathy, seen at bronchoscopy or on chest X-ray [36,37]. Nemir et al. studied 117 children in a placebo-controlled, blinded and randomized study published in 1965 [37]. Serial bronchoscopies were performed by the same investigator and judgements were made (while blinded to treatment group) regarding progression of disease as an ordinal categorical variable. Improvement was reported in 67.2% of the prednisone group and in 45.7% of the placebo group (p<0.05). Of note is that stratification of the results, while insufficiently powered, suggested that earlier initiation of corticosteroids led to a greater beneficial effect. A randomized placebo controlled trial in 120 Indian adults on standard TB treatment showed improved symptom relief (p<0.001), less frequent localized complications (abscess or sinus formation or new node formation) (p<0.001) and more complete resolution of cervical lymphadenopathy at six months of follow up (p<0.001) in patients receiving 4 weeks of prednisolone in addition to TB treatment compared to patients on placebo [11,38].
In the post-rifampicin era, the first prospective, placebo-controlled study of adjuvant oral corticosteroids in tuberculous pleurisy showed that in participants prescribed corticosteroids, symptom relief was seen at a mean of 2.4 days compared to 9.2 days in the placebo group (p<0.05) [39]. In addition, complete resolution of pleural effusion on chest X-ray was seen in the corticosteroid group at 54.5 days, compared to 123.2 days in the placebo group (p<0.01). However, it is noteworthy that thoracocentesis in this study was only performed for diagnostic purposes and therefore limited to less than 50 ml. A subsequent trial, published in 1996 adopted a similar protocol but all participants, irrespective of study group, received therapeutic thoracocentesis [40]. The authors noted that once complete aspiration of effusion was achieved, participants in both groups experienced a marked immediate improvement in clinical symptoms. There was no difference in improvement of clinical symptoms, residual pleural thickness, or improvement in pulmonary function between the groups. Overall the evidence, of moderate quality, therefore suggests that when early complete drainage of pleural fluid is performed alongside effective antitubercular chemotherapy, oral corticosteroids do not provide additional benefit in adults with pleural tuberculosis.
A subsequent study from 2004 investigated whether similar findings applied in HIV-1 co-infected adults with pleural TB [41]. The authors did not specify what percentage of their participants were prescribed ART or whether therapeutic thoracocentesis was performed. Results favoured corticosteroids, showing a faster resolution of symptoms in this group, although there was no effect on mortality. However the authors observed a higher incidence of Kaposi’s Sarcoma in the follow up of those prescribed corticosteroids. A systematic review and meta-analysis published in 2013 that assessed mortality in all forms of tuberculosis, found there was no evidence for a beneficial effect on survival (RR 0.92, 95% CI 0.65–1.32) when adjuvant corticosteroids were prescribed for pleural tuberculosis [3].
No studies have adequately estimated the effect of corticosteroids on mortality from peritoneal tuberculosis. A controlled trial of 47 participants with peritoneal TB was conducted in the 1960s with prednisone given to alternate patients over a four-month period [42]. Three of the 24 controls developed intestinal obstruction due to adhesive bands, while this did not occur in any of those in the steroid group (p=0.234). A further study of peritoneal tuberculosis was reported in 1998, but again the evidence provided is limited by retrospective design, small sample size, and inclusion of participants with serious comorbidities in the control group [43]. The results, however, suggested a trend towards a reduction in pain, obstruction and need for laparotomy in those prescribed adjunctive corticosteroids.
2.5. Role in paradoxical deterioration in tuberculosis and HIV-tuberculosis
Paradoxical deterioration in tuberculosis is characterized by worsening of pre-existing tuberculous lesions, or the appearance of new tuberculous lesions despite effective anti-tuberculosis treatment in patients who had demonstrated initial improvement during therapy [44]. In HIV-1 infection, where paradoxical worsening occurs following the introduction of ART the phenomenon is known as TB-IRIS. Two types of IRIS are recognized: paradoxical and unmasking. Paradoxical TB-IRIS manifests with new or recurrent tuberculosis symptoms or signs in patients being treated for tuberculosis during early ART, and unmasking TB-IRIS is characterized by an exaggerated, unusually inflammatory initial presentation of tuberculosis during early ART [45]. Common clinical features include fever, lymphadenitis and pulmonary manifestations [46]. A systematic review in HIV-1 co-infected patients with known tuberculosis reported a pooled incidence of paradoxical-IRIS of 18% (95%, CI 16–21%) [47]. In HIV-1 uninfected people paradoxical worsening is thought to be less common, with some studies reporting an incidence as low as 2.6% [48]. Although rarely fatal, even in the context of HIV where mortality of TB-IRIS is estimated to be 2% (95% CI 1–3%), TB-IRIS leads to significant morbidity with 25% of cases requiring prolonged hospitalization [47,49].
