Introduction
The Receptor for Advanced Glycation Endproducts (RAGE) is an immunoglobulin-type, transmembrane receptor that is expressed on numerous cell types in the Central Nervous System (CNS) and periphery, such as neurons, astrocytes, microglia, mononuclear phagocytes, epithelial, and endothelial cells (ECs). RAGE binds a discrete repertoire of ligands, including nonenzymatically glycated proteins and lipids also known as advanced glycation endproducts (AGEs), for which the receptor is named, in addition to multiple members of the S100/calgranulin family, oligomeric forms of Aβ, high mobility group box 1 (HMGB1), phosphatidylserine (PS), and lysophosphatidic acid [1–8]. Extensive evidence has implicated RAGE as a critical player in regulating inflammation, as well as oxidative and cellular stress in a variety of organ niches and disease settings, including the CNS during neurodegeneration [5, 6, 9–14].
This review will focus on the current state of knowledge regarding RAGE and neurodegeneration. Specifically, we will detail the effect of RAGE signal transduction on cellular stress, pinpoint clues into RAGE pathophysiology in the context(s) of increased RAGE ligand burden, discuss the systemic consequences of RAGE-driven inflammation in the CNS as a whole, and report on the increasing number of published genome wide association study (GWAS) findings that evoke strong indications for RAGE as a putative driver of cellular and systemic dysfunction during key neurodegenerative pathologies, most specifically Alzheimer’s disease (AD), Parkinson’s disease (PD), Amyotrophic Lateral Sclerosis (ALS), and Multiple Sclerosis (MS).
RAGE Signal Transduction
Our laboratory recently discovered that upon ligand engagement of the extracellular domains of RAGE, the RAGE cytoplasmic domain binds to its intracellular effector molecule, Diaphanous 1 (DIAPH1) [15, 16]. DIAPH1 has subsequently been shown to be required for signal transduction induced by RAGE ligand binding, including the activation of mitogen activated protein kinases (MAPK), Rho GTPases, and phosphatidylinositol 3-kinase (PI3K)/Akt signaling. RAGE-DIAPH1 signaling effects are dependent on many factors, including, but not limited to: cell-type, ligand form and ligand concentration, and the duration of signal induction (acute vs. chronic) [17–22]. The implications of activation of these signaling cascades are substantial, and predominantly pathological. The RAGE-DIAPH1 interaction drives the generation of reactive oxygen species (ROS), the induction of cellular migration, the upregulation of inflammatory cytokines, and subsequent downregulation of ATP binding cassette (ABC) cholesterol transporters, such as ABCA1 and ABCG1, thereby mediating intracellular lipid accumulation and consequent cellular dysfunction [9, 23–25].
Besides its role in RAGE-DIAPH1-mediated inflammation, DIAPH1 is a dynamic mediator of actin cytoskeleton stability and rearrangement, as well as regulation of transcription factors [15, 26–28]. It was recently reported that DIAPH1 was highly expressed in human gliomas; however, the specifics of DIAPH1 expression, including the cellular localization and the potential DIAPH1-mediated mechanisms of in vivo dysfunction in the rodent or human CNS have not been elucidated [29]. Beyond this report, very little is known about DIAPH1 expression pattern and functions in the CNS of normal or degenerating models of humans; there are no known SNPs in DIAPH1 that increase or decrease neurodegenerative disease risk. However, the impact of RAGE-DIAPH1 signal transduction in peripheral cells exhibits prominent overlap with the patterns of cellular dysfunction observed in neurodegeneration, including the increased production of ROS and pro-inflammatory cytokines and the downregulation of homeostatic molecules such as neurotrophins and cholesterol/lipid handlers. This signaling culminates in significant alterations in critical cellular functions, such as migration, phagocytosis, replication, and cell death, particularly in cells of myeloid and endothelial origin, but also in neurons [4, 12, 30–32].
Connecting the dots: potential RAGE mechanisms in Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disorder that impacts millions of people worldwide and is not curable. While the primary risk factor for AD is advanced age, recent insights from genomic technology implicate inflammatory lipid and cytokine signaling in microglia, the myeloid cells of the CNS, as a prominent correlate of disease. Specifically, human GWAS suggest a powerful link between inflammatory pathways, including complement, chemokines, and influential lipid and cholesterol molecules, such as Triggering Receptor Expressed on Myeloid Cells 2 (TREM2), ABCA7, Apolipoprotein E variant 4 (APOE4), and others with AD susceptibility [14, 20, 33–41]. Additional analyses within animal models have illuminated various molecules critical to the innate immune system as major contributors to increased or decreased rate of AD progression, such as Chemokine Receptor Type 2 (CCR2), Chemokine Receptor 1 (CX3CR1 or GPR1), complement components (C1q and C3), and Chemokine Ligand 8 (CXCL8) [35, 42–55].
