Abstract
Objective
Lesinurad (LESU) is a selective urate reabsorption inhibitor approved at 200 mg daily for use with a xanthine oxidase inhibitor (XOI) to treat hyperuricaemia in gout patients failing to achieve target serum urate on XOI. The aim of the study was to investigate the long-term safety of LESU + XOI therapy.
Methods
Safety data were pooled from three 12-month phase III (core) trials evaluating LESU 200 and 400 mg/day combined with an XOI (LESU200+XOI and LESU400+XOI), and two 12-month extension studies using descriptive statistics. To adjust for treatment duration, treatment-emergent adverse events (TEAEs) were expressed as exposure-adjusted incidence rates (patients with events per 100 person-years).
Results
In the core studies, exposure-adjusted incidence rates for total and total renal-related TEAEs were comparable for XOI alone and LESU200+XOI but higher with LESU400+XOI. Exposure-adjusted incidence rates for serum creatinine (sCr) elevations ⩾1.5×baseline were 2.9, 7.3 and 18.7, respectively. Resolution (sCr ⩽1.2×baseline) occurred in 75–90% of all events, with 66–75% occurring without any study medication interruption. Major adverse cardiovascular events were 3, 4 and 9 with XOI, LESU200+XOI and LESU400+XOI, respectively. Longer exposure in core+extension studies did not increase rates for any safety signals.
Conclusion
At the approved dose of 200 mg once-daily combined with an XOI, LESU did not increase renal, cardiovascular or other adverse events compared with XOI alone, except for sCr elevations. With extended exposure in the core+extension studies, the safety profile was consistent with that observed in the core studies, and no new safety concerns were identified.
Keywords: gout, lesinurad, serum creatinine, treatment-emergent adverse events, xanthine oxidase inhibitor, combination therapy, renal adverse events, cardiovascular adverse events
Rheumatology key messages
Gout patients had comparable renal-related treatment-emergent adverse events with xanthine oxidase inhibitors ± lesinurad 200 mg after 12 months.
In gout patients, adding lesinurad to xanthine oxidase inhibitor monotherapy increased serum creatinine elevations vs monotherapy alone.
Treating gout patients with lesinurad 200 mg + xanthine oxidase inhibitors for 24 months revealed no new safety concerns.
Introduction
Gouty arthritis is a significant public health problem, driven by excess body stores of uric acid, reflected in sustained hyperuricaemia [1, 2]. If hyperuricaemia in patients with gout is not adequately treated, deposition of monosodium urate crystals commonly progresses in joints and periarticular tissues, and promotes increased symptoms and joint damage [1, 2]. Long-term therapy for gout, advocated by multiple Rheumatology Society guidelines, includes pharmacologic measures to reduce serum urate (sUA) levels to <6.0 mg/dl and even lower (<5.0 mg/dl) for severe disease [3, 4]. Success of this therapeutic approach, as demonstrated in both early and advanced stages of gout, promotes dissolution of deposited urate crystals and eventual reduction of acute gouty arthritis flares and synovitis [5, 6]. The recommended first-line urate-lowering therapy (ULT) approach in treatment of gout is use of a xanthine oxidase inhibitor (XOI), either allopurinol or febuxostat [3, 4]. The XOIs inhibit urate production [3, 4]; however, many patients with gout fail to achieve their serum urate target using only XOI monotherapy [7–9], often due to poor compliance or failure to up-titrate the dose. In those circumstances, treatment recommendations include use of a uricosuric alone (probenecid or benzbromarone) or combination ULT, using XOI and uricosuric agents to provide complementary mechanisms of action [3, 4]. As the amount of uric acid renally excreted is decreased by XOI therapy, combination XOI and uricosuric therapy can more effectively lower body uric acid stores than an XOI alone, by increasing the major pathway of uric acid excretion by the kidney [3, 4].
Lesinurad (LESU) is a selective uric acid reabsorption inhibitor approved in the United States and Europe at a 200 mg daily dose in combination with an XOI for the treatment of hyperuricaemia associated with gout in patients not achieving target sUA levels on an XOI alone [10]. LESU reduces sUA by inhibiting uric acid transporter 1 (URAT1), which is responsible for the majority of the reabsorption of filtered urate from the renal tubular lumen [11]. LESU increases the excretion of uric acid by the kidney and lowers sUA levels [12, 13].
The LESU clinical programme included three pivotal placebo-controlled, 12-month phase III (core) studies (CLEAR 1, CLEAR 2 and CRYSTAL) that evaluated LESU 200 mg (LESU200) and LESU 400 mg (LESU400), combined with an XOI [14–16]. In these trials, greater proportions of patients treated with LESU200 or LESU400 combined with an XOI achieved sUA targets at 6 and 12 months vs an XOI alone. However, concerns were raised about the safety of combining an XOI with LESU 400 mg. LESU 400 mg monotherapy significantly lowered sUA compared with placebo for up to 18 months [13]. However, there was a high incidence of serum creatinine (sCr) elevations and renal-related adverse events, including serious adverse events. Therefore, it was important to obtain data with the combination therapy over longer periods. Patients completing the core studies were eligible to enter extension studies, in which patients treated with LESU at 200 mg and 400 mg doses in combination with an XOI for up to 2 years, exhibited continued improvements in signs and symptoms of gout, including reductions in tophi and gout flares, while maintaining lower sUA levels [17–19]. We investigated the safety of LESU200+XOI and LESU400+XOI in the three core studies and the two extension studies for a total treatment period of up to 2 years.