In HIV-1 uninfected patients research into the use of corticosteroids has focused on their use to modulate the host inflammatory response described in tuberculosis pathogenesis. Their use as treatment in the context of a paradoxical reaction per se is less well defined. Good evidence for the use of corticosteroids in paradoxical reactions is therefore solely in the context of TB-IRIS. These recommendations are based on results of a randomized double-blind placebo-controlled trial conducted in South Africa [22]. In this study, 110 patients with HIV-tuberculosis and symptoms consistent with standardized case definitions for paradoxical-IRIS were enrolled. Patients randomized to the treatment arm received a total of 4 weeks of prednisone (1.5mg/kg/day for 2 weeks followed by 0.75mg/kg/day for 2 weeks). Results demonstrated a significant reduction in hospital stay compared to placebo (median hospital days 0 (IQR 0–3) and 3 (IQR 0–9) respectively p=0.04). There was also more rapid improvement in i) symptoms in the treatment arm at 2 weeks (p=0.001) and 4 weeks (p=0.03), as well as ii) chest radiograph appearances at 2 weeks (p=0.002) and 4 weeks (p=0.02). To further investigate inflammatory markers in TB-IRIS blood specimens were drawn at 0, 2 and 4 weeks. Results demonstrated a significant decrease in serum concentration of C-reactive protein, interleukin (IL-) 6, IL-10, IL-12p40, tumour necrosis factor-α, interferon-γ, and interferon γ-inducible protein 10 (CXCL-10) in participants on prednisone suggesting that the beneficial effect of corticosteroids in TB-IRIS was mediated at least in part through the attenuation of pro-inflammatory cytokine responses [50]. A previous study had demonstrated these same cytokines are differentially elevated in the plasma of HIV-associated tuberculosis patients who develop TB-IRIS compared to control subjects who start ART and do not develop the condition [51].
In TBM, IRIS is more frequent and more often fatal. One study reported an incidence of 47% and a mortality rate of 25% with severe disability at 9 months observed in 23% of those who survived [52]. In an observational study, patients who developed TBM-IRIS demonstrated an increase in markers of cerebrospinal fluid inflammation despite corticosteroids. Even when corticosteroids were increased in dose, or restarted in the context of TBM-IRIS, there was relatively little change in inflammatory mediators [53]. Similarly poor modulation of the cytokine response in TBM has been observed previously [54], and this raises the question whether corticosteroids are sufficiently potent as a treatment for paradoxical TBM-IRIS.
Trials investigating the timing of ART in HIV-1 associated tuberculosis recommend early ART initiation to improve survival, especially in patients with low CD4 counts, which in turn causes an increased incidence of paradoxical TB-IRIS [55–57]. One randomized placebo-controlled trial has tested prednisone co-administration at ART initiation in patients at high risk of TB-IRIS (CD4 count <100 cells/μL and on tuberculosis treatment for less than one month). This showed a decreased risk of developing paradoxical TB-IRIS in the prednisone versus placebo arm, with a relative risk of 0.70 (95% CI 0.51–0.96) [Meintjes et al, NEJM accepted]. There was no increased risk of infections or other adverse events in patients receiving prednisone prophylaxis.
3. Adverse effects of corticosteroids in tuberculosis
3.1. Adverse events and side-effects
Corticosteroid related adverse events in people with tuberculosis although well-recognised are inconsistently reported across studies. One study that captured this in detail used high doses of corticosteroids (2.75mg/kg per day for 4 weeks and then tapered over 4 weeks) [41]. There were similar numbers of adverse events between the steroid arm and those not receiving steroids, but significantly increased steroid related side-effects; (i) hyperglycaemia in 10% vs 3%, p=0.036, (ii) fluid retention in 30% vs 4%, p<0.001 and (iii) hypertension in 13% vs 4%, p=0.039. Interestingly there were two cases of Kaposi’s sarcoma in the group who did not receive steroids and none in the steroid group. There were no differences between groups in occurrence of candidiasis, Herpes simplex, Herpes zoster, pneumonia or urinary tract infections. A higher proportion of patients on steroids developed hepatitis, although this was not statistically significant with 13% vs 6%, p = 0.09 [10]. Another study similarly showed no overall difference in occurrence of adverse events between groups, but significantly increased steroid related side-effects with (i) gastro-intestinal disturbances (6% vs 0.3%, p < 0.001), (ii) swelling of feet or face (18% vs 0.9%, p < 0.001) and hyperglycaemia (0.6% vs 0%, p value not reported). The risk of HIV-1 associated malignancy associated with adjunctive corticosteroid therapy in persons not prescribed antiretroviral therapy has already been highlighted [34,41].
3.2. Corticosteroid interaction with antitubercular drugs
Concerning corticosteroid effects on antitubercular drug levels, isoniazid concentrations are reduced by prednisolone therapy by two mechanisms: a) enhanced rate of acetylation and b) increased renal clearance. However, rifampicin increases steroid metabolism and decreases its half-life by induction of liver enzymes [58,59]. Isoniazid concentrations are therefore maintained if rifampicin is co-administered with prednisolone in rapid acetylators, but not in slow acetylators (rate of acetylation is genetically determined, with people classified as either rapid or slow acetylators) [60]. Prednisone increases pyrazinamide concentration in pleural fluid (in the absence of rifampicin), but does not affect serum pyrazinamide concentrations [61]. Importantly, rifampicin decreases corticosteroid concentrations dramatically (~50%) by induction of the CYP3A4 enzyme complex which metabolizes corticosteroids and results in increased plasma clearance [62].
4. Mechanism of corticosteroid action in tuberculosis
Although there is a significant body of research on the molecular and cellular actions of corticosteroids, less is known about their mechanisms in disease-specific contexts. The general mode of action of endogenous corticosteroids has been studied extensively and there are three basic mechanisms that inhibit inflammatory signals:
regulation of gene expression via interaction with glucocorticoid-responsive element (GRE).
direct protein-protein interference with transcription factors such as NF-κB.
cellular signal transduction cascades with secondary messengers.