The most prominent risk alleles and impairments were observed in humans and mice with loss-of-function mutations or deletions of the aforementioned chief lipid handling molecules. However, burgeoning data in humans and rodent models also indicate that systemic inflammation and transient infections in the periphery are sufficient to increase production of RAGE ligands, particularly AGEs and oligomeric Aβ. In contexts in which these ligands accumulate in the CNS, RAGE signaling is causally implicated in exacerbating ongoing neurodegenerative disease. Atop these multiple mechanisms of augmented RAGE ligand production in AD, there is also prominent downregulation of specific detoxification mechanisms, which inhibit production of pre-AGEs such as methylglyoxal (MG) [56]. Glyoxalase 1 (GLO1), the principal enzyme that detoxifies MG, mitigates AGE production and is upregulated in the early and mid-stages of AD in human subjects. However, in the late and progressive stages of AD dysfunction, depletion of the enzyme’s chief and essential cofactor, glutathione, reduces overall activity of the GLO1-AGE detoxifying system, thus facilitating increased AGE production and accumulation [56, 57]. Altogether, these findings underscore a potentially profound link between inflammation, both peripheral and central, which prompts the question: To what extent might anti-AGE/RAGE therapies provide protective measures for neurodegeneration and AD, given the prominence of cellular stress driven by increased RAGE ligand burden [58–60]?
Population-based studies have emerged suggesting links between RAGE, dementia, and AD. Genetic sequence variations in 20 genes associated with inflammatory signaling were recently probed for possible associations with dementia risk. From 1,462 Swedish dementia cases and 1,929 controls that were composed of twin and unrelated case-control samples, investigators identified a potential association of sequence variations near the gene encoding RAGE (AGER), to increased risk for dementia and AD, in two independent samples. Further, a recent structural analysis utilizing MRI technology revealed that atrophy of the right hippocampus substructure CA1 during AD progression was significantly correlated to the single nucleotide polymorphism (SNP) variant rs2070600 within AGER [61]. Notably, this variant has been previously associated with increased affinity to ligands and increased ligand-stimulated inflammation in cultured cells, in conjunction with decreased levels of circulating soluble RAGE (sRAGE) [62]. sRAGE is a short, soluble isoform of RAGE, and putative “decoy” receptor. Because it lacks the intracellular and cytoplasmic domains required for signaling, sRAGE is predicted to protect against inflammation and RAGE-dependent cellular stress by sequestering RAGE ligands and preventing their engagement of the full-length, transmembrane RAGE [63, 64]. Thus, in humans bearing this SNP, lower sRAGE concentrations may directly amplify ligand burden and availability for signal transduction through full-length RAGE. This increases cellular stress, impairs lipid and cholesterol handling for the cells, in addition to promoting increased ROS production, thereby forging a feed-forward, self-perpetuating loop of inflammatory cellular stress in ECs, myeloid cells, and others within the CNS niche, including astrocytes, neurons, and oligodendrocytes.
Many of the mechanistic studies of RAGE in AD-like mouse models have been conducted in animals that are globally devoid of Ager and animals with dominant negative-RAGE (DN-RAGE) targeted to myeloid cells, using the macrophage scavenger receptor. DN-RAGE is composed of the extracellular RAGE domains and the transmembrane domain; hence, although ligand binding to this construct is intact and it is tethered to the cell membrane, signaling is abrogated on account of deletion of the cytoplasmic domain. These DN-RAGE studies have indicated that RAGE signal abrogation confers a benefit for AD progression and suggest a role for RAGE in myeloid cells during AD [10, 12, 39, 65]. However, there are possible caveats to these studies, particularly since it is plausible that DN-RAGE may also act as a decoy receptor and “ligand sink”, much like sRAGE, and mice devoid of Ager or expressing DN-RAGE constitutively from birth may develop differently than a wild-type animal. Therefore, further investigation utilizing greater cell type- and temporal specificity would be key for definitively determining a role for RAGE in AD.
RAGE molecules expressed on ECs are also known to facilitate the transport of Aβ into and across the blood brain barrier (BBB) during AD, implicating RAGE in mediating the increased pools of ligand concentrations found during disease progression [9, 66]. Since AGE production is increased in oxidized environments and RAGE engagement drives ROS production, there are additional entry points into the aforementioned feed-forward loop in which RAGE ligand binding drives increased RAGE ligand abundance, increased RAGE-DIAPH1 signaling, and therefore increased ROS and AGEs. Together, this AGE-generating loop and the reduced expression of Low Density Lipoprotein Receptor-related Protein 1 (LRP1), the chief molecule responsible for transporting Aβ out of the brain, in AD, collectively dysregulate the flux and trapping of AGEs and Aβ within the CNS as degeneration progresses [67]. Collectively, these data provide strong evidence for the RAGE-DIAPH1 signaling axis as a prominent mediator of inflammation and cellular dysfunction in a variety of cell types during AD, particularly by igniting an unconstrained iterative loop of signal propagation driving cell-intrinsic and cell-to-cell stress signals that mediate prominent impairments during AD.
