Abstract
Objective
Tofacitinib is an oral Janus kinase inhibitor for treatment of RA. We compared tofacitinib modified-release (MR) 11 mg once daily (QD) with tofacitinib immediate-release (IR) 5 mg twice daily (BID) in Japanese patients with RA and inadequate response to MTX.
Methods
Phase III, randomized, double-blind, double-dummy, 12-week study. Patients were randomized to tofacitinib MR 11 mg QD (n = 104) or IR 5 mg BID (n = 105), with stable MTX. Compliance was based on returned pill counts. The primary objective was to demonstrate non-inferiority of MR 11 mg QD to IR 5 mg BID. Non-inferiority was declared if the upper bound of the two-sided 95% CI for the difference in change from baseline in DAS28-4(CRP) at week 12 was <0.6.
Results
At week 12, with tofacitinib MR 11 mg QD and IR 5 mg BID, respectively, the change from baseline in least squares mean DAS28-4(CRP) was −2.43 and −2.85; the mean difference was 0.43 (95% CI 0.17, 0.69). Non-inferiority of MR 11 mg QD to IR 5 mg BID was not met. Improvement of DAS28-4(CRP) ⩾1.2 was observed in 89 and 85% of patients, respectively, corresponding to a clinically important, significant change in both groups. The frequency of adverse events (52.9 and 51.4%, respectively) and serious adverse events (4.8 and 3.8%, respectively) was generally similar between treatments. No deaths were reported.
Conclusion
Non-inferiority of MR 11 mg QD to IR 5 mg BID was not met in this study. However, clinically meaningful improvements in RA were observed with both tofacitinib formulations in Japanese patients. The safety profile was similar with both formulations.
Trial registration
ClinicalTrials.gov, http://clinicaltrials.gov, NCT02281552.
Keywords: Japan, non-inferiority, once daily, RA, tofacitinib, twice daily
Rheumatology key messages
Non-inferiority of tofacitinib once daily to twice daily was not demonstrated in Japanese patients with RA.
Once- and twice-daily tofacitinib formulations both provided clinically meaningful improvements in Japanese patients with RA.
Safety profiles were similar for once- and twice-daily tofacitinib formulations in patients with RA over 12 weeks.
Introduction
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. An immediate-release (IR) formulation of tofacitinib is widely available and requires a twice-daily (BID) dosing regimen. The efficacy and safety of tofacitinib 5 and 10 mg BID in patients with moderately to severely active RA have been demonstrated in phase II [1–5], phase III [6–11] and long-term extension studies [12–14].
An extrudable core system osmotic delivery technology [15] has been used to develop a modified-release (MR) formulation of tofacitinib. The MR tablet formulation was developed to provide a once-daily (QD) dosing option for patients treated with tofacitinib, thereby enhancing patient convenience and providing another treatment option for the management of RA, a chronic and heterogeneous disease. The MR formulation might improve treatment compliance in patients who can better adhere to a QD regimen, potentially improving disease control and health outcomes in these patients [16–18]. The MR formulation at a dose of 11 mg has demonstrated equivalence in key exposure parameters, including the area under the plasma concentration–time profile (AUC) and the maximum plasma concentration, compared with the IR 5 mg BID formulation [19].
A series of exposure–response analyses, evaluated using clinical end points from the tofacitinib RA development programme, supported the importance of AUC to the efficacy and safety of tofacitinib [20]. These analyses, in conjunction with demonstrated equivalence in AUC, provided foundational rationale to translate clinical trial outcomes from the IR to the MR formulation and formed the basis of regulatory registration in the USA and other countries around the world [20].
In order to meet Japanese regulatory requirements, a direct comparison of the efficacy and safety of MR and IR formulations of tofacitinib in Japanese patients with RA in a randomized controlled clinical study was conducted. The primary objective of this clinical study was to demonstrate the non-inferiority of tofacitinib MR 11 mg QD relative to IR 5 mg BID based on change from baseline (CFB) in DAS in 28 joints with CRP (DAS28-4[CRP]) after 12 weeks of treatment, using a non-inferiority margin of 0.6 (which is also the DAS measurement error based on EULAR criteria [21]).
Methods
Patients
Eligible patients were ⩾20 years of age, had a diagnosis of RA based on the ACR 1987 revised criteria [22] for ⩾6 months before screening, with active disease at both screening and baseline defined as ≥6 tender/painful joints (68-joint assessment) and ≥6 swollen joints (66-joint assessment), and either CRP >0.7 mg/dl or ESR >28 mm/h at screening. These criteria were similar to those in the tofacitinib IR phase III studies for RA. Patients must have had a prior inadequate response to MTX. Key exclusion criteria are detailed in the Methods section (Patient exclusion criteria) of the supplementary data, available at Rheumatology online.
Study design
This was a phase III, randomized, double-blind, double-dummy, parallel-group, 12-week study (ClinicalTrials.gov: NCT02281552). Patients were randomized 1:1 to receive oral tofacitinib MR 11 mg QD or IR 5 mg BID, both with stable background MTX (6–16 mg/week for ⩾6 weeks before baseline; supplemented with folic acid or folinic acid), using an automated Web/telephone system provided by Pfizer Inc. Details of treatment administration, blinding and assessment of patient compliance are provided in the Methods section (Treatment administration and blinding) of the supplementary data, available at Rheumatology online. The study comprised four visits: screening, baseline and on-treatment visits at week 4 and week 12 (or early termination). Patients receiving concomitant glucocorticoids were required to remain on the same dose throughout the study.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation guidelines on Good Clinical Practice and applicable local regulatory requirements. The study protocol and documentation were reviewed by the institutional review board and independent ethics committees of each study centre, and all patients provided informed consent.
