Abstract
Background:
Hypertension (HTN) and diabetes mellitus (DM) both are rapidly emerging as public health problems among geriatric population in developing countries. HTN can lead to stroke, myocardial infarction, congestive heart failure, and chronic kidney diseases among geriatric population. DM increases the risk of coronary heart disease, cerebrovascular disease, peripheral vascular disease, retinopathy, nephropathy, and neuropathy among geriatric population.
Methodology:
A community-based, cross-sectional study was conducted during 2015–2016 in District Nainital, Uttarakhand. A list of all villages with their population in the district was developed. From this list, 30 villages were identified using population proportionate to size sampling method. From each village, 30 geriatric subjects were selected. A total of 1003 geriatric subjects age 60 years and above were included in the study. Data were collected on sociodemographic profile, blood pressure, fasting blood glucose, anthropometry, and lipid profile from all the enrolled subjects. The prevalence of HTN and DM was assessed. Univariate and multivariate analyses were done to identify risk factors associated with HTN and DM.
Results:
The prevalence of HTN and DM was found to be 54.5% and 14.6%, respectively. For HTN, advancing age, high educational level and body mass index (BMI) (≥25 kg/m2) and for DM higher education level and BMI (≥25 kg/m2) were found to be significant risk factors.
Conclusion:
A high prevalence of HTN and DM was found in geriatric population residing in rural area of Uttarakhand.
Keywords: Diabetes, geriatric, high-altitude, hypertension
Introduction
Hypertension (HTN) and diabetes mellitus (DM) both are rapidly emerging as public health problems in developing countries.[1,2] HTN and DM are high in geriatric population across all geographical area and sociodemographic groups in India.[3,4] HTN and DM affect 1 billion[5] and 422 million[6] people worldwide, respectively. Prevalence of HTN among geriatric population was found to be 67.2% in Delhi and 63.6% in Assam.[7,8] Prevalence of DM among geriatric population was found to be 24.0% in Delhi.[7] Earlier studies have reported that the prevalence of HTN and DM was higher among elderly compared with middle age or young adults.[9,10] HTN can lead to cardiovascular disease, stroke, myocardial infarction, congestive heart failure, and chronic kidney diseases.[11] Likewise, DM increases the risk of coronary heart disease, cerebrovascular disease, peripheral vascular disease, retinopathy, nephropathy, and neuropathy.[12] According to World Health Organization (WHO), HTN is one of the most important causes of premature death globally.[13] Epidemiology of HTN and DM varies in India because it is a diverse country and people follow different lifestyle practices in different states. There is a lack of scientific evidence on the prevalence and risk factors associated with HTN and DM among geriatric population living at high-altitude region of India. Hence, this study was conducted.
Methodology
A community-based, cross-sectional study was conducted during 2015–2016 in district Nainital, Uttarakhand state, India. The district is situated at an altitude of 2084 m. A total of 1003 geriatric population were enrolled from 30 clusters (villages) identified using population proportionate to size sampling methodology. After reaching the village, the village president member was contacted. From the selected village, one lane was selected randomly. From the selected lane, one household was selected randomly. The survey was initiated from the selected first household and contiguously covered all the required number of subjects from that cluster. Thirty geriatric subjects in the age group of 60 years and above were selected from each cluster by house-to-house visit. The geriatric subjects were identified with the help of village-level health and nutrition functionaries such as anganwadi workers. However, they did not participate in data collection. The objectives and procedure of data collection were explained to each subject. An informed written consent was obtained from each subject prior to data collection.
An oral questionnaire was administered to obtain information on sociodemographic profile such as age, gender, caste, religion, financial dependency, educational qualification, occupation, family monthly income, type of house, type of family, marital status, and living arrangement. Information on alcohol use, tobacco consumption, Mini Nutritional Assessment (MNA), physical activity, and Barthel scale was obtained through oral questionnaire. Estimation of blood pressure (BP), fasting blood glucose, and lipid profile was undertaken using standard procedure.
BP was measured using digital Omron HEM-7080[14] BP apparatus in the sitting position. Participants were asked to restrict alcoholic or caffeinated beverages and smoking at least 30 min prior to measurements. Before starting BP measurements, the participants were advised to relax in a sitting position for at least 5 min. The participant's left arm was placed on the table. The respondent's arm was positioned so that it is resting at the level of the heart. Two readings of BP were taken at 5 min intervals on the same arm. The mean of the two measures was taken as final reading. Medical records of the subjects were checked and subjects taking hypertensive drug were considered as hypertensive.