Methods
Patient population
Patients with a diagnosis of gout who entered the core studies were aged 18–85 years [11]. The inclusion criteria for CLEAR 1 and CLEAR 2 were sUA levels ⩾6.5 mg/dl while on allopurinol and ⩾2 gout flares in the previous 12 months. The inclusion criteria for CRYSTAL were sUA levels ⩾6.0 mg/dl for patients taking ULT or ⩾8.0 mg/dl for patients not taking ULT, and ⩾1 measurable tophi on hands/wrists and/or feet/ankles. Patients with creatinine clearance <30 ml/min calculated by Cockcroft-Gault formula were excluded from all three studies.
Study design
The three core studies evaluated LESU200 and LESU400 combined with an XOI, either allopurinol (CLEAR 1 and 2; NCT01510158, NCT01493531) or febuxostat (CRYSTAL, NCT01510769) [14–16]. The primary efficacy end point in CLEAR1 and CLEAR2 was the proportion of patients with sUA <6.0 mg/dl by month 6 in each treatment group. In the CRYSTAL study, patients with tophaceous gout were treated with febuxostat for 3 weeks before being randomized to febuxostat alone, LESU200+XOI or LESU400+XOI, and the primary end point was the proportion of patients with sUA <5.0 mg/dl by month 6 in each treatment group. In the CLEAR (NCT01808131) and CRYSTAL (NCT01808144) extension studies, LESU-treated patients who completed the core studies continued to receive LESU+XOI at the same dose (200CONT or 400CONT). Patients initially treated with only an XOI in the core studies were randomized 1:1 to receive LESU200 or LESU400 in addition to the XOI provided in the core trial (Supplementary Fig. S1, available at Rheumatology online). Safety was assessed throughout both the core and extension studies.
Safety assessments
Safety assessments included treatment-emergent adverse events (TEAEs); major adverse cardiovascular events (MACE): cardiovascular (CV) death, myocardial infarction or stroke [20]; CV and vascular TEAEs; and renal-related and kidney stone TEAEs. All TEAEs were reported by investigators and captured in the study case report forms. Investigators were instructed to provide a clinical diagnosis whenever possible. The diagnosis was determined solely by the investigator, not predefined by the sponsor. Laboratory abnormalities (e.g. serum creatinine 1.5 × baseline) were considered a TEAE only if reported so by the investigator, therefore not all abnormalities were TEAEs. Any clinical diagnosis associated (e.g. renal impairment, renal failure) was provided by the investigator. Severity was determined by the investigator according to the Rheumatology Common Terminology Criteria for Adverse Events, as was the relationship to study drug. A predefined list of 36 diagnoses to capture all renal-related adverse events (AEs) and 11 diagnoses to capture all kidney stone-related AEs [Medical Dictionary for Regulatory Activities (MedDRA) version 14.0 preferred terms; see Supplementary Table S1, available at Rheumatology online] was used during safety analysis, utilizing the diagnoses provided by the investigators. For example, if an investigator reported a serum creatinine elevation as a TEAE, the MedDRA term used would be blood creatinine increase. Serious AEs were defined as AEs that resulted in death, hospitalization or prolongation of existing hospitalization; or persistent or significant disability or congenital anomaly; were life threatening; or were considered an important medical event. An adjudication committee, blinded to treatment assignment, prospectively reviewed all deaths and serious CV events.
Institutional review boards (IRBs)/ethics committees (ECs) from each country reviewed and approved the protocol for each study. The central IRBs/ECs were Schulman Associates Institutional Review Board, Inc., Cincinnati, OH, US; Comité d’Éthique Hospitalo-Facultaire – Hôpital Erasme, Brussels, Belgium; Health and Disability Ethics Committees, Wellington, New Zealand; and Komisja Bioetyczna przy Okregowej Izbie Lekarskiej w Gdańsku, Gdańsk, Poland. The studies were conducted in accordance with Independent Ethics Committee E6 Good Clinical Practice, the Declaration of Helsinki (October 2008), and applicable local regulatory requirements. Patients provided written informed consent; they were permitted to withdraw from the medication or the study at any time.
Statistical analysis
Data were pooled for each treatment group from the three core studies and two extension studies. To adjust for varying durations of treatment, TEAEs were expressed as exposure-adjusted incidence rates (EAIRs), defined as the number of patients with events per 100 person-years (PY). No statistical analyses were performed, other than for MACE where the Poisson exact method was used to calculate the 95% CI.
