Abstract
Background
Natural infections of the endosymbiont bacteria Wolbachia have recently been discovered in populations of the malaria mosquito Anopheles gambiae (s.l.) in Burkina Faso and Mali, West Africa. This Anopheles specific strain wAnga limits the malaria parasite Plasmodium falciparum infections in the mosquito, thus it offers novel opportunities for malaria control.
Results
We investigated Wolbachia presence in Anopheles arabiensis and Anopheles funestus, which are the two main malaria vectors in the Kilombero Valley, a malaria endemic region in south-eastern Tanzania. We found 3.1% (n = 65) and 7.5% (n = 147) wAnga infection prevalence in An. arabiensis in mosquitoes collected in 2014 and 2016, respectively, while no infection was detected in An. funestus (n = 41). Phylogenetic analysis suggests that at least two distinct strains of wAnga were detected, both belonging to Wolbachia supergroup A and B.
Conclusions
To our knowledge, this is the first confirmation of natural Wolbachia in malaria vectors in Tanzania, which opens novel questions on the ecological and genetic basis of its persistence and pathogen transmission in the vector hosts. Understanding the basis of interactions between Wolbachia, Anopheles mosquitoes and malaria parasites is crucial for investigation of its potential application as a biocontrol strategy to reduce malaria transmission, and assessment of how natural wAnga infections influence pathogen transmission in different ecological settings.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-3249-y) contains supplementary material, which is available to authorized users.
Keywords: Wolbachia, Anopheles, Malaria parasite, Endosymbiont, Pathogen interference, Maternal transmission, Vector control, Tanzania
Background
The maternally inherited endosymbiont bacteria Wolbachia infects an estimated 40 to 66% of all insect species worldwide [1, 2]. To ensure its transmission and spread in naive insect populations, Wolbachia has, in some species, been found to alter reproduction of the insect host to favour female progeny. For example, it induces production of only female progeny, parthenogenesis and cytoplasmic incompatibility (CI) (i.e. the embryonic death of offspring) from Wolbachia-infected males and uninfected females [3]. Wolbachia has been proposed as a biocontrol tool against vector-borne diseases because it can reduce the pathogens developing within insect vectors. For example, Aedes aegypti mosquitoes that were laboratory infected with Wolbachia are unable to sustain infections with dengue (DENV) [4] and Zika (ZIKV) viruses [5]. By exploiting the CI phenotype of Wolbachia, endosymbiont infected Ae. aegypti have since been introduced and subsequently spread into natural mosquito populations with the aim of reducing dengue and Zika transmission [6, 7].
While Ae. aegypti mosquitoes are naturally uninfected with Wolbachia [8], other mosquito species carry natural infections of this endosymbiont, for example Culex pipiens [9] and Aedes albopictus [10]. Recently, the major African malaria vectors of the Anopheles gambiae (s.l.) complex [including An. gambiae (s.s.), An. coluzzii and An. arabiensis] were found to be infected in Burkina Faso [11–13] and Mali [14]. Additional investigations detected Wolbachia in other malaria vectors also in Central and East Africa [15, 16]. These findings suggest that in addition to artificially introduced Wolbachia strains in the laboratory [17], natural infections in Anopheles mosquitoes should be exploited to identify any opportunities for malaria control. Indeed, negative associations between wAnga (the Anopheles-specific Wolbachia strain/s) and the human malaria parasite Plasmodium falciparum were found in An. gambiae (s.l). [13, 14]. Additionally, An. coluzzii with natural wAnga infections were at least two times less likely to harbour the malaria parasite once experimentally infected with P. falciparum, suggesting a protective effect of the endosymbiont against this pathogen in the mosquito [14]. These early findings raise prospects for the future application of wAnga for malaria control. However, such a strategy will require extensive knowledge of the biology of natural wAnga infections in malaria vectors, including the genetic and ecological basis of the induced phenotypes and the mechanisms of parasite interference.