Of note, the extracellular RAGE inhibitor, Azeliragon, is currently in Stage 3 clinical trials to investigate the therapeutic potential of RAGE inhibition in AD patients. Initially, in an 18-month Stage 2 clinical trial of 399 patients, the trial was preemptively halted when Azeliragon (then by the name of TPP488) was shown to be deleterious to patients at high doses (60 mg for 6 days followed by 20 mg), but protective at low doses (15 mg for 6 days followed by 5 mg) [68, 69]. Currently, a Stage 3 study granted Fast Track designation by the United States Food and Drug Administration, is being conducted that utilizes the low dose (5 mg for 18 months) vs. placebo. This trial, entitled the STEADFAST Study, was recently extended for an optional 2 year continuation in multiple countries across the world [70].
RAGE and Parkinson’s Disease, a second manifestation of the cellular dysfunction
Parkinson’s disease (PD) is another common neurodegenerative disorder that impacts millions of people worldwide and is characterized by the specific loss of nigrostriatal dopaminergic neurons and locomotor deficits [71]. While the cerebral location and neuronal subsets that degenerate in PD are distinct from AD, there are prominent cellular activation mechanisms driving inflammation and perturbation of neurons at the nexus of the two disorders. Akin to AD, the initiation of PD pathogenesis is still not clearly elucidated. However, there are many disease processes correlated to PD and AD pathogeneses, which could potentially be related to RAGE-DIAPH1 signaling, such as enhanced oxidative stress, innate immune activation, protein aggregation, and neuronal death.
Multiple lines of evidence suggest a potential role for RAGE and its ligands in the pathogenesis of PD. First, the same AGER rs2070600 SNP that was implicated in CA1 atrophy during AD, was also correlated to the highest risk for PD development of all known AGER SNPs in a Turkish cohort GWAS (N=174 PD patients and N=150 healthy controls) [72]. In addition, when compared to healthy controls, PD patients have recently been shown to possess higher concentrations of S100B and HMGB1, two known RAGE ligands, in the substantia nigra and cerebral spinal fluid (CSF) [73–75]. In rodent models, numerous studies have indicated that animals derive prominent protection from PD-like impairments when RAGE signaling was blocked through genetic ablation of S100B/RAGE or by the administration of a RAGE inhibitor, FPS-ZM1, a blood-brain barrier (BBB) permeable, high affinity, multimodal blocker of RAGE [73]. Either strategy was sufficient to abrogate a variety of impairments observed in the PD-like rodent models, such as apoptosis of dopaminergic cells; locomotor defects; neuroinflammatory microgliosis and astrogliosis, as measured by increased ionized calcium binding adaptor molecule 1 (IBA1) and glial fibrillary acidic protein (GFAP) staining, respectively; tyrosine hydroxylase (and therefore dopamine) deficits; NF-KB activation; and tumor necrosis factor alpha (TNFα) upregulation in the presence of PD-like syndromes induced by toxins. While many of these benefits only partially rescued cellular deficits or delayed the onset of disease, it is possible that RAGE-based interventions in AD and PD may provide meaningful avenues for therapeutic intervention in either condition of neurodegeneration.
Amyotrophic Lateral Sclerosis, another inflammatory syndrome of the CNS?
Amyotrophic Lateral Sclerosis (ALS) is a fatal neurodegenerative disorder characterized by progressive motor function loss and muscle atrophy. Much like AD and PD, many of the gene mutations linked to ALS have also been shown to drive inflammatory glial activation, oxidative stress, and neuronal loss. There is prominent overlap of disease phenotypes in ALS to other disorders with regard to the cellular consequences of RAGE-DIAPH1 signaling, although further investigation is required to elucidate these mechanisms. [76]. Several studies have reported increased concentrations of RAGE ligands in the spinal cord [77–79] and CSF of ALS patients [80]. Conversely, serum sRAGE was decreased in human ALS patients, thereby putatively increasing ligand burden available for binding to and inducing signaling through full-length RAGE [81]. While little mechanistic evidence is available linking RAGE and ALS, our laboratory recently showed that RAGE and its ligands are increased in the spinal cord of ALS patients [78].
Furthermore, this increase of RAGE and its ligands was recapitulated in one of the most commonly employed ALS rodent models, murine lines containing the familial G93A mutation in superoxide dismutase 1 (SOD1), as discovered in human ALS populations [3, 82, 83]. In these models, nerve growth factor (NGF) is post-translationally modified by oxidation and contributes to RAGE signaling-induced motor neuron death when normal motor neurons are co-cultured with SOD1 G93A astrocytes [84]. In addition, C6 rat astrocytoma cells overexpressing mutant SOD1 G93A protein displayed significantly increased RAGE ligand S100B expression and, intriguingly, inhibition of this process by siRNA targeting S100b ameliorated the inflammatory profile of these cells [3].
