Abstract
While nuclear actin was reported ~50 years ago, it’s in vivo prevalence and structure remain largely unknown. Here we use Drosophila oogenesis, i.e. follicle development, to characterize nuclear actin. We find that three different reagents – DNase I, anti-actin C4, and anti-actin AC15 – recognize distinct pools of nuclear actin. DNase I labels monomeric or G-actin, and, during follicle development, G-actin is present in the nucleus of every cell. Some G-actin is recognized by the C4 antibody. In particular, C4 nuclear actin colocalizes with DNase I to the nucleolus in anterior escort cells, follicle stem cells, some mitotic follicle cells, and a subset of nurse cells during early oogenesis. C4 also labels polymeric nuclear actin in the nucleoplasm of the germline stem cells, early cystoblasts, and oocytes. The AC15 antibody labels a completely distinct pool of nuclear actin from that of DNase I and C4. Specifically, AC15 nuclear actin localizes to the chromatin in the nurse and follicle cells during mid-to-late oogenesis. Within the oocyte, AC15 nuclear actin progresses from localizing to puncta surrounding the DNA, to forming a filamentous cage around the chromosomes. Together these findings reveal that nuclear actin is highly prevalent in vivo, and multiple pools of nuclear actin exist and can be recognized using different reagents. Additionally, our localization studies suggest that nuclear actin may regulate stemness, nucleolar structure and function, transcription, and nuclear structure. Such findings call for further studies to explore the prevalence, diversity, and functions of nuclear actin across tissues and organisms.
Keywords: nuclear actin, oogenesis, Drosophila, oocyte, nucleolus
Introduction
In addition to its well-established cytoskeletal roles, actin also localizes to and functions within the nucleus. Nuclear actin was first reported over 50 years ago (Ohnishi et al., 1963; Ohnishi et al., 1964; Lane, 1969). These finding were met with skepticism. These studies largely relied on subcellular fractionation, raising concerns about the purity of the isolated nuclei. The levels of actin in the nucleus were also low compared to that in the cytoplasm. Additionally, nuclear filamentous actin (F-actin) could not be visualized, and functions for nuclear actin had not been identified. More recent studies have overcome these issues. Indeed, the active mechanisms regulating the nuclear localization of actin have been identified (Wada et al., 1998; Stuven et al., 2003; Dopie et al., 2012; Munsie et al., 2012). Additionally, functional studies have revealed nuclear actin regulates transcription (Fomproix and Percipalle, 2004; Hofmann et al., 2004; Hu et al., 2004; Philimonenko et al., 2004), chromatin remodeling (reviewed in (Venit et al., 2018)), nuclear organization and structure (Sasseville and Langelier, 1998; Wasser and Chia, 2000; Krauss et al., 2003; Holaska et al., 2004), DNA damage repair (Andrin et al., 2012; Belin et al., 2015; Serebryannyy et al., 2017; Wang et al., 2017), cell cycle (Goyal et al., 2011; Baarlink et al., 2017), and meiosis (Bogolyubova and Ginzburg, 2013; Mogessie and Schuh, 2017). Nuclear actin has also been implicated in diseases, including cancer (Spencer et al., 2011; Fiore et al., 2017), neurodegeneration (Minamide et al., 2000; Maloney et al., 2005; Lim et al., 2007; Maloney and Bamburg, 2007; Munsie et al., 2011), and myopathies (Goebel and Warlo, 2001; Domazetovska et al., 2007a; Domazetovska et al., 2007b; Serebryannyy et al., 2016c). Thus, actin has many critical functions within the nucleus that are important not only for key cellular functions but also for human health.
As the functions of actin within the cytoplasm depend upon its structure, it is likely that the structure of nuclear actin similarly impacts its activities. However, insight into the structures of nuclear actin remains limited. This limitation is due to the complexities involved in visualizing actin. Actin can exist as monomers (G-actin), short polymers, filaments (F-actin), and networks of filaments. It also binds to a large number of proteins. These different structures and complexes make it unlikely that any tool or reagent, such as an antibody, will recognize all of the actin within the cell. Indeed, a study using fluorescent recovery after photobleaching (FRAP) and fluorescence correlation spectroscopy of green fluorescent protein (GFP)-tagged actin indicates there are at least two pools of nuclear actin with different dynamics, suggesting nuclear actin exists as both monomers and polymers (McDonald et al., 2006). Another study generated nuclear actin probes using actin binding domains from different proteins, and identified probes that differentially label monomeric versus polymeric nuclear actin. Monomeric actin was observed in nuclear speckles and sites of RNA processing, while polymeric actin seemed to generate a viscoelastic structure (Belin et al., 2013). Polymeric nuclear actin visualized by this same tool has recently been implicated in DNA damage repair (Belin et al., 2015; Wang et al., 2017). Numerous antibodies have also been used to examine nuclear actin. Gonsior et al. (1999) generated an actin antibody (2G2) that labels nuclear dots in myogenic cells and fibrils in the nuclei of Xenopus oocytes (Gonsior et al., 1999). Similarly, another study generated an additional actin antibody (1C7) and found that it labeled distinct nuclear structures when compared to 2G2 (Schoenenberger et al., 2005). The finding that different antibodies label distinct subsets or pools of nuclear actin has been widely observed across cell types and organisms (Grenklo et al., 2004; Jockusch et al., 2006; Cruz and Moreno Diaz de la Espina, 2009; Asumda and Chase, 2012). It is important to note that fixation conditions dramatically affect the ability of actin antibodies to bind to the different actin structures; for example, methanol fixation denatures actin filaments, making them accessible to antibodies, but prevents phalloidin labeling. While the last two decades have significantly increased our understanding of the structures of nuclear actin, much remains to be determined, including how the structure of nuclear actin impacts its different functions.
Both the research into the structures of nuclear actin and its functions have largely been restricted to cultured cells, oocytes, or unicellular organisms; thus, the prevalence, structures, and functions of nuclear actin in in vivo, multicellular, and developmental contexts remain largely unknown. To address this knowledge gap and advance our understanding of nuclear actin, here we utilize the robust and well-characterized process of Drosophila oogenesis, i.e. follicle development. Oogenesis is an excellent system to explore the developmental functions of nuclear actin, as the whole process, from germline and somatic stem cells to a mature ready to ovulate eggs, is easily observed (Fig. 1). See below for a detailed description of follicle development. Briefly, each developing follicle is composed of multiple cells types, including 16-germline-derived cells – 15 nurse or support cells and one oocyte – and a somatic epithelium of follicle cells (Fig. 1B, D). The temporal progression of both cellular events and morphogenesis occurring within these different cell types is well-established. Thus, this knowledge can be used to provide insight into the potential functions of nuclear actin.
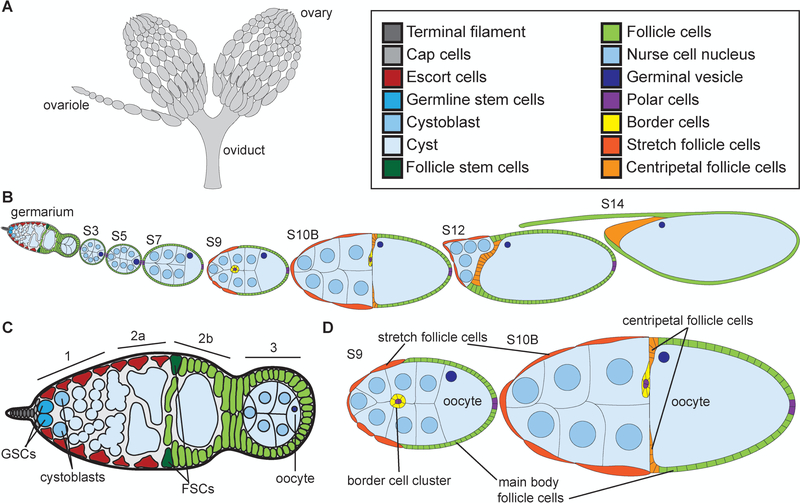
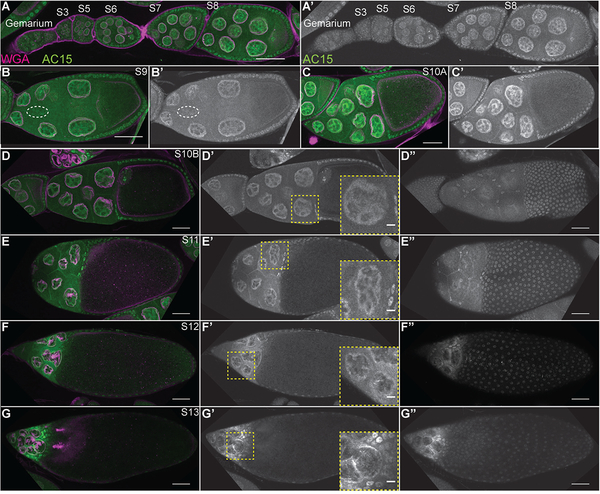
Figure 1:
Overview of Drosophila oogenesis. (A) Schematic of ovaries and oviduct. (B) Schematic of ovariole with the stages of development indicated. (C) Schematic of germarium. (D) Schematic of S9 and S10B follicles. Adult female fly has two ovaries that are comprised of ~15 ovarioles, or chains of sequentially maturing follicles (A). Follicle development is separated into 14 morphological stages (B), from the germarium to S14. The germarium is at the anterior tip of the ovariole (B), is broken into 4 regions (1, 2a, 2b, and 3; C) and contains both germline (bright cyan) and somatic or follicle stem cells (dark green). Two to three germline stem cells (GSCs, bright cyan) reside at the anterior of region 1 in a niche comprised of the terminal filament (dark gray), cap (light gray), and anterior escort (red) cells (C). The GSCs divide asymmetrically to self-renew and generate a cystoblast (C, light cyan). The cystoblast goes on to have four incomplete and synchronous cell divisions to generate a 16-cell cyst (light blue). In region 3, the cyst will differentiate into 15 nurse cells (nuclei are light blue) and one oocyte (nucleus is dark blue) (C). At the 2a/2b boundary in the germarium (C), the follicle stems cells (FSCs, dark green) reside and give rise to all of the somatic, follicle cells (light green) that will encase the 16-cell cysts. These follicle cells will subsequently differentiate into a number of subtypes. By S9 (D), four different types of follicle cells are observed: the purple polar cells which specify the poles, the yellow migrating border cell cluster (comprised of the anterior purple polar cells and surrounding yellow border cells), the squamous, dark orange stretch follicle cells, and the green main body follicle cells. By S10B (D), another follicle cell subtype is observed, the centripetal follicle cells in light orange. An additional type of follicle cells, stalk cells, are not shown but connect the follicles to each other.
To visualize nuclear actin during Drosophila oogenesis, we utilize three reagents – fluorescently conjugated-DNase I, anti-actin C4 and anti-actin AC15. DNase I binds to and, thus, can be used to label monomeric or G-actin (Hitchcock, 1980). Anti-actin C4 is a broad specificity actin antibody (Lessard, 1988) that has been used to examine nuclear actin in other systems (Parfenov et al., 1995; Hofmann et al., 2004; Gedge et al., 2005; Lenart et al., 2005; Spencer et al., 2011; Maslova and Krasikova, 2012). Additionally, we recently uncovered that anti-actin C4 labels some nuclear actin during Drosophila oogenesis (Kelpsch et al., 2016). Anti-actin AC15 (Gimona et al., 1994) also recognizes nuclear actin in other systems, including cultured Drosophila cells (Hofmann et al., 2004; Cruz and Moreno Diaz de la Espina, 2009; Miyamoto et al., 2011; Dopie et al., 2012). Here we define the cellular and subnuclear localization of the nuclear actin recognized by the three reagents. By comparing the labeling of nuclear actin by the two antibodies to that of DNase I, we can gain insight into the structure of the nuclear actin recognized by the different antibodies. Additionally, the subnuclear, cell-type, and developmental/temporal localization of the reagents provides insight into potential functions of nuclear actin.
We find that while nuclear G-actin is ubiquitous throughout follicle development, as DNase I labels a nuclear structure in every cell, the two antibodies recognize more restricted and distinct pools of nuclear actin. Indeed, C4 nuclear actin is observed in a subset of cells during early oogenesis, where it both overlaps with DNase I and labels distinct nuclear regions. This finding suggests that anti-actin C4 recognizes both monomeric and polymeric actin. The G-actin labeled by DNase I and anti-actin C4 is primarily within the nucleolus, suggesting roles of nuclear actin in the function of this nuclear body. Furthermore, the cell-type and pattern of polymeric C4 nuclear actin suggests it may regulate differentiation and nuclear structure. The nuclear actin recognized by anti-actin AC15 is different from that labeled with either of the other reagents. AC15 nuclear actin is observed in all cells from mid to late oogenesis, largely associates with the DNA, and does not colocalize with DNase I. These findings indicate AC15 nuclear actin is likely polymeric and suggests it may have roles in transcription. Together our study reveals that multiple pools of nuclear actin are observed during an in vivo, developmental process, and provides insight into the potential functions of nuclear actin. Such findings are likely to be applicable to other developmental processes and systems.
Materials and Methods
Fly stocks
Fly Stocks were maintained on cornmeal-agar-yeast food at 21°C. Prior to immunofluorescence analysis, flies were fed wet yeast paste daily for 3–4 days. yw was used as the wild-type background. Fly lines expressing CRISPR-mediated enhanced GFP (eGFP)-tagged nucleolar proteins, Fibrillarin and Nopp-140, were a generous gift from Eric Wieschaus (Falahati and Wieschaus, 2017).
Immunofluorescence
Whole-mount Drosophila ovary samples were dissected into Grace’s insect media (Lonza, Walkersville, MD) and were fixed for 10 minutes at room temperature in 4% paraformaldehyde in Grace’s insect media. Briefly, samples were blocked by washing in Triton antibody wash (1X phosphate-buffered saline, 0.1% Triton X-100, and 0.1% bovine serum albumin) six times for 10 minutes each at room temperature. Primary antibodies were incubated for a minimum of 20 hr at 4°C. The following antibodies and concentrations were used: mouse anti-actin C4 1:50 (EMB Millipore, Billerica, MA); mouse anti-actin AC15 1:50–1:100 (Sigma-Aldrich, St. Louis, MO); rabbit anti-GFP 1:5000 (pre-absorbed on yw ovaries at 1:20 and used at 1:500; Torrey Pines Biolabs, Inc., Secaucus, NJ). After 6 washes in Triton antibody wash (10 minutes each), secondary antibodies were incubated from 12–36 hr at 4°C. The following secondary antibodies were used at 1:500: AF488::goat anti-mouse, AF568::goat anti-mouse, or AF488::goat anti-rabbit (Life Technologies, Grand Island, NY). For DNase I staining, AF488-DNase I 1:500 (Life Technologies) was included in both the primary and secondary antibodies. AF555- or AF647-conjugated wheat germ agglutinin (Life Technologies) was included with the secondary antibody at a concentration of 1:500. Following six washes in Triton antibody wash (10 minutes each), DAPI (5 mg/ml) staining was performed at a concentration of 1:5000 in 1X PBS for 10 minutes at room temperature. Ovaries were mounted in 1 mg/ml phenylenediamine in 50% glycerol, pH 9 (Platt and Michael, 1983). All experiments were performed a minimum of three independent times.