Study end points
The primary end point was the CFB in DAS28-4(CRP) at week 12. DAS28-4(CRP) was chosen as the primary end point because it is a validated end point that can be used to assess changes over time and was requested by the Japanese Pharmaceuticals and Medical Devices Agency. Secondary end points included CFB in DAS28-4(ESR), the proportions of patients achieving ⩾20, ⩾50 and ⩾70% improvement in ACR criteria (ACR20, ACR50 and ACR70 response rate, respectively), the proportion of patients achieving DAS28-4(CRP) <2.6 and ⩽3.2, the proportion of patients achieving DAS28-4(ESR) <2.6 (remission) and ⩽3.2 (low disease activity [LDA]), and changes from baseline in HAQ-Disability Index (HAQ-DI), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), EuroQol five dimensions questionnaire (EQ-5D) and short form 36 scores. Rates of remission and LDA based on Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI) and ACR-EULAR Boolean (remission only) definitions were analysed post hoc. All end points were assessed at baseline, week 4 and week 12, with the exceptions of FACIT-F and EQ-5D, which were assessed only at baseline and week 12.
Safety and tolerability were evaluated based on adverse event (AE) reporting, laboratory observations, physical examination and vital signs.
Statistical analysis
Primary and secondary efficacy end points were analysed for the full analysis set: all patients who received at least one dose of their randomized study treatment. The primary objective was to demonstrate the non-inferiority in efficacy of tofacitinib MR 11 mg QD relative to IR 5 mg BID. A non-inferiority margin of 0.6 for the treatment difference of tofacitinib MR 11 mg QD vs IR 5 mg BID was requested by the Japanese Pharmaceuticals and Medical Devices Agency. The DAS measurement error based on EULAR criteria is 0.6, whereas a clinically significant change is ⩾1.2 [21, 23]. Typically, a non-inferiority margin that represents ⩾50% of the placebo-adjusted control drug effect is suggested [24]; thus, a particularly stringent non-inferiority margin was used in the present study. Non-inferiority was to be declared if the upper bound of the two-sided 95% CI for the difference in DAS28-4(CRP) between treatment groups was <0.6.
The study sample size was determined based on the following assumptions for treatment comparisons: one-sided type I error of 0.025; patient assignment to treatment in a 1:1 ratio; the true difference in treatment means between the tofacitinib formulations of zero; and an s.d. of 1.3 (based on previous tofacitinib studies in Japanese patients). Based on these assumptions, a total of ⩾200 patients (100 per treatment arm) were required to demonstrate non-inferiority with a power of 90%.
A prespecified sensitivity analysis was carried out for the primary end point based on the per protocol analysis set. The per protocol analysis set was a subset of the full analysis set that included all randomized patients who completed the 12-week study with no protocol deviations that could impact the efficacy analysis.
Continuous data were analysed using a linear mixed-effect model with repeated measures, which included treatment, visit and treatment by visit interaction as fixed effects and patients as a random effect. Binary data were analysed using the normal approximation for the difference in binomial proportions, with non-responder imputation for missing values. No formal hypothesis tests were conducted except for the non-inferiority in primary end point. The P-values for all secondary end points were not adjusted for multiplicity (i.e. type I error was not controlled) and were exploratory in nature; caution should be used when interpreting the statistical significance. Post hoc analyses are detailed in the Methods section (Post hoc efficacy analyses) of the supplementary data, available at Rheumatology online.
Using efficacy responses, as measured by DAS28-3(CRP), a metric consistently used across all trials of tofacitinib, the dose–response relationship was characterized at week 12 across phase II, double-blind, placebo-controlled dose-ranging studies of tofacitinib (NCT00413660; NCT00550446; NCT00603512; NCT00687193) [19, 20]. Efficacy responses for the MR formulation from the present study were overlaid to examine the consistency with the dose–response profile of tofacitinib. For additional sensitivity, these analyses were conducted by pooling across all phase II studies (n = 4) and by stratifying by Japanese phase II studies (n = 2). Details of the modelling analyses are included in the Methods section (Modelling of the dose-response relationship for change from baseline to week 12 in DAS28-3(CRP)) of the supplementary data, available at at Rheumatology online.
For this study, the safety analysis set was equivalent to the full analysis set. Safety data are summarized descriptively for each treatment group.
Results
Patients
The study was conducted from 18 November 2014 to 15 March 2017, in 36 rheumatology clinics/departments in Japan. In total, 209 patients participated in this study: 104 received tofacitinib MR 11 mg QD, and 105 received tofacitinib IR 5 mg BID. Of these, 195 patients (93.3%) completed the 12-week study. Among patients who received tofacitinib MR 11 mg QD, three discontinued due to AEs and one due to a medication error (without associated AE). Among patients who received tofacitinib IR 5 mg BID, nine discontinued due to AEs and one due to insufficient clinical response.
Patient demographics were generally well balanced between groups (Table 1), with the exceptions that the tofacitinib MR 11 mg BID group included a higher proportion of female patients vs the IR 5 mg BID group (83 vs 71%, respectively) and numerically higher CRP (17.5 vs 13.2 mg/l).
Table 1.
