Abstract
Duration of colonization by extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) and factors associated with it were studied in 20 newborns in Seville, Spain. Median duration of colonization was 7.5 months; factors associated with prolonged colonization were delivery by caesarean section, colonization of the mother, and phylogroup B2 Eschericha coli isolate.
Keywords: colonization, Escherichia coli, extended-spectrum β-lactamases, newborns, risk factors
Extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) have rapidly spread worldwide. Extended-spectrum β-lactamase-producing Enterobacteriaceae are usually resistant to penicillins and cephalosporins, and because ESBL production is frequently associated with resistance to other non-β-lactam drugs, they are also multidrug resistant. Intestinal colonization with ESBL-E is important because colonized persons are key reservoirs of these organisms, and colonization generally precedes infection [1, 2].
The natural history of intestinal colonization by ESBL-E has mostly been studied in adults. In our area, the prevalence of colonization with ESBL-E in pregnant women at delivery is 6.7% (95% confidence interval [CI], 5.2–8.7) [3].
A systematic review showed that colonization was maintained for at least 6 months in 19% (95% CI, 9%–34%) of adults in the community, mostly travelers returning from endemic countries colonized with Escherichia coli [4]. However, the data on duration of colonization in neonates are limited, to the best of our knowledge, to 2 studies including only patients discharged after hospital outbreaks [5, 6]. After a literature search in PubMed, no data were found on healthy newborns, and there is very limited information about the human and microbiological factors associated with prolonged colonization [5]. The objective of our study was to investigate the duration of colonization with ESBL-E in healthy newborns during the first year of life and the risk factors for prolonged colonization.
METHODS
A prospective cohort study of children colonized by ESBL-E during the first year of life was undertaken. This study is part of a project investigating different aspects of the epidemiology of ESBL-E during pregnancy and in children. In summary, rectal colonization with ESBL-E was studied in a convenient sample of newborns and their mothers (see below) attended at Virgen Macarena University Hospital (Seville, Spain), a 900-bed tertiary hospital serving a population of 550000, with 3200 births per year. All pregnant women who gave birth at the hospital (and their children) were eligible if attended for delivery on predefined random days and offered to participate. No exclusion criteria applied. The recruitment was completed from August 1, 2013 to June 30, 2014.
Rectal swabs were taken from the children and their mothers in the first 48 hours of life (or the first 48 hours after delivery in the mothers). Subsequent routine visits were made at 3, 6, 9, and 12 months of life, during which time the data were collected, the babies were explored, and a rectal sample was taken from the children and their mothers. The data were collected using a predesign questionnaire in a secured electronic database. Children detected as colonized with ESBL-E in any rectal sample were included in this analysis. The study was approved by the local ethics committee. All of the mothers provided written informed consent.
The main outcome variable was clearance of colonization (CoC) of ESBL-E in colonized children, which was defined as 2 negative rectal swabs after any positive one. The CoC date was arbitrarily considered as the intermediate date between the last positive sample and the first negative one (because samples were taken every 3 months, this was typically 6 weeks after the last positive sample). Negative samples with the same clone between 2 positives were considered false negatives. Exposure to children, mother-related variables, and microbiologic features of the isolates were collected.
Rectal swab specimens were directly inoculated onto MacConkey agar with 4 mg/L cefotaxime and after 18 hours enrichment in peptone-enriched broth. All colonies morphotypes isolated compatible with Enterobacteriaceae (with or without enrichment) were identified by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Extended-spectrum β-lactamase screening was performed by the combination disc test [7]. Characterization of ESBL genes was determined by in-house polymerase chain reaction (PCR) with group-specific primers for the CTX-M-1, CTX-M-9, TEM, and SHV groups [8] and Sanger sequencing in all cases. XbaI pulsed-field gel electrophoresis was used to study genetic relationships among the isolates [9]. Fingerprinting 2.0 was used to generate dendograms, using the Dice index under 1% tolerance. Isolates with >2 band differences were considered clonally unrelated [10]. Escherichia coli phylogroups were assigned by quadruplex PCR for chuA, yjaA, TspE4.C2, and arpA genes [11]. B2 isolates were screened for belonging to the O25b:H4/ST131 clonal group, using PCR for O25b rfb and allele 3 of the pabB gene and multiplex PCR for phylogroup B23 typing [12].
