Abstract
Background
A number of Pyricularia species are known to infect different grass species. In the case of Pyricularia oryzae (syn. Magnaporthe oryzae), distinct populations are known to be adapted to a wide variety of grass hosts, including rice, wheat and many other grasses. The genome sizes of Pyricularia species are typical for filamentous ascomycete fungi [~ 40 Mbp for P. oryzae, and ~ 45 Mbp for P. grisea]. Genome plasticity, mediated in part by deletions promoted by recombination between repetitive elements [Genome Res 26:1091-1100, 2016, Nat Rev Microbiol 10:417-430,2012] and transposable elements [Annu Rev Phytopathol 55:483-503,2017] contributes to host adaptation. Therefore, comparisons of genome structure of individual species will provide insight into the evolution of host specificity. However, except for the P. oryzae subgroup, little is known about the gene content or genome organization of other Pyricularia species, such as those infecting Pennisetum grasses.
Results
Here, we report the genome sequence of P. penniseti strain P1609 isolated from a Pennisetum grass (JUJUNCAO) using PacBio SMRT sequencing technology. Phylogenomic analysis of 28 Magnaporthales species and 5 non-Magnaporthales species indicated that P1609 belongs to a Pyricularia subclade, which is genetically distant from P. oryzae. Comparative genomic analysis revealed that the pathogenicity-related gene repertoires had diverged between P1609 and the P. oryzae strain 70–15, including the known avirulence genes, other putative secreted proteins, as well as some other predicted Pathogen-Host Interaction (PHI) genes. Genomic sequence comparison also identified many genomic rearrangements relative to P. oryzae.
Conclusion
Our results suggested that the genomic sequence of the P. penniseti P1609 could be a useful resource for the genetic study of the Pennisetum-infecting Pyricularia species and provide new insight into evolution of pathogen genomes during host adaptation.
Electronic supplementary material
The online version of this article (10.1186/s12864-018-5222-8) contains supplementary material, which is available to authorized users.
Keywords: Pennisetum, Pyricularia, PacBio sequencing, Pathogenesis-related genes, Comparative genomic analysis
Background
Pyricularia was established by Saccardo to accommodate a type of fungal species based on pyriform conidia when the first species of this pathogen, Pyricularia grisea, was isolated from crabgrass (Digitaria sanguinalis L.) [1]. Pyricularia became an important research focus due to rice blast disease and now wheat blast caused by Pyricularia oryzae (syn. Magnaporthe oryzae) [2–4]. To date, 100 plant genera comprising 256 species have been documented as hosts of Pyricularia species (https://nt.ars-grin.gov/fungaldatabases/), among which 54 genera belong to the Poaceae family. Seven Pyricularia species (including one unidentified species) have been isolated from Pennisetum spp., a large genus in the Poaceae family, and more than one Pyricularia species can be found on the same Pennisetum species. For instance, 4 Pyricularia species, namely, P. penniseti, P. penniseticola, P. setariae and Pyricularia sp. have been found on P. typhoides [5, 6].
The genome sequence of P. oryzae strain 70–15, a strain produced by an initial cross of rice-infecting isolate 104–3 and the weeping love grass isolate AR4 [7]. A series of selections from crosses, including three generations crossing to the rice isolate Guy11 generated fertile rice infecting strains that have facilitated studies of developmental and pathogenic mechanisms of the blast fungus and helped P. oryzae to become one of the most important fungal models of plant pathogenesis [8, 9]. Since the publication of the P. oryzae strain 70–15 genome, more field isolates were sequenced and assembled, including Ina168, HN9311, FJ81278, Y34, P131, 98–06 and Guy11 [10–14]. Comparative genomic analyses and functional studies of these strains revealed genome plasticity and the involvement of the lineage specific genes in pathogenicity [12, 14]. More recently, facilitated by the fast development of sequencing technologies, a number of field isolates from rice, as well as isolates from different grass and cereal hosts, were sequenced and subjected to population-level analyses, revealing host immunity as a major force driving specialization [2, 15–18]. However, genomes of most of the species of the Pyricularia complex remain unexplored. For example, among the 7 identified Pyricularia species isolated from Pennisetum grasses [5], only P. pennisetigena was recently sequenced [2].
Here, we reported the whole-genome sequence of P. penniseti [19] isolated from a Pennisetum grass JUJUNCAO (Pennisetum giganteum Z. X. Lin). JUJUNCAO was originally developed as a biomass crop for the cultivation of edible mushrooms by Lin et al, and later became a versatile grass that is used as forage for cattle and sheep, material for biofuel production, and a tool for the prevention of soil erosion [20–22]. We have recently isolated a fungus, producing pyriform-shaped conidia from leaf spots of JUJUNCAO. One isolate, P1609, caused a typical blast fungal disease symptom on JUJUNCAO, showing small, round or elliptical lesions as an initial symptom with spindle shaped, grayish to tan necrotic lesion centers, and yellow halos at a later disease stage. The morphologic and phylogenetic analyses distinguished P1609 from other identified Pyricularia species, but it was not clearly distinguishable from the P. penniseti reported in 1970 in India [5, 23]. We therefore identified this fungus as P. penniseti [19]. In this study, we performed genome sequencing of this strain, aiming for a proper classification of this fungus in the Pyricularia population and identification of genes that may be involved in the adaptation of this fungus to JUJUNCAO.
Results
Genome sequencing and assembly
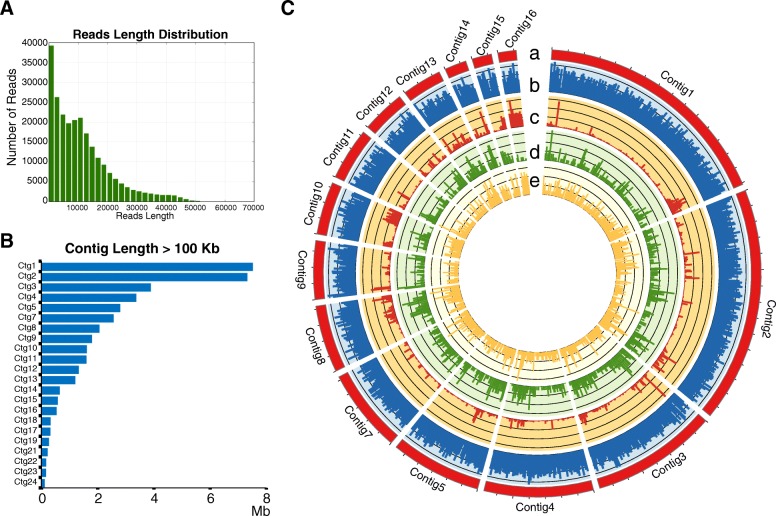
We sequenced the P1609 genome with the long-read PacBio technology. In total, 312,061 reads with 8.6 Kb average lengths were obtained, representing about 60-fold coverage of the genome (Fig. 1a). The genome sequence was assembled with the HAGP pipeline [24], resulting in a total assembly space of 41.82 Mb (Table 1), similar to assemblies of other isolates sequenced by PacBio [10]. The assembly contains 53 contigs, with the N50 of 3.4 Mb and the largest contig of 7.56 Mb (Fig. 1b). Contigs > 1 Mb cover 89.7% and contigs > 100 Kb cover 98.5% of the genome (Fig. 1b; Table 1), indicating long-continuity of the assembly. The GC content of the assembly is 50.3%, similar to genomes of Pyricularia isolates from different host plants, which range from 48.6 to 51% [18]. Genome annotation identified 13,102 genes with average gene size of 1758 bp, fairly evenly dispersed on contigs (Table 2, Fig. 1c, track b).