In the SOD1 G93A mice, daily administration of recombinant sRAGE extended lifespan and duration of healthy body weight, while slowing the onset of motor function loss [82]. Importantly, sRAGE treatment not only reduced motor neuron death but also decreased astrogliosis, indicating a more homeostatic profile in multiple cell types [82]. Altogether, a burgeoning body of literature suggests that RAGE activation, driven by an increased availability of ligands, is likely a contributing factor to ALS pathology. However, further work utilizing established BBB-permeable inhibitors of RAGE-DIAPH1 would be paramount in elucidating the value of targeting this signaling axis as a potential therapeutic target for slowing the progression inflammatory and neuron-perturbing signaling in ALS.
Multiple Sclerosis and experimental autoimmune encephalopathy (EAE)
Multiple Sclerosis (MS) is a debilitating neurodegenerative disease in which autoimmune tissue-destructive processes are implicated. In human subjects, the AGER rs2070600 SNP was associated with MS in several studies [63, 85]. However, in a different study of a Hungarian community, this SNP was not present. Although, another SNP within the AGER promoter suggested altered transcription, rather than differences in ligand binding and sRAGE production, may be contributing to the risk of MS within this population [86].
With respect to sRAGE, akin to other inflammatory neurodegenerative syndromes discussed above, MS patients display lower serum levels of sRAGE relative to control patients and this decreased sRAGE inversely correlates with disease progression [87]. In addition, RAGE ligands are also increased in active MS lesions, as observed by immunohistochemistry. Further, AGER mRNA and RAGE ligand protein concentrations were increased in serum, CSF, and mononuclear cells in both niches during MS [87–91]. Interestingly, patients treated with disease-modifying drugs display a prominent reduction of serum HMGB1 when compared to untreated MS patients, which correlated to a better disease prognosis [90]. Fingolimod, a sphingosine-1P (S1P) analogue, has also been utilized to treat relapse-remitting MS in human patients, and induces a significant reduction in serum HMGB1 after 6 months of treatment while increasing sRAGE, albeit this study was conducted in a small patient cohort (n=17) [91].
Induction of experimental autoimmune encephalomyelitis (EAE), in which mice are immunized with myelin basic protein (MBP), has been utilized to study the molecular mechanisms underlying MS. Studies have reported increased RAGE in the spinal cords of mice with EAE [88, 92], whereas blockade of RAGE signaling by recombinant sRAGE administered concomitantly with EAE induction in mice, significantly reduced immune cell infiltration into the brain and the severity of the disease [92]. However, controversy arose after a report that Ager deficient mice with EAE displayed no differences in disease severity [58].
Three distinct, but not exclusive possibilities may explain these seemingly conflicting results. First, recombinant sRAGE may exert some of its effects independent of RAGE signaling. It is possible that sRAGE is functioning as a pathological ligand sink in this instance that not only reduces RAGE signaling but other inflammatory signals as well through different receptors to which RAGE ligands may also bind. Second, the deletion of Ager from every cell may imbue detrimental effects due to unknown roles of RAGE in homeostatic functions and thus, a complete blockade of this signaling, as opposed to dampening, may reduce the benefits of RAGE inhibition. Third, it is well established that MS and EAE models in mice are characterized by periods of exacerbation vs. remittance of disease; hence, the timing of RAGE inhibition or Ager deletion in vivo may critically impact phenotypic outcomes.
Collectively, these considerations suggest that RAGE signaling is likely contributing to inflammatory perturbation in MS. Potential therapeutic interventions should investigate the possibilities of abrogating disease pathology by quenching RAGE ligands and/or preventing RAGE inflammatory signaling as well, although a much more detailed analysis of when and how to do so would still need to be conducted.
Conclusions
As summarized in the Figure, the manifestations of AD, PD, ALS and MS are distinct in nature, impacting differential subsets of neurons and regional variability within the CNS. However, there are common underlying threads that strongly suggest similarities among these neurodegeneration syndromes, including increased accumulation of RAGE ligands and expression of RAGE, processes that trigger oxidative and cellular stress, and myeloid, neuronal, astrocytic, and endothelial dysfunction. The last decade of research has generated a strong body of evidence to suggest that RAGE signaling plays a prominent role in the pathophysiology of these inflammatory neurodegenerative syndromes, although many of the specific details remain to be fully elucidated. Although these findings are illuminative, multiple questions remain to be addressed, such as, does RAGE signaling participate in disease induction and/or as a potentiation/progression mechanism in these disorders? Why do we sometimes discern differential outcomes upon the use of sRAGE, RAGE inhibitors, or, in animal models, DN-RAGE expression or genetic ablation of Ager? To what extent does RAGE play time-dependent roles during discrete periods of disease and in distinct cell types, in models vs. humans, and are the effects of RAGE specific to aging or prominent across the lifespan? Are there specific patient populations for which RAGE-based therapies would be most or least beneficial? To this end, the future application of recent insights from human GWAS data for the use of genetic testing in conjunction with measuring circulating sRAGE levels might be the first steps to determine the subpopulations in which the administration of RAGE inhibitors may increase healthspan. RAGE presents itself as an attractive target for inhibition, when aiming for therapies that assuage cognitive decline during neurodegeneration through interfering with feed-forward loops of inflammation and oxidative and cellular stress.