Due to the difficulties for screen, recruit, and follow healthy newborns, we planned to include at least 20 colonized by ESBL-E. To do so, we screened for ESBL-E colonization the newborns of 50 ESBL-E colonized mothers and the newborns of 50 randomly selected noncolonized mothers which had been studied for another project investigating ESBL-E colonization in pregnancy [3]. Time until CoC was studied by Kaplan-Meier curves. The association between different exposures (considered until CoC or censoring) and time to CoC was investigated by Cox regression. Different multivariate models were constructed using a forward stepwise process. Variables included initially were those with a crude P ≤ .01. Those causing significant changes in the hazard ratio were kept, and those with an adjusted P value ≥0.1 were excluded at each step. Interactions were also investigated. SPSS 17.0 was used for the analyses.
RESULTS
Twenty children were detected as colonized with ESBL-E and were included (Supplementary Figure S1): 11 (55%) were colonized at birth, and 9 (45%) acquired the colonization during the follow-up; acquisition of ESBL-E after birth was detected after a median of 6 months (range, 4.5–9 months). Among the 20 colonized newborns, 13 were detected among the 46 screened children with a colonized mother (4 with a colonized mothers were excluded because of missing data), and 7 among the 50 screened with a noncolonized mother (randomly selected among the 756 noncolonized mothers studied).
Thirty species/clones of ESBL-E were isolated: 2 were Klebsiella pneumoniae and the rest were E coli; 5 children were colonized with 2 different ESBL-E E coli clones, 1 child had 3 clones and 1 had 4 different clones. Overall, 13 isolates obtained from the children were clonally related to ESBL-E isolated from their mothers (43.3% of isolates). The ESBL types were CTX-M-14 in 14 isolates (46.6%), SHV-12 in 8 isolates (26.6%), and CTX-M-1 in 7 isolates (23.3%). One isolate produced both CTX-M-1 and SHV-12. Among the E coli tested, 12 isolates (42.8%) belonged to phylogroup A, 7 (25%) belonged to D, 6 (21.4%) belonged to B2 (1 of them belonged to ST131), and 3 (10.8%) belonged to B1.
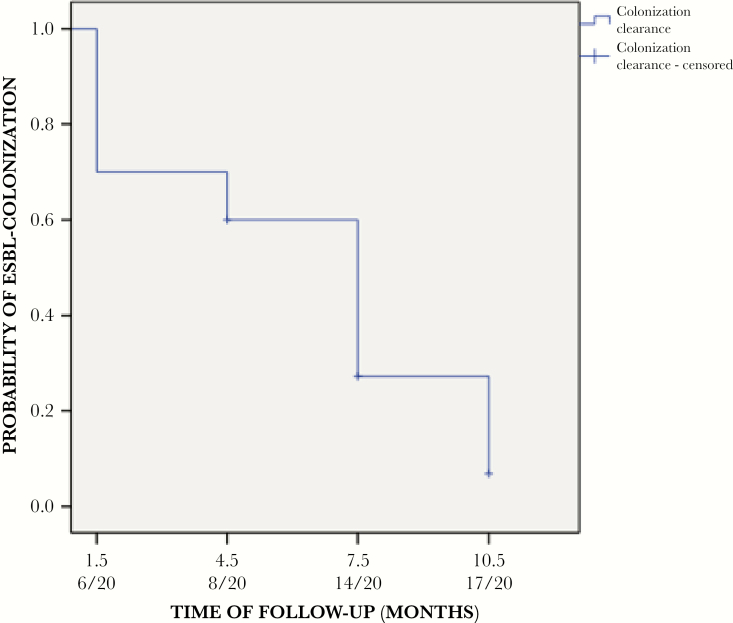
Duration of colonization is shown in Figure 1. Overall, 17 children cleared colonization during their follow up: 6 at 1.5 months, 2 at 4.5 months, 6 at 7.5 months, and 3 at 10.5 months; 3 remained colonized at the end of follow up. Overall, the mean and median times to clearance were 5.5 and 7.5 months, respectively. Table 1 shows the univariate analysis of child, mother, and microbiologic variables associated with CoC. Multivariate analysis showed that delivery by caesarean section, a colonized mother, and being colonized with phylogroup B2 ESBL-producing E coli were associated with prolonged colonization, whereas the opposite was found for colonization with CTX-M-producing ESBL-E (Table 1). No infections due to ESBL-E were detected.
