Abstract
RP is the most common manifestation of SSc and a major cause of disease-related morbidity. This review provides a detailed appraisal of the patient experience of SSc-RP and potential implications for disease classification, patient-reported outcome instrument development and SSc-RP clinical trial design. The review explores the clinical features of SSc-RP, the severity and burden of SSc-RP symptoms and the impact of SSc-RP on function, work and social participation, body image dissatisfaction and health-related quality of life in SSc. Where management of SSc-RP is concerned, the review focuses on the ‘patient experience’ of interventions for SSc-RP, examining geographic variation in clinical practice and potential barriers to the adoption of treatment recommendations concerning best-practice management of SSc-RP. Knowledge gaps are highlighted that could form the focus of future research. A more thorough understanding of the patient experience could support the development of novel reported outcome instruments for assessing SSc-RP.
Keywords: systemic sclerosis, Raynaud’s phenomenon, patient experience, health-related quality of life, disability, function, impact
Rheumatology key messages
RP is the most common and typically earliest clinical manifestation of SSc.
SSc-RP causes distressing physical symptoms, impaired function, body image dissatisfaction and reduced health-related quality of life.
Patient-reported outcome measures that more fully capture the patient experience of SSc-RP are needed.
Introduction
RP is the most common manifestation of SSc, affecting ∼96% of patients [1]. SSc-RP is also typically the earliest clinical manifestation of SSc, with a lag period that can last several years before additional organ-specific disease manifestations emerge [1, 2]. Many comprehensive and valuable reviews have been prepared on the subject of SSc-RP, the majority of which have focused on current advances in elucidating the pathogenesis of SSc-RP and evidence-based approaches to management. A broad definition of RP (episodic digital ischaemia, characterized by pain, numbness and digital colour changes, and provoked by cold exposure and/or emotional stress) is typically recited, alongside reference to the significant morbidity associated with SSc-RP. This review provides a more detailed appraisal of the patient experience of SSc-RP and potential implications for disease classification, patient-reported outcome instrument development and SSc-RP clinical trial design. The focus and scope of the review was not amenable to systematic review methods, but individual comprehensive literature searches (including detailed grey searches of cited papers) were undertaken for each of the sub-headings applied to ensure a comprehensive appraisal of the patient experience of SSc-RP was achieved using a diverse range of sources that included cross-sectional studies, observational studies, registry analyses and clinical trial data. References to primary RP, when applied, are primarily used to compare and contrast the patient experience of primary RP with that of SSc-RP, or where evidence for SSc-RP is lacking. Where management of SSc-RP is concerned, this review focuses on the patient experience of interventions for SSc-RP, examining geographic variation in clinical practice and potential barriers to the adoption of treatment recommendations. Where applicable, knowledge gaps are highlighted that could form the focus of future research.
Sensory symptoms of SSc-RP
Population-based studies of RP (mainly primary) have identified numbness of the fingers as the subjective symptom most commonly associated with RP attacks (93.7%), with a lower rate of tingling (53.2%) and comparatively low levels of pain (27.6%) [3]. In contrast, pain appears to be the predominant symptom associated with SSc-RP, perhaps reflecting a greater degree of tissue ischaemia in SSc compared with primary RP [4]. Median pain visual analogue scores (VAS) are higher in SSc-RP compared with suspected CTD-RP and primary RP [4]. Importantly, the same study reported lower overall RP severity scores in SSc compared with suspected CTD-RP, possibly indicating some degree of habituation to peripheral vascular symptoms in SSc, and also highlighting the impact of item wording on responses generated for any given conceptual framework [4]. More frequent episodes of SSc-RP was one of a small number of disease-specific variables [alongside digital ulcers (DU), gastrointestinal symptoms and synovitis] that were independently associated with increased pain (assessed using an 11-point numerical rating scale) in SSc [5]. Moreover, pain VAS scores aligned with SSc-RP activity scores during factor analysis of data obtained from a large clinical trial of SSc-RP, indicating they have strong inter-correlations and are measuring conceptually similar aspects of disease activity [6]. In addition to pain, a number of additional sensory symptoms that might be attributable to SSc-RP have emerged from previous qualitative research examining the patient experience in SSc (mainly focusing on quality of life and function) including impaired touch function, numbness, sensations related to skin, increased sensibility, loss of sensory functions and reduced tactile sensations in the fingers [7–9]. The physical symptoms of SSc-RP are important to patients with SSc, with RP and difficulties experienced in cold weather listed as two of the three most frequently stated physical symptoms present in SSc [10]. No qualitative research studies to date have exclusively explored the patient experience of SSc-RP. Little is known about the evolution of sensory symptoms of SSc-RP with disease progression.
Digital colour changes of SSc-RP
Maurice Raynaud provided the first detailed description of the digital colour changes that accompany impaired digital perfusion in the phenomenon to which he is eponymously linked [11]. Digital pallor (ischaemic blanching secondary to vasoconstriction of the pre-capillary arterioles), cyanosis (deoxygenation of sequestered blood following constriction of the post-capillary venules) and rubor (a post-ischaemic reactive hyperaemic phenomenon) comprise the tri-phasic digital colour change response that might occur during RP attacks as they develop and abate (Fig. 1). In practice, tri-phasic colour changes are not typical in RP, and certainly not essential for diagnosis. A large community-based questionnaire study estimated the overall prevalence of RP (based on cold sensitivity with white and/or blue digital colour changes) at 4.6%; however, insistence on a tri-phasic digital colour response would have seen the prevalence fall to ∼0.1% [12]. Population-based assessment of digital colour changes have identified uni-phasic blanching, bi-phasic blanching with cyanosis and bi-phasic blanching with rubor as the most common combinations of digital colour changes reported across the spectrum of RP [3]. The UK Scleroderma Study Group proposed a consensus classification approach to RP that was subsequently tested in a small (n = 30) cohort of healthy controls, and primary and secondary RP [13]. The proposed presence of repetitive episodes of bi-phasic (unspecified) colour changes in either cold or normal environments has subsequently been adopted in proposed classification criteria for RP and SSc [14–16]. A recent international Delphi exercise of SSc-RP experts (n = 12), meanwhile, specified the presence of ‘biphasic blanching and cyanosis’ of the digits to diagnose RP [17].
