Abstract
Background:
The iron requirements increase during the second and third trimesters of pregnancy. Maternal anemia is a leading cause of adverse perinatal outcome.
Objectives:
This study was designed to evaluate the efficacy of the heme-bound iron in treatment of pregnancy-associated iron deficiency anemia (IDA).
Materials and Methods:
In all, 122 women with IDA during pregnancy and hemoglobin ≤10 g/dL were studied. The studied women were treated with heme-bound iron tablets for ≥3 months. Pretreatment hemoglobin, ferritin, mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) were compared with the posttreatment values to detect the efficacy of heme-bound iron (Optifer®) in treatment of IDA during pregnancy.
Results:
The mean pretreatment hemoglobin significantly increased from 8.4 ± 2.7 to 11.2 ± 2.1 g/dL and the mean pretreatment ferritin level significantly increased from 22.6 ± 5.6 to 112.8 ± 4.8 μg/L (P < 0.003 and 0.04; respectively) 3 months after heme-bound iron treatment. In addition, the mean pretreatment red blood cells’ MCV and MCH significantly increased from 74.2 ± 4.8 fL and 24.2 ± 7.8 pg, respectively, to 92.0 ± 4.1 fL and 32.6 ± 6.2 pg) (P = 0.04 and 0.007, respectively) 3 months after heme-bound iron treatment.
Conclusion:
Heme-bound iron (Optifer®) is an effective oral iron preparation to treat IDA during pregnancy and to replace the depleted iron store.
Keywords: Deficiency, heme-bound, iron, pregnancy
Introduction
Hemoglobin <11 g/dL is defined as anemia, and iron deficiency anemia (IDA) is the commonest type of nutritional deficiencies.[1,2] The iron requirements during pregnancy are high and increase furthermore during the second and third trimesters.[3]
In addition, blood loss during vaginal and cesarean deliveries increases maternal anemia and increases the need for blood transfusion.[4,5] Maternal anemia is a leading cause of adverse perinatal outcome.[6,7,8,9] Recently, Froessler et al. reported that iron deficiency and its related anemia are associated with adverse outcomes such as reduced maternal cognitive activities and increased maternal depressive disorders. While they reported preterm labor (PTL), intrauterine growth retardation, intrauterine fetal death, and neonatal infection as adverse neonatal outcomes for iron deficiency and IDA.[10]
Peripartum anemia increases the need for red blood cells’ (RBCs) transfusion, which is independently associated with increased morbidity.[11,12] In addition, RBCs’ transfusion corrects hemoglobin temporarily and not the underlying condition.[13] Adequate and effective iron supplementation is crucial during pregnancy to reduce the adverse perinatal outcome related to IDA.[14]
Oral iron therapy is effective in treatment of IDA. The conventional non-heme iron salts are associated with gastric discomfort and constipation which adversely affect the compliance.[15,16]
Heme iron is a tolerable, effective oral iron preparation, improves the compliance, and ensures continuous iron intake.[15,16,17] Nissenson et al. concluded that the use of heme iron preparations in patients undergoing hemodialysis was effective in treatment of IDA and replaced the intravenous iron preparations.[18]
Abdelazim et al. concluded that heme iron is tolerable, effective, and replaces the intravenous iron for treatment of IDA during pregnancy.[17,19] In addition, Hoppe et al. concluded that heme iron dietary-based treatment can improve the reproductive age women's iron store.[20]
Heme-bound iron (Optifer®) is a new genuine heme-bound iron supplement made under Hazards Analysis and Critical Control Points (HACCP) and Good Manufacturing Practice (GMP) standards in Sweden. This study was designed to evaluate the efficacy of new heme-bound iron (Optifer®) in treatment of pregnancy-associated IDA.
Materials and Methods
This was a prospective comparative study conducted in Ahmadi Hospital, Kuwait Oil Company, for a period of 1 year from August 2017 to August 2018, after approval of the study by the Ethical Committee of the Department of Obstetrics and Gynecology.