One key aspect of wAnga biology that needs to be elucidated is its mechanism of persistence and transmission in the mosquito populations. Maternal transmission seems to be incomplete [11], suggesting that this strain is associated with a strong fitness benefit to the female progeny, or that additional factors may be required to ensure successful transgenerational transmission and survival. Nevertheless, laboratory investigations using wAnga infected Anopheles mosquitoes showed that the endosymbiont does not induce CI [13, 14] or distortion of sex ratio [13]. Further work is required to understand if the lack of CI would also occur under natural settings. One apparent fitness advantage of wAnga is the observed accelerated oviposition timing, which could increase the number of gonotrophic cycles and therefore the total number of progeny; nevertheless, this increased oviposition rate might be associated with a decrease in lifespan [18], thus the actual fitness benefit of this induced phenotype is still not resolved.
The identification of natural infections under different ecological settings and in different vector species is crucial to understand the potential impact of this endosymbiont on disease transmission dynamics, and how it could be exploited for vector control. As Wolbachia-induced phenotypes depend on the co-evolutionary history of the host and endosymbiont [19], exploiting the natural Wolbachia-induced parasite interference in Anopheles might result in a more sustainable biological control tool than using artificial infections. Consequently, it is paramount to detect and characterise natural Wolbachia infections in Anopheles populations. Here, we investigated the presence of Wolbachia in An. arabiensis and An. funestus in the Kilombero Valley, south-eastern Tanzania, where these two species are the dominant malaria vectors [20, 21].
Methods
Mosquito collection and Wolbachia detection
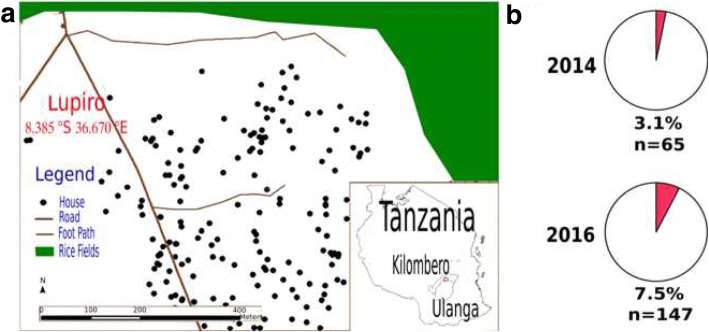
Collections were performed in Lupiro village (8°22'59"S, 36°40'00"E) in Ulanga district, south-eastern Tanzania (Fig. 1a), in November 2014 and in July 2016, during rainy and dry seasons, respectively. Major Anopheles in the area include the An. funestus (s.l.) group [including An. funestus (s.s.) Giles, An. leesoni and An. rivulorum] and the An. gambiae (s.l.) complex (consisting primarily of An. arabiensis), An. coustani, An. pharoensis, An. squamosus, An. ziemanni and An. wellcomei. Of these the main malaria vectors include An. funestus (s.s.) and An. arabiensis with minor contributions from An. rivulorum. Overall the entomological inoculation rate (EIR) was last estimated at 4.2 and 11.7 infectious bites/person/year by An. arabiensis and An. funestus, respectively. There are also culicine species, mainly Mansonia, Aedes and Culex mosquito species [20, 22]. Adult female Anopheles mosquitoes were collected either inside houses with CDC light traps (Prevention, C.f.D.C.a., Model 512, John Hock, Gainesville, FL, USA) or outdoor with backpack aspirators (Prevention, C.f.D.C.a., Model 1412, John Hock). Mosquitoes were sampled from collections from 10 houses. An. gambiae (s.l.) complex and An. funestus (s.l.) group were morphologically identified and DNA extracted from individual whole fresh mosquitoes using a DNeasy kit (Qiagen, Manchester, UK) and eluted in 50 μl of water. Forty to 120 ng of DNA was used to amplify the Wolbachia-specific 16S rDNA region using an established nested PCR approach for natural wAnga infections in An. gambiae (s.l.) [13]. All 13 amplified 412-bp fragments were confirmed to correspond to Wolbachia by Sanger sequencing (Eurofins Genomics, Ebersberg, Germany) (GenBank accession numbers MH596693-MH596703). PCR was used to identify species in An. gambiae (s.l.) complex [23] and An. funestus (s.l.) group [24].