Baseline demographics and disease characteristics (full analysis set)
| Demographics and disease characteristics | Tofacitinib MR 11 mg QD | Tofacitinib IR 5 mg BID |
|---|---|---|
| n = 104 | n = 105 | |
| Females, n (%) | 86 (82.7) | 75 (71.4) |
| Age, mean (s.d.), years | 57.1 (11.4) | 58.9 (10.2) |
| Weight, mean (s.d.), kg | 55.9 (11.2) | 57.5 (11.1) |
| BMI, mean (s.d.), kg/m2 | 22.6 (4.0) | 22.7 (3.2) |
| RA disease duration, mean (range), years | 9.5 (0.5–36.6) | 9.4 (0.6–52.1) |
| RF+, n (%) | 80 (76.9) | 78 (74.3) |
| DAS28-4(CRP), mean (s.d.) | 5.1 (0.9) | 5.0 (0.9) |
| DAS28-4(ESR), mean (s.d.) | 5.9 (0.8) | 5.8 (0.9) |
| HAQ-DI, mean (s.d.) | 1.0 (0.7) | 0.9 (0.7) |
| Tender joint count, mean (s.d.) | ||
| 68-joint count | 15.2 (8.2) | 13.9 (7.4) |
| 28-joint count | 10.3 (4.7) | 9.9 (5.5) |
| Swollen joint count, mean (s.d.) | ||
| 66-joint count | 12.4 (5.3) | 11.3 (5.5) |
| 28-joint count | 9.2 (3.6) | 8.8 (4.1) |
| Pain VAS, mean (s.d.), mm | 54.0 (24.8) | 51.7 (26.5) |
| PtGA, mean (s.d.), mm | 53.1 (23.8) | 52.2 (25.8) |
| PGA, mean (s.d.), mm | 56.6 (19.4) | 56.0 (19.5) |
| CRP, mean (s.d.), mg/l | 17.5 (22.6) | 13.2 (14.5) |
| ESR, mean (s.d.), mm/h | 47.2 (24.8) | 42.8 (22.3) |
| Prior DMARD use, n (%) | 24 (23.1) | 22 (21.0) |
| csDMARD excluding MTX TNFi | 4 (3.8) | 8 (7.6) |
| bDMARD excluding TNFi | 2 (1.9) | 2 (1.9) |
| Concomitant MTX use, n (%) | 104 (100) | 105 (100) |
| Concomitant MTX dose, mean (s.d.), mg/week | 9.7 (2.5) | 9.3 (2.4) |
| Baseline glucocorticoid use, n (%) | 49 (47.1) | 54 (51.4) |
bDMARD: biologic DMARD; BID: twice daily; csDMARD: conventional synthetic DMARD; DAS28: DAS in 28 joints; HAQ-DI: HAQ-Disability Index; IR: immediate-release; MR: modified-release; PGA: Physician's Global Assessment; PtGA: Patient's Global Assessment; QD: once daily; TNFi: tumour necrosis factor inhibitor; VAS: visual analog scale.
Primary efficacy analysis: DAS28-4(CRP)
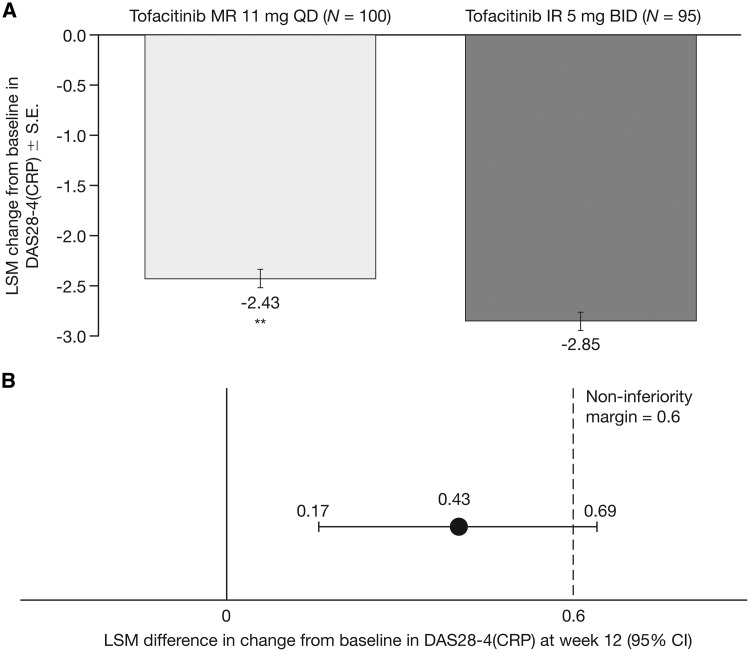
The least squares mean (LSM) CFB in DAS28-4(CRP) was −2.43 for the tofacitinib MR 11 mg QD group and −2.85 for the IR 5 mg BID group (Fig. 1A), and the difference between groups was 0.43 (95% CI 0.17, 0.69; P < 0.01). The upper bound of the 95% CI was greater than the prespecified non-inferiority margin of 0.6; hence, non-inferiority of tofacitinib MR 11 mg QD relative to IR 5 mg BID was not met (Fig. 1B). Results for the per protocol sensitivity analysis set were consistent with the primary analysis. Improvements in DAS28-4(CRP) were also observed with both tofacitinib MR 11 mg QD (−1.76) and IR 5 mg BID (−2.05) at the week 4 assessment (Supplementary Table S1, available at Rheumatology online).
Fig. 1.