Figure 1.
Probability of clearance of colonization in children colonized with extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae.
Table 1.
Univariate and Multivariate Analysis of Variables Associated With Clearance of ESBL-E Colonization in Childrena
| Variable | Category | Colonization Clearance/Exposed (Percentage) |
Crude HR (95% CI) |
P | Adjusted HR (95% CI) |
P |
|---|---|---|---|---|---|---|
| Child Variables | ||||||
| Gender | Male Female |
9/10 (90) 8/10 (80) |
2.01 (0.75–5.36) | .1 | ||
| Delivery by caesarean section | Yes No |
2/4 (50) 15/16 (93.8) |
0.43 (0.09–1.88) | .2 | 0.18 (0.03–0.91) | .03 |
| Travel outside Spain | Yes No |
2/2 (100) 15/18 (83.3) |
0.69 (0.15–3.06) | .6 | ||
| New household member | Yes No |
1/1 (100) 16/19 (84.2) |
0.53 (0.06–4.17) | .5 | ||
| Pets | Yes No |
6/7 (85.7) 11/13 (84.6) |
0.89 (0.32–2.44) | .8 | ||
| Breastfeeding | Yes No |
11/11 (100) 6/9 (66.7) |
1.17 (0.43–3.22) | .7 | ||
| Sterilization of feeding bottles | Yes No |
11/13 (84.6) 6/7 (85.7) |
0.50 (0.17–1.48) | .2 | ||
| Fed with homemade puree | Yes No |
14/17 (82.4) 3/3 (100) |
0.73 (0.20–2.62) | .6 | ||
| Nursery attendee | Yes No |
2/4 (50) 15/16 (87.5) |
0.67 (0.15–2.99) | .6 | ||
| Any chronic disease | Yes No |
1/2 (50) 16/18 (88.9) |
0.67 (0.08–5.17) | .7 | ||
| Any pediatric visit for acute illnessb | Yes No |
5/7 (71.4) 12/13 (92.3) |
0.64 (0.22–1.84) | .4 | ||
| Hospitalizationb | Yes No |
1/2 (50) 16/18 (88.9) |
0.32 (0.04–2.44) | .2 | ||
| Antibiotic useb | Yes No |
3/5 (60) 14/15 (93.3) |
0.39 (0.11–1.38) | .1 | ||
| Colonization with ESBL-E at birth | Yes No |
11/11 (100) 6/9 (66.7) |
1.15 (0.42–3.17) | .7 | ||
| Time of acquisition of ESBL-E | ≤3 months >3 months | 12/13 (92.3) 5/7 (71.4) |
0.46 (0.14–1.50) | .2 | ||
| Mother colonized with the same ESBL-E clone | Yes No |
9/10 (90) 8/10 (80) |
1.4 (0.53–3.69) | .5 | ||
| Mother Variables | ||||||
| Caregiver of a disabled person | Yes No |
2/3 (66.7) 15/17 (88.2) |
0.72 (0.16–3.22) | .6 | ||
| Any chronic disease | Yes No |
2/3 (66.7) 15/17 (88.2) |
0.47 (0.10–2.08) | .3 | ||
| Any doctor’s visit for acute illness | Yes No |
1/1 (100) 16/18 (88.9) |
0.21 (0.02–1.69) | .1 | ||
| Hospitalization | Yes No |
0/0 (0) 17/20 (85) |
-- | -- | ||
| Antibiotic use | Yes No |
1/2 (50) 16/18 (88.9) |
0.21 (0.02–1.69) | .1 | ||
| Mother colonized | Yes No |
11/13 (84.6) 6/7 (85.7) |
0.62 (0.22–1.71) | .3 | 0.31 (0.10–1.01) | .05 |
| Microbiologic Variables | ||||||
| CTX-M producer | Yes No |
12/15 (80) 5/5 (100) |
1.23 (0.42–3.55) | .6 | 5.15 (1.29–20.55) | .02 |
| SHV-12 producer | Yes No |
7/8 (87.5) 10/12 (83.3) |
0.70 (0.25–1.91) | .4 | ||
| Phylogroup A Eschericha coli | Yes No |
10/11 (90.9) 7/9 (77.8) |
1.06 (0.40–2.79) | .9 | ||
| Phylogroup B1 E coli | Yes No |
2/3 (66.7) 15/17 (88.2) |
1.18 (0.26–5.31) | .8 | ||
| Phylogroup B2 E coli | Yes No |
4/6 (66.7) 13/14 (92.9) |
0.51 (0.16–1.58) | .2 | 0.20 (0.05–0.81) | .02 |
| Phylogroup D E coli | Yes No |
6/7 (85.7) 11/13 (84.6) |
1.01 (0.37–2.77) | .9 |
Abbreviations: CI, confidence interval; ESBL-E, extended-spectrum β-lactamase-producing Enterobacteriaceae; HR, hazard ratio.