Fig. 1.
a Map showing Lupiro village (8°22'59"S, 36°40'00"E) in Ulanga district, south-eastern Tanzania, from where the Anopheles specimens were obtained (Courtesy of Alex J. Limwagu, Ifakara Health Institute). b The prevalence of Wolbachia in An. arabiensis in Lupiro village, in 2014 and 2016, is indicated
Phylogenetic analysis
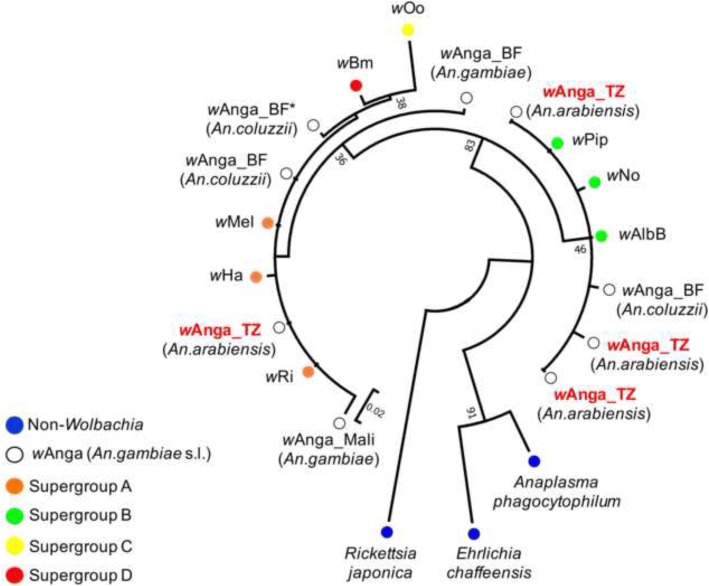
Wolbachia 16S rRNA sequences were aligned using Clustal Omega [25]. Other Wolbachia sequences comprising members of the supergroups A (wMel AE017196.1, wRi CP001391.1, wHa CP003884.1), B (wPip AM999887.1, wAlbB KX155506.1, wNo CP003883.1), C (wOo AJ010276.1), D (wBm AE017321.1) and wAnga (wAnga_BF: KP089991 in An. coluzzii [12], KJ728740.1 and KJ728755.1 in An. coluzzii [11], KJ728754.1 in An. gambiae [11], wAnga_Mali: MF944114.1 in An. gambiae [14], wAnga_TZ: MH596693, MH596696, MH596697, MH596703 in An. arabiensis) sequences were included (Additional file 1: Figure S1).
The sequences of the endosymbionts Rickettsia japonica (CP032049.1), Ehrlichia chaffeensis (NR_074500.2) and Anaplasma phagocytophilum (KY114936.1) were included as non-Wolbachia reference outgroups. The general time reversible (GTR+G) model was used to calculate sequence divergences [26]. A maximum likelihood tree using 1000 bootstrap replicates of GTR+G distances was created to provide a graphic representation of the patterning of divergences among the sequences obtained from the samples.
Results
All 212 An. gambiae (s.l.) females collected in 2014 and 2016 were identified as An. arabiensis by PCR. Wolbachia-specific 16S rRNA nested PCR followed by sequencing (GenBank accession numbers MH596693-MH596703) identified Wolbachia in 3.1% (2/65) and 7.5% (11/147) of the samples collected in 2014 and 2016, respectively (Fig. 1). All 41 An. funestus (s.l.) females collected in 2014 were identified as An. funestus (s.s.) and Wolbachia infection was not detected. The 2016 analysis did not include any An. funestus mosquitoes.