Figure. Working model of RAGE signaling in microglia and the pathogenesis of neurodegenerative disease.
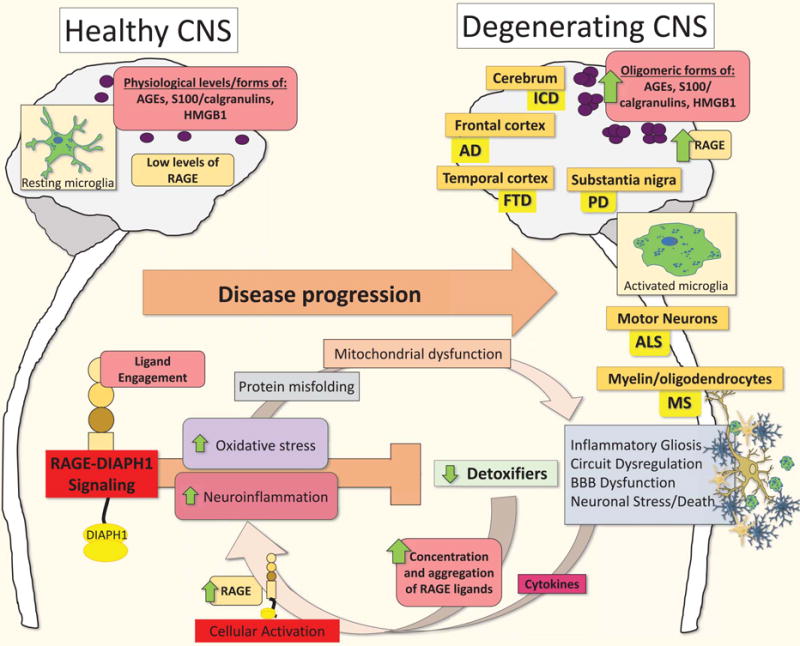
Increases in pathological levels and oligomeric forms of RAGE ligands characterize the transition from health and homeostasis to disease- mediating activities in microglia during the progression of AD, PD, ALS and MS. These pathological RAGE ligands promote RAGE-DIAPH1 signaling-induced oxidative stress, cytokine production, gliosis, and inflammation. Inflammatory cell activation further induces increased RAGE and RAGE ligand expression, while decreasing innate detoxifiers, thereby promoting an inflammatory feed-forward loop resulting in strikingly higher degrees of inflammatory gliosis, neuronal stress, BBB dysfunction and, eventually, neuronal death. We posit that activation of the AGE-RAGE-DIAPH1 axis in microglia is an amplifying event and critical final common pathway driving increased cellular stress and inflammation leading to neurodegeneration and the progression of AD, PD, ALS and MS. Abbreviations: AD, Alzheimer’s disease; AGEs, advanced glycation end products; ALS, Amyotrophic lateral sclerosis; BBB, blood-brain barrier; HMGB1, high mobility group box protein 1; MS, Multiple Sclerosis; and PD, Parkinson’s disease.
A new age in science is upon us where we are poised to integrate these varied questions. Excitingly, the novel discoveries that have revealed the genetics of disease susceptibility have occurred while many laboratories are concurrently flourishing in their revelations on the cellular and molecular mechanisms of RAGE signal transduction, and novel fields have developed to optimize cell targeting and isolation technology, RAGE inhibitors, and more nuanced approaches for clinical trials. Does this mounting evidence suggest a prominent role for RAGE signal transduction in accelerating the pathogenesis of inflammatory neurodegeneration, irrespective of the disease subtype? Further work will undoubtedly be required to determine to what extent and in which specific contexts inhibiting RAGE signaling will protect the CNS from neurodegeneration. However, these developing studies have shown clear benefits of RAGE abrogation, and the future shows promise, particularly as we begin to take a more integrative approach to understanding the complex mechanisms of these devastating diseases and the possibilities of relief through meaningful interventions.
| RAGE SNPs | Animal Findings | Human Findings | |
|---|---|---|---|
|
|
|||
| Alzheimer’s disease | rs2070600 |
|
|
| Ischemic Cerebrovascular disease | rs2070600 |
|
|
| Parkinson’s disease | rs2070600 |
|
|
| Amyotrophic Lateral Sclerosis and FTD | No known SNPs associated |
|
|
| Multiple Sclerosis | rs2070600 RAGE promoter |
|
|
Acknowledgments
We thank Ms. Latoya Woods for the expert assistance in preparation of this review. In addition, we are appreciative of our funding organizations: The National Institute of Health (National Institute on Aging), U.S. Department of Defense, and the Alzheimer’s Association.