Fig. 1.
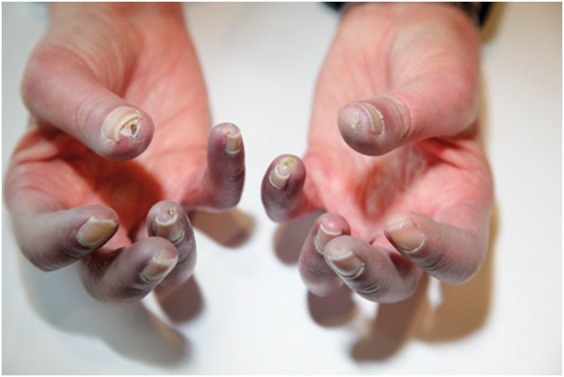
Acrocyanosis in the digits of a patient with limited cutaneous systemic sclerosis
In addition to the typical cyanosis of SSc-RP, there is also evidence of active digital ulceration affecting the right thumb tip and digital pitting affecting the right fifth digit, left thumb and left fifth digit.
Few studies have specifically reported the digital colour changes associated with SSc-RP compared with other forms of RP. It has been suggested that cyanosis without blanching is more common in patients with SSc-RP than primary forms of RP [18]. Reactive hyperaemia, meanwhile, appears to be less common in SSc-RP than in primary RP, and may reflect an irreversible obliterative microangiopathy incapable of post-occlusive vasodilation [19]. A recent small study (n = 20) identified uni-phasic digital colour changes (blanching in 91%, cyanosis in 9%) in over half (55%) of patients with SSc, which has implications for our current approach to disease classification [4]. There was, however, a higher rate of tri-phasic RP symptoms in SSc in comparison with primary RP (20 vs 5.1%) [4]. The impact of strict adherence to bi-phasic digital colour changes on disease classification in early SSc and estimates of prevalence of SSc-RP has not previously been explored. The clinical correlates of specific digital colour changes, and combinations thereof, in SSc (such as associations with SSc-RP severity, disease duration and presence of DU disease) is unknown, but might provide a readily assessed, and hitherto unused, tool for assessing peripheral vascular risk in SSc. For example, digital cyanosis has been shown to correlate with the presence of giant capillaries and microhaemorrhages on nailfold capillaroscopy in one study [4]. The clinical features present within a small group of patients negative for both ANA and SSc-RP from the European Scleroderma Trials And Research (EUSTAR) registry raised the possibility of alternative pathology and the absence of RP symptoms should prompt further diagnostic inquiry to exclude sclerosing skin conditions that can mimic SSc [20]. Gender-specific differences in digital colour changes of RP have not been explored in SSc. Population-based studies of primary RP symptoms suggest uni-phasic blanching is more common in females (75 vs 58%) ,whereas bi-phasic blanching with rubor is more common in males (29 vs 10%) [3].
Body areas affected
The fingers are the most commonly affected body area in RP and symptoms are bilateral in 90% of patients [4]. Asymmetry can be a predictor of secondary RP [21]. Relative sparing of the thumbs occurs across all forms of RP [4, 22], although the extent of thumb involvement assessed using thermal imaging appears to be more exaggerated in secondary RP compared with primary RP [23]. Symptoms affecting the earlobes and nose, meanwhile, appear to be more common in primary RP [19].
Precipitating and aggravating factors
Typical descriptions of RP describe episodic vasospasm occurring in response to cold exposure and/or emotional stress. Cold appears to be the more important precipitating event in SSc-RP. A questionnaire-based study of SSc-RP reported cold being a precipitating factor in all participants (n = 18), whereas only one-third also reported attacks provoked by emotion [24]. These findings were replicated in a recent larger survey of a mixed population of RP (n = 443) that reported cold exposure as the trigger for RP attacks in 91% of subjects overall (95% in secondary RP) and emotional stress in 30% [25]. Changes in ambient temperature were an associated event in 87% of SSc-RP attacks in an early physiological study that incorporated ambulatory temperature measurement, compared with only 65.9% of attacks in primary RP where a higher proportion of attacks appeared to have been precipitated by emotional stress [26]. It was also noted that patients with primary RP reporting higher stress ratings prior to RP attacks often had higher digital skin temperatures throughout RP attacks [26]. The apparent relationship between pre-RP attack stress and skin temperature during RP attacks was not replicated in SSc patients [26]. Physiological studies have also identified an increase in physiological markers of stress (such as muscle tension and tachcardia) during SSc-RP attacks that is not observed in primary RP attacks [26]. In this regard, emotional stress might propagate rather than precipitate attacks of SSc-RP. Thematically relevant emotional stressors might specifically aggravate RP. For example, imagination of the threat of cold exposure (subjects were asked to imagine loss of gloves and car keys during a snowstorm) has been shown to induce reduced finger temperature in RP patients but not healthy controls [27]. Differences in precipitating events of attacks in primary RP and SSc-RP might explain the disparity in responses to behavioural intervention for RP. For example, finger temperature biofeedback intervention (patients trained to augment the temperature of the fingers using a sinusoidal tone that varied according to finger temperature) resulted in reductions in RP attack frequency in primary RP but no such response to biofeedback intervention was observed in SSc [28].