To determine the genetic variation and diversity of the identified Wolbachia strain/s, which we will refer to as wAnga_TZ, we conducted phylogenetic analyses on 4 samples based on the conserved 16S rRNA region amplified and sequenced. For comparison, we included other wAnga sequences identified in An. gambiae and An. coluzzii in Burkina Faso (wAnga_BF) [11, 12] and Mali (wAnga_Mali) [14], and sequences from arthropod-specific (subgroups A: wMel, wHa, wRi; and B: wPip, wAlbB, wNo) and nematode-specific (subgroups C: wOo; and D: wBm) Wolbachia (Additional file 1: Figure S1). Most of the wAnga_TZ sequences (3 out of 4) clustered with supergroup B, and only one with supergroup A. Conversely, wAnga_Mali and most of wAnga_BF clustered with supergroup A and only one wAnga_BF from An. coluzzii belonged to supergroup B (Fig. 2). This phylogenetic analysis suggests that wAnga belongs to the supergroups A or B and exhibits a relatively high genetic diversity which is widespread in both West and East Africa.
Fig. 2.
Phylogenetic analysis of the Wolbachia-specific 16S rRNA conserved region. The sequences identified in this study in An. arabiensis in Tanzania (wAnga_TZ) (highlighted in red) clustered with Wolbachia strains from the supergroup A or B. Sequences from other wAnga from An. gambiae (s.l.) in Burkina Faso [11] (the asterisk indicates a sequence from Buck et al. [12]) and Mali [14] were also included. Other non-Wolbachia proteobacteria (R. japonica, E. chaffeensis and A. phagocytophilum) were also included, and the R. japonica sequence was used as the reference outgroup
Discussion
Here we detected natural Wolbachia infections in An. arabiensis population in south-eastern Tanzania. To our knowledge, this is the first identification of this endosymbiont in natural populations of malaria vectors in Tanzania and highlights need for further investigation of its distribution and importance in the region. Until recently, Wolbachia had not been detected in natural populations of Anopheles mosquitoes [27–30], the vectors of human malaria. This lack of identification was probably due to a general low infection prevalence and Wolbachia density within species of this mosquito genus, which could have prevented the detection in the low sample sizes tested by single PCR. Both the nested PCR approach [13], which was used here, and quantitative PCR [14] increase sensitivity and are therefore more appropriate for the detection of low prevalence and low density endosymbiont loads typical of wAnga. Here, Wolbachia infection prevalence in An. arabiensis (3.1–7.5%, Fig. 1) was lower than wAnga in West Africa, where up to 33% of An. arabiensis were infected in the Soumousso village in Burkina Faso [13]. Furthermore, other species of the An. gambiae (s.l.) complex in West Africa (Burkina Faso and Mali) show higher infection prevalence ranging between 19–78% [13, 14]. These results suggest that natural Wolbachia infections are widespread in species of the An. gambiae (s.l.) complex in Africa, although their prevalence is highly variable.
We did not detect Wolbachia in any of the 41 An. funestus specimens examined. However, given the low prevalence rates observed in An. funestus in another study (5%) [15], the failure to detect Wolbachia in the An. funestus mosquitoes in the present study should not be interpreted as absence of the endosymbiont in this species. Larger sample sizes of An. funestus will need to be tested before any such conclusion can be made. However, one possible hypothesis worth investigating is that the potential absence of wAnga in An. funestus and its presence in An. arabiensis, coupled with proven interference of P. falciparum infections in some mosquitoes by wAnga, may be associated with the differential importance of these two species in the malaria transmission dynamics in East Africa. Indeed, although it occurs in far lower densities than An. arabiensis, An. funestus now mediates more than 80% of malaria transmission in the Kilombero Valley [20]. Future studies should thus investigate interactions and differential effects on vector competence.
As wAnga might have an effect on mosquito vectorial capacity [13, 14], it is crucial to understand the ecological and genetic determinants of wAnga infection dynamics. For example, laboratory investigations showed that in An. stephensi maternal transmission of an artificially introduced Wolbachia strain (wAlbB) is prevented by some components of the mosquito microbiota [31]. Furthermore, in Drosophila, environmental factors such as temperature and diet influence Wolbachia density [32, 33], potentially affecting infection dynamics by influencing maternal transmission efficiency [34] and reproduction manipulation [35]. It is therefore possible that environmental variation including microbiome composition can impede or sustain Wolbachia transmission in Anopheles. Additionally, variation in the genetic background and physiology of mosquito populations might affect Wolbachia persistence; indeed, in the mosquito Culex pipiens, the physiological costs associated with insecticide resistance results in decreased ability to control Wolbachia infection and consequently increased endosymbiont density [36, 37]. Thus, the widespread insecticide resistance occurring in malaria vectors in Africa [38] could also be responsible for the spread of Wolbachia into Anopheles populations, possibly reducing malaria transmission. Additional investigations under different ecological settings and mosquito host genetic backgrounds (including presence and absence of different insecticide resistance mechanisms) are therefore required to understand which factors are affecting wAnga infection dynamics and ultimately the vectorial capacity of its malaria vector hosts.