Results
Patients
A total of 1332 patients were exposed to LESU during the core and extension study periods: LESU200+XOI (n = 666) and LESU400+XOI (n = 666). The patients were predominately male (95%) with an average age at baseline of the core studies of 52 years and body mass index of 34 kg/m2. The mean duration of gout was 12 years with an average experience of six gout flares in the previous 12 months (Table 1). At screening, one third of the patients had tophi, two thirds had hypertension, almost half had hyperlipidaemia and ∼17% had diabetes mellitus. ∼40% of patients had an estimated creatinine clearance (eCrCl) >90 ml/min, 41% had eCrCl 60–<90 ml/min and 19% had eCrCl 30–<60 ml/min. The average sUA at baseline was 6.5 mg/dl.
Table 1.
Demographic and clinical characteristics at screening/baseline in pooled core phase III studies
| XOI alone (n = 516) | LESU 200 mg +XOI (n = 511) | LESU 400 mg +XOI (n = 510) | |
|---|---|---|---|
| Male, n (%) | 492 (95.3) | 489 (95.7) | 482 (94.5) |
| Age, mean (s.d.), years | 52.2 (11.1) | 51.9 (11.0) | 52.1 (11.3) |
| Weight, mean (s.d.), kg | 105.5 (22.3) | 108.0 (22.4) | 106.2 (23.7) |
| Body mass index, mean (s.d.), kg/m2 | 33.7 (6.2) | 34.3 (6.2) | 33.8 (6.9) |
| Duration since gout diagnosis, mean (s.d.), years | 12.2 (9.6) | 13.2 (10.2) | 11.5 (9.3) |
| Tophi at screening, n (%) | 183 (35.5) | 184 (36.0) | 185 (36.3) |
| Number of gout flares in the past 12 months, mean (s.d.) | 5.5 (4.7) | 6.0 (7.1) | 5.8 (5.5) |
| Renal function at baseline, n (%), ml/min | |||
| eCrCl ≥90 | 180 (34.9) | 200 (39.1) | 203 (39.8) |
| eCrCl 60–<90 | 229 (44.4) | 208 (40.7) | 213 (41.8) |
| eCrCl 30–<60 | 101 (19.6) | 101 (19.8) | 92 (18.0) |
| eCrCl <30 | 4 (0.8) | 1 (0.2) | 0 |
| sUA level at baseline, mean (s.d.), mg/dl | 6.6 (1.50) | 6.6 (1.5) | 6.5 (1.45) |
| Comorbidities, n (%) | |||
| Hypertension | 340 (65.9) | 330 (64.6) | 325 (63.7) |
| Hyperlipidaemia | 221 (42.8) | 230 (45.0) | 241 (47.3) |
| Diabetes mellitus | 80 (15.5) | 96 (18.8) | 78 (15.3) |
| Kidney stones | 82 (15.9) | 58 (11.4) | 51 (10.0) |
| Myocardial infarction | 19 (3.7) | 26 (5.1) | 22 (4.3) |
eCrCl: estimated creatinine clearance; LESU: lesinurad; sUA: serum urate; XOI: xanthine oxidase inhibitor.
TEAE rates
The EAIRs of TEAEs in the core studies and the core + extension studies are presented in Table 2. In the core studies, EAIRs were similar with LESU200+XOI and XOI alone and were generally higher with LESU400+XOI for any TEAE, any TEAE with Rheumatology Common Toxicity Criteria grade 3 or 4, those possibly related to randomized study medication, possibly related to XOI, possibly related to prophylaxis, serious TEAEs, fatal TEAEs, and those leading to discontinuation of LESU. The number of these TEAEs increased with longer exposure to LESU200+XOI or LESU400+XOI in the core + extension studies, but EAIRs were lower than in the core studies.
Table 2.