Fig. 1.
PacBio sequencing and genome assembly of P1609. a Reads length distribution. b Contig length of assembled contig > 100 Kb. c Overview of P1609 genome. (Track a) Contig1 to contig16 of P1609, (track b) gene density, (track c) Transposon elements density, (track d) secreted proteins density, (track e) unique gene (compared with P. oryzae, N. crassa, F. graminearum and C. gloeosporioides) density of P1609 per 50 Kb
Table 1.
Details of sequencing reads and genome assembly of P1609
| Feature | Value |
|---|---|
| Number of Subreads | 312,061 |
| Total Length of Subreads (bp) | 2,688,966,115 |
| Mean Coverage | 59 |
| Polished Contigs | 53 |
| Maximum Contig Length (bp) | 7,555,856 |
| N50 Contig Length (bp) | 3,411,241 |
| Sum of Contig Lengths (Mb) | 41.82 |
| GC level | 50.30% |
| length > 1 Mb | 89.70% |
| length > 100 Kb | 98.50% |
Table 2.
Details of genome annotation of P1609
| Category | Value |
|---|---|
| Total TE | 7.67% |
| LINEs | 1.50% |
| LTR elements | 4.27% |
| DNA elements | 0.63% |
| Unclassified | 1.27% |
| Gene Number | 13,102 |
| Average Gene Length (bp) | 1758 |
| Secreted Protein Number | 1409 |
De novo repeat sequence analysis identified 7.67% repeat sequences, among which 4.27% were Gypsy and Copia, the two long terminal repeats (LTR)-type retrotransposons. Although the overall content of repeat sequences in P1609 is less than that of the sequenced P. oryzae strains [8, 10, 12], the high proportion of Gypsy and Copia in repeat sequence is similar to that of the reported P. oryzae strains [25]. The TEs are not evenly distributed along the contigs, with some contigs being highly enriched with TEs (Fig. 1c, track c), suggesting that the P1609 genome also underwent transposon expansion as observed in other plant pathogens [26].
Comparative and phylogenetic analysis
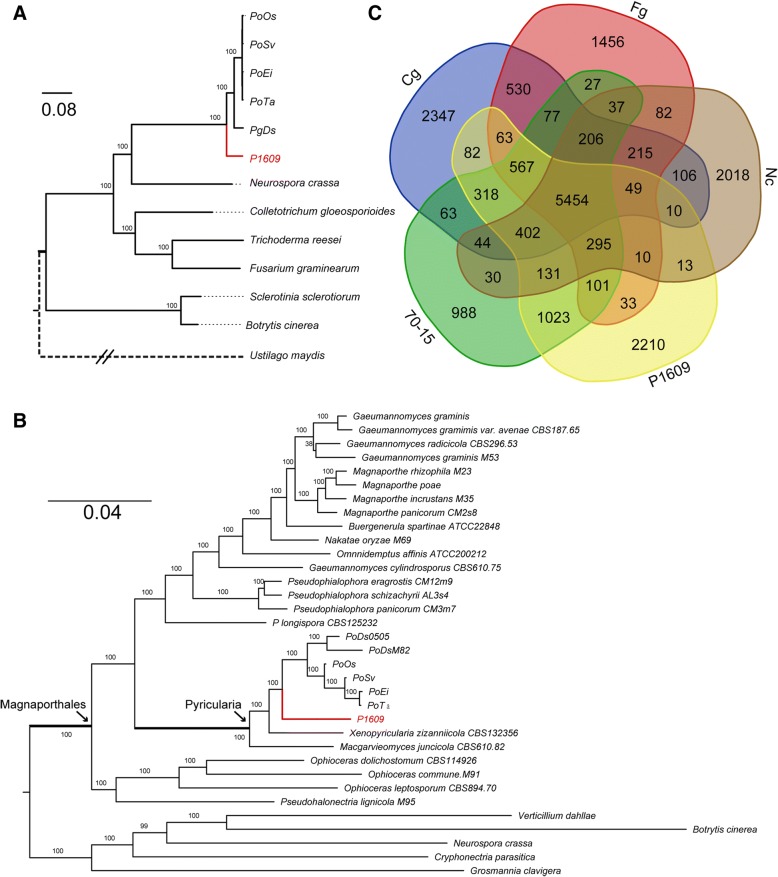
To understand the genetic relationship of P1609 with other fungal phytopathogens, we generated a phylogenetic tree of P1609 with Botrytis cinerea, Colletotrichum gloeosporioides, Fusarium graminearum, Neurospora crassa, Pyricularia grisea (Pg), Pyricularia oryzae (Po), Sclerotinia sclerotiorum, Trichoderma reesei and Ustilago maydis. Since P1609 showed a close morphological relationship with P. oryzae isolates, we included Pyricularia isolates collected from different host plants, including Oryza sativa (PoOs), Triticum aestivum (PoTa), Digitaria sanguinalis (PgDs), Setaria viridis (PoSv), and Eleusine indica (MoEi). In total, 2051 single-copy genes shared by all the examined genomes were selected to infer phylogeny [27]. The resulting phylogenetic tree indicated that P1609 is more distantly related to PoOs, PoTa, PoSv, and PoEi (P. oryzae) than PgDs (P. grisea) (Fig. 2a). We then estimated divergence time of P1609 and Pyricularia isolates by assuming a constant molecular clock calibrated in a previous study [28, 29], which estimated the divergence of Neurospora and Pyricularia at about 200 million years ago (MYA). The estimation indicated that P1609 and Pyricularia isolates diverged at about 31 MYA, earlier than the divergent time of rice- and S. viridis-infecting isolates (about 10,000 years ago) [28, 30]. The phylogenetic tree also indicated that P1609 is a member of Magnaporthales, but is distantly related to the P. oryzae. To further determine exactly where P1609 localized in Magnaporthales, we also generated a phylogenomic tree of P1609 with 28 Magnaporthales species and 5 non-Magnaporthales species using amino acid sequences of 226 conserved orthologous genes as described [31]. The result showed that P1609 was localized in the Pyricularia subclade, and was closer to P. oryzae than Xenopyricularia zizaniicola (Fig. 2b), indicating that P1609 is a Pyricularia species.
Fig. 2.
Phylogenetic and comparative genomic study of P1609. a Phylogenomic tree of P1609 with Botrytis cinereal, Colletotrichum gloeosporioides, Fusarium graminearum, Neurospora crassa, Sclerotinia sclerotiorum, Trichoderma reesei and Ustilago maydis as well as Pyricularia isolates collected from O. sativa (70–15), T. aestivum (PoTa), D. sanguinalis (PgDs), S. viridis (PoSv), and E. indica (PoEi) based on 2051 single copy genes. The values of all of the branches are 100. b Maximum likelihood tree of P1609 and 28 Magnaporthales species, as well as 5 Sordariomycetes used as outgroup species based on 82,715 amino acid positions derived from 226 genes. c Venn diagram showed an overlap of gene families among P1609, Pyricularia rice isolates (PoOs), C. gloeosporioides (Cg), F. graminearum (Fg) and N. crassa (Nc)
We next conducted a comparative genomic study of P1609 with 70–15 (the reference isolate of P. oryzae), N. crassa (Nc), F. graminearum (Fg) and C. gloeosporioides (Cg) using OrthoFinder [27]. The five organisms share 5454 of gene families with each other, which covers 50.2% of the gene set of P1609 (Fig. 2c). Notably, the number of P1609 specific genes in this comparison was 2210. This is about twice the number of 70–15 specific genes. Although most of these P1609 specific genes had no functional annotations, Pfam annotation indicated that some encode carbohydrate-active enzymes (CAZymes) involved in polysaccharide metabolism pathways (Additional file 1: Figure S1; Additional file 2: Table S1).