Microscope image acquisition and processing:
Microscope images of fixed Drosophila follicles were obtained using Zen software on a Zeiss 880 mounted on Zeiss Axio Observer.Z1 using Plan-Apochromat 20×/0.8 WD=0.55 M27, Plan-Apochromat 40×/1.3 Oil DIC WD=2.0, or Plan-Apochromat 63×/1.4 Oil DIC f/ELYRA objectives (Carl Zeiss Microscopy, Thornwood, NY) or LAS AF SPE Core software on a Leica TCS SPE mounted on a Leica DM2500 using an ACS APO 20×/0.60 IMM CORR -/D or an ACS APO 63×/1.30 Oil CS 0.17/E objective (Leica Microsystems, Buffalo Grove, IL). Maximum projections (two to four confocal slices), merged images, rotation, and cropping were performed using ImageJ software (Abramoff et al., 2004).
Results
Overview of Drosophila oogenesis
The prevalence of nuclear actin, its structure, and its subnuclear localization during Drosophila oogenesis are largely unknown. Here we utilize three reagents – DNase I, anti-actin C4, and anti-actin AC15 – to characterize nuclear actin throughout follicle development. DNase I binds to and labels monomeric or G-actin (Hitchcock, 1980), while the two antibodies are reported to have the ability to label both monomeric and filamentous actin (F-actin). Here we define the developmental, cell-type and subnuclear distribution of the pools of nuclear actin recognized by the different reagents. This information, in conjunction with the vast knowledge of the processes occurring during oogenesis, provides insight into the potential functions of nuclear actin.
Drosophila oogenesis is a well-characterized and organized process of development (Spradling, 1993). Each adult female has a pair of ovaries (Fig. 1A). These ovaries are comprised of ~15 ovarioles, chains of sequentially developing follicles (Fig. 1A-B). This means every stage of follicle development is seen multiple times from a single female. At the anterior tip of the ovariole is a structure called the germarium (Fig. 1B-C). The germarium houses the stem cells that give rise to the individual follicles. The germarium is broken into four regions – 1, 2a, 2b, and 3. In region 1, at the anterior of the germarium, there are two to three germline stem cells (GSCs, Fig. 1C, bright cyan) that are supported by a somatic niche including the terminal filament (dark gray), cap (light gray), and escort cells (red). The GSCs give rise to a cystoblast (light cyan) that goes on to have 4 incomplete cell divisions to generate an interconnected 16-cell cyst (light blue, Fig. 1C, regions 2a/b and 3); the cells are connected by ring canals which are remnants of the incomplete cytokinetic furrows. As the cysts develop they are wrapped by the escort cells (red) through region 2a (Fig. 1C). At the 2a-2b boundary, two follicle stem cells (FSCs, dark green) reside (Fig. 1C). The FSCs give rise to all the follicle cells (light green) that go on to surround and encase the 16-cell cysts (Fig. 1C). Within the 16-cell cyst, one cell will become the oocyte (dark blue nucleus), while the other 15 cells become nurse cells (light blue nuclei), which support the growing oocyte; this specification of cell fate occurs in region 3 of the germarium (Fig. 1C). The 16-cell cyst covered in follicle cells (light green) then buds off the germarium to generate a Stage 3 (S3) follicle (Fig. 1B).
During the early stages of oogenesis, the follicles grow in length and in somatic or follicle cell number (Fig. 1B). The follicle cells proliferate through S6 and then endocycle from S7–9, replicating their DNA without dividing (Claycomb and Orr-Weaver, 2005). The follicle cells differentiate into 6 major subtypes: stalk, polar, border, stretch follicle, centripetal follicle and main body follicle cells (Dobens and Raftery, 2000). The stalk cells are the cells that connect each of the developing follicles and are not shown in Fig. 1 or examined in this study. The polar cells are 4 cells, two at the anterior and two at the posterior tip of each follicle (purple, Fig. 1B, D). During S9, the anterior polar cells specify a group of 6–8 surrounding cells to be border cells (yellow border cells surround purple polar cells, Fig. 1D). The border cells then migrate posteriorly between the nurse cells to the nurse cell-oocyte boundary (see yellow border cell cluster in S10B in Fig. 1B, D); the border cells ultimately move dorsally and will secrete the eggshell structure called the micropyle, which is where the sperm enters (Montell, 2003). During S9, while the border cells are migrating, the majority of the other follicle cells will become the main body follicle cells (light green), which ultimately cover the oocyte (Fig. 1D). The remaining ~50 cells become the squamous stretch follicle cells (dark orange) that cover the nurse cells (Fig. 1B, D). During S10B, the anterior main body follicle cells differentiate into centripetal follicle cells (light orange) and migrate to enclose the anterior edge of the oocyte (Fig. 1B, D). During S10B-12, the border, centripetal follicle and main body follicle cells undergo gene amplification to over-replicate the regions of the genome encoding eggshell genes and begin to secrete the eggshell towards the oocyte (Claycomb and Orr-Weaver, 2005).
The germline nurse cells do not divide, but undergo endocycling during S3–10 (Spradling, 1993). The nurse cells are responsible for producing mRNA, proteins, and organelles that will be supplied to the oocyte in S10B-11, in a process termed nurse cell dumping. During nurse cell dumping (S11), the nurse cells squeeze their cytoplasmic contents into the oocyte. This process results in the nurse cells decreasing in size and the oocyte growing in length (see S10B-S12, Fig. 1B). During the subsequent S12–13, the nurse cells undergo a complex process of cell death and are phagocytized by the stretch follicle cells (dark orange), while the centripetal (light orange), border (not illustrated) and main body (green) follicle cells secrete the eggshell structures (Fig. 1B). It is important to note that the process of germline cells supplying their contents into the surviving oocyte is conserved to mammals (Lei and Spradling, 2016). The final stage of oogenesis, S14, is the mature, ready to ovulate egg, which is comprised of the oocyte, eggshell, and surrounding follicle cells (Fig. 1B). During ovulation, these follicle cells will be sluffed off and remain at the base of the ovariole, where they serve as an endocrine organ, much like the corpus luteum in mammals (Deady et al., 2015). Using this knowledge, we can gain significant insight into the potential functions of nuclear actin by simply assessing which cells and when during follicle development the different reagents label nuclear actin.
Nuclear G-actin is ubiquitous
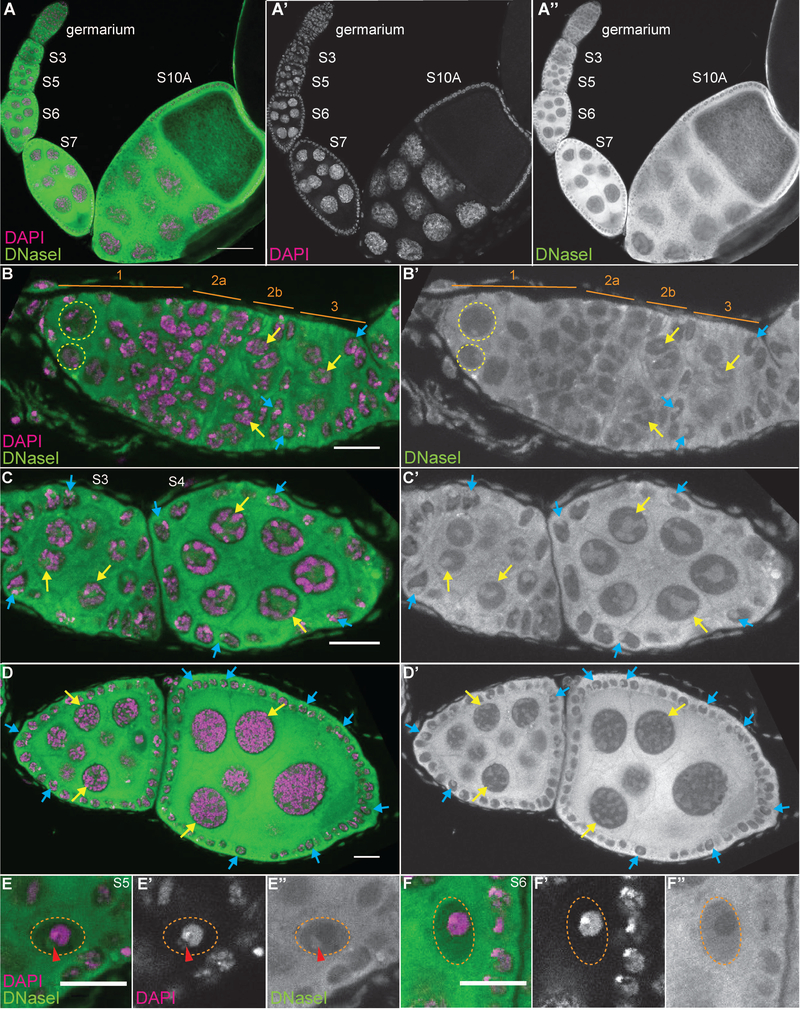
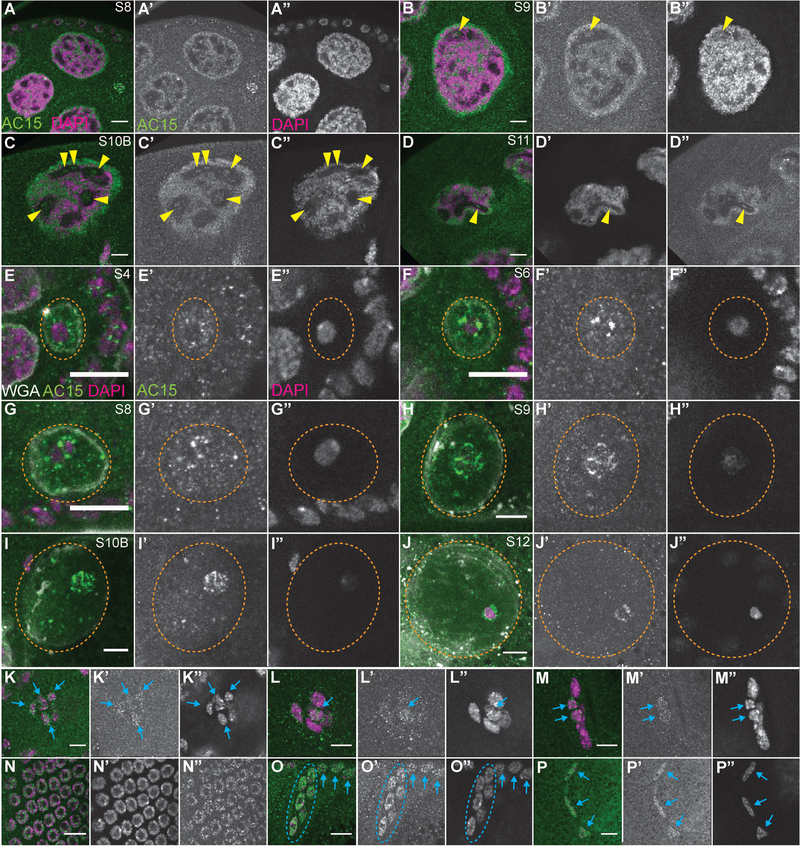
DNase I labeling reveals that while high levels of G-actin are seen in the cytoplasm, lower levels of G-actin are present in the nuclei of all cells throughout follicle development (Fig. 2A-A” and 3). Note that while all the cells exhibit nuclear G-actin, marks are used to point out specific cells in which the DNase I-labeled structure is readily apparent in the focal plane shown. In the germarium (Fig. 2B-B’), DNase I labels a single nuclear spot in all the early germ cells from the GSCs (yellow dashed circles) to the 16-cell cysts (yellow arrows). These 16-cell cysts will differentiate into one oocyte and 15 nurse cells. Upon differentiation, in region 3 of the germarium, the nuclear structure labeled within the nurse cells becomes more irregular in shape (Fig. 2B-B’, yellow arrow). This irregular appearance gets more pronounced and takes on a tubular structure during S3–10 (Fig. 2A-A” and C-D’, yellow arrows), and then becomes progressively more diffuse in the final stages of oogenesis, S11–13 (Fig. 3, yellow arrows). Notably, this nuclear G-actin does not overlap with the chromatin.
Figure 2:
DNase I reveals nuclear actin is ubiquitous throughout early follicle development. (A-F’) Maximum projections of 2–4 slices of confocal stacks of the indicated stages of wild-type (yw) follicles. (A-F) Magenta = DAPI, Green = DNase I. (A’, E’-F’) White = DAPI. (A”, B-D’, E”-F”) White = DNase I. (A-A”) Germarium through S10A. (B-B’) Germarium, regions are indicated in orange. (C-C’) S3–4. (D-D’) S6–7. (E-F”) Oocyte nuclei from S5 (E-E”) and S6 (F-F”). DNase I staining is strong in the cytoplasm and weaker in the nuclei throughout oogenesis and does not overlap with the DNA (A-A’). Note that while all the cells exhibit nuclear G-actin, marks are used to point out specific cells in which the DNase I-labeled structure is readily apparent in the focal planes shown. DNase I reveals that within the germline cells there is a single spot of G-actin from the germline stem cells (B-B’, yellow dashed circles) through the 16 cell cysts (B-B’, yellow arrows). Upon differentiation, the nurse cells exhibit an irregularly shaped DNase I positive nuclear structure (C-C’, yellow arrows) that gets more tubular through mid-oogenesis (D-D’, yellow arrows). The oocyte nucleus (orange dashed circle) exhibits a single DNase I spot adjacent to the chromatin from specification through S5 (E-E”, red arrowhead), and then only labels a nuclear haze (F-F”). Within the somatic cells, both escort (B-B”) and follicle cells (B-D’, blue arrows), DNase I labels a single spot that is in a region devoid of DNA. Scale bars = 50μm in A-A”, and 10μm in B-F”.
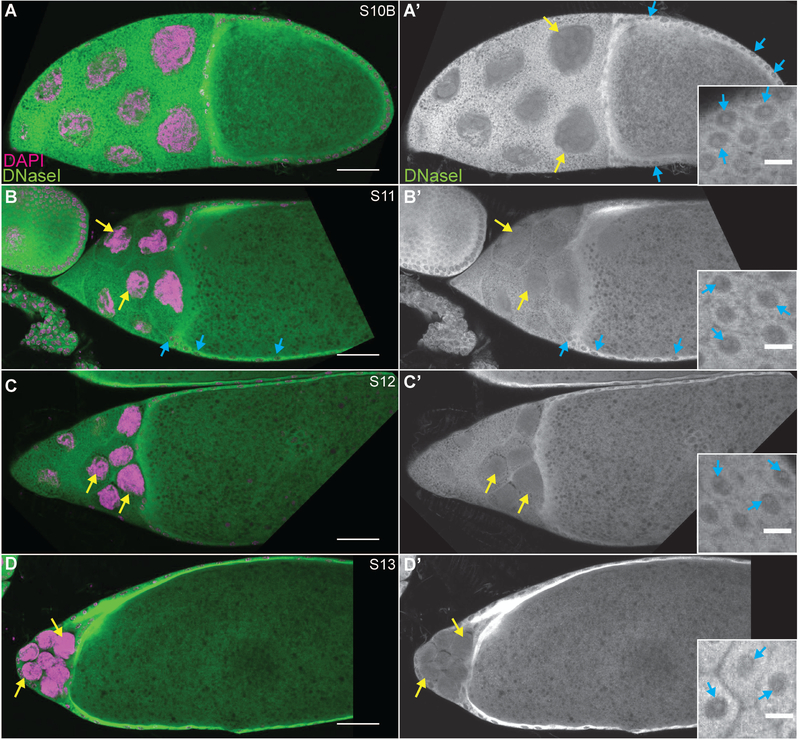
Figure 3:
DNase I labels nuclear actin during late stage follicle development. (A-D’) Maximum projections of 2–4 slices of confocal stacks of the indicated stages of wild-type (yw) follicles. (A-D) Magenta = DAPI, Green = DNase I. (A’-D’) White = DNase I. Insets are a zoom in of a different focal plane of the same follicle revealing the localization of DNase I within the follicle cells. While all the cells exhibit nuclear G-actin, marks are used to point out specific cells in which the DNase I-labeled structure is readily apparent in the focal planes shown. During the late stages of follicle development, DNase I staining in the nurse cells (yellow arrows) goes from tubular (S10B, A-A’) to diffuse (S11–13, B-D’). Conversely, DNase I remains as a single spot within the follicle cells (blue arrows and insets in A-D’) throughout the end of oogenesis. Scale bars = 50μm in A-D’, and 10μm in insets in A’-D’.