In all, 122 women with pregnancy-associated IDA and hemoglobin ≤10 g/dL (8–10 g/dL) were studied after informed consent. The studied women were treated with heme-bound iron (Optifer®) tablets for correction of pregnancy-associated IDA twice daily for ≥3 months.
The inclusion criteria included pregnant women ≥20 years old and 14–26 weeks’ gestation with hemoglobin ≤10 (8–10 g/dL). Pregnant women with anemia other than IDA and/or received blood transfusion during current pregnancy were excluded from this study.
Diagnosis of IDA was based on hemoglobin concentration (g/dL), serum ferritin (μg/L), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH).[7,8,9,21] The heme-bound iron (Optifer®) tablets (MediTec NutriCare Division, MediTec Intern, FairLife Group, Sweden; L’Avenir Med., Kuwait and Qatar) contain 18 mg of heme-bound iron. The heme iron content of the Optifer® tablets has unique intestinal carrier receptors Heme Carrier Protein-1 (HCP-1).
The studied women received heme-bound iron (Optifer®) tablets twice daily (one tablet morning and one tablet evening) not related to meals for ≥3 months till hemoglobin level of 11–12 g/dL (according to the manufacturer's instructions) and then one tablet daily as maintenance dose.[18]
The iron content of the Optifer® tablets absorbed by the HCP-1 receptors of the intestine and each tablet of Optifer® increases the serum iron by 3.15 mg.[9] The studied women received oral folic acid with Optifer® to avoid folic deficiency, and the participants were asked during each antenatal care visit for the side effects related to Optifer® such as metallic taste, constipation, and/or intolerance.
Pretreatment hemoglobin, ferritin, and RBCs’ MCV and MCH were compared with the 3 months’ posttreatment values to detect the efficacy of Optifer® in treatment of pregnancy-associated IDA.[22,23]
Primary outcome measures the efficacy of the Optifer® in treatment of pregnancy-associated IDA. While the secondary outcome measures the tolerability and the side effects related to the Optifer®.
Sample size calculation
The required sample size was calculated using data from previous studies[18,19] and G*Power software for sample size calculation (Heinrich Heine Universität, Germany). An effective sample of ≥120 women was needed to produce a statistically acceptable figure.
Statistical analysis
Collected data were analyzed using Statistical Package for Social Sciences (SPSS) version 20 (Chicago, IL, USA). The mean and standard deviation (±SD) were used to present the numerical values, while the number (n) and percentage (%) were used to present the categorical values. Student's t-test was used to compare the pretreatment hemoglobin, ferritin, and RBCs’ MCV and MCH with the 3 months’ posttreatment values to evaluate the efficacy of heme-bound iron in treatment of pregnancy-associated IDA. P value <0.05 was considered significant.
Results
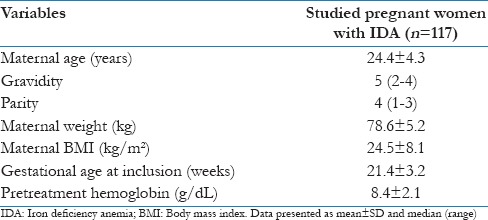
A total of 122 women with pregnancy-associated IDA and hemoglobin ≤10 g/dL (8–10 g/dL) were included in this prospective comparative study and treated with heme-bound iron tablets for ≥3 months. Five women were excluded from the final statistical analysis of this study due to PTL (three women) and (traveling two women), and the study was completed with final analysis of data from 117 pregnant women with IDA. Table 1 shows the demographic data of the studied pregnant women with IDA.
Table 1.
Demographic data of the studied pregnant women with IDA
The mean pretreatment hemoglobin significantly increased from 8.4 ± 2.7 to 11.2 ± 2.1 g/dL and the mean pretreatment ferritin level significantly increased from 22.6 ± 5.6 to 112.8 ± 4.8 μg/L (P < 0.003 and 0.04, respectively) 3 months after heme-bound iron treatment.