Tobacco use has implications for peripheral vascular complications of SSc. Smokers with SSc are also three to four times more likely to require surgical or pharmacological intervention for digital ischaemia than non-smokers [29]. The relationship between smoking and peripheral vascular compromise appears to extend to RP severity. Use of the Comprehensive Smoking Index identified a significant association between smoking intensity (packs/day) and SSc-RP severity, but the effects dissipated within 1 year of smoking cessation, highlighting the importance of this non-pharmacological intervention in SSc-RP [30].
Frequency of RP attacks
Analysis of clinical trial data provides some insight into the average daily frequency of RP attacks experienced (or at least captured using diary monitoring) by patients with SSc, although trials are often enriched with patients with more severe SSc-RP (e.g. eligibility criteria mandating exceeded thresholds for mean daily average RP attack frequency prior to randomization), which means the data might be less applicable to real-life [31–33]. Some studies have also incorporated the presence of DUs as an inclusion criterion for study entry into SSc-RP trials [34]. For example, diary returns from randomized controlled trials of SSc undertaken during the winter months and/or requiring a minimum of >4–8 RP attacks per week prior to enrolment have reported a mean daily frequency of RP attacks of between 3.3 and 4.1 attacks/day [6, 31, 32, 35, 36], or ∼28 attacks/week [34]. Studies of mixed populations of primary and secondary RP, sometimes applying similar approaches, have revealed a slightly lower mean daily attack frequency of 1.9–2.8 attacks/day [37, 38]. In contrast, a cross sectional study of SSc that enrolled patients with SSc throughout the year and did not require a minimum threshold number of RP attacks prior to enrolment revealed a lower mean daily number of attacks of only 2 attacks/day [39]. Unsurprisingly, seasonal variation in weather influences attack burden in SSc-RP. A small (n = 18) longitudinal study identified doubling in the daily frequency of RP attacks (2.9 vs 1.5 attacks/day) during winter compared with summer despite similar rates of outdoor exposure across seasons [24]. This study also highlighted the persistent nature of SSc-RP, with only 16.7% of respondents reporting no attacks during assessment in the summer [24]. Seasonal variation in weather and temperature fluctuations induced by air-conditioning have emerged as contributing to SSc-RP symptom burden in qualitative research [40, 41]. The relationship between RP attack frequency and Raynaud’s classification has varied between studies, with individual papers reporting higher, similar and lower RP attack frequency in primary RP compared with secondary RP [26, 39, 42]. Gender may influence frequency of RP attacks, with significantly fewer RP attacks reported in males in one study of primary RP and SSc [39]. The frequency of RP attacks does not appear to be higher in patients with DU [6]. Diary monitoring of SSc-RP symptoms is laborious for patients and SSc-experts have expressed concerns regarding the respondent burden and value of this approach [43].
Duration of RP attacks
The duration of RP attacks over a 2-week Raynaud’s Condition Score (RCS) diary collection has been relatively consistent across studies, with studies reporting mean daily aggregate duration spent in RP attacks of between 37 and 95 min/day, equating to average attack duration of ∼15–20 min per attack [6, 31, 32, 44]. Seasonal variation is again relevant, with a lower mean daily duration of attacks of ∼20 min daily in a study whose enrolment spanned winter and summer [36]. The aforementioned longitudinal study examining the impact of seasonal variation on SSc-RP symptoms identified an approximate doubling in the aggregate daily duration (70 vs 35 min/day) of attacks during winter compared with summer, despite similar rates of outdoor exposure [24]. The duration of RP attacks does not appear to be higher in patients with active DU [6]. Diary methods of assessing the frequency and duration of SSc-RP assume a paradigm of episodic RP attacks and preclude adequate capture of a phenomenon familiar to SSc clinicians and previously described by Jill Belch as ‘what is for many patients the worst feature of the disease – continual digital ischaemia’ [45]. The phrase ‘my constant companion’ was used by a patient to describe SSc-RP in one qualitative research study to allude to the persistent threat and/or presence of digital ischaemic symptoms experienced by many patients with SSc [8].
Ability to prevent and manage RP attacks
Management of RP usually includes advice on the use of gloves and hand warmers, but evidence examining the adoption and efficacy of such self-management approaches in preventing/shortening RP attacks is lacking. Measures to avoid or ameliorate SSc-RP attacks might influence diary returns concerning RP attack frequency and duration, which might have implications for the value of such parameters as clinical trial endpoints. Approximately two-thirds of patients with secondary RP report the ability to predict the occurrence of at least half of their RP attacks, with a similar proportion being able to predict attack severity based on environmental factors surrounding an attack [25]. Nonetheless, the majority of patients with secondary RP report difficulty preventing or controlling the occurrence of RP attacks [25]. This might indicate that preventative therapeutic approaches might be preferable to treatment strategies designed to ameliorate an attack when it occurs; for example, application of topical vasodilating gels that have been the subject of clinical trials for SSc-RP [37]. The use of gloves and hard-warming devices are considered helpful, but it has been noted that no intervention prevents all attacks, and barriers to wearing gloves such as sclerodactyly and dressings have been identified [8].