Change from baseline in DAS28-4(CRP) at week 12
(A) LSM change from baseline DAS28-4(CRP) at week 12 (primary end point). (B) Difference (95% CI) between groups in LSM change from baseline in DAS28-4(CRP) at week 12 (FAS, longitudinal model). *P<0.05; **P<0.01 vs tofacitinib IR 5 mg BID. BID: twice daily; DAS28-4(CRP): DAS in 28 joints with CRP; FAS: full analysis set; IR: immediate-release; LSM: least squares mean; MR: modified-release; QD: once daily.
Clinically significant reductions from baseline in DAS28-4(CRP) were observed in both treatment groups at week 12; in patients who received tofacitinib MR 11 mg QD and IR 5 mg BID, improvement in DAS28-4(CRP) ⩾0.6 was achieved by 94.2 and 88.5%, respectively, and a decrease ⩾1.2 was achieved by 89.3 and 84.6%, respectively (based on EULAR criteria for a minimum clinically important difference). Response rates are comparable to CIs of the differences between the two groups including zero.
Analysis of the contribution of DAS28-4(CRP) components to the primary outcome at week 12 showed that no single DAS28-4(CRP) component influenced the overall DAS28-4(CRP) score based on unweighted scores (Supplementary Fig. S1A, available at Rheumatology online). Similar changes from baseline in CRP were observed with both tofacitinib formulations (MR 11 mg QD: −10.2 mg/l; IR 5 mg BID: −11.5 mg/l). The weighted contribution based on the DAS28-4(CRP) equation (Supplementary Fig. S1B, available at Rheumatology online) indicated that the greatest contribution (55%) was from the tender joint count. A subset analysis of CFB in DAS28-4(CRP) stratified by baseline patient characteristics [(gender, body weight, age, RF, disease duration, MTX dose, DAS28-4[CRP] and CRP) showed that potential differences at baseline between treatment groups did not impact the treatment differences observed for DAS28-4(CRP) (Supplementary Fig. S2, available at Rheumatology online).
Secondary and exploratory efficacy end points
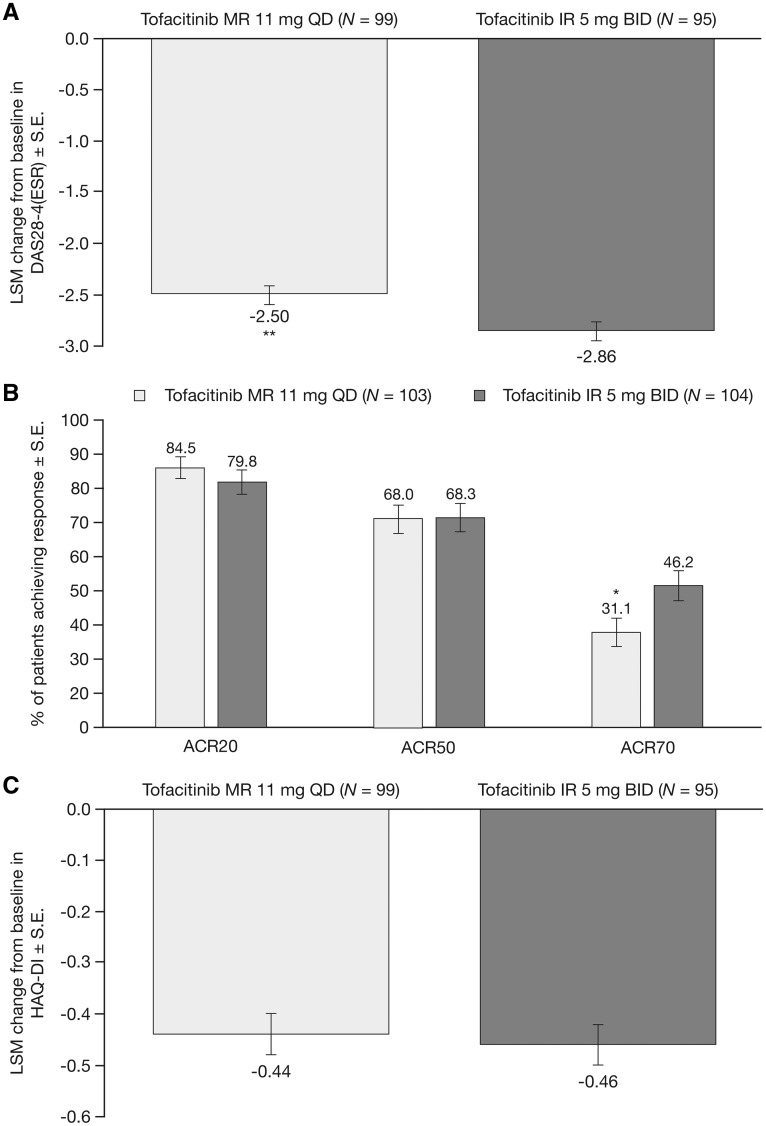
Improvements from baseline in DAS28-4(ESR) were observed with both tofacitinib 11 mg QD (LSM: −2.50) and IR 5 mg BID (LSM: −2.86) formulations at week 12, and were greater with tofacitinib IR 5 mg BID vs MR 11 mg QD (P < 0.01; P-values for secondary end points were not adjusted for multiplicity; Fig. 2A).
Fig. 2.