Understanding the mechanisms and genetic basis of wAnga induced parasite interference is also imperative. Elucidating wAnga genetic variation and association with parasite infection could be a first step to unravel the molecular bases of this phenotype and any associated drivers of parasite interference. Here, phylogenetic analysis of the conserved 16S rRNA region showed that at least two strains infect An. arabiensis in Tanzania, and that both strains belong to either supergroup A or B (Fig. 2). Multilocus sequence typing (MLST) and/or whole genome sequencing of different wAnga isolates will be required to fully characterize the genetic diversity of the circulating strains. Genetic characterization is crucial, as different strains can have opposite effects on malaria parasites, as observed in Anopheles species that were artificially infected with different Wolbachia strains and experimentally challenged with Plasmodium in the laboratory [39] (Table 1). Indeed, pathogen inhibition may not be a consistent consequence of Wolbachia infection. For example, natural Wolbachia infections can increase the susceptibility of Aedes and Culex mosquitoes and Simulium blackflies to avian malaria parasites [40–42]. Therefore, it will be crucial to assess the impact of wAnga on malaria infections and vectorial capacity under natural, ecologically variable conditions.
Table 1.
Wolbachia dependent phenotypes in Anopheles. The phenotypes of different Wolbachia strains infecting Anopheles species are summarized. ↑, ↓, = indicate increased, decreased or stable associations or influence on the trait/phenotype, respectively. CI indicates cytoplasmic incompatibity. One asterisks refers to induced maternal transmission by microbiome suppression [31], two asterisks to a temperature dependent phenotype [43], three asterisks refer to the present study
| Wolbachia strain | Anopheles species | Type of infection | Maternal transmission | CI | Plasmodium infection | Other phenotypes | Reference |
|---|---|---|---|---|---|---|---|
| wAlbB | An. stephensi | Artificial | Yes | Yes |
P. falciparum: ↓oocysts; ↓ sporozoites |
↑ immune response | [17, 44] |
|
P. berghei: ↓ oocysts; ↓ sporozoites | |||||||
| wAlbB | An. gambiae | Artificial | No/Yes* | No |
P. falciparum: ↓ oocysts |
↑/↓immune response | [31, 45, 46] |
|
P. berghei: ↑ oocysts | |||||||
| wAlbB | An. stephensi | Artificial | No/Yes* | No |
P. yoelii: ↑/↓ oocysts**; ↑/↓ sporozoites** |
↑ immune response** | [31] |
| wMelPop | An. gambiae | Artificial | No | No |
P. falciparum: ↓ oocysts |
↑ immune response | [45–47] |
|
P. berghei: ↓/= oocysts |
↓ survival | ||||||
| wAnga_BF | An. gambiae; An. coluzzii; An. arabiensis | Natural | Yes | No |
P. falciparum: ↓ prevalence |
↑ oviposition rate | [11–13] |
| wAnga_Mali | An. gambiae; An. coluzzii | Natural | Yes | No |
P. falciparum: ↓ oocysts; ↓ sporozoites |
? | [14] |
| wAnga_TZ | An. arabiensis | Natural | ? | ? | ? | ? | *** |
In combination with previous evidence from West [11–14], Central and East Africa [15, 16], this confirmation of Wolbachia infection in An. arabiensis in Tanzania indicates that this endosymbiont may be widespread and ubiquitous in malaria vector populations across the continent. Absence of Wolbachia in the 41 An. funestus specimens should not be interpreted as absence of the endosymbiont in the species, and that future surveys may find it. This finding should encourage future exploitation of this strain as an agent of malaria control through its potential impact on the transmission capacity of malaria vectors. Further work is crucially needed to understand the ecological, genetic and mechanistic bases of Wolbachia-parasite interactions in different Anopheles vectors and in different ecological settings. Indeed, this knowledge is required for: (i) the development of this strain as a bio-control agent, similar to ongoing trials for dengue control; (ii) the prediction of how variation of natural wAnga infection prevalence influences disease transmission in mosquito populations.