The severity of SSc-RP
Raynaud’s severity is a broad and challenging concept to measure and attempts to achieve this in the clinical trial setting have relied upon patient-reported outcome instruments such as the RP VAS global assessment (focus on difficulty with Raynaud’s), the Raynaud’s severity score and the RCS (Table 1). The Raynaud’s severity score and RCS are single-item scales that ask patients to consider the difficulty they have had because of their SSc-RP, considering the frequency and duration of attacks, pain, numbness and impact of SSc-RP on function when considering their score. It is not known which domains have the greatest influence on patient response and whether different patients focus on different domains when choosing their score. Despite differences in item wording and recall period (Table 1), mean values of these global assessments are remarkably consistent at around 4.4 on a 0–10 numerical rating scale (NRS) across a number of studies (generally undertaken in winter and requiring a minimum number of RP attacks during run-in phase) irrespective of the instrument used [Raynaud’s severity scale ∼4.4 [34], RCS (UK) ∼4.2 [32], RCS (US) 4.3 [6, 31] and an RCS of 46 mm when captured using 0–100 mm VAS [45]]. Furthermore, patient and physician global assessments of RP produce similar weighting for RP severity (1.37 and 1.36 on 0–3.0 scale; also equating to ∼4.5 on 0–10 NRS) [6, 31]. Mean RCS scores of ∼2.0 have been obtained from studies that enrolled SSc patients throughout the year and did not mandate a minimum threshold of RP attacks to be experienced prior to study entry [39]. Principal components factor analysis identified strong inter-correlation between the RCS score and other patient-reported RP VAS subscales [e.g. the Scleroderma HAQ (SHAQ) RP VAS] but not with physician assessment of RP by VAS, which appeared to have stronger inter-correlation with reported frequency and duration of RP attacks [6]. Furthermore, factor analysis did not identify strong inter-correlation between the RCS score and the frequency/duration of RP attacks, suggesting that patient assessment of the overall severity of RP and the frequency/duration of RP attacks are separable conceptually [6]. Raynaud’s severity assessed using the RCS was noted to be higher in patients with DU (5.03 vs 4.1), although the wording of the item question in this work specifically asked subjects to include symptoms arising from painful sores when choosing their score (Table 1) [6]. The severity of RP (based on mean baseline RCS) has been shown to be the same in patients whether or not they are receiving vasodilator therapy for their RP (∼45 mm on 100 mm VAS) [47] and similar for patients with primary RP and SSc [39]. Severity of SSc-RP may differ among different ethnic groups, with one study identifying higher severity of SSc-RP in native North-American populations [48].
Table 1 Existing patient- and clinician-reported outcome instruments for assessing SSc-RP
| Name | Study | Item metric | Recall period (days) | Score | Item wording |
|---|---|---|---|---|---|
| Patient-reported outcomes | |||||
| Raynaud’s Severity Score | Wigley et al. [34] | 11-point NRS (0–10) | 1 | Mean daily score over 3-week period | Patients were asked to consider in their Raynaud severity score the number and duration of attacks; symptoms, such as numbness, burning, and pain and tingling; hand disability; and influence of cold on daily activity. An attack was defined as an episode of pallor followed by cyanosis with or without associated pain |
| Raynaud’s Condition Score | Black et al. [32] | 11-point NRS (0–10); 0 none–10 very severe | 1 | Mean daily score over 2-week perioda | Please rate the difficulty you had today with your Raynaud’s condition. Please consider the following when choosing your score: the number of Raynaud’s attacks; the duration of the attacks; whether you had, for example, numbness, burning and tingling, and the effect cold had on your ability to use your hands and to perform other activities |
| Raynaud’s Condition Score | Wigley et al. [31] | 11-point NRS (0–10); 0 no difficulty–10 extreme difficultya | 1 | Mean daily score over 2-week perioda | The Raynaud’s Condition Score is your rating of how much difficulty you had with your Raynaud’s TODAY. Consider how many attacks you had and how long they lasted. Consider how much pain, numbness, or other symptoms the Raynaud’s caused in your fingers (including painful sores) and how much the Raynaud’s ALONE affected the use of your hands today |
| Patient global assessment | Wigley et al. [31] | 0–15 cm VAS (0 no disease activity and 100 very severe) | 7 | 15 cm rescaled to continuous 0–3 scale to match SHAQ | In the past week, how severe was your Raynaud’s disease? |
| SHAQ RP VAS subscale | Steen and Medsger [46] | 0–15 cm VAS (0 does not interfer and 100 very severe) | 7 | 15 cm rescaled to continuous 0–3 scale | In the past week how much have your Raynaud’s problems interfered with your activities? |
| Clinician-reported outcomes | |||||
| Physician global assessment | Wigley et al. [34] | 4-point NRS (0–3) | 7 or 1 | Score either on day of assessment of taking into account last 7 days | The study physician evaluated the severity of patients’ Raynaud phenomenon using a scoring system in which 0 represented no attack, 1 represented a mild attack, 2 represented a moderate attack and 3 represented a severe attack |
| Physician global assessment | Wigley et al. [31] | 0–15 cm VAS (anchored at 0 no disease activity, and at 100 very severe disease activity) | 7 | 15 cm rescaled to continuous 0–3 scale to match SHAQ | How severe would you rate the patient’s Raynaud’s disease for the past week? |
Sometimes adapted from its original form as a 0–100 mm VAS with mean values obtained over 1 week of assessment. VAS: visual analogue scale; SHAQ: scleroderma HAQ; NRS: numerical rating scale.