Subjects were classified as hypertensive when systolic blood pressure (SBP) was ≥140 mm Hg or diastolic blood pressure (DBP) was ≥90 mm Hg according to Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.[15] Subjects with elevated BP were referred to a medical practitioner for treatment.
Fasting blood sugar was measured using Accu-Chek Active glucometer with measuring range of 10–600 mg/dL.[16] One day before the investigation, participants were instructed to remain fasting for blood glucose estimation. Each participant was asked to clean and rub his or her hands against each other to stimulate blood flow. Third (middle) or fourth (ring) finger of each subject was selected for collecting the blood. The finger tip was cleaned and punctured with a sterilized disposable lancet and a full drop of blood was allowed to form on finger. The first drop of the free flowing of blood was wiped off using a sterile swab. The second drop of blood was used to fill up the blood glucose strip for estimation of fasting blood glucose.
Medical records of the subjects were checked and subjects taking antidiabetic drug were considered as diabetic. Diagnosis of DM was done based on WHO criteria. Geriatric subjects having fasting blood glucose ≥126 mg/dL were considered as diabetic.[17] Subjects with elevated fasting blood glucose were referred to a medical practitioner for treatment.
Estimation of total serum cholesterol (TC) and triglyceride (TG) was done using Dried Blood Spot methodology.[18,19,20] Biochemical estimation of TC was done by cholesterol oxidase method, whereas TGs were estimated by glycerophosphate oxidase–peroxidase method using enzymatic kits from Randox Laboratories, Ltd. (UK).
The cutoff for TG (mg/dL) was used as normal <150 (normal), 150–199 (borderline high), and 200–499 (high). Similarly, TC (mg/dL) cutoff used was <200 (desirable), 200–239 (borderline high), and ≥240 (high). These cutoffs have been recommended by the third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult treatment panel III).[21]
Weight: Weight (kg) was measured with an electronic weighing scale SECA model-813 to the nearest 100 g. Elderly subjects were asked to be barefoot and wear light clothing. They were asked to stand straight on a firm horizontal flat surface of scale, and weight on the screen was recorded. Height: Height (cm) was measured using SECA-213 portable stadiometer to the nearest 0.1 cm. The individual was asked to stand upright without shoes with his or her back against the vertical backboard, heels together, and eyes directed forward. Body mass index (BMI) was calculated using the following formula: BMI (kg/m2) = weight (kg)/height (m2). The cutoff of BMI (kg/m2) used was <18.5 (underweight), 18.5–24.9 (normal), 25–29.9 (overweight and pre-obese), and ≥30 (obese) as per WHO classification.[22] High BMI level (≥25 kg/m2) included overweight and obesity category. The research was approved by the ethical committee of All India Institute of Medical Sciences, New Delhi.
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 20.0 was used for conducting statistical analysis of data (IBM SPSS statistics for Windows, version 20; IBM Corp, Armonk, NY, USA). Chi-square test was applied to analyze the association of various parameters with HTN and DM among the geriatric population. Stepwise logistic regression analysis was applied to assess the independent contribution of different factors to the presence of HTN and DM.
Results
A total of 1003 geriatric subjects were approached for the study. BP and fasting blood sugar could be done for 994 and 1002 geriatric subjects; respectively.