Conclusions
In the Kilombero Valley (Tanzania), malaria mosquito populations of An. arabiensis are naturally infected with Wolbachia (wAnga_TZ). Understanding its impact on mosquito vectorial capacity is paramount for the development of novel bio-control tools based on this endosymbiont.
Additional file
Figure S1. Multiple sequence alignment of 16S rRNA conserved region used for phylogenetic analysis. The consensus sequence is reported together with the consensus and occupancy histograms (using Jalview). Nucleotides are colour coded for clarity. Sequences are ordered based on their pairwise similarity. (TIF 4248 kb)
Acknowledgments
We thank all the technicians at Ifakara Health Institute who participated in the mosquito collections and Milali P. Masabho for his support in the 2014 collection. We also wish to thank Lisa Ranford-Cartwright for support with laboratory equipment and Kathryn Elmer for advice on phylogenetic analysis.
Funding
This work was supported by an AXA RF fellowship (14-AXA-PDOC-130) and an EMBO LT fellowship (43-2014) to FB. FO was also funded by a Wellcome Trust Intermediate Fellowship in Public Health and Tropical Medicine (grant number: WT102350/Z/13). KK was supported by DELTAS Africa Initiative (Afrique One-ASPIRE/ DEL-15-008).
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional file. The 16S rRNA Wolbachia sequences obtained in this study are available in the GenBank repository under the accession numbers MH596693-MH596703.
Abbreviations
- CI
Cytoplasmic incompatibility
- EIR
Entomological inoculation rate
- GTR +G
General time reversible model
- MLST
Multilocus sequence typing
Authors’ contributions
FB, HMF, NG and FOO conceived the study. KK, GM and SAM performed the mosquito collections. FB and MSL carried out DNA extraction. FB and JR performed PCRs. FB performed phylogenetic analysis and wrote the manuscript with contributions from all authors. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Before the study began, meetings were held with community leaders in the village during which they were informed about the purpose of the study and their participation requested. After their permission was given, the study team visited the village and informed consent was obtained from each head of household where mosquito trapping was conducted. The study was previously approved by the Ifakara Health Institutional Review Board (certificate number IHI/IRB/No: 16-2013) and by the National Institute for Medical Research in Tanzania (NIMR/HQ/R.8c/Vol. II/304).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Francesco Baldini, Email: Francesco.Baldini@glasgow.ac.uk.
Justine Rougé, Email: 2426980R@student.gla.ac.uk.
Katharina Kreppel, Email: kkreppel@ihi.or.tz.
Gustave Mkandawile, Email: gmkandawile@ihi.or.tz.
Salum Abdallah Mapua, Email: smapua@ihi.or.tz.
Maggy Sikulu-Lord, Email: maggy.lord@uq.edu.au.
Heather M. Ferguson, Email: Heather.Ferguson@glasgow.ac.uk
Nicodem Govella, Email: govella@ihi.or.tz.