Impact of SSc-RP on functional capacity
SSc-RP has consistently emerged as the highest-impact disease-specific manifestation of SSc in terms of both frequency and impact on ability to carry out everyday activities in patient surveys undertaken in North America, Europe and South America [49–51]. The strong association between patient-reported assessment of RP severity and measures of function such as the HAQ-Disability Index and Scleroderma Functional Score are cited as evidence of the contribution of SSc-RP to disability, particularly concerning domains concerning hand function [6, 52]. The impact of RP on functional capacity appears to be greatest for secondary RP, with a higher proportion of patients making adjustments to activities of daily living to accommodate RP symptoms (87% vs 71%) compared with primary RP [25]. Clinical trials of SSc-RP have demonstrated beneficial effects on functional capacity, sometimes in the absence of improvement in RCS diary parameters, suggesting functional impairment secondary to SSc-RP might be responsive to vasodilator therapy [34, 53]. The impact of SSc-RP on function is captured with the SHAQ RP VAS subscale, which enquires about the extent to which SSc-RP interferes with activities. Clinical trial data have identified a mean score of 1.15 (on a 0–3 scale), allowing some quantification of the impact of SSc-RP on activities of daily living [6, 46]. The SHAQ RP VAS is higher in SSc patients with digital pitting scars, digital tip ulcers and digital gangrene [6, 46].
Impact of SSc-RP on quality of life
A large patient survey ranked SSc-RP the highest of the organ-specific manifestations of SSc in terms of impact on quality of life and perception of illness severity [54]. Quality of life appears to be affected to a greater extent by secondary RP than primary RP (6.5 vs 5.2 on 0–10 NRS) [25]. Furthermore, people with secondary RP also predict a greater improvement in quality of life when asked to imagine life without RP than people with primary RP [25].
The psychosocial impact of SSc-RP
No qualitative research studies to date have exclusively explored the patient experience of SSc-RP, but SSc-RP themes have emerged in studies of SSc exploring quality of life, functioning and body image dissatisfaction. The socially isolating and psychological impact of SSc-RP is evident in the following quote taken from Stamm et al. [55]:
‘If it’s 20 degrees below zero outside, you don’t go out at all … then I was really depressed, because it was so cold for such a long time. I didn’t go out to get my mail from the mail box for almost three weeks.’
Body image dissatisfaction related to SSc-RP (and the reaction of others) has also emerged in qualitative research, as the following quotes attest:
‘What bothers me most? Of course the symptoms of the Raynaud’s syndrome bother me, the fact that my hands become blue, the fact that I don’t look at them when I go out in cold weather, the fact that I look at the hands of all people. But mostly the psychological issues bother me.’ [55]
‘When I’m on public transport and I pay for my bus ticket, when I stretch out my purple hand and the seller looks strangely at me, I no longer want to stretch out my hand.’ [7]
The appearance of SSc-RP might have influenced the inclusion of items developed for the Body Concealment Scale for Scleroderma such as ‘I wear gloves to hide my hands’, ‘I wear make-up to hide skin discoloration’, ‘I avoid shaking hands with people’ and ‘I hide my hands so that people don’t see them’, and it should not be assumed that body image dissatisfaction pertains solely to the disfiguring effects of skin fibrosis and cutaneous ulceration in SSc [56].
SSc-RP is a factor influencing work participation and often requires adapting the work environment and wearing adequate clothing to avoid cold exposure [40]. Some patients avoid disclosing their SSc diagnosis to avoid feeling different from other colleagues, and because of concern about employer reaction and/or possible impact on career trajectory [40, 57]. Travelling to and from work can represent a barrier to work participation due to the distressing effects of cold exposure, for example, de-icing the car [40].
Barriers to therapeutic intervention
Considering the prevalence and impact of SSc-RP, registry data suggest the range of therapeutic options available for the management of SSc-RP is not fully exploited in terms of initiation of treatment and optimal therapeutic dosing [58]. There is marked variation in the patient experience of therapeutic intervention for SSc-RP, which relates to a number of factors. Physician attitudes to therapeutic intervention might be relevant, with one survey reporting 20% of scleroderma experts considering less than half of their patients requiring treatment for their SSc-RP [59]. Such attitudes might offer an explanation for the wide variation in prescribing practices for SSc-RP [60]. Calcium channel blockers are generally considered first-line therapy for SSc-RP [59, 61] and yet registry analyses suggest that only 47–60.9% of patients with SSc ever receive calcium channel blockers therapy for SSc-RP [62, 63]. Vasoactive drug use varies according to disease-specific organ manifestations and is higher in patients with pulmonary arterial hypertension (84.7%) and DU disease (76.4%) than in patients with SSc-RP alone (58.1%), possibly reflecting physician attitudes to the relative importance of therapeutic intervention for SSc-RP [63]. Marketing authorization approval and challenges securing reimbursement has also contributed to marked geographic variation in practice, with intravenous iloprost use for SSc-RP ranging from 1.3% in North America to 21.1% of patients in Europe [62, 63]. Patient surveys suggest that secondary RP is more readily treated with vasoactive drugs than primary RP (64% vs 33%), but the effectiveness of vasoactive treatment is rated modestly with only 21% of secondary RP respondents considering their RP treatment effective [25]. Smoking does not appear to influence vasoactive therapy use for SSc-RP [30]. Surveys have identified limited utilization of validated patient-reported outcome instruments for SSc-RP in clinical practice, which has limited the emergence of practice-based evidence to ascertain the comparative efficacy of different vasodilator approaches to SSc-RP management [43, 58].