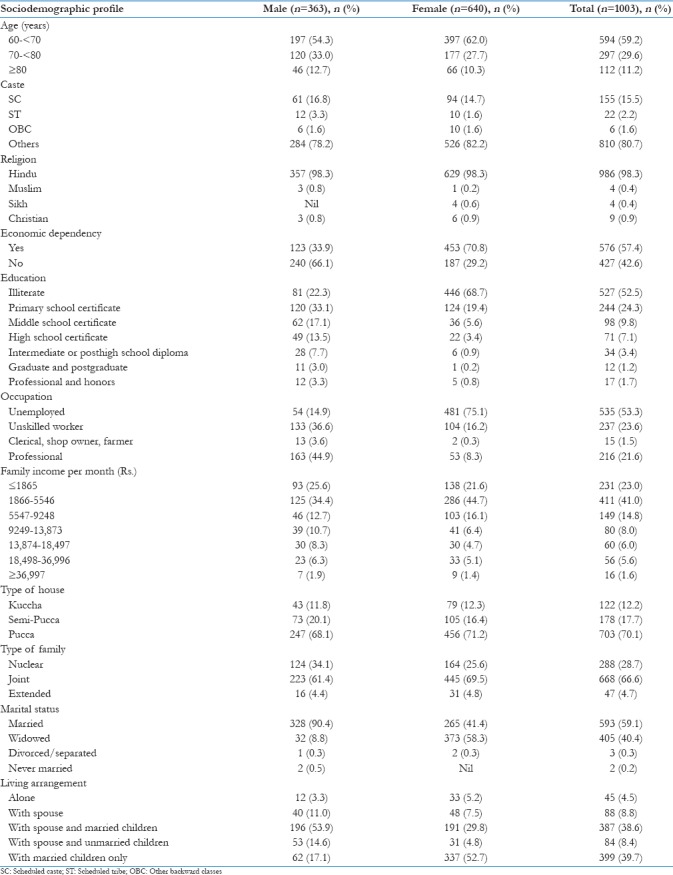
The sociodemographic details of the geriatric subjects are depicted in Table 1. The mean age of the males was 69.5 ± 7.4 years and for female was 67.8 ± 7.2 years. Overall, 52.5% subjects were illiterate. In all, 576 (57.4%) subjects were economically dependent on other family members [Table 1].
Table 1.
Distribution of elderly subjects according to sociodemographic profile (n=1003)
In this study, 542 subjects were found to be diagnosed with HTN. Of these, 383 were detected during the present survey and 159 were earlier known cases on the basis of their medical records. Similarly, 146 geriatric subjects were found to be diagnosed with diabetes. Of these, 77 were earlier known cases on the basis of their medical records and 69 were detected during the present survey [Table 2].
Table 2.
Distribution of elderly subjects according to prevalence of HTN and diabetes mellitus

A total of 8.6% (86/994) geriatric subjects had both HTN and DM. This concomitant prevalence of HTN and DM was higher in female (58.1%) compared with male (41.9%).
The overall prevalence of HTN was 54.5%; males (20.3%) and females (34.2%). The overall prevalence of DM was 14.6%; males (5.2%) and females (9.4%) [Tables 3 and 4].
Table 3.
Profile of HTN and impaired fasting glucose among geriatric subjects
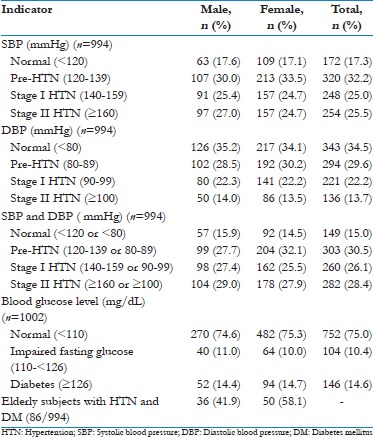
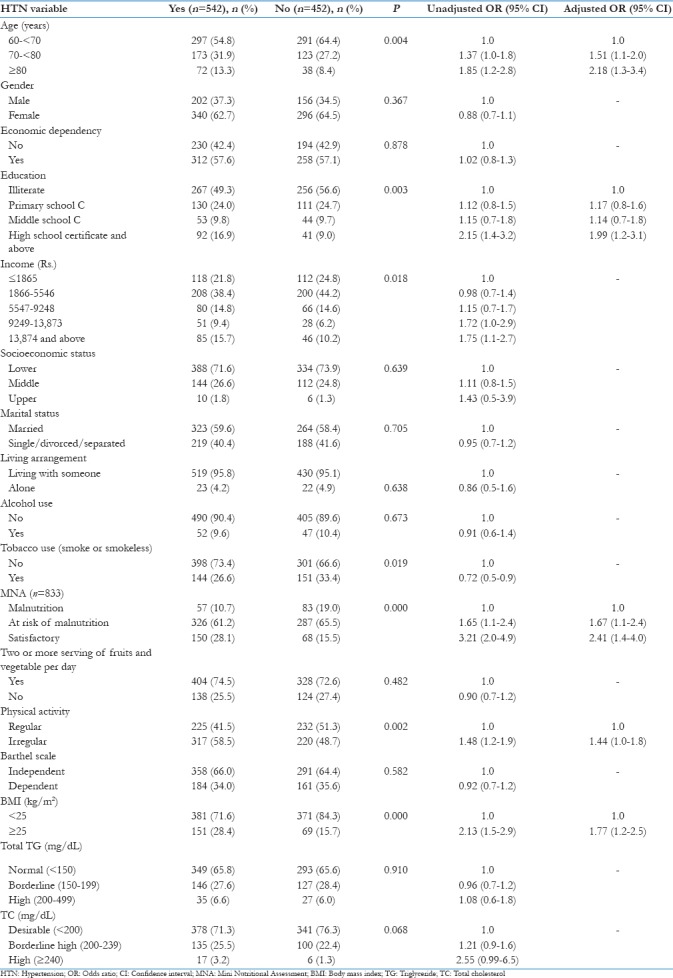
Table 4.