Fredros O. Okumu, Email: fredros@ihi.or.tz
References
- 1.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One. 2012;7:e38544. [DOI] [PMC free article] [PubMed]
- 2.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? - A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 4.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci Rep. 2016;6:28792. [DOI] [PMC free article] [PubMed]
- 6.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 7.Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gloria-Soria A, Chiodo TG, Powell JR. Lack of evidence for natural Wolbachia infections in Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2018;55:1354–1356. doi: 10.1093/jme/tjy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hertig M. The Rickettsia, Wolbachia pipiens (gen. et sp. n.) and associated inclusions of the mosquito, Culex pipiens. Parasitology. 1936;28:453–486. doi: 10.1017/S0031182000022666. [DOI] [Google Scholar]
- 10.Wright JD, Barr AR. The ultrastructure and symbiotic relationships of Wolbachia of mosquitoes of the Aedes scutellaris group. J Ultrastruct Res. 1980;72:52–64. doi: 10.1016/S0022-5320(80)90135-5. [DOI] [PubMed] [Google Scholar]
- 11.Baldini F, Segata N, Pompon J, Marcenac P, Robert Shaw W, Dabiré RK, et al. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat Commun. 2014;5:3985. doi: 10.1038/ncomms4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck M, Nilsson LKJ, Brunius C, Dabiré RK, Hopkins R, Terenius O. Bacterial associations reveal spatial population dynamics in Anopheles gambiae mosquitoes. Sci Rep. 2016;6:22806. doi: 10.1038/srep22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw WR, Marcenac P, Childs LM, Buckee CO, Baldini F, Sawadogo SP, et al. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat Commun. 2016;7:11772. doi: 10.1038/ncomms11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes FM, Hixson BL, Tyner MDW, Ramirez JL, Canepa GE, Alves e Silva TL, et al. Effect of naturally occurring Wolbachia in Anopheles gambiae s.l. mosquitoes from Mali on Plasmodium falciparum malaria transmission. Proc Natl Acad Sci USA. 2017;114:12566–12571. doi: 10.1073/pnas.1716181114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayala D, Akone-Ella O, Rahola N, Kengne P, Ngangue MF, Mezeme F, et al. Natural Wolbachia infections are common in the major malaria vectors in Central Africa. bioRxiv. 2018;Article ID:343715. doi: 10.1111/eva.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffries CL, Lawrence GG, Golovko G, Kristan M, Orsborne J, Spence K, et al. Novel Wolbachia strains in Anopheles malaria vectors from sub-Saharan Africa. Wellcome Open Res. 2018;3:113. doi: 10.12688/wellcomeopenres.14765.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 18.Harshman LG, Zera AJ. The cost of reproduction: the devil in the details. Trends Ecol Evol. 2007;22:80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Caragata EP, Dutra HLC, Moreira LA. Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol. 2016;32:207–18. [DOI] [PubMed]
- 20.Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS One. 2017;12:e0177807. doi: 10.1371/journal.pone.0177807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaindoa EW, Mkandawile G, Ligamba G, Kelly-Hope LA, Okumu FO. Correlations between household occupancy and malaria vector biting risk in rural Tanzanian villages: implications for high-resolution spatial targeting of control interventions. Malar J. 2016;15:199. [DOI] [PMC free article] [PubMed]
- 22.Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, et al. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar J. 2014;13:331. doi: 10.1186/1475-2875-13-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163. doi: 10.1186/1475-2875-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamau L, Coetzee M, Hunt RH, Koekemoer LL. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 25.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nei M, Kumar S. Molecular Evolution and Phylogenetics. New York: Oxford University Press; 2000. [Google Scholar]
- 27.Kittayapong P, Baisley KJ, Baimai V, O’Neill SL. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae) J Med Entomol. 2000;37:340–345. doi: 10.1093/jmedent/37.3.340. [DOI] [PubMed] [Google Scholar]
- 28.Tiawsirisup S, Sripatranusorn S, Oraveerakul K, Nuchprayoon S. Distribution of mosquito (Diptera: Culicidae) species and Wolbachia (Rickettsiales: Rickettsiaceae) infections during the bird immigration season in Pathumthani Province, central Thailand. Parasitol Res. 2008;102:731–5. [DOI] [PubMed]