To identify genes under positive selection, Ka/Ks ratios of the orthologous genes in P1609 versus 70–15 were calculated, with the assumption that Ka/Ks ratio > 1 suggested a positive selection of the gene [11]. Among the 5991 pairs of orthologs of P1609 and 70–15 scanned, 6 genes with Ka/Ks > 1 were identified in P1609 (Table 3), suggestive of positive selection on these genes in P1609 during the adaptation to JUJUNCAO. These genes are involved in different secondary metabolic pathways (See Discussion).
Table 3.
Positively selected genes in P1609
| Protein ID | Ka | Ks | Ka/Ks | P-Value (Fisher) | Description |
|---|---|---|---|---|---|
| P1609_7184 | 0.707979 | 0.440748 | 1.60631 | 0.00697146 | Unknown |
| P1609_5032 | 0.550905 | 0.343609 | 1.60329 | 1.98443E-06 | Isoamyl alcohol oxidase |
| P1609_3360 | 0.183224 | 0.118517 | 1.54597 | 0.00293936 | Glycerol uptake protein 1 |
| P1609_791 | 0.302231 | 0.199786 | 1.51277 | 9.75093E-06 | Folylpolyglutamate synthase |
| P1609_7869 | 0.491873 | 0.328109 | 1.49911 | 0.0296442 | Thioredoxin reductase |
| P1609_1006 | 0.40206 | 0.275456 | 1.45962 | 0.0288585 | Spore surface glycoprotein BclB |
Gene categories involved in pathogenicity
Plant pathogenic fungi employed diverse gene repertoires to invade host plants and subvert host immune systems, which include effectors, carbohydrate-active enzymes (CAZymes), other secreted enzymes and fungal secondary metabolisms [32, 33]. To understand the differences between P. penniseti and P. oryzae secretomes, we examined the 1409 putative secreted proteins of P1609 predicted by SignalP [34], and found 236 were unique in P1609 (Additional file 3: Table S2). By contrast, 165 putative effectors identified in the genome of 70–15, the rice blast reference fungal genome, were absent in the P1609 genome (Additional file 4: Table S3). Notably, all known avirulence genes (AVRs) from the rice-infecting isolate genomes were absent in P1609 genome.
Given the important roles of CAZymes in enabling plant pathogens to break down the plant cell wall [35], we next compared CAZymes between P1609 and 70–15. Our BLASTp search results showed that P1609 contains more predicted CAZyme-coding genes than 70–15 (Additional file 2: Table S1). Detailed analysis showed that the P1609 genome encodes five unique CAZymes belonging to five families, namely CBM61, GH117, GH35, GH65 and PL24, respectively (Additional file 2: Table S1). While it has six copies of GH28 pectinases (three copies in P. oryzae and P. grisea; Additional file 5: Figure S2). The high representation of carbohydrate-active enzymes may be related to the adaptation to the host P. giganteum. We then further analyzed the distribution of annotated PHI genes in P1609. In total, we identified 1692 potential PHI genes belonging to 1154 gene families (Additional file 6: Table S4). Interestingly, we found that several PHI genes exhibited great expansion in P1609 genome. For instance, MGG_12656, a gene involved in virulence in P. oryzae, has 107 homologs in P1609, and ChLae1 contributing to toxin production and virulence in the maize pathogen Cochliobolus heterostrophus has 17 homologs in P1609 [36, 37]. Compared with 70–15, 35 PHI genes were unique in P1609 (Table 4), most of which had highly similar homologs in Fusarium (Gibberella).
Table 4.
Unique Pathogen Host Interaction (PHI) genes in P1609
| Protein ID | PHI ID | Reference Organism | Phenotypes |
|---|---|---|---|
| P1609_2497_494aa | PHI:115 | Cochliobolus carbonum | Unaffected pathogenicity |
| P1609_11506_262aa, P1609_12781_266aa, P1609_4501_233aa, P1609_683_218aa | PHI:698 | Vibrio cholerae | Reduced virulence |
| P1609_11592_161aa, P1609_4614_892aa | PHI:1284 | Fusarium graminearum | Unaffected pathogenicity |
| P1609_11335_610aa | PHI:1803 | Fusarium graminearum | Unaffected pathogenicity |
| P1609_9374_326aa | PHI:2394 | Fusarium graminearum | Increased virulence |
| P1609_1007_600aa | PHI:225 | Fusarium solani | Reduced virulence |
| P1609_13007_833aa | PHI:1714 | Fusarium graminearum | Lethal |
| P1609_3609_435aa, P1609_5044_426aa, P1609_8181_460aa, P1609_960_144aa | PHI:1455 | Fusarium graminearum | Unaffected pathogenicity |
| P1609_11548_383aa, P1609_5843_397aa | PHI:1823 | Fusarium graminearum | Unaffected pathogenicity |
| P1609_2665_64aa | PHI:1421 | Fusarium graminearum | Lethal |
| P1609_2607_206aa, P1609_2608_820aa | PHI:3418 | Staphylococcus saprophyticus | Mixed outcome |
| P1609_11380_688aa | PHI:1809 | Fusarium graminearum | Lethal |
| P1609_7767_257aa | PHI:317 | Aspergillus fumigatus | Reduced virulence |
| P1609_6736_447aa | PHI:323 | Verticillium fungicola | Reduced virulence |
| P1609_13_169aa | PHI:1147 | Fusarium pseudograminearum | Unaffected pathogenicity |
| P1609_2376_825aa | PHI:1871 | Fusarium graminearum | Lethal |
| P1609_10320_326aa | PHI:1522 | Fusarium graminearum | Lethal |
| P1609_11485_355aa, P1609_2130_433aa | PHI:3116 | Pseudomonas syringae | Mixed outcome |
| P1609_10203_738aa | PHI:1815 | Fusarium graminearum | Unaffected pathogenicity |
| P1609_11600_2486aa | PHI:2628 | Salmonella enterica | Reduced virulence, 663 |
| P1609_11388_1000aa | PHI:3076 | Candida parapsilosis | Mixed outcome |
| P1609_11869_369aa | PHI:2375 | Alternaria brassicicola | Mixed outcome |
| P1609_5035_383aa | PHI:233 | Cochliobolus carbonum | Reduced virulence |
| P1609_5702_488aa | PHI:1271 | Fusarium graminearum | Unaffected pathogenicity |
| P1609_11864_596aa | PHI:2380 | Alternaria brassicicola | Mixed outcome |
Chromosome rearrangements
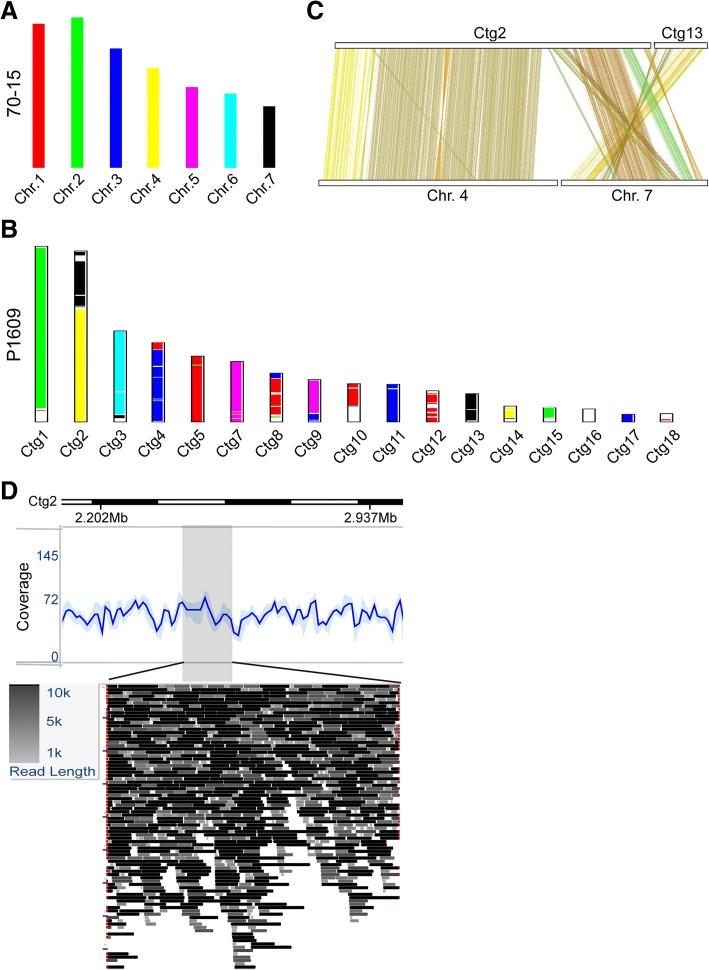
Chromosome rearrangements were broadly documented and had been reported to play important roles in fungal evolution, host adaptation and pathogenicity of phytopathogens [38, 39]. To explore genome collinearity and rearrangement between P1609 and 70–15, the identified collinear gene blocks that linked with different chromosomes in 70–15 (Fig. 3a) were visualized in the contigs (ctgs) of P1609 (Fig. 3b). P1609 and 70–15 overall displayed high genome collinearity. Ctg1, ctg3, ctg5 and ctg7 in P1609 correspond to chr.2, chr.6, chr.1 and chr.5 of 70–15, respectively. We found that the second largest contig in the P1609, ctg2, is a combination of chr.4 and chr.7 of 70–15 (Fig. 3b). The joining region was spanned by multiple single PacBio long reads (Fig. 3d), excluding the possibility that the rearrangement was an artifact due to assembly errors. Meanwhile, notably, the contig end regions of P1609 showed a higher level of chromosome deletion and rearrangement when compared to 70–15 (Fig. 3c). For instance, ctg4 and ctg8 were merged with blocks homologous to 70–15 chr.1 and chr.3 at the end of the contig, while ctg9 was merged with blocks from chr.3 and chr.5 at the end of the contig (Fig. 3b).