Factors associated with HTN: Results of bivariate and stepwise multivariate logistic regression analysis (n=994)
Risk factors of hypertension
Age, education, income, tobacco consumption, MNA, physical activity, and BMI were found to be significantly associated with HTN (P < 0.05). The prevalence of HTN was found to be higher (58.5%) in subjects who were doing irregular physical activity than those who were doing regular physical activity (41.5%) [Table 4].
In stepwise multivariate analysis, age (70–≥80 years), education of high school and above, satisfactory MNA, and BMI ≥25 kg/m2 were observed to be significant and independent risk factors of HTN [Table 4].
Subjects with age 80 years and above were found to be two times at higher risk of developing HTN [adjusted odds ratio (AOR) = 2.18, 95% confidence interval (CI) =1.3–3.4] when compared with age group 60–<70 years. Geriatric subjects having satisfactory nutritional status were found to be at more risk of developing HTN (AOR = 2.41, 95% CI = 1.4–4.0) in comparison to subjects having poor nutritional status. The odds of becoming hypertensive increased with education of high school and above (AOR = 1.99, 95% CI = 1.2–3.1) and BMI ≥25 kg/m2 (AOR = 1.77, 95% CI = 1.2–2.5) [Table 4].
Risk factors of diabetes
In this study, education, income, socioeconomic status, MNA, BMI, and total cholesterol were found to be significantly associated with DM (P < 0.05). The majority of geriatric subjects who had high cholesterol value (74%) were diabetic compared with subjects who had cholesterol in normal range (52.5%) [Table 5].
Table 5.
Factors associated with diabetes: Results of bivariate and stepwise multivariate logistic regression analysis (n=1002)
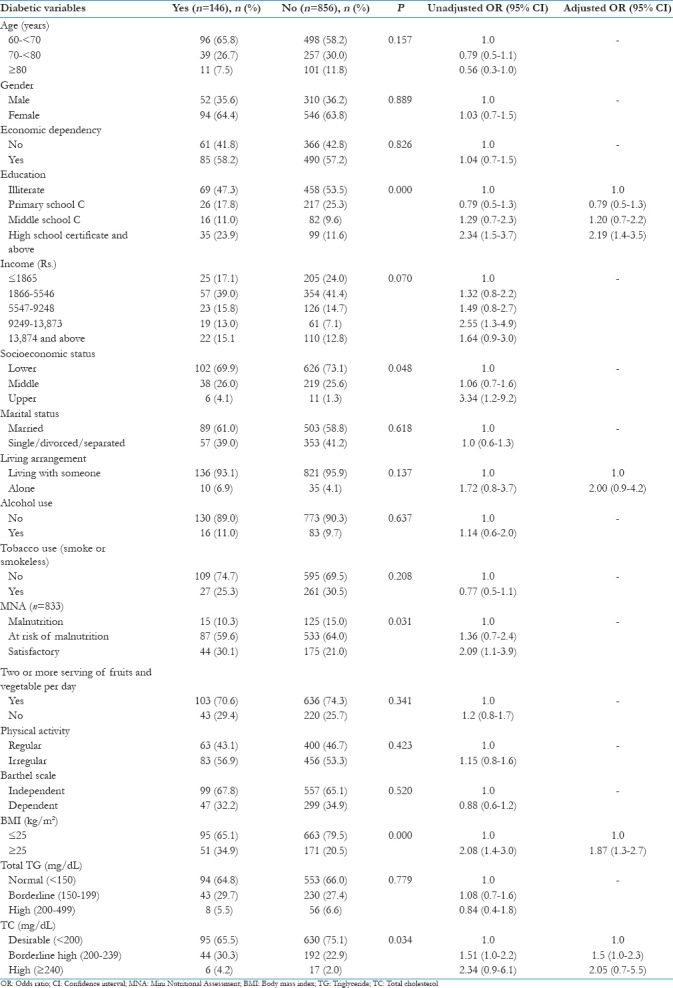
In multivariate logistic regression analysis, education of high school and above and BMI ≥25 kg/m2 were found to be significant and independent risk factors of developing DM. Living alone and high cholesterol level were found as an independent but not significant risk factors of DM [Table 5].