Conclusions
SSc-RP is the most common disease-specific manifestation of SSc and is associated with considerable disease-related morbidity across a broad set of domains including pain, impaired hand function, reduced social participation, body image dissatisfaction, increased reliance on others and reduced quality of life. There are limitations to the current approaches for assessing SSc-RP. An enhanced understanding of the patient experience of SSc-RP might support the development of novel approaches to the assessment of SSc-RP that more fully capture the patient experience of SSc-RP and facilitate a more discerning appraisal of the efficacy of therapeutic intervention.
Acknowledgements
We wish to thank Professor Ariane Herrick for her helpful comments drafting the manuscript.
Funding: This work was funded by the Scleroderma Clinical Trials Consortium as part of a project to develop a conceptual framework for a novel patient-reported outcome instrument for assessing RP in SSc.
Disclosure statement: J.D.P. has undertaken consultancy work and received unrestricted grant support (£25 000), honoraria and meeting sponsorship from Actelion Pharmaceuticals. D.K. is supported by the National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) K24 AR063120 award and has received investigator-initiated grants and acts as a consultant to Actelion, Boehringer Ingelheim, Bristol Myers Squibb, Bayer, Corbus, Cytori, Eicos, GlaxoSmithKline, Genentech/Roche, Sanofi and UCB. All other authors have declared no conflicts of interest.
References
- 1. Walker UA, Tyndall A, Czirjak L. et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis 2007;66:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matucci-Cerinic M, Kahaleh B, Wigley FM.. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum 2013;65:1953–62. [DOI] [PubMed] [Google Scholar]
- 3. Maricq HR, Carpentier PH, Weinrich MC. et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5 region comparison. J Rheumatol 1997;24:879–89. [PubMed] [Google Scholar]
- 4. Ingegnoli F, Gualtierotti R, Orenti A. et al. Uniphasic blanching of the fingers, abnormal capillaroscopy in nonsymptomatic digits, and autoantibodies: expanding options to increase the level of suspicion of connective tissue diseases beyond the classification of Raynaud’s phenomenon. J Immunol Res 2015;2015:371960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schieir O, Thombs BD, Hudson M. et al. Prevalence, severity, and clinical correlates of pain in patients with systemic sclerosis. Arthritis Care Res 2010;62:409–17. [DOI] [PubMed] [Google Scholar]
- 6. Merkel PA, Herlyn K, Martin RW. et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum 2002;46:2410–20. [DOI] [PubMed] [Google Scholar]
- 7. Stamm T, Hieblinger R, Boström C. et al. Similar problem in the activities of daily living but different experience: a qualitative analysis in six rheumatic conditions and eight European countries. Musculoskeletal Care 2014;12:22–33. [DOI] [PubMed] [Google Scholar]
- 8. Kocher A, Adler S, Spichiger E.. Skin and mucosa care in systemic sclerosis–patients’ and family caregivers’ experiences and expectations of a specific education programme: a qualitative study. Musculoskeletal Care 2013;11:168–78. [DOI] [PubMed] [Google Scholar]
- 9. Suarez-Almazor ME, Kallen MA, Roundtree AK, Mayes M.. Disease and symptom burden in systemic sclerosis: a patient perspective. J Rheumatol 2007;34:1718–26. [PubMed] [Google Scholar]
- 10. Cinar FI, Unver V, Yilmaz S. et al. Living with scleroderma: patients’ perspectives, a phenomenological study. Rheumatol Int 2012;32:3573–9. [DOI] [PubMed] [Google Scholar]
- 11. Raynaud M, translated by Barlow T. Selected monographs: Raynaud's two essays on local asphyxia of the extremities. London: The New Sydenham Society, 1888. [Google Scholar]
- 12. Maricq HR, Weinrich MC, Keil JE, LeRoy EC.. Prevalence of Raynaud phenomenon in the general population. A preliminary study by questionnaire. J Chronic Dis 1986;39:423–7. [DOI] [PubMed] [Google Scholar]
- 13. Brennan P, Silman A, Black C. et al. Validity and reliability of three methods used in the diagnosis of Raynaud’s phenomenon. The UK Scleroderma Study Group. Br J Rheumatol 1993;32:357–61. [DOI] [PubMed] [Google Scholar]
- 14. LeRoy EC, Medsger TA Jr.. Raynaud’s phenomenon: a proposal for classification. Clin Exp Rheumatol 1992;10:485–8. [PubMed] [Google Scholar]
- 15. LeRoy EC, Medsger TA Jr.. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001;28:1573–6. [PubMed] [Google Scholar]
- 16. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maverakis E, Patel F, Kronenberg DG. et al. International consensus criteria for the diagnosis of Raynaud’s phenomenon. J Autoimmun 2014;48-49:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maricq HR, Weinrich MC.. Diagnosis of Raynaud’s phenomenon assisted by color charts. J Rheumatol 1988;15:454–9. [PubMed] [Google Scholar]