Fig. 3.
Chromosome rearrangement and splitting between P1609 and 70–15. a Bar plot showing the chromosomes in 70–15. b Bar plot showing the assembled contigs of P1609. Colinear chromosomes of 70–15 and contigs of P1609 are indicated by the same color. c Dual synteny plot showing splitting of Ctg2 of P1609 into chr. 4 and chr. 7 in 70–15. d PacBio long-read coverage from 2.02 to 2.94 Mb of Ctg 2. Color of reads indicate different read lengths.
Discussion
Here we sequenced the genome of P. penniseti using PacBio SMRT technology. Comparative genomic analysis revealed several chromosome rearrangement events relative to P. oryzae and large differences in pathogenicity-related gene repertoires between the P. penniseti strain P1609 and the P. oryzae strain 70–15.
Chromosome fission and fusion
Long-read sequencing greatly improved genome assembly and thus provided more valuable details on genome structures at a chromosome level. By comparing the chromosome structure between P1609 and 70–15, we found several chromosome splitting and rearrangement events. Chromosomal rearrangements have been reported to be associated with virulence evolution in several pathogens due to the loss of AVR gene(s) [26, 40, 41]. In addition to rearrangements, the telomere regions were also rearranged relative to P. oryzae. Further study is required to investigate the role of the chromosome recombination during the adaptation to JUJUNCAO as well as the variation that occurs between individuals in the P. penneseti population.
Unique genes and positively selected genes
In this study, we found 2210 unique genes in P. penniseti that were not present in the 70–15 genome. The P1609 genome encodes more CAZymes than 70–15 genome. For example, P1609 contains twice the number of genes encoding glycosyl hydrolase family 28 (GH28) pectinases in 70–15. Generally, necrotrophic plant pathogens possess more GH28 enzymes than biotrophic and non-pathogens fungi [42]. These results suggested that P1609 might heavily rely on CAZymes in the interaction with JUJUNCAO.
To identify genes under positive selection, the Ka/Ks ratios in the 5991 orthologous gene pairs between P1609 and 70–15 were calculated, and only 6 genes with Ka > Ks in P1609 were identified. Functional annotation showed that most of these genes appear to be involved in secondary metabolic pathways. For example, P1609_5032 encodes an isoamyl alcohol oxidase that turns isoamyl alcohol into isovaleraldehyde [43]. In Saccharomyces cerevisiae, isoamyl alcohol could induce filament formation [44]. P1609_5032 might play a role in fungal development through controlling the level of isoamyl alcohol. P1609_791 encodes a folylpolyglutamate synthase involved in biosynthesis of folate, required for protein synthesis in bacteria, mitochondria, and chloroplasts, and biosynthetic pathways for purines, dTMP, methionine, and formyl-methionyl-tRNA [45]. P1609_3360 encodes a glycerol uptake protein, which is an important intracellular osmolyte participating in osmotic stress response [46] and critical for the function of appressorium in P. oryzae [47]. P1609_7869 encodes a homolog of a spore surface glycoprotein shown to be involved in spore adhesion to hydrophobic surfaces in several Colletotrichum species [48–50]. Adhesion of spore tip mucilage is important for infection in P. oryzae [51]. P1609_1006 encodes a BclB glycoprotein (collagen-like protein). Its homologs in Bacillus anthracis are important components of the infection-associated structure exosporium [52–54], although its role in filamentous pathogens remains unknown. The positive selection on these genes suggested that they might play roles in the interaction between P1609 and JUJUNCAO.
Putative secreted proteins
Increasingly, studies of pathogen populations support the view that plant immunity is the major force driving the specialization of adapted pathogens [2, 15–18]. It was previously proposed by Schulze-Lefert and Panstruga that effector-triggered immunity (ETI) is the major force driving the host specificity of pathogens [55]. Our previous study focusing on AVR gene evolution of the Pyricularia species also revealed that directional selection exerted by host plants is the direct force driving host specificity in Pyricularia species [18]. Recent studies in the interaction between M. oryzae and rice revealed that both ETI and pathogen-associated molecular patterns (PAMPs)-triggered immunity (PTI) are involved in determining effector repertoires and specialization to the two subspecies of P. oryzae [15–17]. This opinion was supported by the results that the japonica-isolate genomes contain many effectors to overcome the strong basal immunity posed by japonica rice, but prevent them from infection of indica rice through the triggering of ETI. By contrast, the indica-isolates deserted most of the effectors to avoid recognition by R genes encoded by indica plants, but are disabled in conquering the elevated basal immunity in japonica rice [16]. Our comparative genomic analysis showed that P1609 contains a large number of unique effector candidates compared with 70–15, but lost (or never possessed) many putative effectors found in the 70–15 genome, including all known AVR effectors. One possible explanation is that the JUJUNCAO harbors a high level of basal immunity, as well as an arsenal of resistance genes, which drove P1609 to gain host-specific effectors to overcome the robust basal immunity posed by JUJUNCAO. The large difference in effector/Avr gene content between P. penniseti and P. oryzae suggest the selection driving evolution of these fungi can help direct investigation of the molecular mechanisms underlying the interaction between Pyricularia species and their hosts.