In addition, the mean pretreatment RBCs’ MCV and MCH significantly increased from 74.2 ± 4.8 fL and 24.2 ± 7.8 pg, respectively, to 92.0 ± 4.1 fL and 32.6 ± 6.2, pg) (P = 0.04 and 0.007, respectively) 3 months after heme-bound iron treatment [Table 2].
Table 2.
Pretreatment hemoglobin, ferritin, MCV, and MCHC compared with the posttreatment values

Only 1.7% (2/117) of the studied women developed intolerance and gastric upset to heme-bound iron tablets (insignificant difference using Chi-square (χ2) test) and no other side effects were recorded with heme-bound iron tablets.
Discussion
IDA is the third leading cause for years lived with disability since 1990.[24] In addition, IDA ranked 13 for disability-adjusted life-years.[25] IDA affects more than 40% of women globally.[26,27]
IDA in the context of peripartum hemorrhage increases the maternal mortality and morbidity.[28] In addition, blood loss during vaginal and cesarean deliveries increases maternal anemia and increases the need for blood transfusion.[29]
In all, 122 pregnant women with pregnancy-associated IDA and hemoglobin ≤10 g/dL were studied and received heme-bound iron tablets for ≥3 months for correction of pregnancy-associated IDA. Five women were excluded from final statistical analysis of this study, and the study was completed with final analysis of data from 117 pregnant women with IDA.
The mean pretreatment hemoglobin significantly increased from 8.4 ± 2.7 to 11.2 ± 2.1 g/dL and the mean pretreatment ferritin level significantly increased from 22.6 ± 5.6 to 112.8 ± 4.8 μg/L (P < 0.003 and 0.04, respectively) 3 months after heme-bound iron treatment. In addition, the mean pretreatment RBCs’ MCV and MCH significantly increased from 74.2 ± 4.8 fL and 24.2 ± 7.8 pg, respectively, to 92.0 ± 4.1 fL and 32.6 ± 6.2 ng) (P = 0.04 and 0.007, respectively) 3 months after heme-bound iron treatment.
Barraclough et al. designed a multicenter study to evaluate whether heme iron preparations effectively augment the iron stores than conventional oral iron or not.[30] They concluded that heme iron preparations showed no clear efficacy in peritoneal dialysis patients than the conventional oral iron.[31]
Nissenson et al. found that the intravenous iron was discontinued and replaced with oral heme iron preparations after 6 months of follow-up of hemodialysis patients on heme iron preparations.[18]
In addition, this study concluded that hemoglobin, ferritin, and RBCs’ MCV and MCH significantly increased 3 months after heme-bound iron treatment in pregnant women with IDA. Additionally, Abdelazim et al. concluded that heme iron preparations are effective to treat IDA during pregnancy.[19]
Gastric side effects are common with oral iron and Al-Momen et al. found that 18 (30%) of the oral iron had gastric symptoms and 18 (30%) had poor compliance.[32]
While in this study only 1.7% (2/117) of the studied women developed intolerance and gastric upset with heme-bound iron tablets (insignificant difference) and no other side effects were recorded with heme-bound iron Optifer® tablets.
Heme-bound iron represents a promising treatment option for ID and IDA due to higher bioavailability and lower gastrointestinal irritability.[33] Superior tolerability of the heme-bound iron provides added advantage because compliance to oral iron treatment is the single most important identified obstacle to effective treatment of IDA. Thus, heme-bound iron is recommended as the first choice of iron supplementation to treat IDA during pregnancy and to maintain maternal iron stores.[34]
Anemic pregnant women developed gastric upset when treated with intravenous iron sucrose because they cannot tolerate other oral iron preparations than Optifer®. To the best of our knowledge, this study was the first study conducted to evaluate the efficacy and tolerability of heme-bound iron tablets (Optifer®) in treatment of pregnancy-associated IDA. The limited available data about heme-bound iron (Optifer®) were the only limitation faced during this study. More comparative studies are needed to compare the efficacy of the heme-bound iron (Optifer®) in treatment of pregnancy-associated IDA with the available oral or intravenous iron preparations.