References
- 1.Xu Y, et al. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem. 2010;285(30):23233–40. doi: 10.1074/jbc.M110.117457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker D, et al. Receptor for advanced glycation endproduct modulators: a new therapeutic target in Alzheimer’s disease. Expert Opin Investig Drugs. 2015:1–7. doi: 10.1517/13543784.2015.1001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serrano A, et al. The Astrocytic S100B Protein with Its Receptor RAGE Is Aberrantly Expressed in SOD1(G93A) Models, and Its Inhibition Decreases the Expression of Proinflammatory Genes. Mediators Inflamm. 2017;2017:1626204. doi: 10.1155/2017/1626204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt AM, et al. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest. 1993;91(5):2155–68. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan SD, et al. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci U S A. 1994;91(16):7787–91. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan SD, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–91. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 7.Kislinger T, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274(44):31740–9. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 8.Rai V, et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. The Journal of Experimental Medicine. 2012;209(13):2339. doi: 10.1084/jem.20120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giri R, et al. beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279(6):C1772–81. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- 10.Lue LF, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171(1):29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 11.Mi W, et al. Cystatin C inhibits amyloid-beta deposition in Alzheimer’s disease mouse models. Nat Genet. 2007;39(12):1440–2. doi: 10.1038/ng.2007.29. [DOI] [PubMed] [Google Scholar]
- 12.Fang F, et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2010;24(4):1043–55. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales-Corraliza J, et al. Immunization targeting a minor plaque constituent clears beta-amyloid and rescues behavioral deficits in an Alzheimer’s disease mouse model. Neurobiol Aging. 2013;34(1):137–45. doi: 10.1016/j.neurobiolaging.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauhan G, et al. Association of Alzheimer’s disease GWAS loci with MRI markers of brain aging. Neurobiol Aging. 2015;36(4):1765 e7–16. doi: 10.1016/j.neurobiolaging.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tominaga T, et al. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5(1):13–25. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 16.Hudson BI, et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283(49):34457–68. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manigrasso MB, et al. Small Molecule Inhibition of Ligand-Stimulated RAGE-DIAPH1 Signal Transduction. Scientific Reports. 2016;6:22450. doi: 10.1038/srep22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch M, et al. Structural Basis for Ligand Recognition and Activation of RAGE. Structure. 18(10):1342–1352. doi: 10.1016/j.str.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park H, Boyington JC. The 1.5 Å Crystal Structure of Human Receptor for Advanced Glycation Endproducts (RAGE) Ectodomains Reveals Unique Features Determining Ligand Binding. Journal of Biological Chemistry. 2010;285(52):40762–40770. doi: 10.1074/jbc.M110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lander HM, et al. Activation of the Receptor for Advanced Glycation End Products Triggers a p21 ras -dependent Mitogen-activated Protein Kinase Pathway Regulated by Oxidant Stress. Journal of Biological Chemistry. 1997;272(28):17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 21.McDonald DR, et al. β-Amyloid Fibrils Activate Parallel Mitogen-Activated Protein Kinase Pathways in Microglia and THP1 Monocytes. The Journal of Neuroscience. 1998;18(12):4451–4460. doi: 10.1523/JNEUROSCI.18-12-04451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He M, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO reports. 2011;12(4):358–364. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerterp M, et al. Deficiency of ABCA1 and ABCG1 in Macrophages Increases Inflammation and Accelerates Atherosclerosis in Mice. Circulation research. 2013;112(11) doi: 10.1161/CIRCRESAHA.113.301086. p. 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daffu G, et al. RAGE Suppresses ABCG1-Mediated Macrophage Cholesterol Efflux in Diabetes. Diabetes. 2015;64(12):4046–60. doi: 10.2337/db15-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arancio O, et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23(20):4096–105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posey SC, Bierer BE. Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. J Biol Chem. 1999;274(7):4259–65. doi: 10.1074/jbc.274.7.4259. [DOI] [PubMed] [Google Scholar]