Pooled analysis of exposure-adjusted TEAE incidence in core and core + extension studies
| Adverse event category, n (rate) | Core studies | Core + extension studies | |||
|---|---|---|---|---|---|
| XOI alone | LESU200 mg+XOI | LESU400 mg+XOI | LESU200 mg+XOI | LESU400 mg+XOI | |
| (n = 516) | (n = 511) | (n = 510) | (n = 666) | (n = 666) | |
| (PY = 408.5) | (PY = 396.3) | (PY = 390.5) | (PY = 1251.5) | (PY = 1236.5) | |
| Any TEAE | 363 (88.9) | 386 (97.4) | 407 (104.2) | 546 (43.6) | 568 (45.9) |
| Upper respiratory tract infection | 44 (10.8) | 46 (11.6) | 57 (14.6) | 85 (6.8) | 96 (7.8) |
| Nasopharyngitis | 43 (10.5) | 45 (11.4) | 47 (12.0) | 83 (6.7) | 83 (6.7) |
| Any TEAE with RCTC toxicity grade 3 or 4 | 48 (11.8) | 52 (13.1) | 67 (17.2) | 112 (8.9) | 134 (10.8) |
| Any TEAE possibly related to randomized study medication | 80 (19.6) | 98 (24.7) | 118 (30.2) | 172 (13.7) | 203 (16.4) |
| Any TEAE possibly related to XOI | 52 (12.7) | 49 (12.4) | 66 (16.9) | 84 (6.7) | 111 (9.0) |
| Any TEAE possibly related to prophylaxis | 52 (12.7) | 56 (14.1) | 61 (15.6) | 78 (6.2) | 82 (6.6) |
| Any serious TEAE | 29 (7.1) | 24 (6.1) | 44 (11.3) | 79 (6.3) | 97 (7.8) |
| Acute myocardial infarction | 0 | 1 (0.3) | 4 (1.0) | 3 (0.2) | 5 (0.4) |
| Coronary artery disease | 0 | 3 (1.8) | 2 (0.5) | 4 (0.3) | 4 (0.3) |
| Acute renal failure | 2 (0.5) | 0 | 2 (0.5) | 3 (0.2) | 12 (1.0) |
| Any fatal TEAE | 0 | 2 (0.5) | 3 (0.8) | 10 (0.8) | 8 (0.6) |
| Any TEAE leading to discontinuation of LESU | 28 (6.9) | 32 (8.1) | 48 (12.3) | 84 (6.7) | 107 (8.7) |
Exposure-adjusted incidence rates are expressed as patients with events per 100 person-years.
LESU200/400: lesinurad 200 mg/400; PY: person-years; RCTC: Rheumatology Common Toxicity Criteria; TEAE: treatment-emergent adverse event; XOI: xanthine oxidase inhibitor.
The most common individual TEAEs were upper respiratory tract infections and nasopharyngitis; EAIRs were less in the core + extension studies than the core studies (Table 2). The most common serious TEAEs were acute myocardial infarction, coronary artery disease and acute renal failure. EAIRs were low in the core studies and did not increase in the core + extension studies.
Renal-related adverse event rates
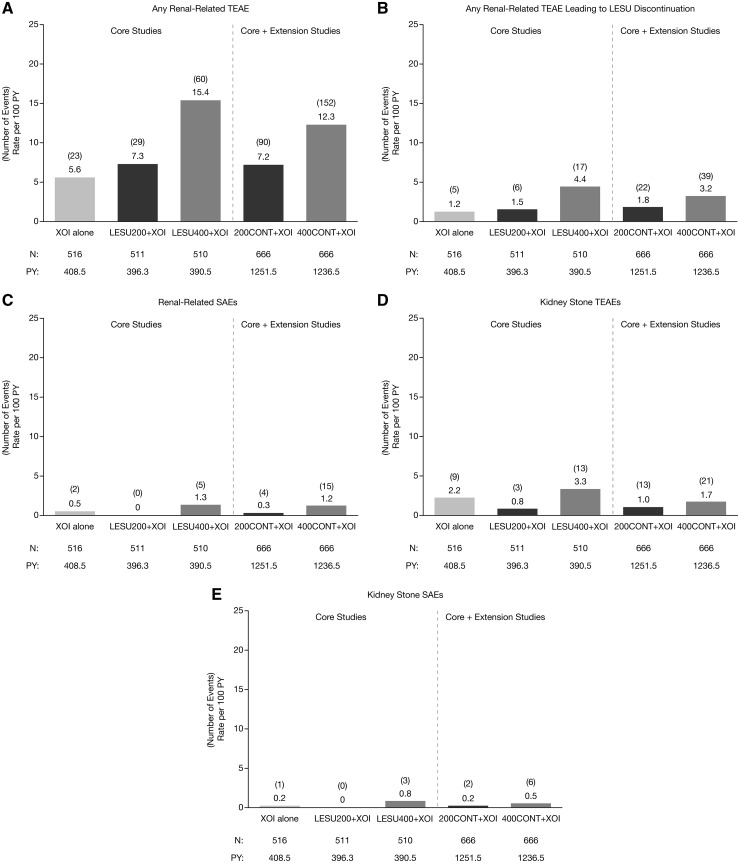
The EAIRs for any renal-related TEAE, any renal-related TEAE possibly related to randomized study medication and any renal-related serious TEAE in the core studies were similar with LESU200+XOI and XOI alone and were higher with LESU400+XOI (Fig. 1). EAIRs for kidney stone TEAEs and serious kidney stone TEAEs in the core studies were less with LESU200+XOI than with XOI alone and were greater with LESU400+XOI. Longer exposure in the extension studies did not increase the EAIRs for any of these TEAEs.
Fig. 1.
Exposure-adjusted rates of renal-related TEAEs
Pooled analysis of exposure-adjusted incidence rates of any renal-related TEAE (A), any renal-related TEAE leading to LESU discontinuation (B), any renal-related serious TEAE (C), kidney stone TEAEs (D), and serious kidney stone TEAEs (E) in core and core + extension studies. LESU200/400: lesinurad 200 mg/400 mg; PY: person-years; TEAEs: treatment-emergent adverse events; XOI: xanthine oxidase inhibitor.