Transcriptional regulation of inflammation begins with corticosteroid binding to glucocorticoid receptor (GR), leading to conformational changes and the release of heat-shock protein 90 complex from the GR [63]. This allows the steroid-GR complex to translocate to the nucleus where it binds the GRE [64] and subsequently co-activators and co-repressors are recruited. All co-activator molecules have intrinsic histone acetyltransferase activities and may modify chromatin structures to initiate transcription of anti-inflammatory genes such as annexin-1, IL-10 and IκB-α (inhibitor of NF-κB) [65]. An absence of co-activators was found to result in excessive local and systemic inflammatory responses and delayed bacterial clearance in mice [66]. Conversely, the mechanisms by which co-repressors silence gene expression are less well understood. It has been reported that the steroid-GR complex could interact with negative GRE to suppress transcription of genes associated with hypothalamic-pituitary-adrenal axis function and inflammation [67], the former of which is also linked to the undesirable side effects of corticosteroids. It has also been proposed that transcription repression occurs by phosphorylating RNA polymerase II [68] and by competing with transcriptional factors (NF-κB and AP-1) for binding to co-activators [69]. Nevertheless, conflicting data have been reported and further investigations are needed to clarify the mechanisms and specific conditions in which repression occurs and if it might be more, or less, preferable as a mechanism to target in corticosteroid therapy than activation.
The corticosteroid-GR complex can also directly interact with NF-κB, AP-1 or other immunomodulatory transcription factors by functioning as an antagonist to physically interfere with their activities [70,71], thereby inhibiting the transcription of a wide array of inflammatory mediators including cytokines, chemokines, adhesion molecules and eicosanoids [72]. This repression typically occurs at lower steroid concentrations than that required for GRE-dependent transcription activation [73]. The binding of GR to the transcription factor alone is insufficient to mediate the repression and requires co-activator molecules such as CBP (CREB-binding protein) for maximal activity and to enhance a mutual transcriptional antagonism [74–76].
Finally, corticosteroids can also exert a rapid cellular effect independent of genomic regulation, which is typically delayed [77]. This has been observed across different tissue compartments, including the rapid suppression of insulin release in the pancreas [77], induction of endothelial nitric oxide in the heart [78], and suppression of histone deacetylase complex to facilitate differentiation of pre-adipocytes into adipocytes [79]. The acute effect is suggested to be initiated by either passive diffusion or receptor-mediated endocytosis of the corticosteroids through the plasma membrane [80,81]. Following intracellular binding to G protein-coupled receptors, various cellular signal transduction pathways and second messenger signaling pathways became activated [82]. The mechanisms through which these signaling pathways induce an anti-inflammatory response are not known and it is also not clear how these pathways are regulated.
Corticosteroids have a profound effect on immune cells as the GR is expressed ubiquitously. In dendritic cells, corticosteroids suppress their maturation with reduced expression of antigen-presenting molecules and direct a subset of cells to differentiate into monocyte/macrophage-like phenotype [83]. Corticosteroids can also reprogram dendritic cells to be tolerogenic, where they become hypo-responsive to T cells and induce CD4+/CD8+ Treg [84]. Similarly, corticosteroids promote monocyte differentiation into macrophage with anti-inflammatory phenotype via inhibition of caspase activities [85] and increased phagocytosis of apoptotic cells [86]. Corticosteroids have also been implicated to regulate T cell differentiation and function by skewing towards a Th2 phenotype while suppressing Th1-mediated immunity through induction of IL-10 secreting Treg [87,88].
Intriguingly, recent studies in zebrafish as well as two Vietnamese clinical studies have identified that a single nucleotide polymorphism in the LTA4H (Leukotriene A4 Hydrolase) gene associates with the treatment response to dexamethasone in TBM patients [12,89]. Given that leukotrienes and other members of the eicosanoid family are implicated in the inflammatory pathogenesis of tuberculosis, it is possible that this polymorphism might have an effect on the GRE of the LTA4H gene, so as to allow better recognition and binding by dexamethasone to suppress LTA4H transcription.
5. Expert commentary
In pulmonary tuberculosis, although there is some evidence for improvement in selected clinical parameters with corticosteroid therapy (reduction in fever, increased weight, shortened duration of hospitalization), these results are all based on findings of a single study in which randomization procedures are not adequately reported to fully assess risk of bias [9]. It could be concluded there is no high-quality evidence showing decreased mortality or sustained improved microbiological or clinical outcomes when comparing adjunctive corticosteroid treatment to placebo in pulmonary tuberculosis.
In TBM there is good evidence for the use of corticosteroids as an adjunct to antitubercular therapy in HIV-1 uninfected people. Since no studies have compared the dose and preparation of corticosteroids, treatment regimens should be based upon those found to be effective in published clinical trials. In the largest study of TBM described in this review [18], patients with mild disease received intravenous dexamethasone 0.3mg/kg/day × 1 week, 0.2mg/kg/day × 1 week and then four weeks of oral tapering therapy. In those with more severe disease intravenous therapy was continued for four weeks (starting dose 0.4mg/kg/day reducing by 0.1mg/kg each week) followed by four weeks of oral tapering therapy. Equivalent doses should be in the form of preparations available locally to reflect this recommendation (Table 2). An ongoing study more rigorously tests whether there is substantial benefit in HIV-1 co-infected tuberculosis patients.