The DNase I labeling in the oocyte is distinct from that of the nurse cells. From region 3 of the germarium to S5, there is a single spot of DNase I staining directly adjacent to the DNA (Fig. 1E-E”, red arrowhead and data not shown). From S6 onward, this spot is no longer observed and only a nuclear G-actin haze is present (Fig. 2F-F”); this nuclear actin haze does not overlap with the chromatin.
DNase I also labels nuclear G-actin within the somatic cells during oogenesis. A single spot is labeled within the escort cells in the anterior of the germarium (data not shown and see Fig. 7K’, blue arrowheads), and in the follicle cells, from the stems cells (data not shown and see Fig. 7L’, blue dashed oval) throughout development (Fig. 2 and insets in Fig. 3A’-D’, blue arrows). Again, these foci do not overlap with the DNA.
Together these findings indicate that during Drosophila follicle development, all cells have nuclear G-actin. Given that this G-actin is within a single nuclear structure within each cell and does not overlap with the chromatin, it suggests G-actin is within a nuclear body.
One actin antibody (C4) labels a subset of nuclear actin during early oogenesis
The C4 actin antibody has broad specificity. While it was raised against chicken gizzard muscle actin, it reacts with all isoforms of vertebrate actin and actins from lower eukaryotes including Dictyostelium and slime mold (Lessard, 1988). The antibody can label both G- and F-actin. The epitope recognized by the C4 antibody is within the N-terminus of actin, and has been reported to be near amino acids 50–70 (Lessard, 1988; Gedge et al., 2005). Importantly, numerous studies have utilized the C4 antibody to examine nuclear actin (Parfenov et al., 1995; Hofmann et al., 2004; Gedge et al., 2005; Lenart et al., 2005; Spencer et al., 2011; Maslova and Krasikova, 2012). Furthermore, we previously found that it labels nuclear actin during the early stages of Drosophila oogenesis (Kelpsch et al., 2016). Thus, the C4 actin antibody is well-suited to study nuclear actin.
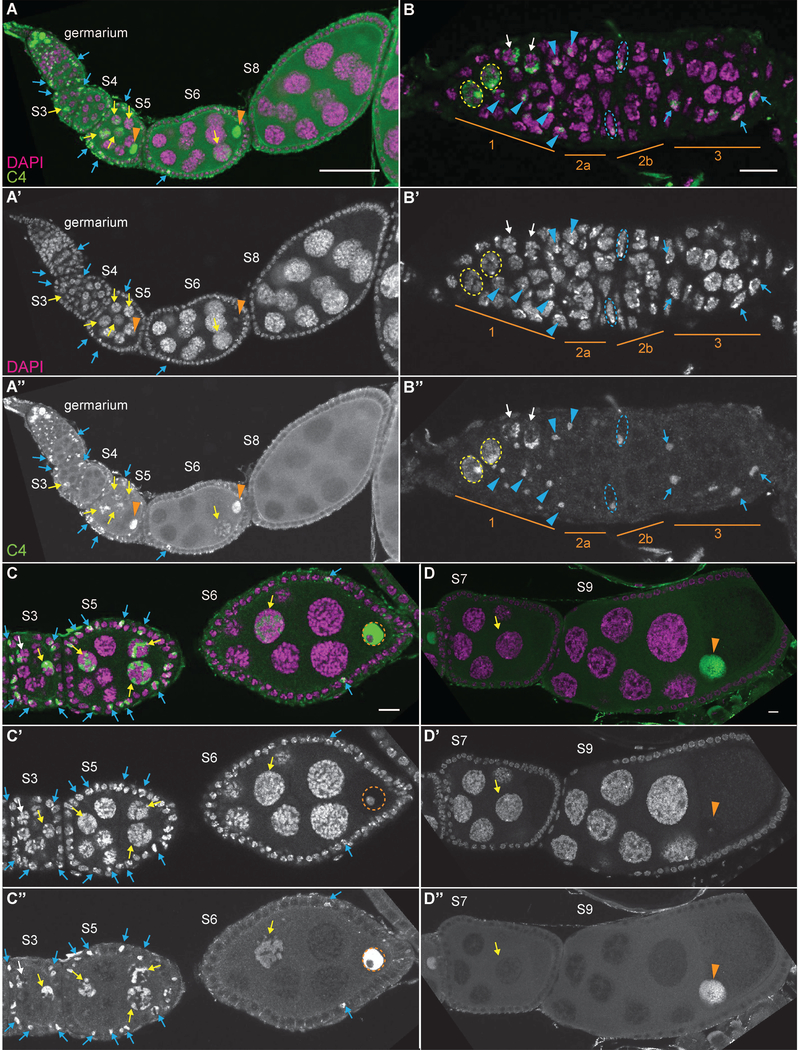
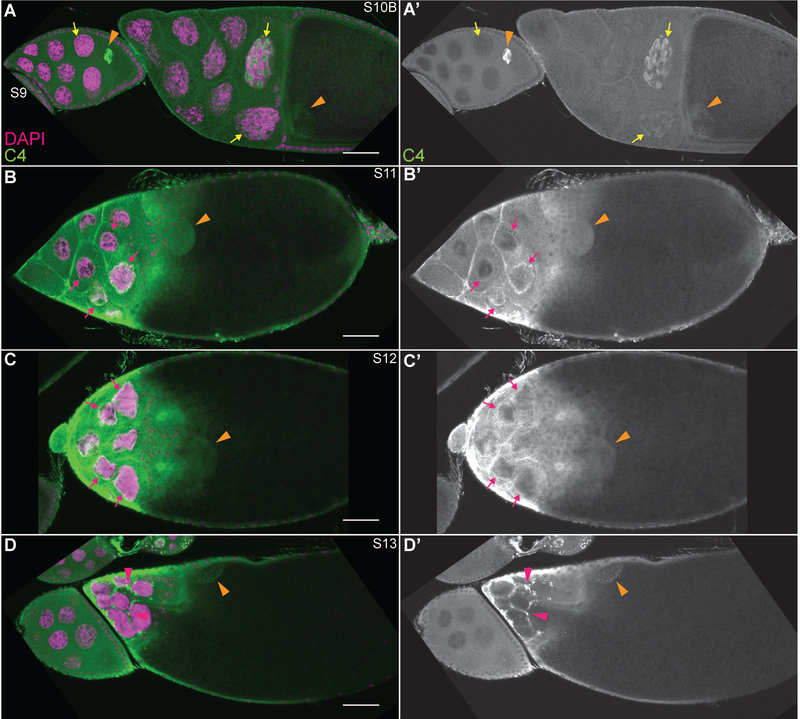
During follicle development, anti-actin C4 weakly labels the cytoplasm and strongly labels a subset of the nuclear actin, subsequently referred to as C4 nuclear actin (Figs. 4–5). In region 1 of the germarium (Fig. 4A-B”), high C4 nuclear actin levels are observed in the GSCs (yellow dashed circles) and the early cystoblasts (white arrows). Conversely, C4 nuclear actin is not observed in the developing cysts in regions 2A and 2B of the germarium. This finding leads us to speculate that C4 nuclear actin may help maintain an undifferentiated state. Upon differentiation of the 16-cell cyst into an oocyte and 15 nurse cells, C4 nuclear actin labels an irregularly shaped structure within a subset of the nurse cells (Fig. 4A-A” and S3 in C-C”, yellow arrows). This C4 nuclear actin is also observed in some of the nurse cells through S10 and exhibits a more tubular appearance as development progresses (Fig. 4C-D” and 5A-A’, yellow arrows). We previously observed that the frequency of nurse cells exhibiting C4 nuclear actin decreases with developmental stage (Kelpsch et al., 2016). Notably, like the DNase I-labeled nuclear G-actin, the C4 nuclear actin does not overlap with the chromatin. During S11 and 12, a ring of C4 nuclear actin is observed just inside the nuclear envelope (Fig. 5B-C’, magenta arrows). Conversely, no C4 nuclear actin is observed during S13, and instead C4 labels puncta either within the nurse cell cytoplasm or the extensions of the stretch follicle cells surrounding them (Fig. 5D-D’, magenta arrowheads).
Figure 4:
C4 nuclear actin is present in a subset of the cells during early oogenesis. (A-D”) Maximum projections of 2–4 slices of confocal stacks of the indicated stages of wild-type (yw) follicles. (A-D) Magenta = DAPI, Green = C4. (A’-D’) White = DAPI. A”-D”. White = C4. (A-A”) Germarium-S8. (B-B”) Germarium, regions are indicated in orange. (C-C”) S3,5, and 6. (D-D”) S7 and S9. C4 nuclear actin is high in the GSCs and undifferentiated cystoblasts (region 1 of germarium, A-B”; yellow dashed circles = GSC nuclei and white arrows = cystoblasts). In the differentiated germline, C4 nuclear actin is observed as an irregular, tubular structure within a subset of the nurse cells (yellow arrows); the frequency of nurse cells with and the level of C4 nuclear actin decreases with development (C-D”). This C4 nuclear actin does not overlap with the chromatin. High levels of C4 nuclear actin are seen within the oocyte nucleus throughout early oogenesis (A-A”, C-D”, orange arrowheads); importantly, it is excluded from the chromatin (C-C”, orange dashed circle). C4 nuclear actin is also observed in a subset of the somatic cells (A-A”). High C4 nuclear actin is observed in the escort cells in region 1, but not region 2a, of the germarium (B-B”, blue arrowheads). Both FSCs exhibit a single spot of C4 nuclear actin (B-B”, nuclei outlined by blue dashed lines). A subset of the follicle cells (blue arrows) from the region 2B of the germarium (B-B”) through S5 exhibit C4 nuclear actin (C-C”), while only a few follicle cells exhibit C4 nuclear during S6 (C-C”). In later stages, no C4 nuclear actin is observed in the follicle cells (D-D”). Note that not all cells with C4 nuclear actin are marked; marks are used to point out specific cells in which the C4 nuclear actin is readily apparent in the focal planes shown. Scale bars = 50μm in A-A”, and 10μm in insets in B-D”.
Figure 5:
C4 nuclear actin is significantly reduced and relocalizes during late oogenesis. (A-D”) Maximum projections of 2–4 slices of confocal stacks of the indicated stages of wild-type (yw) follicles. (A-D) Magenta = DAPI, Green = C4. (A’-D’) White = C4. During the late stages of follicle development, C4 nuclear actin goes from a tubular structure observed at a low frequency in S10B (A-A’, yellow arrows), to localizing to a ring just underneath the nuclear envelope in S11–12 (B-C’, magenta arrows). Finally, during S13, C4 labels puncta that appear to be in either the cytoplasm of the nurse cells or the stretch follicle cells enveloping them (D-D’, magenta arrowheads). C4 nuclear actin is observed throughout the nucleoplasm of the oocyte (A-D’, orange arrowheads). Note that not all cells with C4 nuclear actin are marked; marks are used to point out specific cells in which the C4 nuclear actin is readily apparent in the focal planes shown. Scale bars = 50μm.
C4 nuclear actin is also in the oocyte nucleus, i.e. germinal vesicle. During early oogenesis, very high levels of C4 nuclear actin are observed throughout the nucleoplasm of the oocyte (Fig. 4A-A” and C-D”, orange arrowheads). This labeling is weaker in the later stages (Fig. 5, orange arrowheads). Notably, the nuclear actin within the oocyte is largely excluded from the chromatin (Fig. 4C-C” and see Fig. 7H-J’’’, orange dashed circles).
C4 nuclear actin is also observed as a single spot within a subset of the somatic cells during early oogenesis. Specifically, in the germarium, high C4 nuclear actin levels are seen in the escort cells in region 1, but not those in region 2a (Fig. 4B-B”, blue arrowheads). C4 nuclear actin is also observed in the follicle stem cells at region 2a/2b boundary (Fig. 4B-B”, blue dashed ovals). The findings that all the stem cell populations in the Drosophila ovary have high C4 nuclear actin suggests that nuclear actin may play a role in maintaining stemness. Subsequently, C4 nuclear actin is seen in a subset of the follicle cells from region 3 (Fig. 4B-B”, blue arrows) through S6 (Fig. 4C-C’). The frequency of follicle cells with C4 nuclear actin is high through S5, and only a few cells are labeled in S6 (Fig. 4C-C’). No follicle cells are labeled in the later stages (Fig. 4D-D” and 5). Interestingly, the follicle cells from region 3 to S5 are mitotic, in S6 the cells transition from mitotic to endocycling, and then endocycle through S9 (Claycomb and Orr-Weaver, 2005). Thus, the developmental pattern of C4 nuclear actin within the follicle cells suggests it may regulate or be regulated by the cell cycle. Additionally, like the DNase I labeled nuclear G-actin, the single puncta within all the somatic cells does not colocalize with the DNA.