- 27.Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15(5):590–7. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 28.Toure F, et al. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res. 2012;110(10):1279–93. doi: 10.1161/CIRCRESAHA.111.262519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, et al. Knockdown of Diaph1 expression inhibits migration and decreases the expression of MMP2 and MMP9 in human glioma cells. Biomedicine & Pharmacotherapy. 2017;96(Supplement C):596–602. doi: 10.1016/j.biopha.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Saleh A, et al. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Experimental Neurology. 2013;249(Supplement C):149–159. doi: 10.1016/j.expneurol.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Bucciarelli LG, et al. RAGE and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57(7):1941–51. doi: 10.2337/db07-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouhiainen A, et al. Regulation of monocyte migration by amphoterin (HMGB1) Blood. 2004;104(4):1174–82. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- 33.Guerreiro R, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonsson T, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368(2):107–16. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hickman SE, El Khoury J. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):495–8. doi: 10.1016/j.bcp.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abuznait AH, Kaddoumi A. Role of ABC transporters in the pathogenesis of Alzheimer’s disease. ACS Chem Neurosci. 2012;3(11):820–31. doi: 10.1021/cn300077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villegas-Llerena C, et al. Microglial genes regulating neuroinflammation in the progression of Alzheimer’s disease. Curr Opin Neurobiol. 2015;36:74–81. doi: 10.1016/j.conb.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Cramer PE, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–6. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang ZG, et al. Inflammation in Alzheimer’s Disease and Molecular Genetics: Recent Update. Arch Immunol Ther Exp (Warsz) 2015;63(5):333–44. doi: 10.1007/s00005-015-0351-0. [DOI] [PubMed] [Google Scholar]
- 40.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barzilay JI, et al. The impact of salsalate treatment on serum levels of advanced glycation end products in type 2 diabetes. Diabetes Care. 2014;37(4):1083–91. doi: 10.2337/dc13-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 43.Lee S, et al. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am J Pathol. 2010;177(5):2549–62. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saederup N, et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5(10):e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prinz M, et al. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14(10):1227–35. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 46.Butovsky O, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–43. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsumoto A, et al. Ontogeny and functions of central nervous system macrophages. J Immunol. 2014;193(6):2615–21. doi: 10.4049/jimmunol.1400716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heneka MT, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ransohoff RM, El Khoury J. Microglia in Health and Disease. Cold Spring Harb Perspect Biol. 2015 doi: 10.1101/cshperspect.a020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El Khoury J, et al. Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol Aging. 1998;19(1 Suppl):S81–4. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 51.Hickman SE, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16(12):1896–905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schafer Dorothy P, et al. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron. 74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bilimoria PM, Stevens B. Microglia function during brain development: New insights from animal models. Brain Res. 2015;1617:7–17. doi: 10.1016/j.brainres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 54.Schafer DP, Stevens B. Microglia Function in Central Nervous System Development and Plasticity. Cold Spring Harb Perspect Biol. 2015;7(10) doi: 10.1101/cshperspect.a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Y, et al. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015;36(10):605–13. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhla B, et al. Age- and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer’s disease brains. Neurobiology of Aging. 2007;28(1):29–41. doi: 10.1016/j.neurobiolaging.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 57.More SS, Vartak AP, Vince R. Restoration of Glyoxalase Enzyme Activity Precludes Cognitive Dysfunction in a Mouse Model of Alzheimer’s Disease. ACS Chemical Neuroscience. 2013;4(2):330–338. doi: 10.1021/cn3001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liliensiek B, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113(11):1641–50. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gasparotto J, et al. Receptor for advanced glycation endproducts mediates sepsis-triggered amyloid-β accumulation, tau phosphorylation, and cognitive impairment. Journal of Biological Chemistry. 2017 doi: 10.1074/jbc.M117.786756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holmes C, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang ZX, et al. Genetic Association of HLA Gene Variants with MRI Brain Structure in Alzheimer’s Disease. Molecular Neurobiology. 2017;54(5):3195–3204. doi: 10.1007/s12035-016-9889-z. [DOI] [PubMed] [Google Scholar]
- 62.Hofmann MA, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes And Immunity. 2002;3:123. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- 63.Li K, et al. A functional p.82G>S polymorphism in the RAGE gene is associated with multiple sclerosis in the Chinese population. Mult Scler. 2011;17(8):914–21. doi: 10.1177/1352458511403529. [DOI] [PubMed] [Google Scholar]