Table 2:
Equipotency and half-life of steroids commonly prescribed:
| Equivalent glucocorticoid dose in mg | Anti-inflammatory potency compared to hydrocortisone | Duration of action in hours | |
|---|---|---|---|
| Hydrocortisone | 20 | 1 | 8–12 |
| Prednisone | 5 | 4 | 12–36 |
| Prednisolone | 5 | 4 | 12–36 |
| Dexamethasone | 0.75 | 30 | 36–54 |
| Betamethasone | 0.6 | 30 | 36–54 |
Adapted from Adrenal Cortical Steroids. In Drug Facts and Comparisons. 5th ed. St. Louis, Facts and Comparisons, Inc.:122–128, 1997
On the basis of the available data based on randomized controlled trials, it is reasonable to treat HIV-1 uninfected patients with a diagnosis of pericardial TB with a high dose of oral corticosteroids tapering over eight weeks with an anticipated modest benefit on mortality [35], constrictive pericarditis [90] and recurrent accumulation of pericardial fluid [35]. Although the evidence is less certain, patients who are HIV infected who are prescribed antiretroviral therapy (ART) may derive similar benefit. On the other hand, there is little evidence of benefit amongst HIV infected patients with tuberculous pericarditis not receiving ART and indeed data suggesting harm.
Alongside effective antitubercular chemotherapy, oral corticosteroids do not provide additional benefit in adults with pleural TB. We could not find randomized studies of corticosteroid use in tuberculous lymphadenitis of adults. Drainage of fluctuant lymph nodes appears beneficial in paradoxical deterioration involving the lymph nodes [44].
ART timing trials in HIV-associated tuberculosis indicate early introduction of ART improves survival in several forms of tuberculosis, especially in patients with a low CD4 T-cell count. Guidelines recommend initiation of ART as early as possible, but within two weeks after initiating TB treatment in patients with CD4 counts <50 cells/μl[91,92]. Having a low CD4 count and early initiation of ART are both risk factors for development of paradoxical TB-IRIS it is therefore feasible that TB-IRIS may be observed more frequently in the future. Evidence for the use of corticosteroids exists in the context of paradoxical TB-IRIS where prednisone prescribed for 4 weeks following onset of symptoms reduces hospital admission and improves symptom severity. A recent clinical trial evaluating the role of prednisone in the prevention of paradoxical TB-IRIS has demonstrated a reduction in frequency of TB-IRIS when commencing ART.
5.1. Current recommendations for use of adjunctive steroids in TB treatment
In the 2017 update on treatment of drug susceptible tuberculosis the WHO examined the role of steroids in TB pericarditis and tuberculous meningitis. They issued a conditional recommendation (very low certainty of evidence) for the use of steroids in pericardial tuberculosis and a strong recommendation (moderate certainty in the evidence) for the use of steroids in tuberculous meningitis. The optimal formulation, dose and duration of treatment still needs to be determined. European [93] and South African Western Cape Provincial guidelines [94] recommend the use of steroids to prevent and treat patients with TB-IRIS. This is a complex clinical condition and drug resistant TB, hepatitis B co-infection and Kaposi’s sarcoma need to be excluded prior to steroid therapy.
6. Five-year view
The review highlights that clinical trials have tended to have small sample sizes, heterogeneous endpoints, have been subject to bias and varying antitubercular, and other supportive treatment, regimes. Future trials of steroids and indeed other anti-inflammatory host-directed therapies might best conform to minimal guidelines on desirable data to collect. Trials are also an opportunity to create biobanks of materials in which to investigate potential beneficial effects and mechanisms of action in reverse translational approaches. A number of small studies of host directed therapies other than corticosteroids are underway. A future issue, given the documented efficacy of corticosteroids for some indications, will be comparing or adding those agents to corticosteroids where rationale exists.
The treatment of paradoxical reactions in patients without HIV-1 co-infection remains under researched in the field of tuberculosis. Research in this field would improve understanding of the host inflammatory response not only in the context of paradoxical reactions, but also in the non-paradoxical immune response in tuberculosis. Using new techniques to better describe mechanisms at the transcriptomic and cellular level will lead to better ability to control the host inflammatory response and in turn better outcomes in tuberculosis. Understanding risk factors for the development of paradoxical reactions, including genetic influences, may allow the development and implementation of individualized therapies in tuberculosis.
Research to optimise treatment of TBM should focus on effective antimicrobial therapy and its central nervous system penetration as well as host-directed immune interventions to either enhance protective immunity or regulate pathological tissue-damaging immunity [2]. A larger study is currently underway to address the question of efficacy of corticosteroids in HIV-associated TBM (ClinicalTrials.gov Identifier: NCT03092817). Questions regarding the effect of corticosteroids on long-term survival remain unanswered. Corticosteroids also appear at best only partially able to offset inflammation in neurological TBM-IRIS and the severity of this condition warrants other host-directed approaches. Pre-treatment biomarkers that predict response to host-directed therapies including corticosteroids in TBM would be of great benefit. An observational study documenting a transcriptional signature pre-development of IRIS suggests that genetic variation may predict host immune response [23]. Recent work has also explored the role of the LTA4H genotype whose variation has been linked to distinct phenotypes in pre-treatment CSF markers, survival as well as dexamethasone responsiveness [89,95]. A Leukotriene A4 Hydrolase Stratified Trial of Adjunctive Corticosteroids for HIV-uninfected Adults with Tuberculous Meningitis (ClinicalTrials.gov Identifier: NCT03100786) is underway, and better understanding of the pathological processes that influence the host inflammatory response in TBM will further the development of individualized host-directed therapies including effective use of corticosteroids leading to better outcomes in TBM.