The most common renal-related TEAE was increased blood creatinine, for which the rate was dose-ordered. Others were increased blood urea nitrogen, renal failure and renal impairment where the rates were low and generally similar across treatment groups (Table 3). Rates for these TEAEs were not increased in the core + extension studies. The most common MedDRA-defined serious renal-related TEAEs were acute renal failure, renal failure, chronic renal failure and renal impairment (Table 3). In the core studies, these serious AEs only occurred in the XOI alone (acute renal failure only) and the LESU400+XOI groups, with EAIRs ranging from 0 to 0.5 events per 100 PY. In the core + extension studies, EAIRs for these serious AEs were ⩽0.2 except for acute renal failure in the LESU400+XOI group (1.0).
Table 3.
Exposure-adjusted incidence rates of renal-related adverse events
| Core studies | Core + extension studies | ||||
|---|---|---|---|---|---|
| XOI alone (n = 516) (PY = 408.5) | LESU200+XOI (n = 511) (PY = 396.3) | LESU400+XOI (n = 510) (PY = 390.5) | LESU200+XOI (n = 666) (PY = 1251.0) | LESU400+XOI (n = 666) (PY = 1236.4) | |
| Most common renal-related TEAEs, n (rate) | |||||
| Increased blood creatinine | 12 (2.9) | 22 (5.6) | 40 (10.2) | 70 (5.6) | 105 (8.5) |
| Increased blood urea | 3 (0.7) | 7 (1.8) | 7 (1.8) | 13 (1.0) | 15 (1.2) |
| Renal failure | 6 (1.5) | 4 (1.0) | 6 (1.5) | 5 (0.4) | 13 (1.1) |
| Renal impairment | 0 | 1 (0.3) | 5 (1.3) | 6 (0.5) | 11 (0.9) |
| Acute renal failure | 2 (0.5) | 0 | 4 (1.0) | 4 (0.4) | 17 (1.9) |
| Most common serious renal-related TEAEs, n (%) | |||||
| Acute renal failure | 2 (0.5) | 0 | 2 (0.5) | 3 (0.2) | 12 (1.0) |
| Renal failure | 0 | 0 | 1 (0.3) | 0 | 1 (0.1) |
| Chronic renal failure | 0 | 0 | 1 (0.3) | 0 | 1 (0.1) |
| Renal impairment | 0 | 0 | 1 (0.3) | 1 (0.1) | 1 (0.1) |
Exposure-adjusted incidence rates are expressed as patients with events per 100 person-years.
LESU200/400: lesinurad 200 mg/400 mg; PY: patient years; sCr: serum creatinine; TEAEs: treatment-emergent adverse events; XOI: xanthine oxidase inhibitor.
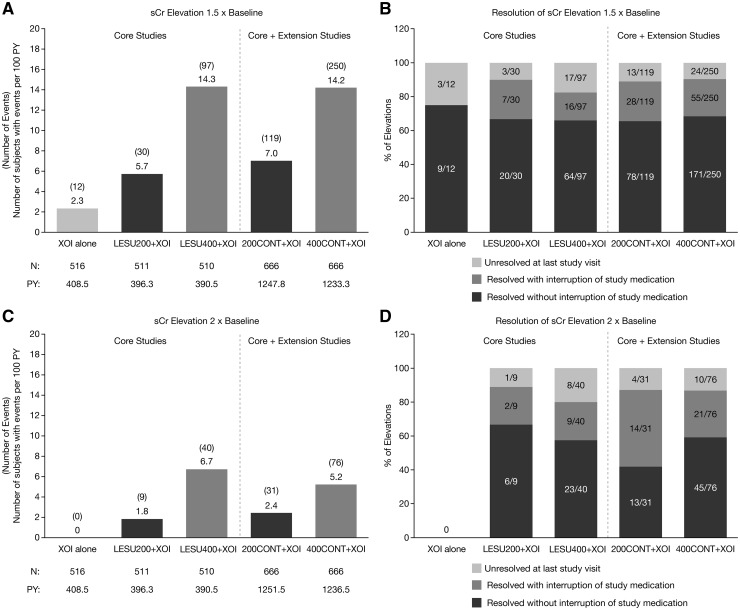
EAIRs for sCr elevations ⩾1.5× baseline in the core studies were dose-ordered, being lowest with XOI alone and highest with LESU400+XOI (Fig. 2A). EAIRs were also dose-ordered in the core + extension studies, with the rate the same in the LESU200+XOI group and lower in the LESU400+XOI group than in the core studies. The percentage of events that resolved during the studies (sCr ⩽1.2× baseline) ranged from 75% (9/12 events) in the core XOI group to 92% (80/87 events) in the core LESU400+XOI group and was 89–90% in the core + extension groups (Fig. 2B). The percentage that resolved without interruption of study medication was 66–68% for all LESU-treated groups and 75% for the core XOI group. EAIRs for sCr elevations ⩾2.0× baseline were lower, but the pattern was similar to elevations ⩾1.5× baseline (Fig. 2C). The percentage of events that resolved ranged from 80% (32/40 events) in the core LESU400+XOI group to 92% (8/9 events) in the core LESU200+XOI group and was 87% in both core + extension groups (Fig. 2D). The percentage that resolved without interruption of study medication ranged from 42% (13/31 events) in the core + extension 200CONT+XOI group to 67% (6/9 events) in the core LESU200+XOI group. The percentage was 58% (23/40 events) in the core LESU400+XOI group and 59% (45/76) in the core + extension 400CONT+XOI group.