The risk for developing DM was found to be 2.19 times higher in subjects who received education of high school and above (95% CI = 1.4–3.5) and almost two times higher risk among subjects having BMI ≥25 kg/m2 (AOR = 1.87, 95% CI = 1.3-2.7) [Table 5].
Discussion
In India, HTN and DM are the major causes of morbidity and mortality in geriatric population and are the risk factors of many other diseases including heart attack, stroke, kidney failure, leg amputation, vision loss, blood vessels, and peripheral nerve damage.
We found that 54.5% of subjects were suffering from HTN; of these, 383 of 542 (70.6%) were not aware about their status of HTN. Earlier studies have found the rate of undiagnosed HTN among geriatric population of Surat,[23] Uttarakhand,[24] Maharashtra,[25] and Kerala[26] to be 52.0%, 40.94%, 36.1%, and 34.6%, respectively.
Similarly, we found that 14.6 subjects were suffering from DM; of these, 69 of 146 (47.2%) were not aware about their presence of DM. Earlier study conducted in the state of Uttar Pradesh[27] reported 35.8% of prevalence of undiagnosed DM.
The prevalence of HTN reported in this study (54.5%) is comparatively higher than reported in earlier studies in Uttarakhand state which documented prevalence of 45.1%,[28] 41.2,[29] and 38.8%.[24] Earlier studies reported lower prevalence of HTN in Maharashtra (46.3%),[25] Andhra Pradesh (32%),[30] Bangalore (31.5%).[31]
We found higher proportion of HTN in females (34.2%) as against their male counterparts (20.3%). Similar findings have been documented among geriatric population by other researchers in rural India (male 47.3%; female 53.3%),[32] Raipur city (male 42.3%; female 55.5%),[33] Wardha district (male 10.4%; female 10.0%),[25] high-altitude region of Ladakh (male 63.8%; female 67.0%),[34] and Punjab (male 38.5%; female 54.4%).[35]
In this study, the prevalence of DM was 14.6%, which was lower than the earlier studies done in Kerala (28.2%),[36] Kashmir (27.3%),[37] Andhra Pradesh (24%),[30] and Delhi (13%).[38] However, a study conducted in Uttarakhand among geriatric population has documented lower prevalence of DM (8.7%)[39] in comparison to this study. With regard to prevalence of DM by sex, we found that females have higher percentage (9.4%) in comparison to male subjects (5.2%). Similar findings have been reported by earlier researchers in India.[10,40] National Family Health Survey-4 has documented a prevalence of impaired fasting glucose of 8.8% among male and 6.1% among female adult population.[41]
HTN in conjunction with DM was found in a total of 8.6% geriatric subjects (in both male and females) in this study which was lower than reported in an earlier study conducted in Karnataka (9.02%).[42] Prevalence of HTN and DM varies in our study compared to earlier studies which might be due to variation in age, genetic background, lifestyle, environmental, methodological, diagnostic criteria, and geographical differences all over the country.
Risk factors associated with HTN were analyzed. We found that with advancing age, the risk of HTN increases and this difference was found to be significant. In the rural community-based, cross-sectional study, risk of HTN was found to be higher among population over age 80 years in comparison to population age 70–80 years of age.[43] Earlier studies reported significant association of HTN with advancing age.[33,44] Vascular stiffening among geriatric leads to increased SBP and SBP, increased pulse pressure, and increased pulse wave velocity which has an important role in the causation of HTN.[45,46,47,48,49] We observed that with increasing educational level, the odds of HTN increased. A similar finding was showed in an earlier study in Uttarakhand among geriatric population.[24] The prevalence of HTN was found to increase with BMI (≥25 mg/m2). Higher BMI has been found to be an important predictor of HTN by other researchers also.[8,31,50,51] Similarly, a study done in rural India among geriatric population reported that higher BMI had twice the risk of having HTN among both males (OR = 1.9; CI = 1.4–2.5) and females (OR = 2.2; CI = 1.7–2.8).[31] We observed HTN higher among geriatric population with irregular physical activity, though this difference was not found significant. An earlier study found a significant positive association of HTN with physical activity.[33]
In this study, it was found that higher educational status and BMI are strongly associated with DM. Higher education influences the lifestyle pattern. Studies have documented that lifestyle modification is one of the most effective tools for primary prevention of diabetes in Asian Indians.[52] This study showed BMI as an independent significant risk factor for the development of diabetes. A study conducted by Bhalerao et al. had reported that BMI is a significant predictor of development of diabetes.[10] DM was found to be more associated with those having high blood cholesterol. A similar finding was observed by Bharti et al. which documented that subjects with hypercholesterolemia level are more likely to develop DM.[53]
The strengths of this resent study include a large population-based sample, representative sampling methodology, and use of standardized data collection protocols, and the study highlights the burden of undiagnosed HTN and DM among geriatric population. This study had a very high response rate (99.1%).