C4 nuclear actin localizes to the spindle during mitosis
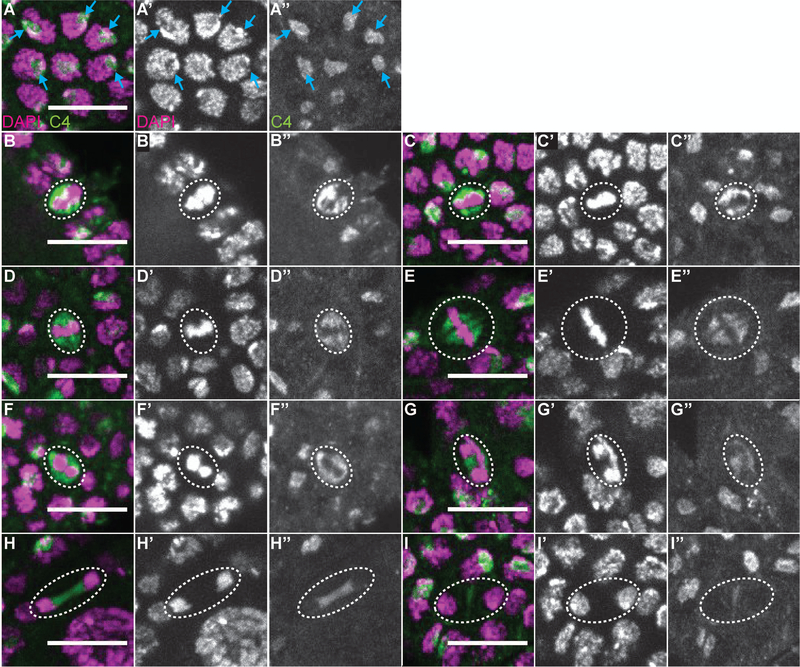
To examine the relationship of C4 nuclear actin within the follicle cells and the cell cycle, we assessed C4 localization during the different stages of the cell cycle. In interphase cells, C4 nuclear actin labels a single spot in a subset of the cells (Fig. 6A-A”, blue arrows). This subnuclear localization pattern changes with the cell cycle. During early metaphase, C4 nuclear actin forms a double bar structure, surrounding the forming metaphase plate (Fig. 6B-C”). Later in metaphase, C4 nuclear actin labels the spindle (Fig. 6D-E”); notably, this nuclear actin appears filamentous. During anaphase, C4 nuclear actin initially forms a ring around each group of segregating chromosomes (Fig. 6F-F”), and later becomes restricted to the region between the chromosomes (Fig. 6G-G”). In early telophase, C4 nuclear actin is seen stretched between the chromosomes (Fig. 6H-H”), while in late telophase only a single vertical bar with low levels of C4 nuclear actin is observed (Fig. I-I”). These data suggest that C4 nuclear actin may regulate chromosome segregation during mitosis.
Figure 6:
C4 nuclear actin is dynamic during mitosis. (A-I”) Maximum projections of 2–4 slices of confocal stacks of zoomed in images of follicle cells, from wild-type (yw) follicles, at different stages of the cell cycle. (A-I) Magenta = DAPI, Green = C4. (A’-I’) White = DAPI. A”-I”. White = C4. (A-A”) Follicle cells in interphase. (B-C”) Follicle cells in early metaphase. (D-E”) Follicle cells in metaphase. (F-G”) Follicle cells in anaphase. (H-I”) Follicle cells in telophase. In interphase follicle cells, a C4 nuclear actin spot is observed in a subset of the cells; this spot appears to be in a nuclear region largely devoid of DNA (A-A”, blue arrows). In early metaphase, C4 nuclear actin begins to form a double bar structure surrounding the aligning chromosomes (B-C”, nuclei circled with white dashed line). Later in metaphase, when the chromosomes are fully aligned, C4 nuclear actin is observed on the spindle in what appear to be filaments (D-E”, nuclei circled with white dashed line). In anaphase, as the chromosomes begin to separate, C4 nuclear actin encircles the DNA, while later in anaphase, C4 nuclear actin is restricted to the region between the separating chromosomes. Early in telophase, C4 nuclear actin appears stretched between the segregated chromosomes; the appearance looks very filamentous (H-H”). In late telophase only a small vertical bar of C4 nuclear actin is observed in the middle of the segregated chromosomes (I-I”). Scale bars = 10μm.
Some C4 nuclear actin colocalizes with DNase I
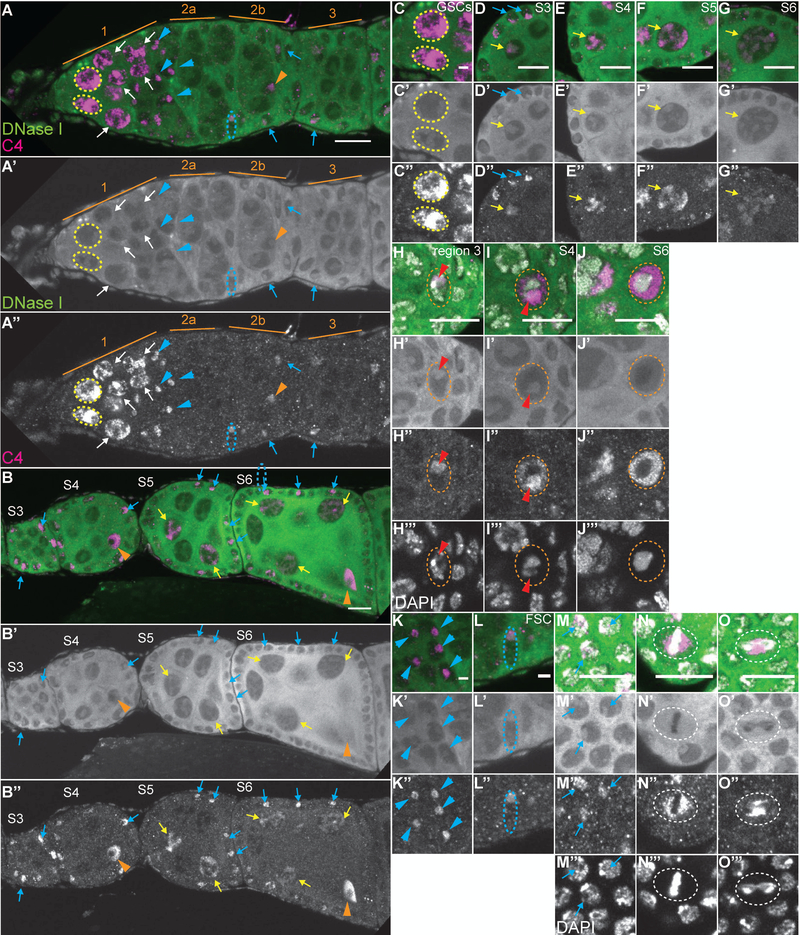
As the C4 antibody is reported to recognize both monomeric and polymeric actin, we next assessed the structure of C4 nuclear actin by examining its localization in relation to DNase I, which recognizes monomeric or G-actin (Hitchcock, 1980). Interestingly, during oogenesis, C4 nuclear actin significantly colocalizes with DNase I in many cells, but is distinct in others (Fig. 7). Specifically, in the region 1 of the germarium, C4 nuclear actin within the GSCs (yellow dashed circles) and early cysts (white arrows) overlaps, but extends significantly beyond the DNase I foci (Fig. 7A-A”, C-C”). This finding suggest that C4 recognizes both monomeric and polymeric actin in the early, undifferentiated germline cells. Note that we define polymeric nuclear actin as nuclear actin that does not co-label with DNase I and also fails to robustly label with phalloidin, a marker of F-actin; this suggests that the polymeric actin may be short filaments or have an altered structure compared to canonical F-actin. In the nurse cells during S3–10, C4 nuclear actin colocalizes with DNase I (Fig. 7B-B” and D-G”, yellow arrows). It is important to note that C4 nuclear actin is only in some of, while DNase I labels the nuclear, tubular structure in all the nurse cells. This finding suggests that C4 nuclear actin labels a subset of the G-actin in the nurse cells.
Figure 7:
C4 nuclear actin largely colocalizes with DNase I. (A-O’’’) Maximum projections of 2–4 slices of confocal stacks of the indicated stages of wild-type (yw) follicles (A-B”) or zoomed in images of specific cell types (C-O’’’). (A-G, K-L) Magenta = C4, Green = DNase I. (A’-G’, K’-L’) White = DNase I. (A”-G”, K”-L”) White = C4. (H-J’’’, M-O’’’) Magenta = C4, Green = DNase I, White = DAPI. (H’-J’, M’-O’) White = DNase I. (H”-J”, M”-O”) White = C4. (H’’’-J’’’, M’’’-O’’’) White = DAPI. (C-C”) GSCs. (D-G”) Nurse cells. (H-J’’’) Oocytes. (K-K”) Escort cells. (L-L”) FSC. (M-O”) Follicle cells in different stages of the cell cycle (interphase = M-M’’’, metaphase = N-N’’’, and anaphase = O-O’’’). In region 1 of the germarium, C4 nuclear actin both overlaps and extends significantly beyond the structure labeled by DNase I in the GSCs (A-A” and C-C”, yellow dashed circles) and early cystoblasts (A-A”, white arrows). In the differentiated nurse cells, C4 nuclear actin colocalizes with DNase I to label an irregular, tubular structure (B-B” and C-G”, yellow arrows). When the oocyte is first specified in region 3 of the germarium, C4 nuclear actin colocalizes with DNase I (A-A”, orange arrowhead, and H-H’’’, oocyte nucleus circled in white dashed line and DNase I spot indicated with red arrowhead). Subsequently, in S4 through early S6 (B-B”, I-I’’’, and data not shown), C4 nuclear actin localizes throughout the nucleoplasm of the oocyte (nucleus circled in orange dashed line), is excluded from the region with the DNA, and is enriched in a circle that encases the DNase I spot (I-I’’’, red arrowhead). In late S6 and later, C4 is bright throughout the nucleoplasm, but is excluded from the chromatin region (J-J’’’, nucleus encircled in orange dashed line and data not shown). C4 nuclear actin also partially overlaps with DNase I in the somatic cells. In region 1 of the germarium, C4 labels a single puncta in the anterior escort cells (A-A”, blue arrowheads); this puncta overlaps, but extends slightly beyond, the puncta labeled with DNase I (K-K”, blue arrows). A similar labeling pattern is seen in the FSCs (A-A” and L-L”, nuclei circled in blue dashed line). While in the differentiated interphase follicle cells C4 nuclear actin is only observed in a subset of cells, when it is seen it largely overlaps the DNase I spot (A-B”, D-D”, M-M’’’, blue arrows). In dividing follicle cells, C4 nuclear actin is distinct from DNase I. DNase I is a haze throughout the nucleoplasm of dividing follicle cells, while C4 exhibits a more restricted and dynamic localization (N-0’’’, nuclei circled in white dashed line). Note that not all cells with C4 nuclear actin are marked; marks are used to point out specific cells in which the C4 nuclear actin is readily apparent in the focal planes shown. Scale bars = 10μm in A-B”, D-J”, M-O’’’ and 2μm in C-C”, K-L”.
The localization of C4 nuclear actin and DNase I changes with oocyte development. In the newly specified oocyte in region 3 of the germarium, C4 nuclear actin and DNase I colocalize (Fig. 7A-A”, orange arrowhead and 7H-H’’’, red arrowhead). This colocalization suggests C4 nuclear actin is monomeric in the early oocyte. However, in S4 through early S6, C4 nuclear actin is high throughout the oocyte nucleoplasm and is enriched in a sphere encasing, but not overlapping the single DNase I puncta (Fig. 7I-I’’’, red arrowhead). In late S6 onward, C4 nuclear actin is throughout the nucleoplasm while DNase I appears as a weak haze (Fig. 7J-J’’’ and data not shown). These data indicate that C4 nuclear actin is likely polymeric within the oocyte nucleus from S4 onward.
C4 nuclear actin largely colocalizes with DNase I in interphase somatic cells (Fig. 7, blue arrows). Specifically, C4 nuclear actin and DNase I label a single spot within the anterior escort cells in region 1 of the germarium (Fig. 7A-A” and K-K”, blue arrowheads) and the FSCs (Fig. 7A-A” and L-L”, blue dashed oval); note that the C4 nuclear actin labeling extends slightly beyond that of DNase I. When C4 nuclear actin is observed in the interphase follicle cells from region 3 of the germarium through S6, it largely colocalizes with DNase I (Fig. 7A-B”, D-D”, and M-M’’’, blue arrowheads). These data suggest that C4 nuclear actin labels a subset of the G-actin within interphase somatic cells. In dividing cells, C4 nuclear actin is dynamic (see Fig. 6), while DNase I is uniform throughout the nucleoplasm but is excluded from the chromatin region (Fig. 7N-O’’’, white dashed circles). This finding indicates that the C4 nuclear actin observed from metaphase to telophase is likely polymeric.
G-actin localizes to the nucleolus
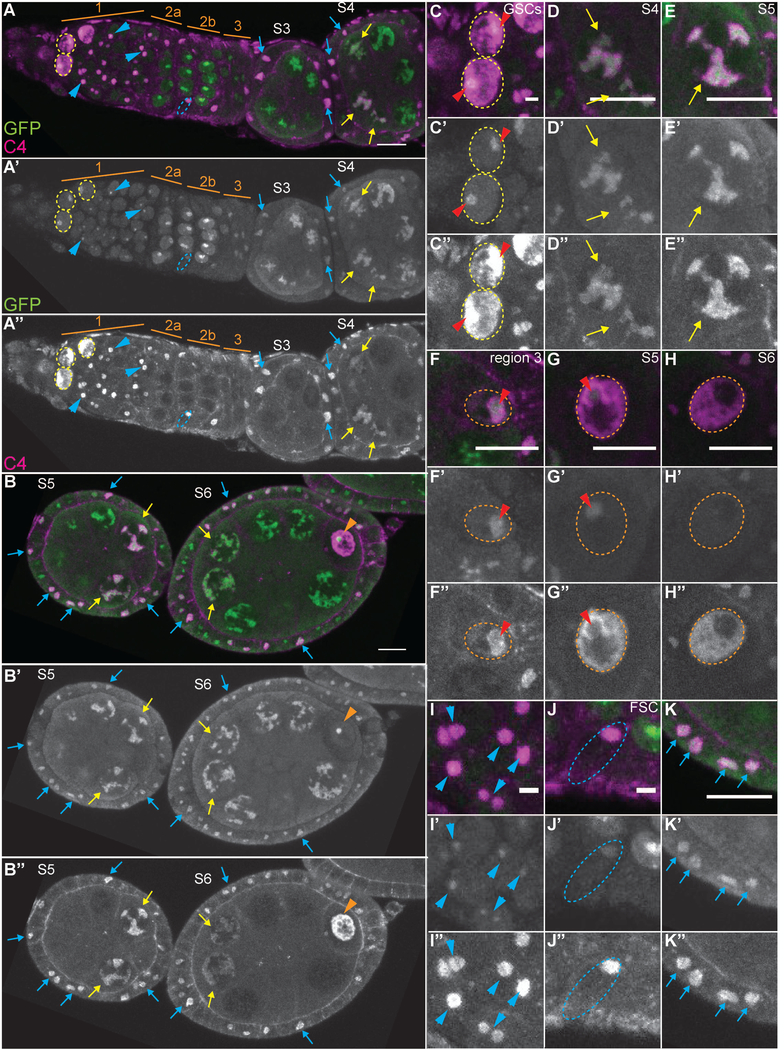
We next wanted to determine where the monomeric or G-actin that is labeled by DNase I and, in some cases, C4, is localizing within the nucleus. As the G-actin was excluded from the DNA, appeared as a single spot in the early germ cells and the somatic cells, and exhibited a tubular appearance within the nurse cells, we hypothesized that the G-actin was within the nucleolus. To test this hypothesis, we compared the localization of C4 nuclear actin to eGFP-tagged nucleolar components, fibrillarin (Fig. 8) and Nopp-140 (data not shown). In the GSCs and cystoblasts (region 1 of germarium), the nucleolus is a single spot; C4 nuclear actin is enriched in the nucleolus, but extends throughout the nucleoplasm (Fig. 8 A-A”, yellow dashed circles, and C-C”, red arrowheads mark the nucleolus). Conversely, in the nurse cells, C4 nuclear actin completely colocalizes with the nucleolus (Fig. 8A-B” and D-E”, yellow arrows). In the oocyte, C4 nuclear actin is initially enriched in the nucleolus (Fig. 8F-F”, red arrowhead). In S4 through early S6, C4 nuclear actin is throughout the nucleoplasm, and is also enriched in the immediate vicinity of the nucleolus (S4 Fig. 8G-G”, red arrowhead, and early S6, B-B”, orange arrowhead). Starting in late S6, no strong nucleolar signal is observed in the oocyte nucleus and C4 nuclear actin is throughout the nucleoplasm (Fig. 8H-H”).
