Abstract
Introduction:
Influenza A (H1N1) virus has caused serious respiratory illness (swine flu) and death over the years. The first confirmed case of swine flu H1N1 in India was documented in May 2009, but huge numbers of cases were reported thereafter. In 2015, swine flu outbreak in India had led to significant morbidity and mortality.
Objective:
to study details of swine flu patients admitted in a rural tertiary care center in western India in 2015 and to identify predictors of mortality.
Methodology:
Retrospective data of swine flu cases admitted at a tertiary care teaching hospital in 2015 and their outcome as either cured or expired was recorded.
Result:
Out of 65 confirmed cases of severe swine flu that required hospitalization, 40(61%) were male. 55 of 65 (84.61%) patients [mean (SD) age: 50(15)] were cured while 10 patients [mean (SD) age 51(15)] expired. Overall mean (SD) age was 50.23(15) years with average (SD) days of hospitalization were 6.32(3.3) days. The commonest symptoms were cough (100%) followed by throat pain (96.9%), common-cold, fever (93.8%), and breathlessness (83.1%). 40% of patients needed non invasive ventilator support while 16.9% patient required invasive ventilator. Mean temperature on presentation was (99.96’F), RR (25.89/min), SpO2 on room air was 82.06%. Average White Blood Cells were 8274/mm3 with neutrophils were 79.58%. Mean procalcitonin was 0.83 ng/ml. It was found through univariate analysis that sputum production (P = 0.013), chest pain (P = 0.04), Respiratory Rate (P = 0.013), SpO2 on presentation at room air (P = 0.001), Days of non invasive ventilator (P = 0.001), intubation and invasive ventilator (P = 0.001) were statistically significantly associated with outcome but through multivariate analysis it was revealed that only requirement of intubation (invasive ventilator) was significantly predicting mortality(Odds ratio=234) (P = 0.0001).
Conclusion:
Requirement of intubation was associated with poor outcome.
Keywords: H1N1 epidemic, Influenza A, Swine flu
Introduction
Influenza is a common human virus which can lead to varying degree of respiratory infections ranging from mild flu to severe and life-threatening pneumonia, bronchitis, acute respiratory distress syndrome, and even death.[1] H1N1 is also known as “swine flu” and it is a novel strain of Influenza A virus that evolved by genetic reassortment.[2,3] It leads to annual epidemics of varying severity. Pandemic of H1N1 first emerged in 2009 April, which was started in Mexico and soon expanded globally.[4,5] In India, the first case was identified on 2009 May 16, in Hyderabad.[1]
There are very limited number of studies on influenza virus-related diseases and morbidity in India. 2015 Indian epidemic of swine flu had led to significant morbidity and mortality in the state of Gujarat. Present study is an effort to reveal the predictors of mortality for better preparation to handle any such epidemics in future.
Aims and Objective
To study clinical, epidemiological, laboratory, and radiological profile of confirmed cases of severe swine flu cases, who required hospitalization and isolation, and to identify the predictors of the outcome, especially in terms of mortality.
Materials and Methods
This analytical study was conducted at our rural tertiary teaching hospital. Retrospective data of 65 confirmed cases of severe swine flu patients requiring hospital isolation of adult age group (>18 years) from January 2015 to April 2016 were retrieved from records. Demographic characteristics, clinical presentation, laboratory parameters, radiological parameters, and outcome were recorded and analyzed. They were divided in two groups as per their outcome – Group A comprised of patients who had positive outcome and had been cured, whereas Group B comprised of patients with negative outcome and had mortality. In addition, various parameters like average days of hospitalization, requirement of oxygen therapy, and requirement of types of ventilators were also recorded. All the parameters between two groups were compared. Statistical analysis was done to find out predictors of mortality. Approval of institutional ethical committee was taken to conduct the study. Data were analyzed using Microsoft Excel Software and basic descriptive statistical measures like mean, median, and percentage.
Results
Out of 65 confirmed cases of severe swine flu patients admitted in hospital isolation, 40 were males.
Overall, 55 (84.62%) patients were cured and got discharged after successful treatment. Among them, 20 were females and 35 were males. Ten patients (15.38%) (five males and five females) had poor outcome and had mortality.
Mean (SD) age was 50.09 (15.41) years in cured group (A) and 51 (15.52) years in mortality group (B). Average (SD) duration of hospitalization was 6.32 (3.3) days in all patients, whereas in cured group, it was 6.44 (3.5) days, and in mortality group, it was 5.7 (2.4) days.
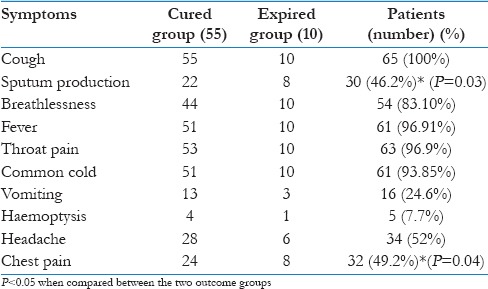
The symptoms on presentation were cough (100%), followed by throat pain (96.9%), common cold (93.85%), fever (93.85%), breathlessness (83.10%), headache (52%), chest pain (49.2%), sputum production (46.2%), vomiting (24.6%), and hemoptysis in 7.7% of cases [Table 1].