Conclusion
Pyricularia species are pathogens of grasses, many of which are either food or forage grasses. The model fungus P. oryzae had been well studied. However, there are only a few whole-genome sequences available for other species, such as those from Pennisetum grasses. Here, we generated long-read PacBio reads and produced a assemblage with long-continuity contig sequences. The phylogenomic and comparative genomic analysis suggested that P1609 is a Pyricularia species genetically distant from P. oryzae, and may have employed diverse mechanisms during the adaptation to JUJUNCAO, including the pathways associated with secondary metabolics (positively selected genes), CAZymes and the putative secreted proteins, as well as other PHI proteins. In summary, the P1609 assembly and genome annotation represents the few available Pyricularia genome resources for studying the pathogenic mechanism of this fungus towards Pennisetum grasses.
Methods
Isolation of the fungal strain
The Pennisetum-infecting strain P1609 was isolated from the leaf spot lesion of JUJUNCAO (Pennisetum giganteum Z. X. Lin), in the nursery of National Engineering Research Center of JUNCAO Technology, Fujian Agriculture and Forestry University located at No. 63 Xiyuangong Road, Minhou County, Fuzhou, Fujian Province, China.
DNA extraction, amplification and sequencing
To prepare the genomic DNA for sequencing, the P1609 isolate was cultured in the liquid complete medium (CM) in a 110-rpm shaker at 25 °C for 3 to 4 days. The mycelia were then collected for the preparation of genomic DNA using a CTAB method as previously described [18]. Sequencing libraries were prepared using the SMRTbellTM Template Prep Kit 1.0 (PACBIO) and sequenced using PacBio Sequel platform (NovoGene, China).
Assembly and annotation
De novo sequence assembly was conducted by SMRTLink v. 5.0.1.10424, HGAP 4 pipeline provided by Pacific Bioscience Company. In HGAP 4 pipeline, the expected genome size was set as 45 Mb based on the reported size of Pyricularia genomes, and default settings were used for other parameters. Gene prediction was conducted using Fgenesh from SoftBerry (MolQuest II v2.4.5.1135, http://linux1.softberry.com/berry.phtml) with Pyricularia additional variants as training organism. Gene functional domain annotation was conducted by InterproScan (version 4.8, http://www.ebi.ac.uk/ interpro/), and PfamScan [56]. Pathogen-Host Interaction (PHI) genes were predicted by performing a whole genome blastp analysis against the PHI database (E < 10− 10) [57, 58]. Putative carbohydrate-active enzymes (CAZymes) were identified using the HMMER 3.1b1 by searching annotated HMM profiles of CAZyme families downloaded from the dbCAN database in protein sequences of P1609 [59].
Repeat analysis
De novo repeat sequence identification was analyzed by using RepeatModeler (version 1.0.8) with default settings. Repeat sequences obtained from RepeatModeler have been used to search for repeat sequences in the P1609 genome by RepeatMasker (version 3.3.0) (http://www.repeatmasker.org/) [60].
Phylogenetic analysis and comparative genomic analysis
Phylogenomic tree of P1609 and B. cinerea [61], C. gloeosporioides [62], F. graminearum [63], N. crassa [64], P. oryzae [8], S. sclerotiorum [61],T. reesei [65] and U. maydis [66] was built based on single copy orthologs from clustering result of OrthoFinder (v0.6.1) [27]. 2051 single copy genes have been selected out from 13 organisms in total (see Fig. 2a) and aligned with MAFFT (mafft-linsi-anysymbol) [67]. The phylogenetic tree was constructed using FastTree based on the alignments of single-copy orthologs with approximately-maximum-likelihood model and 100 bootstrap iterations [68]. For divergence time estimation, the phylogenetic analysis was conducted using r8s (version 1.81), and the divergence time of Pyricularia and Neurospora (200 MYA) was used as a reference [28, 29]. Clustering result of 13 genomes was also used for unique gene identification and comparative genomic analysis. The comparative genomic study of homologs among P1609 and 70–15 (the reference isolate of P. oryzae), N. crassa, F. graminearum and C. gloeosporioides was conducted using the OrthoFinder [27]. The identification of positively selected genes was performed as described previously [11]. Genes were aligned in pairs between P1609 and 70–15. Codeml tool in PAML suite was used to calculate Ka/Ks ratio, with the assumption that Ka/Ks ratio > 1 suggested the gene was under positive selection. For the prediction of secreted proteins, SignalP 4.1 was implemented to predict signal peptides and TMHMM 2.0 have been used to predict the transmembrane domain [34, 69]. Proteins with a signal peptide cleavage site, amino acid length smaller than 400 amino acids, and no transmembrane domain after the region signal peptide cleavage site were defined as putative secreted proteins in this study. We used MCScanX to identified syntenic blocks between P1609 and 70–15. To detect the conserved synteny blocks, the reciprocal best-match paralogs of P1609 and 70–15 were conducted by all-against-all BLASTP comparison, with E-value < 10− 10 [70].
Additional files
Figure S1. Gene copy numbers of CAZymes in Botrytis cinereal, Colletotrichum gloeosporioides, Fusarium graminearum, Neurospora crassa, Sclerotinia sclerotiorum, Trichoderma reesei and Ustilago maydis as well as Pyricularia isolates collected from O. sativa (PoOs), T. aestivum (PoTa), D. sanguinalis (PgDs), S. viridis (PoSv), and E. indica (PoEi). Log2 Copy Number presents variation of copy number with increased red color means increased number of CAZymes. (XLSX 20 kb)
Table S1. The CAZymes identified in P1609 and other fungi. (XLS 47 kb)
Table S2. P1609_vs_7015_unique_secreted. (XLSX 16 kb)
Table S3. 70–15 VS P1609 unique secreted proteins. (XLSX 71 kb)
Figure S2. GH28 of P1609 (P1609_11576, P1609_5879, P1609_2497, P1609_5781, P1609_680 and P1609_5514)) and PoOs (MGG_09608, MGG_08752 and MGG_08938), PgDs (Ds0505_9820). Extra copies of GH28 in P1609 is marked by blue. (JPG 237 kb)
Table S4. Predicted PHI in P1609. (JPG 113 kb)
Acknowledgments
We would like to thank Dr. Sanzhen Liu at Kansas State University and Dr. Daniel Ebbole at Texas A&M University for critically reading the manuscript.
Funding
This work was supported by grants from the Natural Science Foundations of China to Z.W (31770156), the Scientific Research Foundation of the Graduate School of FAFU to Z.Z, and the State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops to H.Z (SKB2017002).
Availability of data and materials
Genome assembly and PacBio reads are available in GenBank under BioProject PRJNA416656. The Whole Genome sequence has been deposited at GenBank under the accession PELF00000000.