- 29.Wiwatanaratanabutr I. Geographic distribution of wolbachial infections in mosquitoes from Thailand. J Invertebr Pathol. 2013;114:337–340. doi: 10.1016/j.jip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Ricci I, Cancrini G, Gabrielli S, D’amelio S, Favia G. Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): large polymerase chain-reaction survey and new identifications. J Med Entomol. 2002;39:562–567. doi: 10.1603/0022-2585-39.4.562. [DOI] [PubMed] [Google Scholar]
- 31.Hughes GL, Dodson BL, Johnson RM, Murdock CC, Tsujimoto H, Suzuki Y, et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc Natl Acad Sci USA. 2014;111:12498–12503. doi: 10.1073/pnas.1408888111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strunov A, Kiseleva E, Gottlieb Y. Spatial and temporal distribution of pathogenic Wolbachia strain wMelPop in Drosophila melanogaster central nervous system under different temperature conditions. J Invertebr Pathol. 2013;114:22–30. doi: 10.1016/j.jip.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Serbus LR, White PM, Silva JP, Rabe A, Teixeira L, Albertson R, et al. The impact of host diet on Wolbachia titer in Drosophila. PLoS Pathog. 2015;11:e1004777. doi: 10.1371/journal.ppat.1004777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurst GDD, Jiggins FM, Robinson SJW. What causes inefficient transmission of male-killing Wolbachia in Drosophila? Heredity (Edinb). 2001;87:220–226. doi: 10.1046/j.1365-2540.2001.00917.x. [DOI] [PubMed] [Google Scholar]
- 35.Bordenstein SR, Bordenstein SR. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS One. 2011;6:e29106. doi: 10.1371/journal.pone.0029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M. High Wolbachia density in insecticide-resistant mosquitoes. Proc Biol Sci. 2002;269:1413–1416. doi: 10.1098/rspb.2002.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Echaubard P, Duron O, Agnew P, Sidobre C, Noël V, Weill M, et al. Rapid evolution of Wolbachia density in insecticide resistant Culex pipiens. Heredity (Edinb). 2010;104:15–19. doi: 10.1038/hdy.2009.100. [DOI] [PubMed] [Google Scholar]
- 38.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387:1785–1788. doi: 10.1016/S0140-6736(15)00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes GL, Rivero A, Rasgon JL. Wolbachia can enhance Plasmodium infection in mosquitoes: implications for malaria control? PLoS Pathog. 2014;10:e1004182. doi: 10.1371/journal.ppat.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baton LA, Pacidônio EC, Gonçalves D da S, Moreira LA. wFlu: Characterization and evaluation of a native Wolbachia from the mosquito Aedes fluviatilis as a potential vector control agent. PLoS One. 2013;8:e59619. doi: 10.1371/journal.pone.0059619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zele F, Nicot A, Berthomieu A, Weill M, Duron O, Rivero A. Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proc Biol Sci. 2014;281:20132837. doi: 10.1098/rspb.2013.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodford L, Bianco G, Ivanova Y, Dale M, Elmer K, Rae F, et al. Vector species-specific association between natural Wolbachia infections and avian malaria in black fly populations. Sci Rep. 2018;8:4188. doi: 10.1038/s41598-018-22550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murdock CC, Blanford S, Hughes GL, Rasgon JL, Thomas MB. Temperature alters Plasmodium blocking by Wolbachia. Sci Rep. 2015;4:3932. doi: 10.1038/srep03932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi D, Pan X, McFadden MJ, Bevins D, Liang X, Lu P, et al. The maternally inheritable Wolbachia wAlbB induces refractoriness to Plasmodium berghei in Anopheles stephensi. Front Microbiol. 2017;8:366. doi: 10.3389/fmicb.2017.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7:e1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes GL, Vega-Rodriguez J, Xue P, Rasgon JL. Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Appl Environ Microbiol. 2012;78:1491–1495. doi: 10.1128/AEM.06751-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kambris Z, Blagborough AM, Pinto SB, Blagrove MSC, Godfray HCJ, Sinden RE, et al. Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6:e1001143. doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Multiple sequence alignment of 16S rRNA conserved region used for phylogenetic analysis. The consensus sequence is reported together with the consensus and occupancy histograms (using Jalview). Nucleotides are colour coded for clarity. Sequences are ordered based on their pairwise similarity. (TIF 4248 kb)
Data Availability Statement
Data supporting the conclusions of this article are included within the article and its additional file. The 16S rRNA Wolbachia sequences obtained in this study are available in the GenBank repository under the accession numbers MH596693-MH596703.