Table 1.
Distribution of various presenting symptoms by outcome
We analyzed various comorbid conditions in patients of swine flu, which depicted that Diabetes mellitus was present in 27.7% of cases, hypertension was there in 36.9% cases, ischemic heart disease was there in 15.4%, chronic obstructive pulmonary disease was found in 4.6%, Tuberculosis was present in 4.6% cases, valvular heart disease was there in 1.5% cases, whereas anemia, pregnancy, and liver diseases were found in 4.6% cases. One patient was positive for HIV (1.5%), asthma was there in 3.1% cases, chronic kidney disease was present in 1.5% of cases, rheumatoid arthritis was there in 3% of cases, and hypothyroidism was there in 3% of cases. Around 73.85% of cases had no comorbidities.
Analysis of various vital parameters at the time of admission showed that mean temperature on presentation was 99.96°F, respiratory rate was 25.89/min, and SpO2 was 82.06% on room air. Thus, most of the patients were febrile, tachypneic, and had hypoxemic.
Overall, 55.4% of patients needed oxygen therapy, 40% of patients needed noninvasive ventilatory support (NIV), whereas 16.9% of patients required invasive ventilator, in addition to routine management.
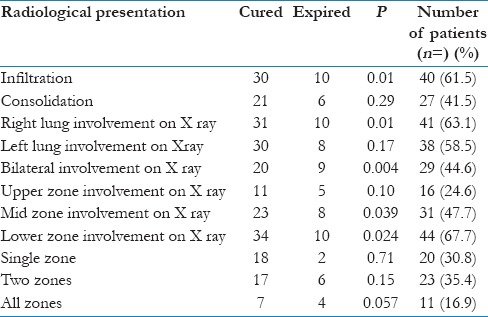
Commonest radiological presentation on x-ray chest was infiltration followed by consolidation. Right lung was affected more as compared with left lung, whereas involvement of both the lungs was less common as compared with unilateral involvement. Lower zone was affected the most, followed by mid-zone and upper zone, respectively [Table 2].
Table 2.
Radiological parameters and outcome
In the blood investigations, we found that average white blood cell (WBC) counts were 8274/mm3 and in differential count neutrophils (79.58%) were predominant as compared with lymphocytes. Mean procalcitonin level in blood at admission was 0.83 ng/mL. Mean hemoglobin was 12.11 g/dL, and mean platelet counts were 218,000/μL.
We have compared various parameters between two groups and have tried to find out predictors of poor outcome [Table 3].
Table 3.
Comparison of various parameters and outcome
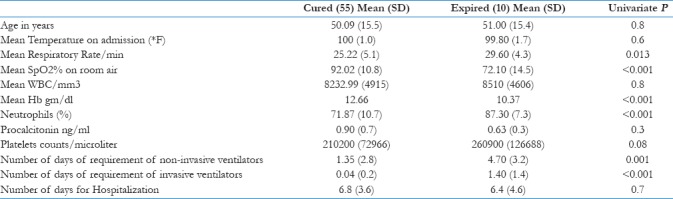
We have analyzed and compared all the variables between two groups that can predict worst outcome. It was found through univariate analysis that sputum production (P = 0.013), chest pain (P = 0.04), respiratory rate (P = 0.013), SpO2 (P < 0.001), days of noninvasive ventilator support (P < 0.001), and intubation and invasive ventilator support (P < 0.001) were having statistically significant difference between two groups, but through multivariable logistic regression analysis, entering sputum production (no/yes), chest pain (no/yes), SPO2, respiratory rate, need for noninvasive ventilation (no/yes), and need for intubation (no/yes) through enter method resulted in a good model with Nagelkerke R-square = 0.773 and correct classification value of 95.4%. This model found only requirement of intubation (invasive ventilator) to be making difference for mortality (odds ratio = 75.89; P = 0.008; with need of ventilation – No as the reference category).
Discussion
In our study mean age was 50.23 years, which was higher as compared with other study. In the study conducted by Kashinkunti et al.[2] in North Karnataka in 2009 epidemic, more number of patients was found to have younger age group. The difference of age might be due to severity change of epidemic or exclusion of pediatric age group in our study. In another study on epidemic of swine flu of 2015 by Dhawale et al., average age was found <50 years, whereas in our study, it was more than 50 years.[6]
In our study, more numbers of male patients were there, which is similar to other study of 2009 swine flu epidemic by Prakash.[7] Study conducted by Revdiwala et al. showed that H1N1 infection was more in males than females,[8] whereas in another study of 2015 by Dhawale et al., females found to have acquired infection more frequently.