Figure 8:
C4 nuclear actin is primarily nucleolar. (A-O’’’) Maximum projections of 2–4 slices of confocal stacks of the indicated stages of eGFP-fibrillarin expressing follicles (A-B”) or zoomed in images of specific cell types (C-K”). (A-K) Magenta = C4, Green = GFP (labels eGFP-Fibrillarin). (A’-K’) White = GFP. (A”-K”) White = C4. (C-C”) GSCs. (D-E”) Nurse cells. (F-H”) Oocytes. (I-I”) Escort cells. (J-J”) FSC. (K-K”) Interphase follicle cells. In region 1 of the germarium, eGFP-Fibrillarin reveals the nucleolus is a single spot within the GSCs and cystoblasts (A-A” and C-C”, yellow dashed circles); C4 nuclear actin is enriched at these spots but significantly extends into other regions of the nucleus. In the differentiated nurse cells, when C4 nuclear actin is present it colocalizes with eGFP-Fibrillarin (A-B” and D-E”, yellow arrows). In the newly specified oocyte (region 3 of the germarium), C4 nuclear actin also colocalizes with eGFP-Fibrillarin (F-F”, nucleus circled by orange dashed line and eGFP-Fibrillarin spot indicated with red arrowhead). As the oocyte develops in S4 through early S6 (B-B”, orange arrowhead, and G-G”, nucleus circled by orange dashed line), C4 nuclear actin is throughout the nucleoplasm and is enriched in a ring around the eGFP-Fibrillarin spot (red arrowhead). From late S6 onward, no strong eGFP-Fibrillarin signal is observed, while C4 nuclear actin is dispersed thoughout the nucleoplasm (H-H”, nucleus circled by white dashed line). C4 nuclear actin also largely overlaps with eGFP-Fibrillarin in the somatic cells. In region 1 of the germarium, C4 nuclear actin labels a single spot within the anterior escort cells that overlaps but extends beyond the eGFP-Fibrillarin spot (A-A” and I-I”, blue arrowheads). In the FSCs, C4 nuclear actin completely colocalizes to the eGFP-Fibrillarin spot (A-A” and J-J”, nuclei circled in blue dashed lines), while in the differentiated follicle cells, C4 nuclear actin overlaps but extends beyond the eGFP-Fibrillarin spot (A-B” and K-K”, blue arrows). Note that not all cells with C4 nuclear actin are marked; marks are used to point out specific cells in which the C4 nuclear actin is readily apparent in the focal planes shown. Scale bars = 10μm in A-B”, D-H”, and K-K”, and 2μm in C-C” and I-J”.
In the somatic cells during Drosophila oogenesis, C4 nuclear actin largely localizes to the nucleolus (Fig. 8, blue arrowheads, arrows and dashed ovals). In region 1 of the germarium, C4 nuclear actin within the escort cells overlaps and extends beyond the nucleolus to form a larger spot (Fig. 8A-A” and I-I”, blue arrowheads). Within the FSCs, C4 nuclear actin completely localizes to the nucleolus (Fig. 8A-A” and J-J”, blue dashed ovals). When C4 nuclear actin is observed in the follicle cells from region 3 of the germarium to S6, it localizes to and extends slightly beyond the nucleolus (Fig. 8A-B”, and K-K”, blue arrows).
Similar to monomeric C4 nuclear actin, DNase I-labeled G-actin localizes to the nucleolus as visualized using either eGFP-tagged nucleolar components or an antibody to fibrillarin (data not shown). These data, in conjunction with the colocalization data between C4 nuclear actin and DNase I, suggest that monomeric nuclear actin is largely found within the nucleolus in both somatic and germline cells.
One actin antibody (AC15) recognizes a pool of nuclear actin during mid and late oogenesis
The AC15 actin antibody specifically recognizes β-, but not β- or β-actin (Gimona et al., 1994). It was raised against a modified N-terminal peptide from β-actin (amino acids 2–16) conjugated to a carrier protein (Keyhole Limpet Hemocyanin, KLH). This peptide is 100% similar to the N-termini of the two ubiquitous Drosophila actins, Actin 5C and 42A. Notably, these two actins are highly expressed during oogenesis (ModENCODE; (Tootle et al., 2011), and we previously found that expression of GFP-Actin 5C or 42A within the germline results in nuclear actin rod formation (Kelpsch et al., 2016). The AC15 antibody has been widely used to examine nuclear actin, including in plants (Cruz and Moreno Diaz de la Espina, 2009), Drosophila cultured cells (Dopie et al., 2012), amphibian oocytes (Miyamoto et al., 2011), and mammalian cells (Hofmann et al., 2004). Thus, AC15 antibody is suitable for examining nuclear actin.
During oogenesis, anti-actin AC15 weakly labels the cytoplasm and recognizes a distinct pool of nuclear actin from mid-oogenesis onward, subsequently referred to as AC15 nuclear actin (Figs. 9–10). During early oogenesis (germarium through S5), there are very low levels of AC15 nuclear actin (Fig. 9A-A’). At S6, weak AC15 nuclear actin is observed in the nurse and follicle cells (Fig. 9A-A’), and the level of AC15 nuclear actin in these cells continues to increase through S10 (Fig. 9A-C’). The AC15 nuclear actin in the nurse cells is structured, with enrichment at the nuclear periphery (Fig. 9B-C’). This peripheral localization increases with follicle development (S10B-12, Fig. 9D-F’). At S13, AC15 nuclear actin is largely absent and AC15 labels a cytoplasmic cytoskeletal structure (Fig. 9G-G”). In the follicle cells, AC15 nuclear actin is observed in the early migrating border cells during S9 (Fig. 9B-B’. white dashed oval), the main body follicle cells from S6 onward (Fig. 9A-C’, D”-G”), and the stretch follicle cells over the nurse cells (Fig. 9D”-G”). Notably, the nuclear actin structures recognized by AC15 in both the nurse and follicle cells is distinct from that of DNase I and C4, as it does not colocalize with DNase I (data not shown). This finding suggests that AC15 nuclear actin is likely polymeric. As phalloidin does not label the AC15 nuclear actin, we speculate that the antibody recognizes short polymers of actin and not canonical F-actin. Based on the distinct localization pattern, we next examined where AC15 nuclear actin is compared to the DNA. In the nurse cells, AC15 nuclear actin is enriched on the chromatin (Fig. 10A-D”), but also labels puncta in regions that are devoid of DNA (yellow arrowheads).
Figure 9:
AC15 nuclear actin is present in all nuclei from mid-oogenesis. (A-G”) Maximum projections of 2–4 slices of confocal stacks of the indicated stages of wild-type (yw) follicles; insets in D’-G’ are a zoomed in image of the yellow boxed region. (A-G) Magenta = Wheat germ agglutinin (WGA, marks nuclear envelope), Green = AC15. (A’-G’ and D”-G”) White = AC15. AC15 nuclear actin is weak during early oogenesis (germarium-S5, A-A’). During S6–8, AC15 nuclear actin is weak but increasing with development in both the nurse cells and follicle cells (A-A’); nurse cell levels appear higher than the follicle cells at these stages. This increase in AC15 nuclear actin continues during S9–10A (B-C’). Within the nurse cells, the AC15 nuclear actin is structured and is enriched at the nuclear periphery (B-C’). During S10B-12, the level of AC15 nuclear actin remains high, but becomes more restricted to the nuclear periphery in the nurse cells (D-F’, insets are zoomed in images of nurse cells). At S13, AC15 nuclear actin is strongly reduced and AC15 labels cytoplasmic actin (G-G’, inset is zoomed in image of nurse cells). AC15 nuclear actin also increases with development in the follicle cells (A-C’). AC15 nuclear actin is weak within the migrating border cells during S9 (B-B’, border cells circled by white dashed line). AC15 also labels both the main body (those over the oocyte) and stretch follicle cells (those over the nurse cells) throughout the end of oogenesis (D”-G”). Scale bars = 50μm, except in the insets in D’-G’ where scale bars = 10μm.
Figure 10:
AC15 nuclear actin localizes to the DNA. (A-P”) Zoomed in images of different cell types from maximum projections of 2–4 slices of confocal stacks of the indicated stages of wild-type (yw) follicles. (A-D, K-P) Magenta = DAPI, Green = AC15. (E-J) Magenta = DAPI, Green = AC15, and White = Wheat germ agglutinin (WGA, marks nuclear envelope). (A’-P’) White = AC15. (A”-P”) White = DAPI. (A-D”) Nurse cells. (E-J”) Oocytes. (K-M”) Border cells. (N-N”) Main body follicle cells. (O-O”) Centripetal follicle cells. (P-P”) Stretch follicle cells. Within the nurse cells, AC15 largely colocalizes with the DNA (A-D”), however it also labels puncta within regions devoid of chromatin in S9–11 (yellow arrowheads). In the oocyte (nuclei circled by orange dashed line), AC15 nuclear actin structure changes with development (E-J). In S4 oocytes, AC15 nuclear actin exhibits a speckled appearance throughout the nucleoplasm, with bright puncta adjacent around the edge of the chromatin (E-E”). This enrichment of AC15 puncta around the DNA in the oocyte increases in S4–8 (F-G”), and then appears to form filaments that encircle the chromatin in S9–11 (H-I”). In S12 oocytes, AC15 nuclear actin becomes more diffuse, but still surrounds the DNA (J-J”). AC15 nuclear actin is also observed in the somatic cells. In the migrating border cells, AC15 nuclear actin is weakly observed in all of the cells (both border and polar cells) in the early stage of migration (K-K”, blue arrows). Towards the end of the border cell migration (late S9 and S10A), AC15 nuclear actin appears restricted to a subset of the cells (L-M”, blue arrows); given the localization of the AC15 positive nuclei, we hypothesize that they are the polar cells. AC15 nuclear actin is observed in all of the other follicle cell populations, including the main body follicle cells (N-N” and blue arrows in O-O’), centripetal follicle cells (circled in blue dashed line), and the stretch follicle cells (P-P”, blue arrows). Scale bars = 10μm.
In the oocyte, AC15 nuclear actin changes with development. In S3–4, AC15 nuclear actin exhibits a speckled pattern throughout the oocyte nucleoplasm, with the brightest spots at the edge of the chromatin (Fig. 10E-E”). As the oocyte develops (S6–8), AC15 nuclear actin becomes restricted to puncta adjacent to the DNA (Fig. 10F-G”). These puncta appear to transition to filamentous structures that surround the DNA starting at S9 and continuing through S11 (Fig. 10H-I” and data not shown). Finally, in S12–13, AC15 nuclear actin is more diffuse but still encircles the chromatin (Fig. 10J-J”).
AC15 nuclear actin within the follicle cells is also on the DNA. Interestingly, AC15 nuclear actin is dynamic in the border cells during their migration. Early in border cell migration, AC15 nuclear actin is weakly observed in the all the cells of the cluster (Fig. 9B-B’, white dashed oval and 10K-K”, blue arrows). Later during both the posterior (Fig. 10L-L”) and dorsal migration (Fig. 10M-M”) of the border cells, AC15 nuclear actin labels one to two cells. Given the location of the labeled cells during the late stages of migration, we speculate that these are the polar cells. Compared to the border cells, the level of AC15 nuclear actin is significantly higher in the other follicle cells (see Fig. 10N-P”). In the main body follicle cells, AC15 nuclear actin exhibits a speckled appearance and the speckles localize to the DNA (Fig. 10N-N”). AC15 nuclear actin is also enriched in the centripetal follicle cells, compared to the adjacent main body follicle cells (Fig. 10O-O”, blue dashed oval compared to blue arrows), as they begin their migration between the nurse cells and oocyte. In the stretch follicle cells, AC15 nuclear actin also localizes to the chromatin (Fig. 10P-P”).
In summary, AC15 nuclear actin generally localizes to the chromatin during mid to late oogenesis in both the germline and somatic cells. These findings lead us to speculate that this pool of nuclear actin may play a role in transcription.
Discussion
Using Drosophila oogenesis as a model to study nuclear actin reveals that multiple pools of nuclear actin exist and provides insight into the potential functions of nuclear actin (Table 1). Thus, while all statements made about the functions of nuclear actin during Drosophila oogenesis are based on both our findings and prior studies, they are speculative and remain to be tested. We find that all cells contain G-actin, recognized by DNase I, in a spot or structure within their nucleus, suggesting that nuclear actin plays a general role within every cell. Intriguingly, one actin antibody, C4, exhibits a more restricted pattern during early oogenesis, but when it is present, it often colocalizes with DNase I. This finding supports the model that C4 nuclear actin recognizes a specific subset or pool of G-actin. This G-actin may be post-translationally modified or in a particular protein complex that results in the antigen on actin being available. Future proteomic studies are needed to address these possibilities. It is also possible that the C4 nuclear actin that colocalizes with DNase I is not monomeric but polymeric actin at the same subnuclear localization. Indeed, polymeric C4 nuclear actin, defined by the lack of colocalization with DNase I, is also observed in specific cells, including the GSCs and oocyte. Such findings support the idea that the structure of nuclear actin impacts its functions. Interestingly, another actin antibody, AC15, labels a distinct pool of polymeric actin. AC15 nuclear actin is observed from mid-to-late oogenesis in almost every cell, and does not colocalize with DNase I. These findings suggest that AC15 nuclear actin is polymeric and mediates completely different processes from DNase I-labeled and C4 nuclear actin. The cell-type, developmental, and subnuclear pattern of the different pools of nuclear actin leads us to speculate that nuclear actin contributes to stemness, transcription, nucleolar structure/function, cell cycle, and nuclear structure.
Table 1:
Summary of the nuclear actin pools during Drosophila oogenesis.
| Labeling tool | Predicted structure | Cell-type | Subnuclear localization | Potential functions |
|---|---|---|---|---|
| DNase I | G-actin | All cells | Nucleolus | General nucleolar structure and functions. Potential functions include: ribosomal gene expression, ribosome production, and response to cellular stress |
| Anti-actin C4 | G-actin | Anterior escort cells* | Nucleolus | Nucleolar structure and/or function (see above) in cells with or at a times of specific protein production needs |
| FSCs | ||||
| Some mitotic interphase follicle cells* | ||||
| Subset of nurse cells (S3–10) | ||||
| Region 3 oocyte | ||||
| Polymers | GSCs and early cystoblasts | Nucleoplasm, including chromatin | Maintenance of undifferentiated state via chromatin remodeling and transcriptional programing | |
| Mitotic follicle cells | Dynamic localization, including to the spindle | Regulation of chromosome segregation | ||
| Oocyte (S3-S13) | Extra chromosomal nucleoplasm | Structural actin meshwork needed for nuclear integrity and organization | ||
| Anti-actin AC15 | Polymers | Follicle cells (S6-S14) | Chromatin | RNAPII-dependent transcription |
| Nurse cells (S6–10) | Chromatin | |||
| Nucleolus | RNAPI- and RNAPIII-dependent transcription | |||
| Migrating border and centripetal follicle cells | Chromatin | MAL/SRF transcription factor regulation | ||
| Oocyte | Puncta near chromosomes | Restricted to puncta and filamentous structures to block oocyte transcription, but have readily available pool of actin for embryonic transcription initiation | ||
| Filamentous structure surrounding chromosomes |
indicates the cell-types where C4 nuclear actin localizes to the nucleolus, but it also extends slightly beyond it.