aExposures are considered for the whole period from detection of colonization to clearance, or censoring, except where specified.
bNone of these were related to infection due to ESBL producers.
DISCUSSION
Mean duration of rectal colonization with ESBL-E during the first year of life in unselected newborns was found to be approximately 6 months, and some children were persistently colonized. Delivery by caesarean section and colonized mothers were factors associated with prolonged colonization. It is interesting to note that ESBL-producing E coli belonging to phylogroup B2 was associated with longer colonization, and the opposite was found in isolates producing CTX-M enzymes.
In our area, the prevalence of colonization with ESBL-E in pregnant women at delivery is 6.7% (95% CI, 5.2–8.7) [3]. Previous studies conducted with newborns were performed in completely different epidemiological situations and included patients discharged from neonatal intensive care units during ESBL-E outbreaks in the unit [5, 6]. Löhr et al [5] found that median duration of carriage was 12.5 months in 51 infants (mostly preterm) colonized with CTX-M-15-producing K pneumoniae; risk factors for prolonged carriage were delivery by caesarean section (as in our study) and use of antibiotics during hospitalization. They also found household transmission of ESBL-E in 32% of households. Nordberg et al [6] found that median duration of colonization was 12.5 months in 14 children (13 colonized with ESBL-producing K pneumoniae). Tandé et al [13] found that median duration of colonization with ESBL-E in 22 adopted children from Mali living in France was 9 months. To the best of our knowledge, this is the first study to evaluate both the duration and the risk factors for clearance of ESBL-E colonization (mostly E coli) in unselected newborns in a non-outbreak situation.
The association with delivery by caesarean section and prolonged carriage could be explained by the potential protective influence of the composition of gut microbiota acquired during vaginal delivery. Babies born vaginally are colonized by the mother’s vaginal and fecal flora during delivery, whereas those born by caesarean section would have more influence from environment to form their microbiota; the latter also have lower counts of Bacteroides spp and bifidobacteria. Such impaired composition of protective gut microbiota might contribute to longer fecal colonization by ESBL-E if colonized early in life [5, 14]. Colonization of the mother was also associated, possibly reflecting person-to-person transmission between mother and newborn or common acquisition from an external source. It is interesting to note that phylogroup B2 ESBL-producing E coli was also associated with longer carriage. Titelman et al [15] also found that isolates from this phylogroup were more frequent among those with carriage at 12 months in a recent unadjusted prospective study of 61 colonized hospitalized adults, many of whom had a previous infection; 14 of the 19 B2 isolates in that study belonged to ST131, which may have been part of an outbreak, whereas this was the case in only 1 of 6 in our study. We did not study the virulence factors of the isolates, although Titelman et al [15] found no association with typical uropathogenic virulence factors. Therefore, B2 isolates may have some features unrelated to E coli uropathogenesis that favor prolonged intestinal colonization in adults and newborns. Finally, we found that isolates producing CTX-M enzymes were associated with shorter duration of colonization in neonates. We do not have an explanation for this; in fact, Titelman et al [15] found that CTX-M-9-producing isolates were associated with prolonged carriage, although it is possible that most of the B2 isolates were CTX-M-9 producers. If so, the phylogroup-associated feature would be more important than the ESBL in this regard. Therefore, the association between type of enzyme and carriage duration requires further studies.