Secondary efficacy end points at week 12
(A) LSM change from baseline in DAS28-4(ESR) (FAS, longitudinal model). (B) ACR20, ACR50 and ACR70 response rates (FAS NRI). (C) LSM change from baseline in HAQ-DI (FAS, longitudinal model). *P<0.05; **P<0.01 vs tofacitinib IR 5 mg BID [P-values were not adjusted for multiplicity]. ACR20, ACR50, ACR70: ≥20, ≥50 and ≥70% improvement in ACR criteria; BID: twice daily; DAS28-4(ESR): DAS in 28 joints with ESR; FAS: full analysis set; HAQ-DI: HAQ-Disability Index; IR: immediate-release; LSM: least squares mean; MR: modified-release; NRI: non-responder imputation; QD: once daily.
At week 12, similar proportions of patients who received tofacitinib MR 11 mg QD and IR 5 mg BID achieved ACR20 (84.5 and 79.8%, respectively) and ACR50 responses (68.0 and 68.3%, respectively; Fig. 2B). The ACR70 response rate was lower with tofacitinib MR 11 mg QD vs IR 5 mg BID (31.1 and 46.2%, respectively; P < 0.05).
At week 12, a similar LSM CFB in HAQ-DI was reported by patients who received tofacitinib MR 11 mg QD (−0.44) and IR 5 mg BID (−0.46; Fig. 2C). Among patients who received MR 11 mg QD or IR 5 mg BID, 63.1 and 57.7%, respectively, achieved a clinically significant improvement in HAQ-DI score (decrease ⩾0.22).
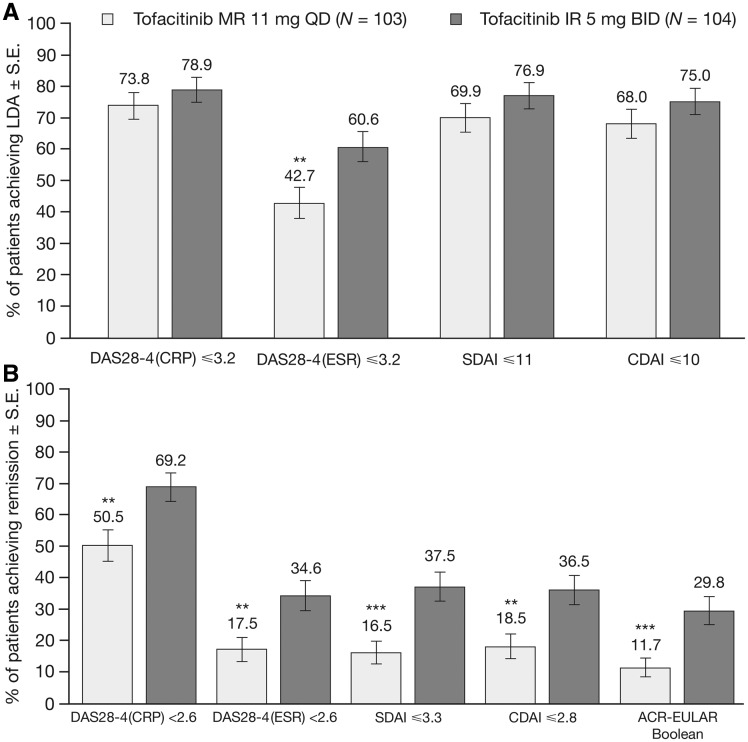
Rates of DAS28-4(CRP) ⩽3.2, and LDA based on SDAI and CDAI definitions were similar between treatment groups, whereas a larger proportion of patients achieved DAS28-4(ESR)-defined LDA with tofacitinib IR 5 mg BID vs MR 11 mg QD (P < 0.01; Fig. 3A). The proportions of patients achieving DAS28-4(CRP) <2.6 and remission based on all definitions at week 12 were greater with tofacitinib IR 5 mg BID vs MR 11 mg QD (all P < 0.01; Fig. 3B).
Fig. 3.
Rates of LDA and remission at week 12
Patients achieving (A) DAS28-4(CRP) ≤3.2 and LDA based on DAS28-4(ESR), CDAI and SDAI definitions and (B) DAS28-4(CRP) <2.6 and remission based on DAS28-4(ESR), CDAI and SDAI definitions at week 12 (FAS, NRI). *P<0.05; **P<0.01; ***P<0.001 vs tofacitinib IR 5 mg BID [P-values were not adjusted for multiplicity]. Rates of remission and LDA based on SDAI, CDAI and ACR-EULAR Boolean criteria were analysed post hoc. BID: twice daily; CDAI: Clinical Disease Activity Index; DAS28-4: DAS in 28 joints; FAS: full analysis set; IR: immediate-release; LDA: low disease activity; MR: modified-release; NRI: non-responder imputation; QD: once daily; SDAI: Simplified Disease Activity Index.
Although there were nominal significant differences, which were small in magnitude, in DAS28-4(ESR) and ACR70 between tofacitinib IR 5 mg BID and MR 11 mg QD, clinically meaningful improvements were observed with both formulations at week 12 for both DAS28-4(ESR) and ACR70, and for all other secondary efficacy end points (Fig. 2). Improvement in all secondary efficacy end points was also observed by the week 4 assessment (Supplementary Table S1, available at Rheumatology online); differences between treatment groups were similar to those observed at week 12.
At week 12, changes from baseline in FACIT-F, EQ-5D utility scores (Supplementary Fig. S3, available at Rheumatology online) and short form 36 scores (Supplementary Table S2, available at Rheumatology online) were generally similar between tofacitinib formulations.