- 64.Miller S, et al. The Ser82 RAGE Variant Affects Lung Function and Serum RAGE in Smokers and sRAGE Production In Vitro. PLOS ONE. 2016;11(10):e0164041. doi: 10.1371/journal.pone.0164041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, et al. Synergistic exacerbation of mitochondrial and synaptic dysfunction and resultant learning and memory deficit in a mouse model of diabetic Alzheimer’s disease. J Alzheimers Dis. 2015;43(2):451–63. doi: 10.3233/JAD-140972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deane R, et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. 2003;9:907. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 67.Qosa H, et al. Mixed oligomers and monomeric amyloid-β disrupts endothelial cells integrity and reduces monomeric amyloid-β transport across hCMEC/D3 cell line as an in vitro blood–brain barrier model. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2014;1842(9):1806–1815. doi: 10.1016/j.bbadis.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burstein A, et al. Effect of TTP488 in patients with mild to moderate Alzheimer’s disease. BMC Neurology. 2014;14(1):12. doi: 10.1186/1471-2377-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galasko D, et al. Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease. Neurology. 2014;82(17):1536–42. doi: 10.1212/WNL.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ongoing Azelirgon Clinical Trial
- 71.Goetz CG. The History of Parkinson’s Disease: Early Clinical Descriptions and Neurological Therapies. Cold Spring Harbor Perspectives in Medicine. 2011;1(1):a008862. doi: 10.1101/cshperspect.a008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oliveira SA, et al. Association study of parkin gene polymorphisms with idiopathic parkinson disease. Archives of Neurology. 2003;60(7):975–980. doi: 10.1001/archneur.60.7.975. [DOI] [PubMed] [Google Scholar]
- 73.Gasparotto J, et al. Targeted inhibition of RAGE in substantia nigra of rats blocks 6-OHDA–induced dopaminergic denervation. Scientific Reports. 2017;7(1):8795. doi: 10.1038/s41598-017-09257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sathe K, et al. S100B is increased in Parkinson’s disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-α pathway. Brain. 2012;135(11):3336–3347. doi: 10.1093/brain/aws250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teismann P, et al. Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. Neurobiology of Aging. 2012;33(10):2478–2490. doi: 10.1016/j.neurobiolaging.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hardiman O, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17085. doi: 10.1038/nrdp.2017.85. [DOI] [PubMed] [Google Scholar]
- 77.Kikuchi S, et al. Detection of N epsilon-(carboxymethyl)lysine (CML) and non-CML advanced glycation end-products in the anterior horn of amyotrophic lateral sclerosis spinal cord. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3(2):63–8. doi: 10.1080/146608202760196020. [DOI] [PubMed] [Google Scholar]
- 78.Juranek JK, et al. Receptor for Advanced Glycation End Products and its Inflammatory Ligands are Upregulated in Amyotrophic Lateral Sclerosis. Front Cell Neurosci. 2015;9:485. doi: 10.3389/fncel.2015.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casula M, et al. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience. 2011;179:233–43. doi: 10.1016/j.neuroscience.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Kaufmann E, et al. The advanced glycation end-product N epsilon-(carboxymethyl)lysine level is elevated in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Neurosci Lett. 2004;371(2-3):226–9. doi: 10.1016/j.neulet.2004.08.071. [DOI] [PubMed] [Google Scholar]
- 81.Ilzecka J. Serum-soluble receptor for advanced glycation end product levels in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2009;120(2):119–22. doi: 10.1111/j.1600-0404.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 82.Juranek JK, et al. Soluble RAGE Treatment Delays Progression of Amyotrophic Lateral Sclerosis in SOD1 Mice. Front Cell Neurosci. 2016;10:117. doi: 10.3389/fncel.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lo Coco D, et al. Distribution and cellular localization of high mobility group box protein 1 (HMGB1) in the spinal cord of a transgenic mouse model of ALS. Neurosci Lett. 2007;412(1):73–7. doi: 10.1016/j.neulet.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 84.Kim MJ, et al. Nitration and Glycation Turn Mature NGF into a Toxic Factor for Motor Neurons: A Role for p75(NTR) and RAGE Signaling in ALS. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2016.6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caillier SJ, et al. Uncoupling the roles of HLA-DRB1 and HLA-DRB5 genes in multiple sclerosis. J Immunol. 2008;181(8):5473–80. doi: 10.4049/jimmunol.181.8.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tiszlavicz Z, et al. RAGE gene polymorphisms in patients with multiple sclerosis. J Mol Neurosci. 2009;39(3):360–5. doi: 10.1007/s12031-009-9291-7. [DOI] [PubMed] [Google Scholar]
- 87.Sternberg Z, et al. Soluble receptor for advanced glycation end products in multiple sclerosis: a potential marker of disease severity. Mult Scler. 2008;14(6):759–63. doi: 10.1177/1352458507088105. [DOI] [PubMed] [Google Scholar]
- 88.Andersson A, et al. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J Leukoc Biol. 2008;84(5):1248–55. doi: 10.1189/jlb.1207844. [DOI] [PubMed] [Google Scholar]
- 89.Barateiro A, et al. S100B as a Potential Biomarker and Therapeutic Target in Multiple Sclerosis. Mol Neurobiol. 2016;53(6):3976–3991. doi: 10.1007/s12035-015-9336-6. [DOI] [PubMed] [Google Scholar]
- 90.Sternberg Z, et al. High-mobility group box 1 in multiple sclerosis. Immunol Res. 2016;64(2):385–91. doi: 10.1007/s12026-015-8673-x. [DOI] [PubMed] [Google Scholar]
- 91.Sternberg Z, et al. Fingolimod anti-inflammatory and neuroprotective effects modulation of RAGE axis in multiple sclerosis patients. Neuropharmacology. 2017;130:71–76. doi: 10.1016/j.neuropharm.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 92.Yan SS, et al. Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat Med. 2003;9(3):287–93. doi: 10.1038/nm831. [DOI] [PubMed] [Google Scholar]