CONCLUSIONS
The most important limitations of this study include the small number of patients, the fact that the date for CoC was arbitrarily chosen as the intermediate between the last positive and first negative samples, and the possible limited sensitivity for detection of colonization. In addition, our sample may have overestimated the estimation for mother colonization. Although these data improve our understanding of the epidemiology of ESBL-E in healthy newborns, more studies in this population are needed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank those who participated in the study for their unconditional support, especially colleagues in the neonatology and pediatric emergency department.
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Financial support. This work was funded by the following: Plan Nacional de I+D+i 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015/0010 and REIPI RD16/0016/0001), cofinanced by European Development Regional Fund “A way to achieve Europe”, Operative Programme Intelligent Growth 2014–2020; Fondo de Investigación Sanitaria; Instituto de Salud Carlos III (Grants 070190, 10/01955, and 10/00795); and Consejería de Innovación, Junta de Andalucía (Grants CTS-5259 and CTS210).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 2008; 8:159–66. [DOI] [PubMed] [Google Scholar]
- 2. Lukac PJ, Bonomo RA, Logan LK. Extended-spectrum β-lactamase-producing Enterobacteriaceae in children: old foe, emerging threat. Clin Infect Dis 2015; 60:1389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiménez-Rámila C, López-Cerero L, Aguilar Martín MA, et al. Vagino-rectal colonization and maternal-neonatal transmission of Enterobacteriaceae producing extended-spectrum β-lactamases or carbapenemases: a cross-sectional study. J Hosp Infect 2018. doi: 10.1016/j.jhin.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 4. Bar-Yoseph H, Hussein K, Braun E, Paul M. Natural history and decolonization strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage: systematic review and meta-analysis. J Antimicrob Chemother 2016; 71:2729–39. [DOI] [PubMed] [Google Scholar]
- 5. Löhr IH, Rettedal S, Natås OB, et al. Long-term faecal carriage in infants and intra-household transmission of CTX-M-15-producing Klebsiella pneumoniae following a nosocomial outbreak. J Antimicrob Chemother 2013; 68:1043–8. [DOI] [PubMed] [Google Scholar]
- 6. Nordberg V, Jonsson K, Giske CG, et al. Neonatal intestinal colonization with extended-spectrum β-lactamase-producing Enterobacteriaceae-a 5-year follow-up study. Clin Microbiol Infect 2018; 24:1004–9. [DOI] [PubMed] [Google Scholar]
- 7. EUCAST guidelines for detection of resistance mechanisms and specific resistance of clinical and/or epidemiological importance Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf. Published 2013. Accessed 2 April 2013.
- 8. Rodríguez-Baño J, López-Cerero L, Navarro MD, et al. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J Antimicrob Chemother 2008; 62:1142–9. [DOI] [PubMed] [Google Scholar]
- 9. Standard Operating Procedure for Pulsenet PFGE of Escherichia coli O157:H7, Escherichia coli Non-O157 (Stec), Salmonella Serotypes, Shigella sonnei and Shigella flexneri Available at: http://www.pulsenetinternational.org/assets/PulseNet/uploads/pfge/PNL05_Ec-Sal-ShigPFGEprotocol.pdf. Accessed April 2, 2013.
- 10. Johnson JR, Nicolas-Chanoine MH, DebRoy C, et al. Comparison of Escherichia coli ST131 pulsotypes, by epidemiologic traits, 1967-2009. Emerg Infect Dis 2012; 18:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 2013; 5:58–65. [DOI] [PubMed] [Google Scholar]
- 12. López-Cerero L, Bellido Mdel M, Serrano L, et al. Escherichia coli O25b:H4/ST131 are prevalent in Spain and are often not associated with ESBL or quinolone resistance. Enferm Infecc Microbiol Clin 2013; 31:385–8. [DOI] [PubMed] [Google Scholar]
- 13. Tandé D, Boisramé-Gastrin S, Münck MR, et al. Intrafamilial transmission of extended-spectrum-beta-lactamase-producing Escherichia coli and Salmonella enterica Babelsberg among the families of internationally adopted children. J Antimicrob Chemother 2010; 65:859–65. [DOI] [PubMed] [Google Scholar]
- 14. Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol 2016; 16:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Titelman E, Hasan CM, Iversen A, et al. Faecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae is common 12 months after infection and is related to strain factors. Clin Microbiol Infect 2014; 20:O508–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.