Safety
The overall frequencies of AEs, severe AEs and serious AEs were similar in patients treated with tofacitinib MR 11 mg QD and IR 5 mg BID (Table 2). The most frequently reported AEs by preferred term were nasopharyngitis, hepatic function abnormal and blood creatine phosphokinase increase (Table 2). All AEs were generally balanced between treatment groups (Table 2; Supplementary Table S3, available at Rheumatology online). No deaths were reported.
Table 2.
Summary of safety up to week 12
| AEs [n (%)] | Tofacitinib MR 11 mg QD | Tofacitinib IR 5 mg BID |
|---|---|---|
| n = 104 | n = 105 | |
| AEs | 55 (52.9) | 54 (51.4) |
| SAEs | 5 (4.8)a | 4 (3.8)b |
| Severe AEsc | 4 (3.8) | 2 (1.9) |
| Discontinuations attributable to AEs | 3 (2.9) | 9 (8.6) |
| Most common AEs by SOC and preferred term (≥3 patients in any group) | ||
| Infections and infestations | 21 (20.2) | 27 (25.7) |
| Nasopharyngitis | 10 (9.6) | 13 (12.4) |
| Bronchitis | 1 (1.0) | 3 (2.9) |
| Investigations | 12 (11.5) | 13 (12.4) |
| Blood CPK increased | 4 (3.8) | 2 (1.9) |
| Blood cholesterol increased | 3 (2.9) | 2 (1.9) |
| AST increased | 0 | 3 (2.9) |
| Gastrointestinal disorders | 15 (14.4) | 11 (10.5) |
| Stomatitis | 2 (1.9) | 3 (2.9) |
| Abdominal pain upper | 3 (2.9) | 2 (1.9) |
| Diarrhoea | 3 (2.9) | 1 (1.0) |
| Abdominal discomfort | 3 (2.9) | 0 |
| Respiratory, thoracic and mediastinal disorders | 3 (2.9) | 7 (6.7) |
| Upper respiratory tract inflammation | 1 (1.0) | 3 (2.9) |
| Hepatobiliary disorders | 4 (3.8) | 4 (3.8) |
| Hepatic function abnormal | 3 (2.9) | 4 (3.8) |
One patient in the tofacitinib MR 11 mg QD group had an SAE (breast cancer) reported 100 days after study completion and is not included here.
One patient in the tofacitinib IR 5 mg BID group had AEs (infective tenosynovitis and bursitis infective) that were judged as SAEs after completion of the study and are not included here.
Investigator reported. AE: adverse event; AST: aspartate aminotransferase; BID: twice daily; CPK: creatine phosphokinase; IR: immediate-release; MR: modified-release; n: number of patients with events; QD: once daily; SAE: serious adverse event; SOC: system organ class.
There were six serious infections (requiring parenteral antimicrobial therapy or hospitalization) reported in five patients across treatment groups: Pneumocystis jirovecii pneumonia (MR 11 mg BID, n = 2; IR 5 mg BID, n = 1), interstitial lung disease and pneumonia bacterial (IR 5 mg BID) and pneumonia (IR 5 mg BID). Two cases of herpes zoster were reported, one moderate (MR 11 mg QD) and one mild event (IR 5 mg BID). Two malignancies were reported (rectal cancer and breast cancer), both in patients who received tofacitinib MR 11 mg QD. No patients had gastrointestinal obstructions, gastrointestinal perforations or hypertension.
A confirmed (based on two sequential measurements) decrease in haemoglobin to <8.0 g/dl, or >30% decrease from baseline, was reported in one patient who received tofacitinib IR 5 mg BID. No patients had confirmed decreases in neutrophil counts <1.0 × 103 cells/mm3 or lymphocyte counts <0.5 × 103 cells/mm3. Confirmed increases in aspartate or alanine aminotransferase ≥ 3 times the upper limit of normal were reported in one patient who received tofacitinib MR 11 mg QD. There were no cases of probable or definite drug-induced liver injury, and no patients met the criteria for Hy’s law. Laboratory results are presented in Supplementary Table S4, available at Rheumatology online.
Discussion
In this phase III study in Japanese patients with RA and an inadequate response to MTX, the non-inferiority of tofacitinib MR 11 mg QD relative to IR 5 mg BID could not be declared based on the primary end point of CFB in DAS28-4(CRP) at week 12. Numerically greater efficacy with tofacitinib IR 5 mg BID vs MR 11 mg QD was observed for DAS28-4(CRP), DAS28-4(ESR) and the more stringent treatment goals of ACR70, rates of DAS28-4(CRP) <2.6 and rates of remission based on DAS28-4(ESR), CDAI, SDAI and Boolean definitions at week 12. The end points including ACR20 and ACR50 were nearly superimposable, and CFB in HAQ-DI and rates of DAS28-4(CRP) ⩽3.2 and LDA based on CDAI and SDAI definitions were similar between the two formulations. Clinically meaningful improvements in RA signs and symptoms were observed with both tofacitinib MR 11 mg QD and IR 5 mg BID, with >84% of patients exceeding the clinically significant change for DAS28-4(CRP) in both groups. Clinically meaningful improvements with both formulations were also observed in secondary efficacy end points, including DAS28-4(ESR) CFB, rates of remission and LDA, and CFB in HAQ-DI and patients achieving the minimum clinically important difference. The observed difference in CFB in DAS28-4(CRP) between treatment groups was not a result of differences in known patient characteristics assessed in the present study and could not be explained by changes in any specific component of the DAS28-4(CRP).