Fig. 2.
Exposure-adjusted rates of sCr elevations and resolution of sCR elevations
Exposure-adjusted rates of serum creatinine 1.5 × baseline (A) and 2.0 × baseline (C) and resolution of the elevations (B & D, respectively) in the core and core + extension studies. LESU200/400: lesinurad 200 mg/400 mg; PY: person-years; sCr: serum creatinine; XOI: xanthine oxidase inhibitor.
MACE rates/CV adverse events
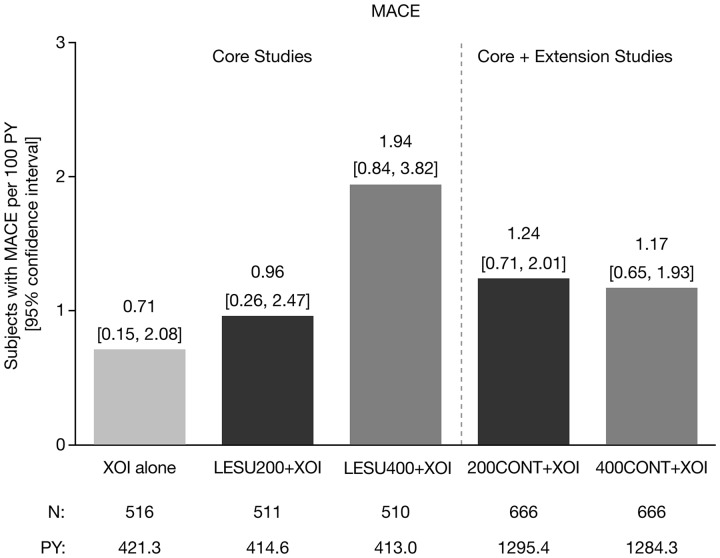
In the core studies, there were four adjudicated MACEs that occurred in 3/516 patients (0.6%) on XOI alone, four events in 4/511 patients (0.8%) on LESU200+XOI, and nine events in 8/510 patients (1.6%) on LESU400+XOI. The higher incidence with LESU400+XOI was driven by a higher incidence of nonfatal myocardial infarction (XOI, 1; LESU200+XOI, 2; LESU400+XOI, 7). In the core + extension studies, there were 17 MACE in 16/666 patients (2.4%) in the LESU200+XOI group and 17 events in 15/66 patients (2.3%) in the LESU400+XOI group. The higher incidence of nonfatal myocardial infarction observed with LESU400+XOI in the core studies was retained in the core + extension studies (LESU200+XOI, 5; LESU400+XOI, 9). Exposure-adjusted MACE rates in the core and core + extension studies are shown in Fig. 3.
Fig. 3.
Exposure-adjusted rates of MACE
LESU200/400: lesinurad 200 mg/400 mg; MACE: major adverse cardiovascular events; PY: patient years; XOI: xanthine oxidase inhibitor.
The most common individual cardiac TEAE was angina. In the core studies, the EAIR was 0.5 patients with an event per 100 PY with an XOI alone, 1.0 with LESU200+XOI and 1.5 with LESU400+XOI, and was 0.5 and 0.6 with LESU200+XOI and LESU400+XOI, respectively, in the core + extension studies. The most common vascular TEAE was hypertension. The EAIRs in the core studies were 6.1, 7.8 and 9.0, respectively, and 5.0 and 6.4 in the core + extension studies, respectively.
Discussion
In this integration of clinical trials, LESU at the sole approved dose of 200 mg/day combined with an XOI demonstrated a consistent safety profile in the core studies with TEAE rates that did not differ from XOI monotherapy. Rates with LESU400+XOI were higher than XOI alone or LESU200+XOI. A similar pattern was observed in the core studies for renal-related AEs, except for higher rates of sCr elevations. Renal safety was of special interest because many gout patients have significant comorbid illnesses, including chronic kidney disease, diabetes, hypertension and heart failure, and certain medications used for these conditions that put them at increased risk for adverse effects related to the kidney [21–23]. That risk might theoretically be increased in patients treated with LESU because the drug is rapidly absorbed and rapidly increases urinary uric acid excretion, although an exact mechanism for the effects of LESU on the kidney is unknown. However, the rates of renal-related TEAEs were similar with XOI alone and LESU200+XOI, except for higher rates of sCr elevations. Rates were higher with LESU400+XOI. The rate of kidney stone TEAEs was actually less with LESU200+XOI than with XOI alone, while rates were higher with LESU400+XOI. Serious kidney stone TEAEs were rare in any treatment group.