Conclusion
Heme-bound iron (Optifer®) is an effective oral iron preparation to treat IDA during pregnancy and to replace the depleted iron store.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors are grateful to women who gave consent and agreed to participate in this study.
References
- 1.Api O, Breyman C, Çetiner M, Demir C, Ecder T. Diagnosis and treatment of iron deficiency anemia during pregnancy and the postpartum period: Iron deficiency anemia working group consensus report. Turk J Obstet Gynecol. 2015;12:173–81. doi: 10.4274/tjod.01700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Iron and Folate Supplementation: Standards for Maternal and Neonatal Care. Integrated Management of Pregnancy and Childbirth (IMPAC). Department of Making Pregnancy Safer. WHO; 2007. [Google Scholar]
- 3.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1 Suppl):257S–64S. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- 4.Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol. 2008;199:519.e1–7. doi: 10.1016/j.ajog.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Breymann C, Bian XM, Blanco-Capito LR, Chong C, Mahmud G, Rehman R. Expert recommendations for the diagnosis and treatment of iron-deficiency anemia during pregnancy and the postpartum period in the Asia-Pacific region. J Perinat Med. 2011;39:113–21. doi: 10.1515/jpm.2010.132. [DOI] [PubMed] [Google Scholar]
- 6.Kalaivani K. Prevalence & consequences of anaemia in pregnancy. Indian J Med Res. 2009;130:627–33. [PubMed] [Google Scholar]
- 7.Shafi D, Purandare SV, Sathe AV. Iron deficiency anemia in pregnancy: Intravenous versus oral route. J Obstet Gynaecol India. 2012;62:317–21. doi: 10.1007/s13224-012-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 95: Anemia in pregnancy. Obstet Gynecol. 2008;112:201–7. doi: 10.1097/AOG.0b013e3181809c0d. [DOI] [PubMed] [Google Scholar]
- 9.Abdelazim IA, Abu-Faza ML, Hamdan SB. Intravenous iron saccharate infusion for treatment of iron deficiency anemia before labor. ARC J Gynecol Obstetr. 2016;1:16–20. [Google Scholar]
- 10.Froessler B, Gajic T, Dekker G, Hodyl NA. Treatment of iron deficiency and iron deficiency anemia with intravenous ferric carboxymaltose in pregnancy. Arch Gynecol Obstet. 2018;298:75. doi: 10.1007/s00404-018-4782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts CL, Nippita TA. International caesarean section rates: The rising tide. Lancet Glob Health. 2015;3:e241–2. doi: 10.1016/S2214-109X(15)70111-7. [DOI] [PubMed] [Google Scholar]
- 12.Patterson JA, Roberts CL, Bowen JR, Irving DO, Isbister JP, Morris JM, et al. Blood transfusion during pregnancy, birth, and the postnatal period. Obstet Gynecol. 2014;123:126–33. doi: 10.1097/AOG.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 13.Froessler B, Palm P, Weber I, Hodyl NA, Singh R, Murphy EM. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: A randomized controlled trial. Ann Surg. 2016;264:41–6. doi: 10.1097/SLA.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the safety of heme iron (blood peptonates) for the proposed uses as a source of iron added for nutritional purposes to foods for the general population, including food supplements. EFSA J. 2010;8:1585. [Google Scholar]
- 15.Pavord S, Myers B, Robinson S, Allard S, Strong J, Oppenheimer C. British Committee for Standards in Haematology. UK Guidelines on the Management of Iron Deficiency in Pregnancy. Br J Haematol. 2012;156:588–600. doi: 10.1111/j.1365-2141.2011.09012.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011;4:177–84. doi: 10.1177/1756283X11398736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelazim IA, Abu-Faza M, Elbiaa AAM, Othman HS, Alsharif DA, Elsawah WF. Heme iron polypeptide (Proferrin®-ES) versus iron saccharate complex (Ferrosac) for treatment of iron deficiency anemia during pregnancy. Acta Med Int. 2017;4:55–60. [Google Scholar]