The data presented here suggest that the differences in efficacy observed between treatment arms in the present study were not due to poor responses among tofacitinib MR 11 mg QD recipients (as illustrated by the absence of treatment group differences at the ACR20, ACR50 and LDA response level), but instead by larger than expected responses among IR 5 mg BID recipients. This might be due to the fact that this study was carried out in Japanese patients, a population that showed numerically greater responses to tofacitinib IR 5 mg BID compared with the global population in phase II studies [25] and thus might be particularly sensitive to this formulation. Therefore, small differences between the profiles of the two tofacitinib formulations might induce observable differences in efficacy in this Japanese population, which might not be representative of a global population.
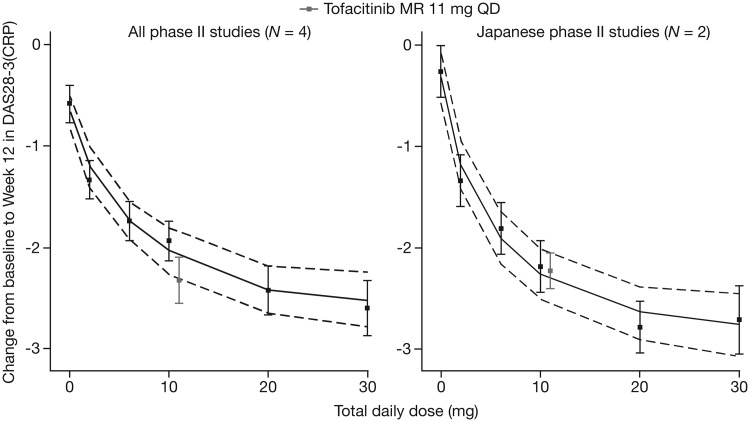
Importantly, the DAS28(CRP) response for the MR 11 mg QD formulation in this study was consistent with the model-predicted dose–response profile of tofacitinib based on a BID regimen (Fig. 4). In the additional sensitivity analyses examining the subset of Japanese studies, a greater degree of alignment was observed between the tofacitinib MR 11 mg QD and IR 5 mg BID responses compared with the analysis using all phase II studies, and the differences between the tofacitinib MR 11 mg QD formulation used in the present study and the predicted profile of the tofacitinib IR 5 mg BID formulations were well within the model variability. These analyses may further support the importance of AUC as the most relevant pharmacokinetic parameter driving the overall efficacy of tofacitinib.
Fig. 4.
Comparison of MR 11 mg QD with predicted dose–response profile of IR 5 mg BID
The left panel shows the external evaluation result based on a model using data from all four phase II studies, and the right panel shows the model subsetting for the two Japanese phase II studies. The grey symbols are the observed mean change from baseline in DAS28-3(CRP) at week 12 for tofacitinib MR 11 mg QD from the present study. As a visual aid, the efficacy of MR 11 mg QD is plotted alongside the IR 5 mg BID dose. Continuous black lines indicate the model-predicted median of the predicted mean change from baseline in DAS28-3(CRP), and dotted lines indicate the 95% CI of the predicted mean. Black symbols are the observed mean change from baseline in DAS28-3(CRP) for each dose at week 12 across the studies. Error bars indicate the 95% CI of the mean observation. DAS28-3(CRP) is compared rather than DAS28-4(CRP) (primary end point in the present study), because the phase II studies did not report DAS28-4(CRP). BID: twice daily; DAS28-3(CRP): DAS in 28 joints with CRP; IR: immediate-release; MR: modified-release; n: number of studies included in model; QD: once daily.
The safety profiles of tofacitinib MR 11 mg QD and IR 5 mg BID were generally similar over 12 weeks, with the exceptions that the frequency of AEs leading to discontinuation was lower in patients who received MR 11 mg QD. Few clinically significant abnormalities in laboratory parameters were observed in either treatment group over 12 weeks. The safety profile of both tofacitinib formulations in this study in Japanese patients was consistent with the known safety profile of tofacitinib for RA in both global and Japanese studies [4–11, 26].
The non-inferiority criteria for tofacitinib MR 11 mg QD relative to IR 5 mg BID was not met in this study, which might be explained, in part, by the limitations of the study design. The first limitation was that, as requested by the Japanese Pharmaceuticals and Medical Devices Agency, the present study used a stringent non-inferiority margin of 0.6. A non-inferiority margin of 0.6 is equivalent to the measurement error for the DAS28-4(CRP) end point, but less than a clinically significant response (decrease of ⩾1.2) when this is considered at the individual patient level [21]. Typically, a non-inferiority margin that represents ⩾50% of the placebo-adjusted control drug effect is suggested by US Food and Drug Administration guidance [24]. For example, a non-inferiority margin of 0.8 would have preserved 59% of the placebo-adjusted control drug effect, based on data from previous placebo-controlled phase II and III studies in Japanese patients [4, 5, 10]. The 0.6 non-inferiority margin used in the present study required preservation of 69% of the placebo-adjusted control drug effect, and tofacitinib MR retained 64% of the placebo-adjusted control drug effect. A second limitation of the study design was that the sample size calculations were based on the assumption that the true difference between the treatment arms was zero, which is also a particularly stringent expectation even for two formulations of the same drug. The study would be significantly underpowered if the true difference was close to the observed treatment difference of 0.4. In addition, the findings of the present study are limited by the relatively small sample size in a Japanese patient population. As described above, differences seen in the results for some of the end points within the present study were probably reflective of variability and study design rather than true clinically meaningful differences between formulations. Therefore, the lack of non-inferiority shown in the present study should be interpreted accordingly, based on these limitations, and caution should be used when extrapolating these data to the clinical use of the tofacitinib MR 11 mg QD and IR 5 mg BID formulations in the global population.