Nuclear actin and stemness
Our findings suggest nuclear actin may play a critical role in maintaining an undifferentiated state. Specifically, high levels of C4 nuclear actin are observed in the GSCs and the undifferentiated cystoblasts, but not in the later cysts within the germarium. This C4 nuclear actin is enriched at and overlaps the small DNase I spot that localizes to the nucleolus, but also extends throughout the nucleoplasm. These data lead us to hypothesize that polymeric C4 nuclear actin regulates germline differentiation (Table 1). Supporting this idea, nuclear actin has been implicated in controlling both cell quiescence, and the escape from it, in mammary epithelial cells. Quiescent or senescent cells have low nuclear actin, and overexpression of nuclear actin restores transcription and DNA synthesis. In these cells, nuclear actin levels are regulated by laminin-111, an extracellular matrix (ECM) component. Interestingly, in the Drosophila germarium, specific ECM, extracellular ligands, and cell adhesions occur between the GSCs and the surrounding niche (reviewed in (Wu et al., 2008; Spradling et al., 2011). Such signals are essential for maintaining the GSCs (Guo and Wang, 2009; Hayashi et al., 2009; Yan et al., 2009; Pearson et al., 2016). In the future, it will be important to determine whether these signals regulate nuclear actin and if nuclear actin is required for GSC maintenance.
A potential mechanism by which C4 nuclear actin may maintain an undifferentiated state is by regulating chromatin remodeling and transcriptional programing. Indeed, actin is found within many, but not all chromatin remodeling complexes. Within such complexes, polymeric actin has been implicated in modulating the complex ATPase activity and/or serving as a scaffold for complex assembly or stability (reviewed in (Venit et al., 2018). This polymeric nuclear actin restricts repressive chromatin marks and limit promoter chromatin compaction (Almuzzaini et al., 2016), regulates the genome-wide organization of the heterochromatin (Xie et al., 2018), and mediates cellular programming and identity (Tondeleir et al., 2012). Additionally, monomeric actin binds to and inhibits histone deacetylases (HDAC1 and 2), which may facilitate opening the chromatin (Serebryannyy et al., 2016a). Interestingly, the Drosophila GSCs, like many stem cells, have a specific epigenetic state that is required for proper differentiation of their daughters. Epigenetic alterations within the GSCs, such as loss of particular histones or alterations in histone modifying enzymes, results in GSC loss (reviewed in (Flora et al., 2017)). Additionally, chromatin remodeling complexes reported to contain nuclear actin are required for Drosophila GSC maintenance (Ables and Drummond-Barbosa, 2010; He et al., 2014). It will be important to determine how altering nuclear actin level and/or structure within the GSCs affects the epigenetic landscape, transcriptional state, and ultimately GSC self-renewal and differentiation. Intriguingly, preliminary studies support the model that nuclear actin is required for GSC maintenance as germline knock down of Drosophila importin 9 (ranbp9), the importin required for the nuclear localization of actin (Dopie et al., 2012), results in an altered structure of the germarium and female sterility (Kelpsch, Jaime, and Tootle, unpublished observations).
Nuclear actin and transcription
The best characterized function of nuclear actin across all systems is its role in mediating transcription. Indeed, actin binds to and regulates all three RNA polymerases (RNAP) (Smith et al., 1979; Fomproix and Percipalle, 2004; Hofmann et al., 2004; Hu et al., 2004; Philimonenko et al., 2004). Nuclear actin promotes transcription as it was purified as an activator of transcription (Egly et al., 1984), and microinjection of antibodies to actin and actin binding proteins inhibits transcription (Scheer et al., 1984). More recent studies have shown that actin is required for RNAPI activity (Fomproix and Percipalle, 2004; Philimonenko et al., 2004; Almuzzaini et al., 2016) and transcriptional initiation and elongation by RNAPII (Hofmann et al., 2004). This actin may be polymeric as mutations in actin and drugs that prevent actin polymerization block in vitro transcription by RNAPI (Ye et al., 2008), and nuclear myosin I is a well-established regulator of transcription (Hofmann et al., 2004; Philimonenko et al., 2004; Ye et al., 2008; Philimonenko et al., 2010). However, monomeric actin may also contribute to transcription, as monomers mediate chromatin remodeling complex recruitment and formation (reviewed in (Grosse and Vartiainen, 2013), and sequestering monomeric nuclear actin by inducing nuclear actin rod formation inhibits transcription (Serebryannyy et al., 2016b). Thus, it remains unclear what form or forms of nuclear actin contribute to transcription.
Our findings suggest that during Drosophila oogenesis multiple pools of polymeric actin may regulate RNAPII transcription (Table 1). Indeed, polymeric C4 nuclear actin overlaps with the DNA in the GSCs and early cystoblasts. This finding leads us to speculate that this polymeric C4 nuclear actin mediates RNAPII transcription to drive the transcriptional program necessary for maintaining an undifferentiated state. Additionally, polymeric AC15 nuclear actin localizes to the chromatin within the nurse cells and follicle cells from mid-to-late oogenesis. During this period of development, the nurse cells massively upregulate transcription to supply the oocyte with mRNA, protein, and organelles necessary for it to complete embryogenesis (Spradling, 1993). The follicle cells undergo gene amplification and express high levels of genes encoding the eggshell (Claycomb and Orr-Weaver, 2005). Thus, we hypothesize that the chromatin localized AC15 nuclear actin mediates the upregulation of transcription in these cells. These hypotheses can be explored by defining the transcriptional changes occurring when nuclear actin levels or dynamics (i.e. actin rod formation) are perturbed.
Nuclear actin also regulates specific transcription factors. For example, myocardin-related transcription factor A (MRTF-A; also referred to as MAL)/serum response factor (SRF) localization and activity is negatively regulated by G-actin in the cytoplasm and the nucleus (Vartiainen et al., 2007). MAL/SRF transcription plays important roles in cell migration, including during cancer metastasis (Medjkane et al., 2009) and Drosophila border cell migration (Somogyi and Rorth, 2004). Interestingly, during early border cell migration, AC15 nuclear actin is observed in all cells within the cluster, both border and polar cells. But later in migration, and when the cluster has reached the oocyte, only one to two cells exhibit AC15 nuclear actin; we presume these are the polar cells. These findings lead us to hypothesize that AC15 nuclear actin may regulate MAL/SRF activity to control the collective and invasive migration of the border cells (Table 1). As these cells are a good model for cancer metastasis (Montell, 2003), examination of the prevalence and level of nuclear actin in the different stages of cancer is warranted. Additionally, we find that AC15 nuclear actin levels are higher in the migrating centripetal follicle cells compared to the neighboring main body follicle cells. Thus, nuclear actin may play a more general role in mediating cell migration (Table 1). Indeed, the level of nuclear actin must be tightly controlled to mediate the transcriptional program necessary for cell motility in keratinocytes (Sharili et al., 2016).
During Drosophila oogenesis, nuclear actin may also regulate RNAPI- and/or RNAPIII- dependent transcription (Table 1). The primary site of RNAPI and RNAPIII activity is the nucleolus, where they mediate the transcription of ribosomal genes. G-actin, recognized by DNase I, is observed in the nucleolus of every cell throughout follicle development. This finding suggests monomeric actin may have a general role in nucleolar function, such as ribosomal gene expression (Table 1). Some C4 nuclear actin also localizes to the nucleolus. Specifically, every GSC, early cystoblast, and FSC has nucleolar C4 nuclear actin. Interestingly, this is the only C4 nuclear actin observed in the FSCs. This finding suggests that in undifferentiated cells, including stem cells, the protein production needs are higher (Table 1). Indeed, the mouse β‐actin knockout studies implicate nuclear actin’s role in RNAPI activity and ribosomal RNA production as a key factor in cell growth and proliferation (Almuzzaini et al., 2015), properties that are particularly important for the rapidly dividing Drosophila GSCs and FSCs. C4 nuclear actin is also observed in the nucleoli of a subset of mitotic follicle cells and the nurse cells during S3–10. We speculate that this nuclear actin might reflect that the cells are in a period where higher RNAPI and RNAPIII activity is needed (Table 1). While AC15 nuclear actin is primarily on the chromatin in the nurse cells, small puncta are observed within the areas devoid of DNA that, based on our prior work (Groen et al., 2015), are likely in the nucleolus. Interestingly, these nucleolar AC15 puncta are most frequently observed during S9–10B. During this period of development, the nurse cells are producing vast quantities of ribosomes, and proteins to be transported into the oocyte. Additionally, the puncta strikingly resemble what is seen for nuclear myosin I, which is required for RNAPI transcription (Fomproix and Percipalle, 2004). Thus, we hypothesize that polymeric AC15 nuclear actin interacts with a nuclear myosin to drive RNAPI transcription in the nurse cells during this critical period of development (Table 1). In the future, it will be important to define the specific roles of the different nuclear actin pools in RNAPI and RNAPIII activity.
Nuclear actin and the nucleolus
Nuclear actin may play other roles in the nucleolus. Actin has been observed in the nucleolus in numerous species, including plants (Cruz and Moreno Diaz de la Espina, 2009), slime mold (Jockusch et al., 1971), Drosophila (this study), and human cells (Belin et al., 2013). Additionally, numerous actin binding proteins are also found in the nucleolus (Hubert et al., 2008; Deng et al., 2012; Kitamura et al., 2015). While it is possible that nuclear actin and its regulators are simply sequestered in the nucleolus until they are needed for their nuclear functions, in a similar manner to cell cycle factors (Visintin and Amon, 2000; Boisvert et al., 2007), it is likely that these proteins also have roles in this nuclear body. Indeed, one of these proteins, ARP6, mediates both ribosomal gene expression and repression, depending upon the cellular environment (Kitamura et al., 2015). This finding leads us to speculate that the dynamic localization of C4 and AC15 nuclear actin to the nucleolus during Drosophila oogenesis may reflect periods of inhibiting and driving ribosome biogenesis. ARP6 also regulates the structure of the nucleolus, as knockdown in HeLA cells results in striking morphological changes in the nucleolus (Kitamura et al., 2015). We find this particularly intriguing, as the actin bundling protein Fascin localizes to the nucleus, regulates nucleolar structure (Groen et al., 2015), and positively influences C4 nuclear actin (Kelpsch et al., 2016). In Drosophila, Fascin localization and function are regulated by lipid signals termed prostaglandins (Groen et al., 2012; Groen et al., 2015). Together these data lead us to hypothesize that prostaglandins tightly control the levels of nuclear Fascin to modulate nuclear actin to ultimately regulate nucleolar structure. Supporting this, preliminary studies indicate that loss of prostaglandin synthesis, which causes an aberrant nucleolar structure (Groen et al., 2015), increases C4 nuclear actin within the nurse cells (Wineland, Kelpsch, and Tootle; unpublished observation).
The potential connection between Fascin, prostaglandins, nuclear actin, and nucleolar structure has interesting implications in the context of cancer, as the nucleolus is a new target for anticancer therapies (reviewed in (Ruggero, 2012; Hein et al., 2013; Quin et al., 2014)). Indeed, pathologists diagnose cancer by alterations in nucleolar morphology (reviewed in (Ruggero, 2012; Hein et al., 2013; Quin et al., 2014)). The changes in the number and structure of nucleoli may affect cell proliferation, differentiation, senescence, apoptosis, and coordination of response to stress. Additionally, the severity the nucleolar morphological changes is used as a marker of cancer aggressiveness (reviewed in (Ruggero, 2012; Hein et al., 2013; Quin et al., 2014)). Notably, increased Fascin expression (Hashimoto et al., 2004; Yoder et al., 2005; Lee et al., 2007; Okada et al., 2007; Li et al., 2008; Chan et al., 2010) and prostaglandin production (Rolland et al., 1980; Chen et al., 2001; Khuri et al., 2001; Denkert et al., 2003) contribute to tumorigenesis and metastasis, and are associated with poor patient prognosis. Finally, recent studies indicate cancer cells have misregulated and higher nuclear actin levels than the normal epithelium (Fiore et al., 2017). Additionally, many actin binding proteins exhibit increased nuclear localization during tumorigenesis and progression (Yang and Lin, 2018). However, the nucleolar versus other nuclear function of actin in cancer development and progression remain to be elucidated.
Nuclear actin and mitosis
Nuclear actin also plays roles in the cell cycle and chromosome segregation. While studies have implicated nuclear actin in mitosis (Jockusch et al., 1971), and even observed it localizing in the vicinity of the mitotic spindle (Woolner et al., 2008; Hubert et al., 2011), clear evidence of its presence and function have remained elusive. Here we find that during mitosis in the follicle cells, C4 nuclear actin is dynamic, forming a double bar around the forming metaphase plate, localizing to the spindle during metaphase, rearranging to ultimately be restricted to the area between the segregating chromosomes in anaphase, and this structure elongating and thinning as the segregation continues in telophase (Table 1). Notably, DNase I is diffuse throughout the extrachromosomal nucleoplasm, indicating that the C4 nuclear actin is likely polymeric. Interestingly, the Drosophila protein EAST is known to bind to nuclear actin (Wasser and Chia, 2000) and exhibits a similar, dynamic localization during mitosis in the embryo (Wasser and Chia, 2000). As EAST is highly expressed during oogenesis, it is tempting to speculate it may regulate nuclear actin localization or function during follicle cell mitosis (Brown et al., 2014). Further studies are need to determine the role(s) of nuclear actin in cell cycle progression and chromosome segregation.
While actin has not been previously shown to be on the spindle or regulate chromosome segregation in mitosis, it has been widely implicated in these processes during meiosis. Indeed, over 30 years ago, actin was observed on the meiotic spindles in plants (Forer and Jackson, 1979; Forer et al., 1979). Subsequently, it was observed on the meiotic spindles of insects (Silverman-Gavrila and Forer, 2000; Fabian and Forer, 2007) and mice (Bogolyubova and Ginzburg, 2013). A recent study extended these findings from observational to functional, showing that in mammalian oocytes, including humans, spindle actin is required to align and segregate the chromosomes (Mogessie and Schuh, 2017). These studies, in conjunction with our findings, lead us to hypothesize that nuclear actin similarly regulates chromosome dynamics during mitosis (Table 1).