- 19. Wollersheim H, Thien T.. The diagnostic value of clinical signs and symptoms in patients with Raynaud’s phenomenon. A cross-sectional study. Neth J Med 1990;37:171–82. [PubMed] [Google Scholar]
- 20. Schneeberger D, Tyndall A, Kay J. et al. Systemic sclerosis without antinuclear antibodies or Raynaud's phenomenon: a multicentre study in the prospective EULAR Scleroderma Trials and Research (EUSTAR) database. Rheumatology (Oxford) 2013;52:560–7. [DOI] [PubMed] [Google Scholar]
- 21. Cardelli MB, Kleinsmith DM.. Raynaud’s phenomenon and disease. Med Clin North Am 1989;73:1127–41. [DOI] [PubMed] [Google Scholar]
- 22. Chikura B, Moore TL, Manning JB, Vail A, Herrick AL.. Sparing of the thumb in Raynaud’s phenomenon. Rheumatology (Oxford) 2008;47:2–219. [DOI] [PubMed] [Google Scholar]
- 23. Chikura B, Moore T, Manning J, Vail A, Herrick AL.. Thumb involvement in Raynaud’s phenomenon as an indicator of underlying connective tissue disease. J Rheumatol 2010;37:783–6. [DOI] [PubMed] [Google Scholar]
- 24. Watson HR, Robb R, Belcher G, Belch JJ.. Seasonal variation of Raynaud’s phenomenon secondary to systemic sclerosis. J Rheumatol 1999;26:1734–7. [PubMed] [Google Scholar]
- 25. Hughes M, Snapir A, Wilkinson J. et al. Prediction and impact of attacks of Raynaud’s phenomenon, as judged by patient perception. Rheumatology (Oxford) 2015;54:1443–7. [DOI] [PubMed] [Google Scholar]
- 26. Freedman RR, Ianni P.. Role of cold and emotional stress in Raynaud’s disease and scleroderma. Br Med J (Clin Res Ed) 1983;287:1499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freedman RR, Ianni P.. Effects of general and thematically relevant stressors in Raynaud’s disease. J Psychosom Res 1985;29:275–80. [DOI] [PubMed] [Google Scholar]
- 28. Freedman RR, Ianni P, Wenig P.. Behavioral treatment of Raynaud's disease. J Consult Clin Psychol 1983;51:539–49. [DOI] [PubMed] [Google Scholar]
- 29. Harrison BJ, Silman AJ, Hider SL, Herrick AL.. Cigarette smoking as a significant risk factor for digital vascular disease in patients with systemic sclerosis. Arthritis Rheum 2002;46:3312–6. [DOI] [PubMed] [Google Scholar]
- 30. Hudson M, Lo E, Lu Y. et al. Cigarette smoking in patients with systemic sclerosis. Arthritis Rheum 2011;63:230–8. [DOI] [PubMed] [Google Scholar]
- 31. Wigley FM, Korn JH, Csuka ME. et al. Oral iloprost treatment in patients with Raynaud’s phenomenon secondary to systemic sclerosis: a multicenter, placebo-controlled, double-blind study. Arthritis Rheum 1998;41:670–7. [DOI] [PubMed] [Google Scholar]
- 32. Black CM, Halkier-Sorensen L, Belch JJ. et al. Oral iloprost in Raynaud’s phenomenon secondary to systemic sclerosis: a multicentre, placebo-controlled, dose-comparison study. Br J Rheumatol 1998;37:952–60. [DOI] [PubMed] [Google Scholar]
- 33. Schiopu E, Hsu VM, Impens AJ. et al. Randomized placebo-controlled crossover trial of tadalafil in Raynaud’s phenomenon secondary to systemic sclerosis. J Rheumatol 2009;36:2264–8. [DOI] [PubMed] [Google Scholar]
- 34. Wigley FM, Wise RA, Seibold JR. et al. Intravenous iloprost infusion in patients with Raynaud phenomenon secondary to systemic sclerosis. A multicenter, placebo-controlled, double-blind study. Ann Intern Med 1994;120:199–206. [DOI] [PubMed] [Google Scholar]
- 35. Shenoy PD, Kumar S, Jha LK. et al. Efficacy of tadalafil in secondary Raynaud’s phenomenon resistant to vasodilator therapy: a double-blind randomized cross-over trial. Rheumatology (Oxford) 2010;49:2420–8. [DOI] [PubMed] [Google Scholar]
- 36. Herrick AL, van den Hoogen F, Gabrielli A. et al. Modified-release sildenafil reduces Raynaud’s phenomenon attack frequency in limited cutaneous systemic sclerosis. Arthritis Rheum 2011;63:775–82. [DOI] [PubMed] [Google Scholar]
- 37. Chung L, Shapiro L, Fiorentino D. et al. MQX-503, a novel formulation of nitroglycerin, improves the severity of Raynaud’s phenomenon: a randomized, controlled trial. Arthritis Rheum 2009;60:870–7. [DOI] [PubMed] [Google Scholar]
- 38. Fries R, Shariat K, von Wilmowsky H, Böhm M.. Sildenafil in the treatment of Raynaud’s phenomenon resistant to vasodilatory therapy. Circulation 2005;112:2980–5. [DOI] [PubMed] [Google Scholar]
- 39. Pauling JD, Shipley JA, Hart DJ, McGrogan A, McHugh NJ.. Use of laser speckle contrast imaging to assess digital microvascular function in primary Raynaud phenomenon and systemic sclerosis: a comparison using the Raynaud Condition Score Diary. J Rheumatol 2015;42:1163–8. [DOI] [PubMed] [Google Scholar]
- 40. Mendelson C, Poole JL, Allaire S.. Experiencing work as a daily challenge: the case of scleroderma. Work 2013;44:405–13. [DOI] [PubMed] [Google Scholar]