In summary, non-inferiority of tofacitinib MR 11 mg QD relative to IR 5 mg BID was not demonstrated for the primary end point (CFB in DAS28-4[CRP] at week 12). However, from a clinical point of view, both the IR and MR formulations provided clinically important improvement across primary and secondary end points, with a similar safety profile for both formulations. The results for tofacitinib MR 11 mg QD from this clinical study show excellent alignment with predictions based on the dose–response model in Japanese patients. There is evidence that drug adherence decreases over time [16] and that QD dosing may result in greater adherence compared with BID dosing for some treatments [16, 17]. Therefore, providing both QD and BID dosing regimens for tofacitinib will allow patients a wider choice to meet their treatment preferences and might improve overall treatment adherence and treatment outcomes. These data suggest that while numerically greater efficacy was shown with tofacitinib IR 5 mg BID vs MR 11 mg QD for some end points, the MR 11 mg QD formulation still provides substantial improvement in RA signs and symptoms, has comparable safety to the IR 5 mg BID formulation and might be a useful option for patients.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the support of all the study patients and investigators. A full list of study investigators can be found in the Supplementary Material, available at Rheumatology online. Medical writing support, under the guidance of the authors, was provided by Alice MacLachlan, PhD, of CMC Connect, a division of Complete Medical Communications Ltd, Glasgow, UK, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461–464).
Funding: This study was sponsored by Pfizer Inc.
Disclosure statement: Y.T. has received consulting fees, speaking fees and/or honoraria from Astellas, Bristol-Myers, Chugai, Daiichi-Sankyo, Eli Lilly, Janssen, Mitsubishi-Tanabe, Pfizer Inc, Sanofi, UCB and YL Biologics, and has received research grants from AbbVie, Astellas, Bristol-Myers, Chugai, Daiichi-Sankyo, Eisai, Kyowa-Kirin, Mitsubishi-Tanabe, MSD, Ono, Pfizer Inc and Takeda. N.S. and H.Y. are employees and shareholders of Pfizer Japan Inc. S.T. is an employee of Pfizer Japan Inc. T.L., R.Z., C.C., T.S., H.V. and H.F. are employees and shareholders of Pfizer Inc. M.L., C.M. and C.D. were employees and shareholders of Pfizer Inc at the time of this analysis.
References
- 1. Fleischmann R, Cutolo M, Genovese MC. et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29. [DOI] [PubMed] [Google Scholar]
- 2. Kremer JM, Bloom BJ, Breedveld FC. et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 2009;60:1895–905. [DOI] [PubMed] [Google Scholar]
- 3. Kremer JM, Cohen S, Wilkinson BE. et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970–81. [DOI] [PubMed] [Google Scholar]
- 4. Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH; Tofacitinib Study Investigators. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res 2011;63:1150–8. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka Y, Takeuchi T, Yamanaka H. et al. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol 2015;25:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burmester GR, Blanco R, Charles-Schoeman C. et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 7. Fleischmann R, Kremer J, Cush J. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 8. Kremer J, Li Z-G, Hall S. et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 9. Lee EB, Fleischmann R, Hall S. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 10. van der Heijde D, Tanaka Y, Fleischmann R. et al. Tofacitinib (CP-690, 550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 11. van Vollenhoven RF, Fleischmann R, Cohen S. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 12. Wollenhaupt J, Silverfield J, Lee EB. et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 13. Wollenhaupt J, Silverfield J, Lee EB. et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open-label, long-term extension studies over 9 years. Arthritis Rheumatol 2017;69:683–4. [DOI] [PubMed] [Google Scholar]
- 14. Yamanaka H, Tanaka Y, Takeuchi T. et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther 2016;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waterman KC, MacDonald BC, Roy MC.. Extrudable core system: development of a single-layer osmotic controlled-release tablet. J Control Release 2009;134:201–6. [DOI] [PubMed] [Google Scholar]
- 16. Coleman CI, Limone B, Sobieraj DM. et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm 2012;18:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC.. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care 2009;15:e22–33. [PubMed] [Google Scholar]
- 18. Richter A, Anton SF, Koch P, Dennett SL.. The impact of reducing dose frequency on health outcomes. Clin Ther 2003;25:2307–35. [DOI] [PubMed] [Google Scholar]
- 19. Lamba M, Wang R, Fletcher T. et al. Extended-release once-daily formulation of tofacitinib: evaluation of pharmacokinetics compared with immediate-release tofacitinib and impact of food. J Clin Pharmacol 2016;56:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamba M, Hutmacher MM, Furst DE. et al. Model-informed development and registration of a once-daily regimen of extended-release tofacitinib. Clin Pharmacol Ther 2017;101:745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Gestel AM, Prevoo ML, van't Hof MA. et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996;39:34–40. [DOI] [PubMed] [Google Scholar]
- 22. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 23. Fransen J, van Riel PL.. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 2005;23:S93–9. [PubMed] [Google Scholar]
- 24.Food and Drug Administration. Guidance for Industry: Non-inferiority Clinical Trials to Establish Effectiveness. 2016: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm202140.pdf (10 July 2017, date last accessed).
- 25. Fleischmann R, Kremer J, Tanaka Y. et al. Efficacy and safety of tofacitinib in patients with active rheumatoid arthritis: review of key Phase 2 studies. Int J Rheum Dis 2016;19:1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen SB, Tanaka Y, Mariette X. et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.