Abbreviations
- AVR gene
Avirulence gene
- B. cinerea
Botrytis cinerea
- CAZymes
Carbohydrate-active enzymes
- CBM
Carbohydrate-binding module family
- Cg
Colletotrichum gloeosporioides (C. gloeosporioides)
- chr
Chromosome
- CM
Complete medium
- CTAB
Cetyltrimethyl ammonium bromide
- ctg
Contig
- ETI
Effector-triggered immunity
- Fg
Fusarium graminearum (F. graminearum)
- GH
Glycosyl hydrolase family pectinases
- LTR
Long terminal repeats
- MYA
Million years ago
- Nc
Neurospora crassa (N. crassa)
- P. giganteum
Pennisetum giganteum
- P. grisea
Pyricularia grisea
- P. penniseti
Pyrucularia penniseti
- P. penniseticola
Pyricularia penniseticola
- P. setariae
Pyricularia setariae
- P. typhoides
Pennisetum typhoides
- PgDs
Pyricularia strain isolated from Digitaria sanguinalis
- PHI
Pathogen-Host Interaction
- PL
Polysaccharide Lyase Family
- Po
Pyricularia oryzae (P. oryzae)
- PoEi
Pyricularia strain isolated from Eleusine indica
- PoOs
Pyricularia strain isolated from Oryza sativa
- PoSv
Pyricularia strain isolated from Setaria viridis
- PoTa
Pyricularia strain isolated from Triticum aestivum
- PTI
Pathogen-associated molecular pattern (PAMPs)-triggered immunity
- R genes
Resistance gene
- S. Sclerotiorum
Sclerotinia sclerotiorum
- SMRT
Single-Molecule Real-Time
- T. reesei
Trichoderma reesei
- TE
Transposable elements
- U. maydis
Ustilago maydis
Authors’ contributions
The study was conceived and designed by ZW and GL. The initial collection and culturing of the strain was performed by HZ, XC, HH, MS, LiZ, YH, TF, YZ, JG, LnZ, JF and HL. Bioinformatics was performed by ZZ, and LL. ZZ, HZ, JN, GL and ZW wrote, revised and approved the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huakun Zheng, Email: huakunzheng@163.com.
Zhenhui Zhong, Email: zhenhuizhong@gmail.com.
Mingyue Shi, Email: 1253985346@qq.com.
Limei Zhang, Email: 996272349@qq.com.
Lianyu Lin, Email: 279035687@qq.com.
Yonghe Hong, Email: 616944462@qq.com.
Tian Fang, Email: 919352540@qq.com.
Yangyan Zhu, Email: 1642575948@qq.com.
Jiayuan Guo, Email: 1053373938@qq.com.
Limin Zhang, Email: 2955180771@qq.com.
Jie Fang, Email: 550316079@qq.com.
Hui Lin, Email: ljuncao@qq.com.
Justice Norvienyeku, Email: nkjustice07@yahoo.com.
Xiaofeng Chen, Email: verry@163.com.
Guodong Lu, Phone: 86-13950480067, Email: gdlufafu@163.com.
Hongli Hu, Phone: 86-13599082338, Email: huhongli7905@gmail.com.
Zonghua Wang, Phone: 86-13706948783, Email: zonghuaw@163.com.
References
- 1.Saccardo P. Fungorum Extra-Europaeorum pugillus. Michelia. 1880;2(6):136–149. [Google Scholar]
- 2.Gladieux P, Condon B, Ravel S, Soanes D, Leodato Nunes Maciel J, Nhani J, Antonio N, Chen L, Terauchi R, Lebrun M-H, Tharreau D, et al. Gene flow between divergent cereal- and grass-specific lineages of the rice blast fungus Magnaporthe oryzae: mBio; 2017. p. e01219-01217. 10.1128/mBio.01219-01217. [DOI] [PMC free article] [PubMed]
- 3.Igarashi S, Utiamada C, Igarashi L, Kazuma A, Lopes R. Pyricularia em trigo. 1. Ocorrência de Pyricularia sp. no estado do Paraná. Fitopatol Bras. 1986;11:351–352. [Google Scholar]
- 4.Wilson RA, Talbot NJ. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol. 2009;7(3):185–195. doi: 10.1038/nrmicro2032. [DOI] [PubMed] [Google Scholar]
- 5.Klaubauf S, Tharreau D, Fournier E, Groenewald JZ, Crous PW, de Vries RP, Lebrun MH. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae) Stud Mycol. 2014;79:85–120. doi: 10.1016/j.simyco.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenne JM. A world list of fungal diseases of tropical pasture species. Australas Plant Pathol. 1991;20(3):122–124. doi: 10.1071/APP9910122. [DOI] [Google Scholar]
- 7.Chao CCT, Ellingboe AH. Selection for mating competence in Magnaporthe grisea pathogenic to rice. Can J Bot. 1991;69(10):2130–2134. doi: 10.1139/b91-267. [DOI] [Google Scholar]
- 8.Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu JR, Pan H, et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434(7036):980–986. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- 9.Yan X, Talbot NJ. Investigating the cell biology of plant infection by the rice blast fungus Magnaporthe oryzae. Curr Opin Microbiol. 2016;34:147–153. doi: 10.1016/j.mib.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Bao J, Chen M, Zhong Z, Tang W, Lin L, Zhang X, Jiang H, Zhang D, Miao C, Tang H. PacBio sequencing reveals transposable element as a key contributor to genomic plasticity and virulence variation in Magnaporthe oryzae. Mol Plant. 2017. 10.1016/j.molp.2017.1008.1008. [DOI] [PubMed]
- 11.Chen C, Lian B, Hu J, Zhai H, Wang X, Venu RC, Liu E, Wang Z, Chen M, Wang B, et al. Genome comparison of two Magnaporthe oryzae field isolates reveals genome variations and potential virulence effectors. BMC Genomics. 2013;14. 10.1186/1471-2164-1114-1887. [DOI] [PMC free article] [PubMed]
- 12.Dong Y, Li Y, Zhao M, Jing M, Liu X, Liu M, Guo X, Zhang X, Chen Y, Liu Y, et al. Global genome and transcriptome analyses of Magnaporthe oryzae epidemic isolate 98-06 uncover novel effectors and pathogenicity-related genes, revealing gene gain and lose dynamics in genome evolution. PLoS Pathog. 2015;11(4):e1004801. doi: 10.1371/journal.ppat.1004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue M, Yang J, Li Z, Hu S, Yao N, Dean RA, Zhao W, Shen M, Zhang H, Li C, et al. Comparative analysis of the genomes of two field isolates of the rice blast fungus Magnaporthe oryzae. PLoS Genet. 2012;8(8):e1002869. doi: 10.1371/journal.pgen.1002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida K, Saitoh H, Fujisawa S, Kanzaki H, Matsumura H, Tosa Y, Chuma I, Takano Y, Win J, Kamoun S, et al. Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell. 2009;21(5):1573–1591. doi: 10.1105/tpc.109.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gladieux P, Ravel S, Rieux A, Cros-Arteil S, Adreit H, Milazzo J, Thierry M, Fournier E, Terauchi R, Tharreau D. Coexistence of multiple endemic and pandemic lineages of the rice blast pathogen. MBio. 2018;9(2):e01806-01817. doi: 10.01128/mBio.01806-01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao J, Huang H, Meusnier I, Adreit H, Ducasse A, Bonnot F, Pan L, He X, Kroj T, Fournier E, et al. Pathogen effectors and plant immunity determine specialization of the blast fungus to rice subspecies. Elife. 2016:5. 10.7554/eLife.19377. [DOI] [PMC free article] [PubMed]
- 17.Zhong Z, Chen M, Lin L, Han Y, Bao J, Tang W, Lin Y, Somai R, Lu L, Zhang W, et al. Population genomic analysis of the rice blast fungus reveals specific events associated with expansion of three main clades. ISME J. 2018;12(8):1867–1878. doi: 10.1038/s41396-018-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Z, Justice N, Chen M, Bao J, Lin L, Chen L, Lin Y, Wu X, Cai Z, Zhang Q. Directional selection from host plants is a major force driving host specificity in Magnaporthe species. Sc Rep. 2016;6:25591. doi: 10.21038/srep25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H, Zheng H, Yang T, Chen X, Ye W, Lu G, Lin Z, Wang Z. First report of Pyricularia leaf spot on Pennisetum giganteum (JUJUNCAO) in China. Plant Dis. 2018. 10.1094/PDIS-05-18-0898-PDN.