- 18.Nissenson AR, Charytan C. Controversies in iron management. Kidney Int. 2003;64:S64–71. doi: 10.1046/j.1523-1755.64.s87.10.x. [DOI] [PubMed] [Google Scholar]
- 19.Abdelazim IA, Abu-Faza M, Elbiaa A, Osman H, Alsharif D, Elsawah W. Heme iron to correct Iron deficiency anemia with pregnancy. Clin Obstet Gynecol Reprod Med. 2017;3:1–3. [Google Scholar]
- 20.Hoppe M, Brün B, Larsson MP, Moraeus L, Hulthén L. Heme iron-based dietary intervention for improvement of iron status in young women. Nutrition. 2013;29:89–95. doi: 10.1016/j.nut.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Short MW, Domagalski JE. Iron deficiency anemia: Evaluation and management. Am Fam Physician. 2013;87:98–104. [PubMed] [Google Scholar]
- 22.Yee J, Besarab A. Iron sucrose: The oldest iron therapy becomes new. Am J Kidney Dis. 2002;40:1111–21. doi: 10.1053/ajkd.2002.36853. [DOI] [PubMed] [Google Scholar]
- 23.Teucher B, Olivares M, Cori H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403–19. doi: 10.1024/0300-9831.74.6.403. [DOI] [PubMed] [Google Scholar]
- 24.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–57. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 26.Sweet MG, Schmidt-Dalton TA, Weiss PM, Madsen KP. Evaluation and management of abnormal uterine bleeding in premenopausal women. Am Fam Physician. 2012;85:35–43. [PubMed] [Google Scholar]
- 27.Bora R, Sable C, Wolfson J, Boro K, Rao R. Prevalence of anemia in pregnant women and its effect on neonatal outcomes in Northeast India. J Matern-Fetal Neonatal Med. 2014;27:887–91. doi: 10.3109/14767058.2013.845161. [DOI] [PubMed] [Google Scholar]
- 28.Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW Nutrition Impact Model Study Group (anaemia) Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ. 2013;346:f3443. doi: 10.1136/bmj.f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barut A, Harma M. Intravenous iron treatment for iron deficiency anaemia in pregnancy. J Turkish-German Gynecol Assoc. 2009;10:109–15. [Google Scholar]
- 30.Barraclough KA, Noble E, Leary D, Brown F, Hawley CM, Campbell SB, et al. Rationale and design of the oral HEMe iron polypeptide Against Treatment with Oral Controlled Release Iron Tablets trial for the correction of anaemia in peritoneal dialysis patients (HEMATOCRIT trial) BMC Nephrol. 2009;10:20. doi: 10.1186/1471-2369-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barraclough KA, Brown F, Hawley CM, Leary D, Noble E, Campbell SB, et al. A randomized controlled trial of oral heme iron polypeptide versus oral iron supplementation for the treatment of anaemia in peritoneal dialysis patients: HEMATOCRIT trial. Nephrol Dial Transplant. 2012;27:4146–53. doi: 10.1093/ndt/gfs372. [DOI] [PubMed] [Google Scholar]
- 32.al-Momen AK, al-Meshari A, al-Nuaim L, Saddique A, Abotalib Z, Khashogji T, et al. Intravenous iron sucrose complex in the treatment of iron deficiency anemia during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;69:121–4. doi: 10.1016/0301-2115(95)02538-3. [DOI] [PubMed] [Google Scholar]
- 33.Nagaraju SP, Cohn A, Akbari A, Davis JL, Zimmerman DL. Iron polypeptide for the treatment of iron deficiency anemia in non-dialysis chronic kidney disease patients: A randomized controlled trial. BMC Nephrol. 2013;14:64. doi: 10.1186/1471-2369-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal B, Deshpande H, Sundari T, Biniwale P, Shah, K, Goel S, et al. Heme iron polypeptid in iron deficiency anemia of pregnancy: Current evidence. Open J Obstetr Gynecol. 2017;7:420–31. [Google Scholar]