Many questions remain to be addressed on the basic mechanisms of action of corticosteroids and how they confer protective effects as an adjunctive therapy in tuberculosis. For instance, do different corticosteroids provide the same effect and do they all share the same mechanism of action? A range of different corticosteroids (predominantly dexamethasone and prednisone or its active metabolic form prednisolone) have been used in tuberculosis. While all corticosteroids are similar in molecular structures, they differ in binding affinity to GR and half-life of functional activities [96]. It is not known if these differences also affect their mode of action and if they inherently target different pro- or anti-inflammatory mediators. Detailed investigation of these mechanisms could provide the basis of understanding on how corticosteroids exert their protective effects in TB and facilitate future regimen design and selection.
Key issues.
Corticosteroids are by far the most widely used adjunctive therapy in TB, although have received little notice in recent literature reviving the idea of adjunctive immunotherapies for tuberculosis in the form of host-directed therapy.
Clinical trials have tended to have small sample sizes, heterogenous endpoints, have been subject to bias and varying antitubercular, and other supportive treatment, regimes.
There is no clear distinction between corticosteroid use at the start of TB therapy or as an intervention should deterioration occur
The assumed equivalence of corticosteroid preparations has not been investigated and dosage and length of treatment not standardised
The specific mechanism by which steroids may be effective in tuberculosis is poorly understood. There is some evidence that their effects may be host genotype specific, raising the possibility that therapy could be personalised.
There is evidence that adjunctive corticosteroids reduce mortality in HIV-1 uninfected patients with pericardial or meningeal tuberculosis.
In HIV-1 co-infected patients corticosteroids relieve symptoms and reduce risk of TB-IRIS. However, benefit in other disease forms in HIV-1 co-infected patients is lacking and they should not be prescribed to those not receiving antiretroviral therapy
Further Clinical Trials and Experimental Medicine studies have the potential to define and refine clinical approaches and are also an opportunity to learn via reverse translational approaches.
Acknowledgments
Funding
This manuscript has received funding from Cancer Research UK (FC00101218), European and Developing Countries Clinical Trials Partnership (SRIA2015–1065), Foundation for the National Institutes of Health (WILKI16PTB), Francis Crick Institute (FC0010218), Research Councils UK Medical Research Council (FC0010218), U.S. Department of Health and Human Services National Institutes of Health National Institute of Allergy and Infectious Diseases (U01AI115940), South African Medical Research Council, Wellcome (104803, 203135, FC00110218)
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Global Tuberculosis Report 22nd edition. World Health Organisation, Geneva, 2017 [Google Scholar]
- 2.Wilkinson RJ. Host-directed therapies against tuberculosis. Lancet. Respir Med, 2(2), 85–87 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Critchley JA, Young F, Orton L, Garner P. Corticosteroids for prevention of mortality in people with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis, 13(3), 223–237 (2013). [DOI] [PubMed] [Google Scholar]; **This is an up to date systematic review of the use of corticosteroids as adjuncts to the chemotherapy of tuberculosis
- 4.Smego RA, Ahmed N. A systematic review of the adjunctive use of systemic corticosteroids for pulmonary tuberculosis. Int J Tuberc Lung Dis, 7(3), 208–213 (2003). [PubMed] [Google Scholar]
- 5.Critchley JA, Orton LC, Pearson F. Adjunctive steroid therapy for managing pulmonary tuberculosis. Cochrane Database Syst Rev, (11), CD011370 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Study of chemotherapy regimens of 5 and 7 months’ duration and the role of corticosteroids in the treatment of sputum-positive patients with pulmonary tuberculosis in South India. Tubercle, 64(2), 73–91 (1983). [DOI] [PubMed] [Google Scholar]
- 7.Toppet M, Malfroot A, Derde MP, Toppet V, Spehl M, Dab I. Corticosteroids in primary tuberculosis with bronchial obstruction. Arch Dis Child, 65(11), 1222–1226 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park IW, Choi BW, Hue SH. Prospective study of corticosteroid as an adjunct in the treatment of endobronchial tuberculosis in adults. Respirology, 2(4), 275–281 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Bilaceroglu S, Perim K, Buyuksirin M, Celikten E. Prednisolone: a beneficial and safe adjunct to antituberculosis treatment? A randomized controlled trial. Int J Tuberc Lung Dis, 3(1), 47–54 (1999). [PubMed] [Google Scholar]
- 10.Mayanja-Kizza H, Jones-Lopez E, Okwera A et al. Immunoadjuvant Prednisolone Therapy for HIV-Associated Tuberculosis: A Phase 2 Clinical Trial in Uganda. J Infect Dis, 191(6), 856–865 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]; *A well-conducted clinical trial of the adjunctive corticosteroids in HIV-1 associated pulmonary tuberculosis
- 11.Yang JY, Han M, Koh Y et al. Effects of Corticosteroids on Critically Ill Pulmonary Tuberculosis Patients With Acute Respiratory Failure: A Propensity Analysis of Mortality. Clin Infect Dis, 63(11), 1449–1455 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Thuong NTT, Heemskerk D, Tram TTB et al. Leukotriene A4 Hydrolase Genotype and HIV Infection Influence Intracerebral Inflammation and Survival From Tuberculous Meningitis. J Infect Dis, 215(7), 1020–1028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Toole RD, Thornton GF, Mukherjee MK, Nath RL. Dexamethasone in tuberculous meningitis. Relationship of cerebrospinal fluid effects to therapeutic efficacy. Ann Intern Med, 70(1), 39–48 (1969). [DOI] [PubMed] [Google Scholar]