- 20.Lin X, Lin Z, Lin D, Lin H, Luo H, Hu Y, Lin C, Zhu C. Effects of planting Pennisetum sp. (Giant Juncao) on soil microbial functional diversity and fertility in the barren hillside. Acta Ecol Sin. 2014;34(15):4304–4312. [Google Scholar]
- 21.Lin ZX, Lin DM, Su DW, Lin H, Jing L, Zheng D, Yu S-K. Effect of different salt-affected soils on biological characteristics of Pennisetum sp. Southwest Chin J Agr Sci. 2015;28(2):675–680. [Google Scholar]
- 22.Shi J, Lin ZX, Lin DM, De-Wei SU, Luo HL, Lin XS, Lin ZS, Zheng D, Chen JH, Yao JX. Enzymolysis conditions of Pennisetum sp. cellulose. Pratacultural Sci. 2014;31(4):760–765. [Google Scholar]
- 23.Prasada R, Goyal JP. A new species of Pyricularia on Bajra. Cur Sci. 1970;39(12):287–288. [Google Scholar]
- 24.Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10(6):563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 25.Muszewska A, Hoffmansommer M, Grynberg M. LTR retrotransposons in Fungi. PLoS One. 2011;6(12):e29425. doi: 10.21371/journal.pone.0029425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raffaele S, Kamoun S. Genome evolution in filamentous plant pathogens: why bigger can be better. Nat Rev Microbiol. 2012;10(6):417–430. doi: 10.1038/nrmicro2790. [DOI] [PubMed] [Google Scholar]
- 27.Emms DM, Kelly S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015;16:157. doi: 10.1186/s13059-13015-10721-13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedges SB. The origin and evolution of model organisms. Nat Rev Genet. 2002;3(11):838–849. doi: 10.1038/nrg929. [DOI] [PubMed] [Google Scholar]
- 29.Sanderson MJ. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics. 2003;19(2):301–302. doi: 10.1093/bioinformatics/19.2.301. [DOI] [PubMed] [Google Scholar]
- 30.Couch BC, Fudal I, Lebrun MH, Tharreau D, Valent B, van Kim P, Notteghem JL, Kohn LM. Origins of host-specific populations of the blast pathogen Magnaporthe oryzae in crop domestication with subsequent expansion of pandemic clones on rice and weeds of rice. Genetics. 2005;170(2):613–630. doi: 10.1534/genetics.105.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo J, Qiu H, Cai G, Wagner NE, Bhattacharya D, Zhang N. Phylogenomic analysis uncovers the evolutionary history of nutrition and infection mode in rice blast fungus and other Magnaporthales. Sci Rep. 2015;5:9448. doi: 10.1038/srep09448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiapello H, Mallet L, Guerin C, Aguileta G, Amselem J, Kroj T, Ortega-Abboud E, Lebrun MH, Henrissat B, Gendrault A, et al. Deciphering genome content and evolutionary relationships of isolates from the fungus Magnaporthe oryzae attacking different host plants. Genome Biol Evol. 2015;7(10):2896–2912. doi: 10.1093/gbe/evv187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubicek CP, Starr TL, Glass NL. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol. 2014;52(1):427–451. doi: 10.1146/annurev-phyto-102313-045831. [DOI] [PubMed] [Google Scholar]
- 34.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Z, Liu H, Wang C, Xu JR. Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genomics. 2013;14:274. doi: 10.1186/1471-2164-1114-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon J, Park SY, Chi MH, Choi J, Park J, Rho HS, Kim S, Goh J, Yoo S, Park JY, et al. Genome-wide functional analysis of pathogenicity genes in the rice blast fungus. Nat Genet. 2007;39(4):561–565. doi: 10.1038/ng2002. [DOI] [PubMed] [Google Scholar]
- 37.Wu D, Oide S, Zhang N, Choi MY, Turgeon BG. ChLae1 and ChVel1 regulate T-toxin production, virulence, oxidative stress response, and development of the maize pathogen Cochliobolus heterostrophus. PLoS Pathog. 2012;8(2):e1002542. doi: 10.1371/journal.ppat.1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehrabi R, Mirzadi Gohari A, Kema GHJ. Karyotype variability in plant-pathogenic Fungi. Annu Rev Phytopathol. 2017;55:483–503. doi: 10.1146/annurev-phyto-080615-095928. [DOI] [PubMed] [Google Scholar]
- 39.Faino L, Seidl MF, Shi-Kunne X, Pauper M, van den Berg GC, Wittenberg AH, Thomma BP. Transposons passively and actively contribute to evolution of the two-speed genome of a fungal pathogen. Genome Res. 2016;26(8):1091–1100. doi: 10.1101/gr.204974.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Jonge R, Bolton MD, Kombrink A, van den Berg GC, Yadeta KA, Thomma BP. Extensive chromosomal reshuffling drives evolution of virulence in an asexual pathogen. Genome Res. 2013;23(8):1271–1282. doi: 10.1101/gr.152660.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann FE, Sanchez-Vallet A, McDonald BA, Croll D. A fungal wheat pathogen evolved host specialization by extensive chromosomal rearrangements. ISME J. 2017;11(5):1189–1204. doi: 10.1038/ismej.2016.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sprockett DD, Piontkivska H, Blackwood CB. Evolutionary analysis of glycosyl hydrolase family 28 (GH28) suggests lineage-specific expansions in necrotrophic fungal pathogens. Gene. 2011;479(1–2):29–36. doi: 10.1016/j.gene.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita N, Motoyoshi T, Nishimura A. Purification and characterization of Isoamyl alcohol oxidase ("Mureka"-forming enzyme) Biosci Biotechnol Biochem. 1999;63(7):1216–1222. doi: 10.1271/bbb.63.1216. [DOI] [PubMed] [Google Scholar]
- 44.Kern K, Nunn CD, Pichova A, Dickinson JR. Isoamyl alcohol-induced morphological change in Saccharomyces cerevisiae involves increases in mitochondria and cell wall chitin content. FEMS Yeast Res. 2004;5(1):43–49. doi: 10.1016/j.femsyr.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 45.DeSouza L, Shen Y, Bognar AL. Disruption of cytoplasmic and mitochondrial folylpolyglutamate synthetase activity in Saccharomyces cerevisiae. Arch Biochem Biophys. 2000;376(2):299–312. doi: 10.1006/abbi.2000.1741. [DOI] [PubMed] [Google Scholar]
- 46.Varela JC, Mager WH. Response of Saccharomyces cerevisiae to changes in external osmolarity. Microbiology. 1996;142(Pt 4):721–731. doi: 10.1099/00221287-142-4-721. [DOI] [PubMed] [Google Scholar]
- 47.Foster AJ, Ryder LS, Kershaw MJ, Talbot NJ. The role of glycerol in the pathogenic lifestyle of the rice blast fungus Magnaporthe oryzae. Environ Microbiol. 2017;19(3):1008–1016. doi: 10.1111/1462-2920.13688. [DOI] [PubMed] [Google Scholar]