Nuclear actin and nuclear structure
Although we did not examine whether nuclear actin regulates meiosis, our data suggests that nuclear actin may play a structural role in the developing Drosophila oocyte. We find that high levels of C4 nuclear actin are present in the nucleoplasm of the oocyte (Table 1). Given that this nuclear actin does not overlap with DNase I staining, we hypothesize this is polymeric actin. Supporting that polymeric or filamentous actin is important for nuclear structure within the oocyte, numerous studies have shown the presence of a filamentous nuclear actin network within the germinal vesicles of organisms with large oocytes. This network is thought to mediate nuclear organization, including chromosome and nuclear body dispersal (Clark and Merriam, 1977; Parfenov et al., 1995; Gonsior et al., 1999; Kiseleva et al., 2004) (Maslova and Krasikova, 2012). For example, Xenopus oocytes accumulate large amounts of nuclear actin because they do not express the specific export factor, Exportin 6 (Stuven et al., 2003), that translocates actin from the nucleus. Ectopic expression of Exportin 6 results in the loss of the intranuclear F-actin scaffold and a fragile nucleus (Bohnsack et al., 2006). This nuclear F-actin scaffold forms a weak viscoelastic network that helps to stabilize the intranuclear localization of the chromatin and nuclear bodies against gravitational forces that affect large cells (Feric and Brangwynne, 2013; Feric et al., 2015). Indeed, if the nuclear actin network is disrupted, the nuclear bodies sediment and fuse (Feric and Brangwynne, 2013). These data lead us to speculate that polymeric C4 nuclear actin within the oocyte nucleus plays a similar role (Table 1).
In addition to C4 nuclear actin, AC15 nuclear actin is also found in the oocyte. During S3–8, AC15 nuclear actin is seen as puncta that are enriched around the chromosomes. While during S9–11, filamentous structures are observed surrounding the DNA, and in S12–13, AC15 nuclear actin forms a more diffuse cage around the chromosomes. Given the association of AC15 nuclear actin with the chromatin in the other cells during Drosophila oogenesis, the well-established role of nuclear actin in transcription (reviewed in (Venit et al., 2018), and the finding that inducing stable nuclear actin filaments blocks transcription (Serebryannyy et al., 2016b), we hypothesize that in the oocyte, AC15 nuclear actin is sequestered into filaments to block transcription (Table 1). These filaments can then be depolymerized during embryogenesis to allow for transcription to resume. If this hypothesis is correct, then altering nuclear actin level or dynamics will have severe effects on embryonic development. Supporting this idea, mutations in the gene encoding the importin (Importin 9) responsible for taking actin into the nucleus results in lethality in Drosophila (Kelpsch, Jaime, and Tootle, unpublished observation) and mice (Blake et al., 2017). Future studies are needed to determine the cause of the lethality.
Conclusions
This study, in conjunction with the last 50 years of research on nuclear actin, strongly supports the ideas that the prevalence of nuclear actin is widespread, and actin within the nucleus likely has a variety of functions that are impacted by its structure and binding partners. Our data leads us to speculate that nuclear actin regulates transcription, nucleolar structure and function, cell cycle, and nuclear structure (Table 1). Such activities are important for regulating cell fate, growth, proliferation, and function. Thus, nuclear actin is predicted to have widespread roles in mediating development, and its misregulation likely contributes to diseases. Future studies are needed to determine nuclear actin prevalence and structure across tissues and organisms. Additional mechanistic studies are required to elucidate the activities of the different forms or pools of nuclear actin.
Acknowledgements
We thank the Westside Fly Group and Dunnwald Lab for helpful discussions, and members of the Tootle Lab for helpful discussions and careful review of the manuscript. Information Technology Services – Research Services, provided data storage support. This project is supported by National Institutes of Health R01GM116885. D.J.K. is partially supported by NINDS T32NS045549.
Grant sponsors: NIH/NIGMS, NIH/NINDS; Grant numbers: R01GM116885, T32NS045549
Literature cited
- Ables ET, and Drummond-Barbosa D (2010). The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell 7, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff M, Magalhaes P, and Ram S (2004). Image processing with ImageJ. Biophotonics Int. 11, 36–42. [Google Scholar]
- Almuzzaini B, Sarshad AA, Farrants AK, and Percipalle P (2015). Nuclear myosin 1 contributes to a chromatin landscape compatible with RNA polymerase II transcription activation. BMC biology 13, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almuzzaini B, Sarshad AA, Rahmanto AS, Hansson ML, Von Euler A, Sangfelt O, Visa N, Farrants AK, and Percipalle P (2016). In beta-actin knockouts, epigenetic reprogramming and rDNA transcription inactivation lead to growth and proliferation defects. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 30, 2860–2873. [DOI] [PubMed] [Google Scholar]
- Andrin C, McDonald D, Attwood KM, Rodrigue A, Ghosh S, Mirzayans R, Masson JY, Dellaire G, and Hendzel MJ (2012). A requirement for polymerized actin in DNA double-strand break repair. Nucleus 3, 384–395. [DOI] [PubMed] [Google Scholar]
- Asumda FZ, and Chase PB (2012). Nuclear cardiac troponin and tropomyosin are expressed early in cardiac differentiation of rat mesenchymal stem cells. Differentiation 83, 106–115. [DOI] [PubMed] [Google Scholar]
- Baarlink C, Plessner M, Sherrard A, Morita K, Misu S, Virant D, Kleinschnitz EM, Harniman R, Alibhai D, Baumeister S, Miyamoto K, Endesfelder U, Kaidi A, and Grosse R (2017). A transient pool of nuclear F-actin at mitotic exit controls chromatin organization. Nature cell biology 19, 1389–1399. [DOI] [PubMed] [Google Scholar]
- Belin BJ, Cimini BA, Blackburn EH, and Mullins RD (2013). Visualization of actin filaments and monomers in somatic cell nuclei. Molecular biology of the cell 24, 982–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin BJ, Lee T, and Mullins RD (2015). DNA damage induces nuclear actin filament assembly by Formin −2 and Spire-(1/2) that promotes efficient DNA repair. [corrected]. eLife 4, e07735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake JA, Eppig JT, Kadin JA, Richardson JE, Smith CL, Bult CJ, and the Mouse Genome Database G (2017). Mouse Genome Database (MGD)-2017: community knowledge resource for the laboratory mouse. Nucleic acids research 45, D723–D729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogolyubova N, and Ginzburg A (2013). Actin Colocalization with Metaphase Chromosomes of the Second Meiosis in Ovulated Mouse Oocytes. Developmental Biology Journal 2013, 6. [Google Scholar]
- Bohnsack MT, Stuven T, Kuhn C, Cordes VC, and Gorlich D (2006). A selective block of nuclear actin export stabilizes the giant nuclei of Xenopus oocytes. Nature cell biology 8, 257–263. [DOI] [PubMed] [Google Scholar]
- Boisvert FM, van Koningsbruggen S, Navascues J, and Lamond AI (2007). The multifunctional nucleolus. Nature reviews. Molecular cell biology 8, 574–585. [DOI] [PubMed] [Google Scholar]
- Brown JB, Boley N, Eisman R, May GE, Stoiber MH, Duff MO, Booth BW, Wen J, Park S, Suzuki AM, Wan KH, Yu C, Zhang D, Carlson JW, Cherbas L, Eads BD, Miller D, Mockaitis K, Roberts J, Davis CA, Frise E, Hammonds AS, Olson S, Shenker S, Sturgill D, Samsonova AA, Weiszmann R, Robinson G, Hernandez J, Andrews J, Bickel PJ, Carninci P, Cherbas P, Gingeras TR, Hoskins RA, Kaufman TC, Lai EC, Oliver B, Perrimon N, Graveley BR, and Celniker SE (2014). Diversity and dynamics of the Drosophila transcriptome. Nature 512, 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Jankova L, Fung CL, Clarke C, Robertson G, Chapuis PH, Bokey L, Lin BP, Dent OF, and Clarke S (2010). Fascin expression predicts survival after potentially curative resection of node-positive colon cancer. The American journal of surgical pathology 34, 656–666. [DOI] [PubMed] [Google Scholar]
- Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, and Yang WK (2001). Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. International journal of cancer. Journal international du cancer 91, 894–899. [DOI] [PubMed] [Google Scholar]
- Clark TG, and Merriam RW (1977). Diffusible and bound actin nuclei of Xenopus laevis oocytes. Cell 12, 883–891. [DOI] [PubMed] [Google Scholar]
- Claycomb JM, and Orr-Weaver TL (2005). Developmental gene amplification: insights into DNA replication and gene expression. Trends in genetics : TIG 21, 149–162. [DOI] [PubMed] [Google Scholar]
- Cruz JR, and Moreno Diaz de la Espina S (2009). Subnuclear compartmentalization and function of actin and nuclear myosin I in plants. Chromosoma 118, 193–207. [DOI] [PubMed] [Google Scholar]
- Deady LD, Shen W, Mosure SA, Spradling AC, and Sun J (2015). Matrix metalloproteinase 2 is required for ovulation and corpus luteum formation in Drosophila. PLoS Genet 11, e1004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Lopez-Camacho C, Tang JY, Mendoza-Villanueva D, Maya-Mendoza A, Jackson DA, and Shore P (2012). Cytoskeletal protein filamin A is a nucleolar protein that suppresses ribosomal RNA gene transcription. Proc Natl Acad Sci U S A 109, 1524–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Winzer KJ, Muller BM, Weichert W, Pest S, Kobel M, Kristiansen G, Reles A, Siegert A, Guski H, and Hauptmann S (2003). Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer 97, 2978–2987. [DOI] [PubMed] [Google Scholar]
- Dobens LL, and Raftery LA (2000). Integration of epithelial patterning and morphogenesis in Drosophila ovarian follicle cells. Dev Dyn 218, 80–93. [DOI] [PubMed] [Google Scholar]
- Domazetovska A, Ilkovski B, Cooper ST, Ghoddusi M, Hardeman EC, Minamide LS, Gunning PW, Bamburg JR, and North KN (2007a). Mechanisms underlying intranuclear rod formation. Brain : a journal of neurology 130, 3275–3284. [DOI] [PubMed] [Google Scholar]
- Domazetovska A, Ilkovski B, Kumar V, Valova VA, Vandebrouck A, Hutchinson DO, Robinson PJ, Cooper ST, Sparrow JC, Peckham M, and North KN (2007b). Intranuclear rod myopathy: molecular pathogenesis and mechanisms of weakness. Annals of neurology 62, 597–608. [DOI] [PubMed] [Google Scholar]
- Dopie J, Skarp KP, Rajakyla EK, Tanhuanpaa K, and Vartiainen MK (2012). Active maintenance of nuclear actin by importin 9 supports transcription. Proc Natl Acad Sci U S A 109, E544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egly JM, Miyamoto NG, Moncollin V, and Chambon P (1984). Is actin a transcription initiation factor for RNA polymerase B? The EMBO journal 3, 2363–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian L, and Forer A (2007). Possible roles of actin and myosin during anaphase chromosome movements in locust spermatocytes. Protoplasma 231, 201–213. [DOI] [PubMed] [Google Scholar]
- Falahati H, and Wieschaus E (2017). Independent active and thermodynamic processes govern the nucleolus assembly in vivo. Proc Natl Acad Sci U S A 114, 1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M, and Brangwynne CP (2013). A nuclear F-actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. Nature cell biology 15, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M, Broedersz CP, and Brangwynne CP (2015). Soft viscoelastic properties of nuclear actin age oocytes due to gravitational creep. Sci Rep 5, 16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore A, Spencer VA, Mori H, Carvalho HF, Bissell MJ, and Bruni-Cardoso A (2017). Laminin-111 and the Level of Nuclear Actin Regulate Epithelial Quiescence via Exportin-6. Cell Rep 19, 2102–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora P, McCarthy A, Upadhyay M, and Rangan P (2017). Role of Chromatin Modifications in Drosophila Germline Stem Cell Differentiation In: Signaling-Mediated Control of Cell Division : From Oogenesis to Oocyte-to-Embryo Development, ed. Arur S, Cham: Springer International Publishing, 1–30. [DOI] [PubMed] [Google Scholar]
- Fomproix N, and Percipalle P (2004). An actin-myosin complex on actively transcribing genes. Exp Cell Res 294, 140–148. [DOI] [PubMed] [Google Scholar]
- Forer A, and Jackson WT (1979). Actin in spindles of Haemanthus katherinae endosperm. I. General results using various glycerination methods. J Cell Sci 37, 323–347. [DOI] [PubMed] [Google Scholar]
- Forer A, Jackson WT, and Engberg A (1979). Actin in spindles of Haemanthus katherinae endosperm. II. Distribution of actin in chromosomal spindle fibres, determined by analysis of serial sections. J Cell Sci 37, 349–371. [DOI] [PubMed] [Google Scholar]
- Gedge LJ, Morrison EE, Blair GE, and Walker JH (2005). Nuclear actin is partially associated with Cajal bodies in human cells in culture and relocates to the nuclear periphery after infection of cells by adenovirus 5. Exp Cell Res 303, 229–239. [DOI] [PubMed] [Google Scholar]
- Gimona M, Vandekerckhove J, Goethals M, Herzog M, Lando Z, and Small JV (1994). Beta-actin specific monoclonal antibody. Cell Motil Cytoskeleton 27, 108–116. [DOI] [PubMed] [Google Scholar]
- Goebel HH, and Warlo IA (2001). Surplus protein myopathies. Neuromuscular disorders : NMD 11, 3–6. [DOI] [PubMed] [Google Scholar]
- Gonsior SM, Platz S, Buchmeier S, Scheer U, Jockusch BM, and Hinssen H (1999). Conformational difference between nuclear and cytoplasmic actin as detected by a monoclonal antibody. J Cell Sci 112 ( Pt 6), 797–809. [DOI] [PubMed] [Google Scholar]
- Goyal P, Behring A, Kumar A, and Siess W (2011). STK35L1 associates with nuclear actin and regulates cell cycle and migration of endothelial cells. PloS one 6, e16249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenklo S, Johansson T, Bertilson L, and Karlsson R (2004). Anti-actin antibodies generated against profilin:actin distinguish between non-filamentous and filamentous actin, and label cultured cells in a dotted pattern. Eur J Cell Biol 83, 413–423. [DOI] [PubMed] [Google Scholar]
- Groen CM, Jayo A, Parsons M, and Tootle TL (2015). Prostaglandins regulate nuclear localization of Fascin and its function in nucleolar architecture. Molecular biology of the cell 26, 1901–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen CM, Spracklen AJ, Fagan TN, and Tootle TL (2012). Drosophila Fascin is a novel downstream target of prostaglandin signaling during actin remodeling. Molecular biology of the cell 23, 4567–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse R, and Vartiainen MK (2013). To be or not to be assembled: progressing into nuclear actin filaments. Nature reviews. Molecular cell biology 14, 693–697. [DOI] [PubMed] [Google Scholar]
- Guo Z, and Wang Z (2009). The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development 136, 3627–3635. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Shimada Y, Kawamura J, Yamasaki S, and Imamura M (2004). The prognostic relevance of fascin expression in human gastric carcinoma. Oncology 67, 262–270. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Kobayashi S, and Nakato H (2009). Drosophila glypicans regulate the germline stem cell niche. J Cell Biol 187, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Xuan T, Xin T, An H, Wang J, Zhao G, and Li M (2014). Evidence for chromatin-remodeling complex PBAP-controlled maintenance of the Drosophila ovarian germline stem cells. PloS one 9, e103473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein N, Hannan KM, George AJ, Sanij E, and Hannan RD (2013). The nucleolus: an emerging target for cancer therapy. Trends in molecular medicine 19, 643–654. [DOI] [PubMed] [Google Scholar]