- 14.Girgis NI, Farid Z, Kilpatrick ME, Sultan Y, Mikhail IA. Dexamethasone adjunctive treatment for tuberculous meningitis. Pediatr Infect Dis J, 10(3), 179–183 (1991). [DOI] [PubMed] [Google Scholar]
- 15.Kumarvelu S, Prasad K, Khosla A, Behari M, Ahuja GK. Randomized controlled trial of dexamethasone in tuberculous meningitis. Tuber Lung Dis, 75(3), 203–207 (1994). [DOI] [PubMed] [Google Scholar]
- 16.Chotmongkol V, Jitpimolmard S, Thavornpitak Y. Corticosteroid in tuberculous meningitis. J Med Assoc Thai, 79(2), 83–90 (1996). [PubMed] [Google Scholar]
- 17.Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics, 99(2), 226–231 (1997). [DOI] [PubMed] [Google Scholar]
- 18.Thwaites GE, Nguyen DB, Nguyen HD et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med, 351(17), 1741–1751 (2004). [DOI] [PubMed] [Google Scholar]; **A landmark clinical trial of corticosteroids in TBM
- 19.Malhotra HS, Garg RK, Singh MK, Agarwal A, Verma R. Corticosteroids (dexamethasone versus intravenous methylprednisolone) in patients with tuberculous meningitis. Annals of Tropical Medicine and Parasitology, 103(7), 625–634 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev, 4, CD002244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torok ME, Nguyen DB, Tran TH et al. Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS One, 6(12), e27821 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meintjes G, Wilkinson RJ, Morroni C et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. Aids, 24(15), 2381–2390 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; *Clinical trial evidence that corticosteroids aid the resolution of HIV-tuberculosis associated immune reconstitution inflammatory syndrome
- 23.Marais S, Lai RPJ, Wilkinson KA, Meintjes G, O’Garra A, Wilkinson RJ. Inflammasome Activation Underlying Central Nervous System Deterioration in HIV-Associated Tuberculosis. J Infect Dis, 215(5), 677–686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasipanodya JG, Mubanga M, Ntsekhe M et al. Tuberculous Pericarditis is Multibacillary and Bacterial Burden Drives High Mortality. EBioMedicine, 2(11), 1634–1639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ntsekhe M, Matthews K, Syed FF et al. Prevalence, hemodynamics, and cytokine profile of effusive-constrictive pericarditis in patients with tuberculous pericardial effusion. PLoS One, 8(10), e77532 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syed FF, Mayosi BM. A modern approach to tuberculous pericarditis. Prog Cardiovasc Dis, 50(3), 218–236 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Pandie S, Peter JG, Kerbelker ZS et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-gamma in a high burden setting: a prospective study. BMC Med, 12, 101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ntsekhe M, Mayosi BM. Tuberculous pericarditis with and without HIV. Heart Fail Rev, 18(3), 367–373 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation, 112(23), 3608–3616 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Ntsekhe M, Wiysonge C, Volmink JA, Commerford PJ, Mayosi BM. Adjuvant corticosteroids for tuberculous pericarditis: promising, but not proven. QJM, 96(8), 593–599 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Strang JI, Kakaza HH, Gibson DG, Girling DJ, Nunn AJ, Fox W. Controlled trial of prednisolone as adjuvant in treatment of tuberculous constrictive pericarditis in Transkei. Lancet, 2(8573), 1418–1422 (1987). [DOI] [PubMed] [Google Scholar]
- 32.Strang JI, Nunn AJ, Johnson DA, Casbard A, Gibson DG, Girling DJ. Management of tuberculous constrictive pericarditis and tuberculous pericardial effusion in Transkei: results at 10 years follow-up. QJM, 97(8), 525–535 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Reuter H, Burgess LJ, Louw VJ, Doubell AF. The management of tuberculous pericardial effusion: experience in 233 consecutive patients. Cardiovasc J S Afr, 18(1), 20–25 (2007). [PubMed] [Google Scholar]
- 34.Mayosi BM, Ntsekhe M, Bosch J et al. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med, 371(12), 1121–1130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; *The largest clinical trial of adjunctive corticosteroids in tuberculous pericarditis
- 35.Wiysonge CS, Ntsekhe M, Thabane L et al. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev, 9, CD000526 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; **An important recent systematic review of the use of steroids in pericardial tuberculosis
- 36.Keidan SE, Todd RM. Triamcinolone in primary pulmonary tuberculosis. A controlled trial. Lancet, 2(7214), 1224–1227 (1961). [DOI] [PubMed] [Google Scholar]
- 37.Nemir RL, Cardona J, Lacoius A, David M. Prednisone Therapy as an Adjunct in the Treatment of Lymph Node-Bronchial Tuberculosis in Childhood. A Double-Blind Study. Am Rev Respir Dis, 88, 189–198 (1963). [DOI] [PubMed] [Google Scholar]
- 38.Bunkar ML, Agnihotri SP, Gupta PR, Arya S. Add-on prednisolone in the management of cervical lymph node tuberculosis. Indian J Tuberc, 63(2), 96–99 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Lee CH, Wang WJ, Lan RS, Tsai YH, Chiang YC. Corticosteroids in the treatment of tuberculous pleurisy. A double-blind, placebo-controlled, randomized study. Chest, 94(6), 1256–1259 (1988). [DOI] [PubMed] [Google Scholar]