- 48.Mercure EW, Kunoh H, Nicholson RL. Adhesion of Colletotrichum graminicola conidia to corn leaves, a requirement for disease development. Physiological & Molecular Plant Pathology. 1995;45(6):407–420. doi: 10.1016/S0885-5765(05)80039-6. [DOI] [Google Scholar]
- 49.Sela-Buurlage MB, Epstein L, Rodriguez RJ. Adhesion of ungerminated Colletotrichum musae conidia. Physiological & Molecular Plant Pathology. 1991;39(5):345–352. doi: 10.1016/0885-5765(91)90016-B. [DOI] [Google Scholar]
- 50.Young DH, Kauss H. Adhesion of Colletotrichum lindemuthianum spores to Phaseolus vulgaris hypocotyls and to polystyrene. Appl Environ Microbiol. 1984;47(4):616–619. doi: 10.1128/aem.47.4.616-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamer JE, Howard RJ, Chumley FG, Valent B. A mechanism for surface attachment in spores of a plant pathogenic fungus. Science. 1988;239(4837):288–290. doi: 10.1126/science.239.4837.288. [DOI] [PubMed] [Google Scholar]
- 52.Thompson BM, Hoelscher BC, Driks A, Stewart GC. Assembly of the BclB glycoprotein into the exosporium and evidence for its role in the formation of the exosporium 'cap' structure in Bacillus anthracis. Mol Microbiol. 2012;86(5):1073–1084. doi: 10.1111/mmi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson BMWLN, Fox KF. The BdB glycoprotein of Bacillus anthracis is involved in exosporium integrity. J Bacteriol. 2007;189(18):6704–6713. doi: 10.1128/JB.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Jenkins SA, Gu C, Shree A, Martinez-Moczygemba M, Herold J, Botto M, Wetsel RA, Xu Y. Bacillus anthracis spore surface protein BclA mediates complement factor H binding to spores and promotes spore persistence. PLoS Pathog. 2016;12(6):e1005678. doi: 10.1371/journal.ppat.1005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulze-Lefert P, Panstruga R. A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci. 2011;16(3):117–125. doi: 10.1016/j.tplants.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Mistry J, Bateman A, Finn RD. Predicting active site residue annotations in the Pfam database. BMC Bioinformatics. 2007;8:298. doi: 10.1186/1471-2105-1188-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urban M, Cuzick A, Rutherford K, Irvine A, Pedro H, Pant R, Sadanadan V, Khamari L, Billal S, Mohanty S, et al. PHI-base: a new interface and further additions for the multi-species pathogen-host interactions database. Nucleic Acids Res. 2017;45(D1):D604–D610. doi: 10.1093/nar/gkw1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winnenburg R, Baldwin TK, Urban M, Rawlings C, Kohler J, Hammond-Kosack KE. PHI-base: a new database for pathogen host interactions. Nucleic Acids Res. 2006;34:D459–D464. doi: 10.1093/nar/gkj047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin Y, Mao X, Yang J, Chen X, Mao F, Xu Y. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40(Web Server issue):445–451. doi: 10.1093/nar/gks479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarailo-Graovac M, Chen N. UNIT 4.10Using RepeatMasker to identify repetitive elements in genomic sequences. 2009; Chapter 4(Unit 4):4.10.11–14.10.14. doi: 10.1002/0471250953.bi0410s25. [DOI] [PubMed]
- 61.Amselem J, Cuomo CA, van Kan JA, Viaud M, Benito EP, Couloux A, Coutinho PM, de Vries RP, Dyer PS, Fillinger S, et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011;7(8):e1002230. doi: 10.1371/journal.pgen.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gan P, Ikeda K, Irieda H, Narusaka M, O'Connell RJ, Narusaka Y, Takano Y, Kubo Y, Shirasu K. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 2013;197(4):1236–1249. doi: 10.1111/nph.12085. [DOI] [PubMed] [Google Scholar]
- 63.Cuomo CA, Güldener U, Xu JR, Trail F, Turgeon BG, Pietro AD, Walton JD, Ma LJ, Baker SE, Rep M. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science. 2007;317(5843):1400–1402. doi: 10.1126/science.1143708. [DOI] [PubMed] [Google Scholar]
- 64.Galagan JE, Calvo SE, Borkovich KA, Selker EU, Read ND, Jaffe D, FitzHugh W, Ma LJ, Smirnov S, Purcell S, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422(6934):859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- 65.Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, Chapman J, Chertkov O, Coutinho PM, Cullen D, et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina) Nat Biotechnol. 2008;26(5):553–560. doi: 10.1038/nbt1403. [DOI] [PubMed] [Google Scholar]
- 66.Kamper J, Kahmann R, Bolker M, Ma LJ, Brefort T, Saville BJ, Banuett F, Kronstad JW, Gold SE, Muller O, et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature. 2006;444(7115):97–101. doi: 10.1038/nature05248. [DOI] [PubMed] [Google Scholar]
- 67.Kato H, Yamamoto M, Yamaguchiozaki T, Kadouchi H, Iwamoto Y, Nakayashiki H, Tosa Y, Mayama S, Mori N. Pathogenicity, mating ability and DNA restriction fragment length polymorphisms of Pyricularia populations isolated from Gramineae, Bambusideae and Zingiberaceae plants. J Gen Plant Pathol. 2000;66(1):30–47. doi: 10.1007/PL00012919. [DOI] [Google Scholar]
- 68.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305(3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gene copy numbers of CAZymes in Botrytis cinereal, Colletotrichum gloeosporioides, Fusarium graminearum, Neurospora crassa, Sclerotinia sclerotiorum, Trichoderma reesei and Ustilago maydis as well as Pyricularia isolates collected from O. sativa (PoOs), T. aestivum (PoTa), D. sanguinalis (PgDs), S. viridis (PoSv), and E. indica (PoEi). Log2 Copy Number presents variation of copy number with increased red color means increased number of CAZymes. (XLSX 20 kb)
Table S1. The CAZymes identified in P1609 and other fungi. (XLS 47 kb)
Table S2. P1609_vs_7015_unique_secreted. (XLSX 16 kb)
Table S3. 70–15 VS P1609 unique secreted proteins. (XLSX 71 kb)
Figure S2. GH28 of P1609 (P1609_11576, P1609_5879, P1609_2497, P1609_5781, P1609_680 and P1609_5514)) and PoOs (MGG_09608, MGG_08752 and MGG_08938), PgDs (Ds0505_9820). Extra copies of GH28 in P1609 is marked by blue. (JPG 237 kb)
Table S4. Predicted PHI in P1609. (JPG 113 kb)
Data Availability Statement
Genome assembly and PacBio reads are available in GenBank under BioProject PRJNA416656. The Whole Genome sequence has been deposited at GenBank under the accession PELF00000000.