- 41. Mendelson C, Poole JL.. Become your own advocate: advice from women living with scleroderma. Disabil Rehabil 2007;29:1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gladue H, Maranian P, Paulus HE, Khanna D.. Evaluation of test characteristics for outcome measures used in Raynaud’s phenomenon clinical trials. Arthritis Care Res 2013;65:630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pauling J, Frech TM, Hughes M. et al. Attitudes toward patient-reported outcome instruments for the assessment of Raynaud’s phenomenon in systemic sclerosis. Arthritis Rheumatol 2016;68(Suppl 10): Abstract No. 2909. [Google Scholar]
- 44. Roustit M, Blaise S, Allanore Y. et al. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud’s phenomenon: systematic review and meta-analysis of randomised trials. Ann Rheum Dis 2013;72:1696–9. [DOI] [PubMed] [Google Scholar]
- 45. Belch JJ. Raynaud’s phenomenon: its relevance to scleroderma. Ann Rheum Dis 1991;50 (Suppl 4):839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steen VD, Medsger TA Jr.. The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum 1997;40:1984–91. [DOI] [PubMed] [Google Scholar]
- 47. Khanna PP, Maranian P, Gregory J, Khanna D.. The minimally important difference and patient acceptable symptom state for the Raynaud’s condition score in patients with Raynaud’s phenomenon in a large randomised controlled clinical trial. Ann Rheum Dis 2010;69:588–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bacher A, Mittoo S, Hudson M. et al. Systemic sclerosis in Canada's North American Native population: assessment of clinical and serological manifestations. J Rheumatol 2013;40:1121–6. [DOI] [PubMed] [Google Scholar]
- 49. Willems LM, Kwakkenbos L, Leite CC. et al. Frequency and impact of disease symptoms experienced by patients with systemic sclerosis from five European countries. Clin Exp Rheumatol 2014;32(6 Suppl 86):S-88–93. [PubMed] [Google Scholar]
- 50. Bassel M, Hudson M, Taillefer SS. et al. Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian National Survey. Rheumatology (Oxford) 2011;50:762–7. [DOI] [PubMed] [Google Scholar]
- 51. Leite CC, Maia A.. Symptoms of disease and psychological adaptation in Brazilian scleroderma patients. Rev Bras Reumatol 2013;53:405–11. [PubMed] [Google Scholar]
- 52. Sandqvist G, Hesselstrand R, Eberhardt K.. A longitudinal follow-up of hand involvement and activities of daily living in early systemic sclerosis. Scand J Rheumatol 2009;38:304–10. [DOI] [PubMed] [Google Scholar]
- 53. Nguyen VA, Eisendle K, Gruber I. et al. Effect of the dual endothelin receptor antagonist bosentan on Raynaud’s phenomenon secondary to systemic sclerosis: a double-blind prospective, randomized, placebo-controlled pilot study. Rheumatology (Oxford) 2010;49:583–7. [DOI] [PubMed] [Google Scholar]
- 54. Frantz C, Avouac J, Distler O. et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: a large international survey. Semin Arthritis Rheum 2016;46:115–23. [DOI] [PubMed] [Google Scholar]
- 55. Stamm TA, Mattsson M, Mihai C. et al. Concepts of functioning and health important to people with systemic sclerosis: a qualitative study in four European countries. Ann Rheum Dis 2011;70:1074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jewett LR, Malcarne VL, Kwakkenbos L. et al. Development and validation of the Body Concealment Scale for Scleroderma. Arthritis Care Res 2016;68:1158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sandqvist G, Hesselstrand R, Scheja A, Hakansson C.. Managing work life with systemic sclerosis. Rheumatology (Oxford) 2012;51:319–23. [DOI] [PubMed] [Google Scholar]
- 58. Herrgott I, Riemekasten G, Hunzelmann N, Sunderkotter C.. Management of cutaneous vascular complications in systemic scleroderma: experience from the German network. Rheumatol Int 2008;28:1023–9. [DOI] [PubMed] [Google Scholar]
- 59. Walker KM, Pope J; participating members of the Scleroderma Clinical Trials Consortium (SCTC); Canadian Scleroderma Research Group (CSRG). Treatment of systemic sclerosis complications: what to use when first-line treatment fails–a consensus of systemic sclerosis experts. Semin Arthritis Rheum 2012;42:42–55. [DOI] [PubMed] [Google Scholar]
- 60. Harding S, Khimdas S, Bonner A, Baron M, Pope J; Canadian Scleroderma Research Group. Best practices in scleroderma: an analysis of practice variability in SSc centres within the Canadian Scleroderma Research Group (CSRG). Clin Exp Rheumatol 2012;30(2 Suppl 71):S38–43. [PubMed] [Google Scholar]
- 61. Kowal-Bielecka O, Fransen J, Avouac J. et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017;76:1327–39. [DOI] [PubMed] [Google Scholar]
- 62. Pope J, Harding S, Khimdas S. et al. Agreement with guidelines from a large database for management of systemic sclerosis: results from the Canadian Scleroderma Research Group. J Rheumatol 2012;39:524–31. [DOI] [PubMed] [Google Scholar]
- 63. Moinzadeh P, Riemekasten G, Siegert E. et al. Vasoactive therapy in systemic sclerosis: real-life therapeutic practice in more than 3000 patients. J Rheumatol 2016;43:66–74. [DOI] [PubMed] [Google Scholar]