- 40.Wyser C, Walzl G, Smedema JP, Swart F, van Schalkwyk EM, van de Wal BW. Corticosteroids in the treatment of tuberculous pleurisy. A double-blind, placebo-controlled, randomized study. Chest, 110(2), 333–338 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Elliott AM, Luzze H, Quigley MA et al. A Randomized, Double-Blind, Placebo-Controlled Trial of the Use of Prednisolone as an Adjunct to Treatment in HIV-1-Associated Pleural Tuberculosis. J Infect Dis, 190(5), 869–878 (2004). [DOI] [PubMed] [Google Scholar]; *A well-conducted clinical trial of adjunctive corticosteroids in HIV-1 associated pleural tuberculosis
- 42.Singh MM, Bhargava AN, Jain KP. Tuberculous peritonitis. An evaluation of pathogenetic mechanisms, diagnostic procedures and therapeutic measures. N Engl J Med, 281(20), 1091–1094 (1969). [DOI] [PubMed] [Google Scholar]
- 43.Alrajhi AA, Halim MA, al-Hokail A, Alrabiah F, al-Omran K. Corticosteroid treatment of peritoneal tuberculosis. Clin Infect Dis, 27(1), 52–56 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Hawkey CR, Yap T, Pereira J et al. Characterization and management of paradoxical upgrading reactions in HIV-uninfected patients with lymph node tuberculosis. Clin Infect Dis, 40(9), 1368–1371 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Lai RP, Meintjes G, Wilkinson RJ. HIV-1 tuberculosis-associated immune reconstitution inflammatory syndrome. Seminars in Immunopathology, 38(2), 185–198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meintjes G, Lawn SD, Scano F et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis, 8(8), 516–523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Namale PE, Abdullahi LH, Fine S, Kamkuemah M, Wilkinson RJ, Meintjes G. Paradoxical TB-IRIS in HIV-infected adults: a systematic review and meta-analysis. Future Microbiol, 10(6), 1077–1099 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Cheng SL, Wang HC, Yang PC. Paradoxical response during anti-tuberculosis treatment in HIV-negative patients with pulmonary tuberculosis. Int J Tuberc Lung Dis, 11(12), 1290–1295 (2007). [PubMed] [Google Scholar]
- 49.Yang CH, Chen KJ, Tsai JJ et al. The impact of HAART initiation timing on HIV-TB co-infected patients, a retrospective cohort study. BMC Infect Dis, 14, 304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meintjes G, Skolimowska KH, Wilkinson KA et al. Corticosteroid-modulated Immune Activation in the Tuberculosis Immune Reconstitution Inflammatory Syndrome. Am J Respir Crit Care Med, 186(4), 369–377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tadokera R, Meintjes G, Skolimowska KH et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Resp J, 37(5), 1248–1259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marais S, Meintjes G, Pepper DJ et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis, 56(3), 450–460 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; *A prospective study describing the frequent occurrence of TBM-IRIS despite concomitant corticosteroid therapy
- 53.Marais S, Wilkinson KA, Lesosky M et al. Neutrophil-associated central nervous system inflammation in tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis, 59(11), 1638–1647 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons CP, Thwaites GE, Quyen NT et al. The clinical benefit of adjunctive dexamethasone in tuberculous meningitis is not associated with measurable attenuation of peripheral or local immune responses. J Immunol, 175(1), 579–590 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Abdool Karim SS, Naidoo K, Grobler A et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med, 362(8), 697–706 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanc FX, Sok T, Laureillard D et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med, 365(16), 1471–1481 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luetkemeyer AF, Havlir DV, Currier JS. Complications of HIV disease and antiretroviral therapy. Top Antivir Med, 19(2), 58–68 (2011). [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards OM, Courtenay-Evans RJ, Galley JM, Hunter J, Tait AD. Changes in cortisol metabolism following rifampicin therapy. Lancet, 2(7880), 548–551 (1974). [PubMed] [Google Scholar]
- 59.Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand, 213(5), 339–343 (1983). [DOI] [PubMed] [Google Scholar]
- 60.Sarma GR, Kailasam S, Nair NG, Narayana AS, Tripathy SP. Effect of prednisolone and rifampin on isoniazid metabolism in slow and rapid inactivators of isoniazid. Antimicrob Agents Chemother, 18(5), 661–666 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagay AR, Singhal KC, Bhargava R. Alteration in the levels of pyrazinamide in pleural fluid following simultaneous administration of prednisoline in patients of tubercular pleural effusion. Indian J Physiol Pharmacol, 34(4), 259–262 (1990). [PubMed] [Google Scholar]
- 62.McAllister WA, Thompson PJ, Al-Habet SM, Rogers HJ. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed), 286(6369), 923–925 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev, 18(3), 306–360 (1997). [DOI] [PubMed] [Google Scholar]