- Hitchcock SE (1980). Actin deoxyroboncuclease I interaction. Depolymerization and nucleotide exchange. J Biol Chem 255, 5668–5673. [PubMed] [Google Scholar]
- Hofmann WA, Stojiljkovic L, Fuchsova B, Vargas GM, Mavrommatis E, Philimonenko V, Kysela K, Goodrich JA, Lessard JL, Hope TJ, Hozak P, and de Lanerolle P (2004). Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nature cell biology 6, 1094–1101. [DOI] [PubMed] [Google Scholar]
- Holaska JM, Kowalski AK, and Wilson KL (2004). Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS biology 2, E231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Wu S, and Hernandez N (2004). A role for beta-actin in RNA polymerase III transcription. Genes & development 18, 3010–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert T, Van Impe K, Vandekerckhove J, and Gettemans J (2008). The F-actin filament capping protein CapG is a bona fide nucleolar protein. Biochem Biophys Res Commun 377, 699–704. [DOI] [PubMed] [Google Scholar]
- Hubert T, Vandekerckhove J, and Gettemans J (2011). Unconventional actin conformations localize on intermediate filaments in mitosis. Biochem Biophys Res Commun 406, 101–106. [DOI] [PubMed] [Google Scholar]
- Jockusch BM, Brown DF, and Rusch HP (1971). Synthesis and some properties of an actin-like nuclear protein in the slime mold Physarum polycephalum. J Bacteriol 108, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch BM, Schoenenberger CA, Stetefeld J, and Aebi U (2006). Tracking down the different forms of nuclear actin. Trends Cell Biol 16, 391–396. [DOI] [PubMed] [Google Scholar]
- Kelpsch DJ, Groen CM, Fagan TN, Sudhir S, and Tootle TL (2016). Fascin regulates nuclear actin during Drosophila oogenesis. Molecular biology of the cell 27, 2965–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, Feng L, Hong WK, and Xu XC (2001). Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 7, 861–867. [PubMed] [Google Scholar]
- Kiseleva E, Drummond SP, Goldberg MW, Rutherford SA, Allen TD, and Wilson KL (2004). Actin- and protein-4.1-containing filaments link nuclear pore complexes to subnuclear organelles in Xenopus oocyte nuclei. J Cell Sci 117, 2481–2490. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Matsumori H, Kalendova A, Hozak P, Goldberg IG, Nakao M, Saitoh N, and Harata M (2015). The actin family protein ARP6 contributes to the structure and the function of the nucleolus. Biochem Biophys Res Commun 464, 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss SW, Chen C, Penman S, and Heald R (2003). Nuclear actin and protein 4.1: essential interactions during nuclear assembly in vitro. Proc Natl Acad Sci U S A 100, 10752–10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane NJ (1969). Intranuclear fibrillar bodies in actinomycin D-treated oocytes. J Cell Biol 40, 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Poon RT, Man K, Guan XY, Ma S, Liu XB, Myers JN, and Yuen AP (2007). Fascin over-expression is associated with aggressiveness of oral squamous cell carcinoma. Cancer letters 254, 308–315. [DOI] [PubMed] [Google Scholar]
- Lei L, and Spradling AC (2016). Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science 352, 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenart P, Bacher CP, Daigle N, Hand AR, Eils R, Terasaki M, and Ellenberg J (2005). A contractile nuclear actin network drives chromosome congression in oocytes. Nature 436, 812–818. [DOI] [PubMed] [Google Scholar]
- Lessard JL (1988). Two monoclonal antibodies to actin: one muscle selective and one generally reactive. Cell Motil Cytoskeleton 10, 349–362. [DOI] [PubMed] [Google Scholar]
- Li X, Zheng H, Hara T, Takahashi H, Masuda S, Wang Z, Yang X, Guan Y, and Takano Y (2008). Aberrant expression of cortactin and fascin are effective markers for pathogenesis, invasion, metastasis and prognosis of gastric carcinomas. International journal of oncology 33, 69–79. [PubMed] [Google Scholar]
- Lim MK, Kawamura T, Ohsawa Y, Ohtsubo M, Asakawa S, Takayanagi A, and Shimizu N (2007). Parkin interacts with LIM Kinase 1 and reduces its cofilin-phosphorylation activity via ubiquitination. Exp Cell Res 313, 2858–2874. [DOI] [PubMed] [Google Scholar]
- Maloney MT, and Bamburg JR (2007). Cofilin-mediated neurodegeneration in Alzheimer’s disease and other amyloidopathies. Molecular neurobiology 35, 21–44. [DOI] [PubMed] [Google Scholar]
- Maloney MT, Minamide LS, Kinley AW, Boyle JA, and Bamburg JR (2005). Beta-secretase-cleaved amyloid precursor protein accumulates at actin inclusions induced in neurons by stress or amyloid beta: a feedforward mechanism for Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 25, 11313–11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslova A, and Krasikova A (2012). Nuclear actin depolymerization in transcriptionally active avian and amphibian oocytes leads to collapse of intranuclear structures. Nucleus 3, 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Carrero G, Andrin C, de Vries G, and Hendzel MJ (2006). Nucleoplasmic beta-actin exists in a dynamic equilibrium between low-mobility polymeric species and rapidly diffusing populations. J Cell Biol 172, 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medjkane S, Perez-Sanchez C, Gaggioli C, Sahai E, and Treisman R (2009). Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nature cell biology 11, 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamide LS, Striegl AM, Boyle JA, Meberg PJ, and Bamburg JR (2000). Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nature cell biology 2, 628–636. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Pasque V, Jullien J, and Gurdon JB (2011). Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes. Genes & development 25, 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogessie B, and Schuh M (2017). Actin protects mammalian eggs against chromosome segregation errors. Science 357. [DOI] [PubMed] [Google Scholar]
- Montell DJ (2003). Border-cell migration: the race is on. Nature reviews. Molecular cell biology 4, 13–24. [DOI] [PubMed] [Google Scholar]
- Munsie L, Caron N, Atwal RS, Marsden I, Wild EJ, Bamburg JR, Tabrizi SJ, and Truant R (2011). Mutant huntingtin causes defective actin remodeling during stress: defining a new role for transglutaminase 2 in neurodegenerative disease. Human molecular genetics 20, 1937–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsie LN, Desmond CR, and Truant R (2012). Cofilin nuclear-cytoplasmic shuttling affects cofilin-actin rod formation during stress. J Cell Sci 125, 3977–3988. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Kawamura H, and Tanaka Y (1964). [Actin and Myosin-Like Proteins in the Calf Thymus Cell Nucleus]. J Biochem 56, 6–15. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Kawamura H, and Yamamoto T (1963). [Extraction of a Protein Resembling Actin from the Cell Nucleus of the Calf Thymus]. J Biochem 54, 298–300. [DOI] [PubMed] [Google Scholar]
- Okada K, Shimura T, Asakawa K, Hashimoto S, Mochida Y, Suehiro T, and Kuwano H (2007). Fascin expression is correlated with tumor progression of extrahepatic bile duct cancer. Hepato-gastroenterology 54, 17–21. [PubMed] [Google Scholar]
- Parfenov VN, Davis DS, Pochukalina GN, Sample CE, Bugaeva EA, and Murti KG (1995). Nuclear actin filaments and their topological changes in frog oocytes. Exp Cell Res 217, 385–394. [DOI] [PubMed] [Google Scholar]
- Pearson JR, Zurita F, Tomas-Gallardo L, Diaz-Torres A, Diaz de la Loza Mdel C, Franze K, Martin-Bermudo MD, and Gonzalez-Reyes A (2016). ECM-Regulator timp Is Required for Stem Cell Niche Organization and Cyst Production in the Drosophila Ovary. PLoS Genet 12, e1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philimonenko VV, Janacek J, Harata M, and Hozak P (2010). Transcription-dependent rearrangements of actin and nuclear myosin I in the nucleolus. Histochemistry and cell biology 134, 243–249. [DOI] [PubMed] [Google Scholar]
- Philimonenko VV, Zhao J, Iben S, Dingova H, Kysela K, Kahle M, Zentgraf H, Hofmann WA, de Lanerolle P, Hozak P, and Grummt I (2004). Nuclear actin and myosin I are required for RNA polymerase I transcription. Nature cell biology 6, 1165–1172. [DOI] [PubMed] [Google Scholar]
- Quin JE, Devlin JR, Cameron D, Hannan KM, Pearson RB, and Hannan RD (2014). Targeting the nucleolus for cancer intervention. Biochimica et biophysica acta 1842, 802–816. [DOI] [PubMed] [Google Scholar]
- Rolland PH, Martin PM, Jacquemier J, Rolland AM, and Toga M (1980). Prostaglandin in human breast cancer: Evidence suggesting that an elevated prostaglandin production is a marker of high metastatic potential for neoplastic cells. Journal of the National Cancer Institute 64, 1061–1070. [PubMed] [Google Scholar]
- Ruggero D (2012). Revisiting the nucleolus: from marker to dynamic integrator of cancer signaling. Science signaling 5, pe38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasseville AM, and Langelier Y (1998). In vitro interaction of the carboxy-terminal domain of lamin A with actin. FEBS Lett 425, 485–489. [DOI] [PubMed] [Google Scholar]
- Scheer U, Hinssen H, Franke WW, and Jockusch BM (1984). Microinjection of actin-binding proteins and actin antibodies demonstrates involvement of nuclear actin in transcription of lampbrush chromosomes. Cell 39, 111–122. [DOI] [PubMed] [Google Scholar]
- Schoenenberger CA, Buchmeier S, Boerries M, Sutterlin R, Aebi U, and Jockusch BM (2005). Conformation-specific antibodies reveal distinct actin structures in the nucleus and the cytoplasm. Journal of structural biology 152, 157–168. [DOI] [PubMed] [Google Scholar]
- Serebryannyy LA, Cruz CM, and de Lanerolle P (2016a). A Role for Nuclear Actin in HDAC 1 and 2 Regulation. Sci Rep 6, 28460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebryannyy LA, Parilla M, Annibale P, Cruz CM, Laster K, Gratton E, Kudryashov D, Kosak ST, Gottardi CJ, and de Lanerolle P (2016b). Persistent nuclear actin filaments inhibit transcription by RNA polymerase II. J Cell Sci 129, 3412–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebryannyy LA, Yemelyanov A, Gottardi CJ, and de Lanerolle P (2017). Nuclear alpha-catenin mediates the DNA damage response via beta-catenin and nuclear actin. J Cell Sci 130, 1717–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebryannyy LA, Yuen M, Parilla M, Cooper ST, and de Lanerolle P (2016c). The Effects of Disease Models of Nuclear Actin Polymerization on the Nucleus. Front Physiol 7, 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharili AS, Kenny FN, Vartiainen MK, and Connelly JT (2016). Nuclear actin modulates cell motility via transcriptional regulation of adhesive and cytoskeletal genes. Sci Rep 6, 33893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman-Gavrila RV, and Forer A (2000). Evidence that actin and myosin are involved in the poleward flux of tubulin in metaphase kinetochore microtubules of crane-fly spermatocytes. J Cell Sci 113 ( Pt 4), 597–609. [DOI] [PubMed] [Google Scholar]
- Smith SS, Kelly KH, and Jockusch BM (1979). Actin co-purifies with RNA polymerase II. Biochem Biophys Res Commun 86, 161–166. [DOI] [PubMed] [Google Scholar]
- Somogyi K, and Rorth P (2004). Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Developmental cell 7, 85–93. [DOI] [PubMed] [Google Scholar]
- Spencer VA, Costes S, Inman JL, Xu R, Chen J, Hendzel MJ, and Bissell MJ (2011). Depletion of nuclear actin is a key mediator of quiescence in epithelial cells. J Cell Sci 124, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Fuller MT, Braun RE, and Yoshida S (2011). Germline stem cells. Cold Spring Harbor perspectives in biology 3, a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC (1993). Developmental genetics of oogenesis In: The development of Drosophila melanogaster, ed. Martinez-Arias B: Cold Spring Harbor Laboratory Press, 1–70. [Google Scholar]
- Stuven T, Hartmann E, and Gorlich D (2003). Exportin 6: a novel nuclear export receptor that is specific for profilin.actin complexes. The EMBO journal 22, 5928–5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondeleir D, Lambrechts A, Muller M, Jonckheere V, Doll T, Vandamme D, Bakkali K, Waterschoot D, Lemaistre M, Debeir O, Decaestecker C, Hinz B, Staes A, Timmerman E, Colaert N, Gevaert K, Vandekerckhove J, and Ampe C (2012). Cells lacking beta-actin are genetically reprogrammed and maintain conditional migratory capacity. Mol Cell Proteomics 11, 255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootle TL, Williams D, Hubb A, Frederick R, and Spradling A (2011). Drosophila eggshell production: identification of new genes and coordination by Pxt. PloS one 6, e19943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen MK, Guettler S, Larijani B, and Treisman R (2007). Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752. [DOI] [PubMed] [Google Scholar]
- Venit T, Xie X, and Percipalle P (2018). Actin in the Cell Nucleus In: Nuclear Architecture and Dynamics, vol. 2, eds. Lavelle C and Victor J-M, United Kingdom: Elsevier, 345–367. [Google Scholar]
- Visintin R, and Amon A (2000). The nucleolus: the magician’s hat for cell cycle tricks. Current opinion in cell biology 12, 752. [DOI] [PubMed] [Google Scholar]
- Wada A, Fukuda M, Mishima M, and Nishida E (1998). Nuclear export of actin: a novel mechanism regulating the subcellular localization of a major cytoskeletal protein. The EMBO journal 17, 1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Hariharan A, Bastianello G, Toyama Y, Shivashankar GV, Foiani M, and Sheetz MP (2017). DNA damage causes rapid accumulation of phosphoinositides for ATR signaling. Nature communications 8, 2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser M, and Chia W (2000). The EAST protein of drosophila controls an expandable nuclear endoskeleton. Nature cell biology 2, 268–275. [DOI] [PubMed] [Google Scholar]
- Woolner S, O’Brien LL, Wiese C, and Bement WM (2008). Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol 182, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tanwar PS, and Raftery LA (2008). Drosophila follicle cells: morphogenesis in an eggshell. Semin Cell Dev Biol 19, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Almuzzaini B, Drou N, Kremb S, Yousif A, Farrants AO, Gunsalus K, and Percipalle P (2018). beta-Actin-dependent global chromatin organization and gene expression programs control cellular identity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, fj201700753R. [DOI] [PubMed] [Google Scholar]
- Yan D, Wu Y, Feng Y, Lin SC, and Lin X (2009). The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Developmental cell 17, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, and Lin Y (2018). Functions of nuclear actin-binding proteins in human cancer. Oncol Lett 15, 2743–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Zhao J, Hoffmann-Rohrer U, and Grummt I (2008). Nuclear myosin I acts in concert with polymeric actin to drive RNA polymerase I transcription. Genes & development 22, 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BJ, Tso E, Skacel M, Pettay J, Tarr S, Budd T, Tubbs RR, Adams JC, and Hicks DG (2005). The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clinical cancer research : an official journal of the American Association for Cancer Research 11, 186–192. [PubMed] [Google Scholar]