Conclusion
A high prevalence of HTN and DM was found in geriatric population residing in rural area of Uttarakhand. About 70% of the geriatric subjects were unaware of the presence of HTN and 47.2% were unaware of the presence of DM in Uttarakhand. Hence, routine screening of the presence of BP and impaired fasting blood glucose is required to identify people with undiagnosed HTN and DM so that early treatment can be started to control and prevent complications related to HTN and DM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bhardwaj R, Kandori A, Marwah R, Vaidya P, Singh B, Dhiman P, et al. Prevalence, awareness and control of hypertension in rural communities of Himachal Pradesh. J Assoc Physicians India. 2010;58:423–4. 429. [PubMed] [Google Scholar]
- 2.Verma R, Khanna P, Mehta B. National programme on prevention and control of diabetes in India: Need to focus. Australas Med J. 2012;5:310–5. doi: 10.4066/AMJ.2012.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geldsetzer P, Manne-Goehler J, Theilmann M, Davies JI, Awasthi A, Vollmer S, et al. Diabetes and hypertension in India: A nationally representative study of 1.3 million adults. JAMA Intern Med. 2018;178:363–72. doi: 10.1001/jamainternmed.2017.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanaya AM. India's call to action-prioritize chronic cardiovascular disease. JAMA Intern Med. 2018;178:373–4. doi: 10.1001/jamainternmed.2017.8083. [DOI] [PubMed] [Google Scholar]
- 5.WHO Regional Office for South East Asia. Hypertension Fact Sheet. [Last accessed on 2017 Jun 21]. Available from: http://www.searo.who.int/linkfiles/non_communicable_diseases_hypertension.fs.pdf .
- 6.World Health Organization. Global Report on Diabetes. World Health Organization; 2016. [Last accessed on 2017 Jun 21]. Available from: http://www.apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf . [Google Scholar]
- 7.Goswami AK, Gupta SK, Kalaivani M, Nongkynrih B, Pandav CS. Burden of hypertension and diabetes among urban population aged ≥ 60 years in South Delhi: A community based study. J Clin Diagn Res. 2016;10:LC01–5. doi: 10.7860/JCDR/2016/17284.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazarika NC, Biswas D, Mahanta J. Hypertension in the elderly population of Assam. J Assoc Physicians India. 2003;51:567–73. [PubMed] [Google Scholar]
- 9.Ahmad J, Masoodi MA, Ganie MA, Rashid R, Ahmad R, Ahmad A, et al. Prevalence of diabetes mellitus and its associated risk factors in age group of 20 years and above in Kashmir, India. Al Ameen J Med Sci. 2011;4:38–44. [Google Scholar]
- 10.Bhalerao SD, Somannavar M, Vernekar SS, Ravishankar R, Goudar SS. Risk factors for type 2 diabetes mellitus in rural population of North Karnataka: A community-based cross-sectional study. Int J Pharm Med Biol Sci. 2014;3:1–4. [Google Scholar]
- 11.Garg R. What health problems are associated with hypertension? Indian J Clin Pract. 2014;25:683–4. [Google Scholar]
- 12.Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of diabetes and diabetes complications in the elderly: An emerging public health burden. Curr Diab Rep. 2013;13:805–13. doi: 10.1007/s11892-013-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackay J, Mensah G. Atlas of heart disease and stroke. Geneva: World Health Organization; 2004. [Google Scholar]
- 14.Joshi SR, Anjana RM, Deepa M, Pradeepa R, Bhansali A, Dhandania VK, et al. Prevalence of dyslipidemia in urban and rural India: The ICMR-INDIAB study. PLoS One. 2014;9:e96808. doi: 10.1371/journal.pone.0096808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JNC-VII, editor. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Publication No. 04-5230. US: NIH; 2004. [Google Scholar]