In our study, the symptoms on presentation in descending order were cough (100%), followed by throat pain (96.9%), common cold (93.85%), fever (93.85%), breathlessness (83.1%), headache (52%), chest pain (49.2%), sputum production (46.2%), vomiting (24.6%), and hemoptysis (7.7%), whereas in the study by Dhawale et al., cough (97.72%) and fever (77.27%) were the most common presenting symptoms followed by breathlessness in 29.54% patients,[6] whereas in other study of 2011 conducted in New Delhi, fever (100%), cough (87.5%), sore throat (43.7%), and breathlessness (87.5%) were found to be the most common symptoms in Influenza A H1N1.[9] In the study by Srinivasa et al.,[10] the commonest symptoms identified were cough (100%), fever (90%), sore throat (25%), and difficulties/shortness of breath (50%).[11]
In our study, a significant proportion of hospitalized patients had findings on chest radiography suggestive of infiltrations and right lung involvement was more common with unilateral predominance as compared with the study published by Kashinkunti et al., which showed bilateral involvement more frequently.
In our study, average WBC counts were within normal levels. Study done by Shin Ahan et al.[12] in 2009 epidemic in Korea revealed that serum procalcitonin concentration was significantly higher in patients with mixed infection of pneumonia compared with those with 2009 H1N1 infection alone, indicating that this marker could be useful in differentiating between these conditions. In our study, mean procalcitonin was on lower side, which might be due to less number of patients with mixed bacterial and viral infections.
In our study, the requirement of invasive ventilatory support predicted poor prognosis, in the same way as it was shown in the study by Kinikar et al.[13]. In our study, 10 (15.38%) patients were expired as compared with other study by Singh et al.,[10] which had a mortality rate of 19.08%, whereas Dhawale et al. reported mortality rate of 4.45%.
Conclusion
Fever, cough, throat pain, and breathlessness were the common presenting symptoms of swine flu. Increased respiratory rate (tachypnea), low oxygen saturation (hypoxia), WBC count within normal range, and lower level of procalcitonin in blood were commonly found. Thus, in patients of pneumonia with above features coming in winter season especially in countries like India, we should suspect swine flu, and management must be rationalized as early as possible. Patients who needed invasive ventilation had the worst outcome. Thus, in patients of swine flu with severe pneumonia, hypoxemia and lung injury needing invasive support chances of survival decreased significantly and which could be prevented by early utilization of healthcare resources and proper preventive measures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Khanna M, Kumar P, Choudhary K, Kumar B, Vijayan VK. Emerging influenza virus: A global threat. J Biosci. 2008;33:475–82. doi: 10.1007/s12038-008-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashinkunti MD, Gundikeri SK, Dhananjaya M. Study of clinical profile of patients with H1N1 influenza in a teaching hospital of North Karnataka. Diabetes. 2013;11:50. [Google Scholar]
- 3.Gaikwad LL, Haralkar S. Clinico-epidemiological profile of influenza A H1N1 cases admitted at a tertiary care institute of Western India. Int J Med Sci Public Health. 2014;3:1258–60. [Google Scholar]
- 4.Cheng AC, Kotsimbos T, Reynolds A, Bowler SD, Brown SG, Hancox RJ, et al. Clinical and epidemiological profile of patients with severe H1N1/09 pandemic influenza in Australia and New Zealand: An observational cohort study. BMJ. 2011 doi: 10.1136/bmjopen-2011-000100. bmj open-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virus NS, Team I. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009. 2009:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 6.Dhawale S, Jayant S. Clinical profile, morbidity and mortality among swine flu (H1N1) infected patients: 2015 Gwalior, Madhya Pradesh pandemic, India. Int J Adv Med. 2017;3:324–7. [Google Scholar]
- 7.Gelotar PS. Epidemiological and Clinical profile of patients with swine flu (Influenza A, H1N1) attending Guru Govindsingh Government Hospital, Jamnagar, India. J Res Med Den Sci. 2013;1:1–6. [Google Scholar]
- 8.Revdiwala S, Mulla S, Panwala T, Shah L, Shah A. Clinical characterisation of H1N1 influenza Taqman real time PCR positive cases. Nat J Med Res. 2012;2:12–4s. [Google Scholar]
- 9.Sharma V, Verma PK, Gupta S, Sharma A. Mortality from Influenza A/H1N1 in a tertiary care teaching institution in North India. J Infect Deving Ctries. 2010;4:468–71. doi: 10.3855/jidc.1007. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Sharma S. An epidemiological study of recent outbreak of influenza A H1N1 (Swine Flu) in Western Rajasthan region of India. J Med Allied Sci. 2013;3:48. [Google Scholar]
- 11.Srinivasa R, Malini J, Nandini S, Umapathy B. Profile of H1N1 infection in a tertiary care centre. Ind J Pathol Microbiol. 2011;52:323–5. doi: 10.4103/0377-4929.81618. [DOI] [PubMed] [Google Scholar]
- 12.Ahn S, Kim WY, Kim SH, Hong S, Lim CM, Koh Y, et al. Role of procalcitonin and C-reactive protein in differentiation of mixed bacterial infection from 2009 H1N1 viral pneumonia. Influenza Other Respir Viruses. 2011;5:398–403. doi: 10.1111/j.1750-2659.2011.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinikar AA, Kulkarni RK, Valvi CT, Mave V, Gupte N, Khadse S, et al. Predictors of mortality in hospitalized children with pandemic H1N1 influenza 2009 in Pune, India. Indian J Pediatr. 2012;79:459–66. doi: 10.1007/s12098-011-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