Extended exposure to LESU did not lead to higher EAIRs of TEAEs, renal-related TEAEs, or kidney stone TEAEs in the core + extension studies than those observed in the core studies. Overall, the renal safety profile in the core + extension studies was consistent with that observed in the core studies, and no new safety concerns were identified in the extension studies.
The mechanism underlying the elevation of sCr levels is not fully known, but it may be due to increased excretion and microcrystallization of urinary uric acid in renal tubules. The vast majority of sCr elevations in the core and core + extension studies resolved, most often without an interruption of study medication. When LESU was interrupted, it was resumed when sCr was ⩽0.1 mg/dl greater than the baseline value. There was no apparent association between the incidence of sCr elevations and baseline renal function (eCrCl category ⩾90, <90, ⩾60, ⩾45 and <45 ml/min), and therefore no evidence that patients with impaired renal function at baseline were at an increased risk for sCr elevations. In fact, the incidence of sCr elevations was lower in those with eCrCl <60 ml/min, presumably because less was excreted in the urine. There was also no apparent relationship between the incidence of sCr elevations and either the presence/absence of tophi at screening or gout flare prophylaxis medication (NSAID/colchicine). An exploratory analysis was conducted to identify potential predictors of sCr elevations and to determine whether any subgroup of patients had an increased risk for sCr elevations. ∼300 parameters were evaluated: baseline laboratory values and vital signs, all AEs, concomitant medications, questionnaire scores, physical examination results, medical history, ECG data and gout flare data. No strong predictors of sCr elevations were identified. Baseline urinary uric acid, urinary creatinine and sCr and parameters related to general health (body mass index, smoking, physical functioning) were associated with elevations; however, none of the associations was strong enough to be of practical importance in either predicting sCr elevations or in identifying a subgroup of patients with increased risk.
CV safety was also of special interest because hyperuricaemia and gout are associated with increased CV events and mortality. While the MACE rates in the core studies appeared to be dose-ordered, assessment of treatment-associated differences in risk is limited due to the small number of MACE. Hence, a causal relationship with LESU has not been determined.
The observed MACE rate for the total population of LESU-treated patients was virtually identical to the rate in a prospective study of 1732 patients with gout treated with allopurinol for 6 months [7]. In that study, which included the same entry criteria and prospective adjudication of potential CV events utilized in the LESU clinical programme, the MACE rate was 1.42 events per 100 PY (95% CI: 0.68, 2.62) (10 MACE events, 703.2 PY; 95% CI based on Poisson distribution). In addition, the CV mortality rate in the LESU treatment groups in the core studies (0.48 CV deaths per 100 PY [95% CI: 0.18, 1.29]) was lower than the unadjusted CV mortality rate among patients with gout from the National Health and Nutrition Examination Survey (NHANES) (2.31 CV deaths per 100 PY) [24]. The rates are also broadly similar to other long-term follow-up studies of patients with gout [25, 26].
Limitations to the studies include the inability to follow all dropouts and patients after the study who had sCr elevations. In order to capture the clinical diagnoses provided by investigators, a predefined list of MedDRA terms that included such terms as renal failure, acute renal failure and chronic renal failure was used without a distinct definition for each term.
LESU, at the approved dose of 200 mg once daily combined with an XOI, did not increase renal, CV or other AEs compared with an XOI alone in the core studies, except for a higher rate of sCr elevations. With extended exposure in the extension studies, the renal safety profile was consistent with that observed in the core studies, and no new safety concerns were identified.
Supplementary Material
Acknowledgements
Editorial support was provided by Tom Claus, PhD, of PAREXEL and funded by Ironwood Pharmaceuticals.
Funding: Funding was provided by Ardea Biosciences, Inc., a member of the AstraZeneca group, and Ironwood Pharmaceuticals, Inc.
Disclosure statement: R.T. is consultant for Ardea Bisociences/AstraZeneca, SOBI, Horizon, Revive, Aequus, Relburn, Selecta and ProteoThera. K.G.S. is consultant for Ardea/AstraZeneca, Horizon and Takeda. D.S.G. is consultant for AstraZeneca, Revive, Cymabay and Retrophin and owns the Ravine Group. S.B. is a former employee of Ardea Biosciences, Inc., a member of the AstraZeneca Group. B.M.S. is an employee of Ironwood Pharmaceuticals. R.V. is a former employee of Ironwood Pharmaceuticals. M.P. has consulted for AstraZeneca and Crealta/Horizon and received a research grant from Takeda. W.B.W. is consultant for AstraZeneca. D.J. has declared no conflicts of interest.