- 64.Gallo LI, Ghini AA, Piwien Pilipuk G, Galigniana MD. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry, 46(49), 14044–14057 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem, 70, 81–120 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Chen Q, Chen T, Xu Y et al. Steroid receptor coactivator 3 is required for clearing bacteria and repressing inflammatory response in Escherichia coli-induced septic peritonitis. J Immunol, 185(9), 5444–5452 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dostert A, Heinzel T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des, 10(23), 2807–2816 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev, 14(18), 2314–2329 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Bosscher K, Vanden Berghe W, Haegeman G. Mechanisms of anti-inflammatory action and of immunosuppression by glucocorticoids: negative interference of activated glucocorticoid receptor with transcription factors. J Neuroimmunol, 109(1), 16–22 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A, 91(2), 752–756 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol, 15(2), 943–953 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reichardt HM, Tuckermann JP, Gottlicher M et al. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J, 20(24), 7168–7173 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adcock IM, Nasuhara Y, Stevens DA, Barnes PJ. Ligand-induced differentiation of glucocorticoid receptor (GR) trans-repression and transactivation: preferential targetting of NF-kappaB and lack of I-kappaB involvement. Br J Pharmacol, 127(4), 1003–1011 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheppard KA, Phelps KM, Williams AJ et al. Nuclear integration of glucocorticoid receptor and nuclear factor-kappaB signaling by CREB-binding protein and steroid receptor coactivator-1. J Biol Chem, 273(45), 29291–29294 (1998). [DOI] [PubMed] [Google Scholar]
- 75.Wadgaonkar R, Phelps KM, Haque Z, Williams AJ, Silverman ES, Collins T. CREB-binding protein is a nuclear integrator of nuclear factor-kappaB and p53 signaling. J Biol Chem, 274(4), 1879–1882 (1999). [DOI] [PubMed] [Google Scholar]
- 76.McKay LI, Cidlowski JA. CBP (CREB binding protein) integrates NF-kappaB (nuclear factor-kappaB) and glucocorticoid receptor physical interactions and antagonism. Mol Endocrinol, 14(8), 1222–1234 (2000). [DOI] [PubMed] [Google Scholar]
- 77.Sutter-Dub MT. Rapid non-genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic B cell, the adipocyte and other cell types. Steroids, 67(2), 77–93 (2002). [DOI] [PubMed] [Google Scholar]
- 78.Hafezi-Moghadam A, Simoncini T, Yang Z et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med, 8(5), 473–479 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiper-Bergeron N, Wu D, Pope L, Schild-Poulter C, Hache RJ. Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex. EMBO J, 22(9), 2135–2145 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nykjaer A, Dragun D, Walther D et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell, 96(4), 507–515 (1999). [DOI] [PubMed] [Google Scholar]
- 81.Oren I, Fleishman SJ, Kessel A, Ben-Tal N. Free diffusion of steroid hormones across biomembranes: a simplex search with implicit solvent model calculations. Biophys J, 87(2), 768–779 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C, Liu Y, Cao JM. G protein-coupled receptors: extranuclear mediators for the non-genomic actions of steroids. Int J Mol Sci, 15(9), 15412–15425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matasic R, Dietz AB, Vuk-Pavlovic S. Dexamethasone inhibits dendritic cell maturation by redirecting differentiation of a subset of cells. J Leukoc Biol, 66(6), 909–914 (1999). [DOI] [PubMed] [Google Scholar]
- 84.Raker VK, Domogalla MP, Steinbrink K. Tolerogenic Dendritic Cells for Regulatory T Cell Induction in Man. Front Immunol, 6, 569 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barczyk K, Ehrchen J, Tenbrock K et al. Glucocorticoids promote survival of anti-inflammatory macrophages via stimulation of adenosine receptor A3. Blood, 116(3), 446–455 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest, 101(4), 890–898 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci, 1024, 138–146 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Hawrylowicz CM. Regulatory T cells and IL-10 in allergic inflammation. J Exp Med, 202(11), 1459–1463 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tobin DM, Roca FJ, Oh SF et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell, 148(3), 434–446 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; *An intriguing and elegant study indicating corticosteroid responsiveness is influenced by host (LTA4H) genotype
- 90.Mayosi BM, Ntsekhe M, Bosch J et al. Rationale and design of the Investigation of the Management of Pericarditis (IMPI) trial: a 2 × 2 factorial randomized double-blind multicenter trial of adjunctive prednisolone and Mycobacterium w immunotherapy in tuberculous pericarditis. Am Heart J, 165(2), 109–115e103 (2013). [DOI] [PubMed] [Google Scholar]
- 91.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. Available at https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf Section accessed 27 July 2018, page J14
- 92.Universal antiretroviral therapy (ART) for all HIV-infected TB patients. World Health Organization, Available at http://www.who.int/hiv/topics/tb/art_hivpatients/en/ accessed 27 July 2018
- 93.European AIDS Clinical Society: Guidelines. Version 9, October 2017, Available at http://www.eacsociety.org/files/guidelines_9.0-english.pdf, accessed 27 July 2018
- 94.Prevention of Mother- to- Child Transmission of HIV (PMTCT), Children, Adolescents and Adults. (2018 amended version) Available at https://www.westerncape.gov.za/assets/departments/health/wc_hiv_consolidated_guidelines_march_2018_0.pdf, accessed 27 July 2018
- 95.Singh AK, Malhotra HS, Garg RK et al. Paradoxical reaction in tuberculous meningitis: presentation, predictors and impact on prognosis. BMC Infect Dis, 16, 306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boland EW. Clinical comparison of the newer anti-inflammatory corticosteroids. Annals of the Rheumatic Diseases, 21, 176–187 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]