- 16.Deepthi R, Chandini C, Pratyusha K, Kusuma N, Raajitha B, Shetty G. Screening for Diabetes and their risk factors among adults in Rural Kolar – A community based study. Int J Res Dev Health. 2013;1:152–9. [Google Scholar]
- 17.World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. World Health Organization; 2006. [Last accessed on 207 Jul 06]. Available from: http://www.apps.who.int/iris/bitstream/10665/43588/1/9241594934_eng.pdf . [Google Scholar]
- 18.Lakshmy R, Gupta R, Prabhakaran D, Snehi U, Reddy KS. Utility of dried blood spots for measurement of cholesterol and triglycerides in a surveillance study. J Diabetes Sci Technol. 2010;4:258–62. doi: 10.1177/193229681000400206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quraishi R, Lakshmy R, Prabhakaran D, Mukhopadhyay AK, Jailkhani B. Use of filter paper stored dried blood for measurement of triglycerides. Lipids Health Dis. 2006;5:20. doi: 10.1186/1476-511X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quraishi R, Lakshmy R, Prabhakaran D, Irshad M, Mukhopadhyay AK, Jailkhani BL, et al. Effect of storage temperature on cholesterol measurement from dried blood. Indian J Med Res. 2007;126:228–9. [PubMed] [Google Scholar]
- 21.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Nishida C. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 23.Pawar AB, Bansal RK, Bharodiya P, Panchal S, Patel H, Padariya P, et al. Prevalence of hypertension among elderly women in slums of Surat city. Natl J Community Med. 2010;1:39–40. [Google Scholar]
- 24.Bartwal J, Rawat CS, Awasthi S. Prevalence, awareness, treatment and control of hypertension among the elderly residing in rural area of Haldwani block, in Nainital district of Uttarakhand. J Cardiovasc Dis Res. 2016;7:112–5. [Google Scholar]
- 25.Joshi R, Taksande B, Kalantri SP, Jajoo UN, Gupta R. Prevalence of cardiovascular risk factors among rural population of elderly in Wardha district. J Cardiovasc Dis Res. 2013;4:140–6. doi: 10.1016/j.jcdr.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebastian NM, Jesha MM, Sheela PH, Arya SN. Hypertension in Kerala: A study of prevalence, control, and knowledge among adults. Int J Med Sci Public Health. 2016;5:2041–6. [Google Scholar]
- 27.Singh PS, Sharma H, Zafar KS, Singh PK, Yadav SK, Gautam RK, Pious T. Prevalence of type 2 diabetes mellitus in rural population of India – A study from Western Uttar Pradesh. Int J Res Med Sci. 2017;5:1363–7. [Google Scholar]
- 28.Pooja B, Yashoda M. Prevalence of hypertension among rural population of Doiwala block, Dehradun, Uttarakhand India. Recent Res Sci Technol. 2013;5:21–4. [Google Scholar]
- 29.Bansal SK, Saxena V, Kandpal SD, Gray WK, Walker RW, Goel D, et al. The prevalence of hypertension and hypertension risk factors in a rural Indian community: A prospective door-to-door study. J Cardiovasc Dis Res. 2012;3:117–23. doi: 10.4103/0975-3583.95365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borra S, Kommula VM, Jyothirmai MS. Prevalence of hypertension and diabetes among the rural elderly in South India. Int J Curr Microbiol Appl Sci. 2015;4:251–4. [Google Scholar]
- 31.Srikanth J, Kulkarni S. Hypertension in elderly: Prevalence and health care seeking pattern in an urban slum of Bangalore city. Int J Recent Sci Res. 2015;6:2952–7. [Google Scholar]
- 32.Arlappa N, Laxmmaiah A, Balakrishna N, Harikumar R, Mallikharjuna Rao K, Brahmam GN. Prevalence of hypertension and its relationship with adiposity among rural elderly population in India. Int J Clin Cardiol. 2014;1:1–6. [Google Scholar]
- 33.Alam MN, Soni GP, Jain KK, Verma S, Panda PS. Prevalence and determinants of hypertension in elderly population of Raipur city, Chhattisgarh. Int J Res Med Sci. 2015;3:568–73. [Google Scholar]