References
- 1. Dalbeth N, Bardin T, Doherty M. et al. Discordant American College of Physicians and international rheumatology guidelines for gout management: consensus statement of the Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN). Nat Rev Rheumatol 2017;13:561–8. [DOI] [PubMed] [Google Scholar]
- 2. Neogi T. Clinical practice. Gout. N Engl J Med 2011;364:443–52. [DOI] [PubMed] [Google Scholar]
- 3. Khanna D, Fitzgerald JD, Khanna PP. et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richette P, Doherty M, Pascual E. et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42. [DOI] [PubMed] [Google Scholar]
- 5. Dalbeth N, Saag KG, Palmer WE. et al. Effects of febuxostat in early gout: a randomized, double-blind, placebo-controlled study. Arthritis Rheumatol 2017;69:2386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sundy JS, Baraf HS, Yood RA. et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 2011;306:711–20. [DOI] [PubMed] [Google Scholar]
- 7. Becker MA, Fitz-Patrick D, Choi HK. et al. An open-label, 6-month study of allopurinol safety in gout: the LASSO study. Semin Arthritis Rheum 2015;45:174–83. [DOI] [PubMed] [Google Scholar]
- 8. Cutolo M, Cimmino MA, Perez-Ruiz F.. Potency on lowering serum uric acid in gout patients: a pooled analysis of registrative studies comparing febuxostat vs. allopurinol. Eur Rev Med Pharmacol Sci 2017;21:4186–95. [PubMed] [Google Scholar]
- 9. Singh JA, Akhras KS, Shiozawa A.. Comparative effectiveness of urate lowering with febuxostat versus allopurinol in gout: analyses from large U.S. managed care cohort. Arthritis Res Ther 2015;17:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huneycutt E, Board C, Clements JN.. Lesinurad, a selective URAT-1 inhibitor with a novel mechanism in combination with a xanthine oxidase inhibitor, for hyperuricemia associated with gout. J Pharm Pract 2017; Advance Access published 1 January 2017, doi: 10.1177/0897190017734427. [DOI] [PubMed] [Google Scholar]
- 11. Miner J, Tan PK, Hyndman D. et al. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res Ther 2016;18:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleischmann R, Kerr B, Yeh LT. et al. Pharmacodynamic, pharmacokinetic and tolerability evaluation of concomitant administration of lesinurad and febuxostat in gout patients with hyperuricaemia. Rheumatology (Oxford) 2014;53:2167–74. [DOI] [PubMed] [Google Scholar]
- 13. Tausche AK, Alten R, Dalbeth N. et al. Lesinurad monotherapy in gout patients intolerant to a xanthine oxidase inhibitor: a 6 month phase 3 clinical trial and extension study. Rheumatology (Oxford) 2017;56:2170–8. [DOI] [PubMed] [Google Scholar]
- 14. Bardin T, Keenan RT, Khanna PP. et al. Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann Rheum Dis 2017;76:811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dalbeth N, Jones G, Terkeltaub R. et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with febuxostat in patients with tophaceous aout: findings of a phase III clinical trial. Arthritis Rheumatol 2017;69:1903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saag KG, Fitz-Patrick D, Kopicko J. et al. Lesinurad combined with allopurinol: a randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study). Arthritis Rheumatol 2017;69:203–12. [DOI] [PubMed] [Google Scholar]
- 17. Bardin T, Dalbeth N, Terkeltaub R. et al. Clinical response of tophus and flares to extended use of lesinurad in combination with a xanthine oxidase inhibitor in patients with gout. Arthritis Rheumatol 2016;68(Suppl 10):Abstract 209. [Google Scholar]
- 18. Dalbeth N, Jones G, Terkeltaub R. et al. Efficacy and safety in patients with tophaceous gout receiving lesinurad and febuxostat combination therapy: interim analysis of an extension study. Arthritis Rheumatol 2015;67(suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-in-patients-with-tophaceous-gout-receiving-lesinurad-and-febuxostat-combination-therapy-interim-analysis-of-an-extension-study/ (27 July 2018, date last accessed).
- 19. Saag K, Becker MA, Storgard C. et al. Examination of serum uric acid (sUA) lowering and safety with extended lesinurad + allopurinol treatment in subjects with gout. Arthritis Rheumatol 2016;68(Suppl 10):Abstract 208. [Google Scholar]
- 20. White WB, Faich G, Borer JS, Makuch RW.. Cardiovascular thrombotic events in arthritis trials of the cyclooxygenase-2 inhibitor celecoxib. Am J Cardiol 2003;92:411–8. [DOI] [PubMed] [Google Scholar]
- 21. Chawla LS, Kimmel PL.. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012;82:516–24. [DOI] [PubMed] [Google Scholar]
- 22. Krishnan E, Akhras KS, Sharma H. et al. Serum urate and incidence of kidney disease among veterans with gout. J Rheumatol 2013;40:1166–72. [DOI] [PubMed] [Google Scholar]
- 23. Richette P, Clerson P, Périssin L, Flipo RM, Bardin T.. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis 2015;74:142–7. [DOI] [PubMed] [Google Scholar]
- 24. Stack AG, Hanley A, Casserly LF. et al. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM 2013;106:647–58. [DOI] [PubMed] [Google Scholar]
- 25. Becker MA, Schumacher HR, Espinoza LR. et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010;12:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krishnan E, Svendsen K, Neaton JD. et al. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med 2008;168:1104–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.