- 34.Norboo T, Stobdan T, Tsering N, Angchuk N, Tsering P, Ahmed I, et al. Prevalence of hypertension at high altitude: Cross-sectional survey in Ladakh, Northern India 2007-2011. BMJ Open. 2015;5:e007026. doi: 10.1136/bmjopen-2014-007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta A, Girdhar S, Chaudhary A, Chawla JS, Kaushal P. Patterns of multi morbidity among elderly in an urban area of North India. J Evol Med Dent Sci. 2016;5:936–41. [Google Scholar]
- 36.Vijayakumar G, Arun R, Kutty VR. High prevalence of type 2 diabetes mellitus and other metabolic disorders in rural central Kerala. J Assoc Physicians India. 2009;57:563–7. [PubMed] [Google Scholar]
- 37.Singh K, Kumar S. Prevalence of diabetes mellitus in rural population of Jammu. JK Sci. 2015;7:84–7. [Google Scholar]
- 38.Gupta HL, Yadav M, Sundarka MK, Talwar V, Saini M, Garg P, et al. A study of prevalence of health problems in asymptomatic elderly individuals in Delhi. J Assoc Physicians India. 2002;50:792–5. [PubMed] [Google Scholar]
- 39.Kandpal SD, Kakkar R, Aggarwal P, Bansal S. Pattern of prevalence of risk factors for non-communicable diseases in the geriatric population of district Dehradun. J Indian Acad Clin Med. 2013;14:214–7. [Google Scholar]
- 40.Singh J, Saoji AV, Kasturwar NB, Pitale SP, Deoke AR, Nayse JG. Epidemiological study of diabetes amongst geriatric population in an urban slum, Nagpur. Natl J Community Med. 2011;2:204–8. [Google Scholar]
- 41.State Fact Sheet Uttarakhand. National Family Health Survey-4. Indian Institute for Population Sciences, Mumbai. [Last accessed on 2017 Jul 07]. Available from: http://www.rchiips.org/NFHS/pdf/NFHS4/UT_FactSheet.pdf .
- 42.Venkateshkrishna N, Suresh S. A cross-sectional study on the prevalence of diseases among the geriatric population in the rural field practice areas of a tertiary care hospital in Mangaluru, India. J Pharm Pract Community Med. 2018;4:30–2. [Google Scholar]
- 43.Mohan V, Deepa M, Farooq S, Datta M, Deepa R. Prevalence, awareness and control of hypertension in Chennai – The Chennai urban rural epidemiology study (CURES-52) J Assoc Physicians India. 2007;55:326–32. [PubMed] [Google Scholar]
- 44.Radhakrishnan S, Balamurugan S. Prevalence of diabetes and hypertension among geriatric population in a rural community of Tamilnadu. Indian J Med Sci. 2013;67:130–6. [PubMed] [Google Scholar]
- 45.Barodka VM, Joshi BL, Berkowitz DE, Hogue CW, Jr, Nyhan D. Implications of vascular aging. Anesth Analg. 2011;112:1048. doi: 10.1213/ANE.0b013e3182147e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam study. Circulation. 2006;113:657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 47.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 48.Franklin SS, Gustin W, 4th, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham heart study. Circulation. 1997;96:308–15. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 49.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 50.Naveen KH, Sumanth MM, Manjunatha SN, Hassan MA, Dwivedi S. Prevalence and predictors of hypertension among the elderly population in rural areas of Allahabad district: A cross sectional study. J Med Sci Clin Res. 2014;2:2004–15. [Google Scholar]
- 51.Gupta R, Guptha S, Gupta VP, Prakash H. Prevalence and determinants of hypertension in the urban population of Jaipur in Western India. J Hypertens. 1995;13:1193–200. doi: 10.1097/00004872-199510000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Ramachandran A, Snehalatha C. Current scenario of diabetes in India. J Diabetes. 2009;1:18–28. doi: 10.1111/j.1753-0407.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 53.Bharati DR, Pal R, Kar S, Rekha R, Yamuna TV, Basu M, et al. Prevalence and determinants of diabetes mellitus in Puducherry, South India. J Pharm Bioallied Sci. 2011;3:513–8. doi: 10.4103/0975-7406.90104. [DOI] [PMC free article] [PubMed] [Google Scholar]


