SUMMARY
Objective:
Mutations on chromosomes 5p (CCAL2) and 8q (CCAL1) have been linked to familial forms of calcium pyrophosphate deposition disease (CPDD). Mutations in the ANKH gene account for CCAL2, but the identity of CCAL1 has been elusive. Recently, a single Dutch kindred with a mutation in the Tumor Necrosis Factor Receptor Super Family member 11B (TNFRSF11B) gene coding for osteoprotegerin (OPG) was described as a gain-of-function mutation. Affected family members had premature generalized osteoarthritis (PGOA) and CPDD. As the TNFRSF11B gene is on 8q, we sought additional evidence that TNFRSF11B was CCAL1, and investigated potential disease mechanisms.
Design:
DNA from two novel PGOA/CPDD families was screened for sequence variants in the TNFRSF11B gene. Mutations were verified by genotype analysis of affected and unaffected family members. We also investigated effects of normal and mutant OPG on regulators of CPP crystal formation in porcine cartilage.
Results:
The identical TNFRSF11B mutation described in the Dutch family was present in two novel PGOA/CPDD families. ANKH was normal in affected patient fibroblasts. Exogenous OPG did not alter ANKH mRNA or protein levels, affect translocation of ANKH to the membrane, nor increase [pyrophosphate (PPi)] or other key regulators of CPDD.
Conclusion:
We have firmly established the identity of CCAL1 as TNFRSF11B (OPG). Our findings suggest that this mutation produces disease in an ANKH-independent manner via novel mechanisms not primarily targeting cartilage. This work rationalizes further investigation of OPG pathway components as potential druggable targets for CPDD.
Keywords: Calcium pyrophosphate deposition, Osteoarthritis, Osteoprotegerin
Calcium pyrophosphate deposition disease (CPDD) is a common form of arthritis caused by the deposition of calcium pyrophosphate (CPP) crystals in articular hyaline and fibro-cartilage1. While many older patients with CPDD do not report affected relatives, the original descriptions of CPDD included five patients from a single family2. Subsequently, multiple kindreds with autosomal dominant forms of CPDD have been reported, and two distinct genetic loci were observed in these families. These loci were originally termed CCAL1 and CCAL23,4. CCAL2, on chromosome 5p, is in a region syntenic with the human homolog of the progressive ankylosis gene coding for the ANKH protein5. Eight families with ANKH mutations show missense, insertion, and frameshift mutations in the 5’ end of the ANKH gene generally believed to result in gain-of-function6. Current evidence supports a role for ANKH in transporting critical precursors of CPP crystal formation, including pyrophosphate (PPi)5 and/or its precursor, ATP7, across the cell membrane.
The identity of the gene and protein associated with CCAL1 has proven to be more elusive. The CCAL1 locus on chromosome 8q was initially described in a kindred from Maine, USA, in which the occurrence of CPP crystals in hips and knees of affected members coincided with premature severe generalized osteoarthritis (PGOA). Refinement of the linkage interval and identification of the disease gene in this family was not possible due to the limited number of individuals available for study. Importantly, the phenotype in the CCAL1 Maine family differs from that reported in CCAL2 families in that the CCAL2 individuals displayed evidence of CPP deposition well before the onset of frank OA.
In 2015, a family from the Netherlands displaying the PGOA/ CPDD phenotype was studied by Ramos et al. using a whole exome sequencing approach8. A prioritized candidate in the chromosome 8q region, encompassing the CCAL1 locus, was identified and direct sequencing of affected family members resulted in the discovery of a substitution mutation (Stop402Leu) in the Tumor Necrosis Factor Receptor Super Family member 11B (TNFRSF11B) gene in this family. The mutation was confirmed by linkage analysis across the extended family. The TNFRSF11B gene codes for osteoprotegerin (OPG), a 61 k Dalton glycoprotein best known for its role as a decoy receptor for Receptor Activator of Nuclear Factor κB Ligand (RANKL). In a process which is critical to regulation of bone remodeling, OPG binds to RANKL and blocks RANKL from interacting with its receptor, Receptor Activator of Nuclear Factor κB (RANK). Functionally, OPG inhibits RANKL-induced osteoclasto-genesis. The TNFRSF11B mutation at the stop codon results in an OPG translation product that is 19 amino acids longer than the normal product. In functional studies of mutant OPG, Ramos et al. showed that the conditioned media from HEK293T cells transfected with the mutant OPG mRNA suppressed RANKL-induced osteoclastogenesis slightly more effectively than conditioned media from cells transfected with normal OPG mRNA, suggesting that this was a gain-of-function mutation.
The mechanisms through which OPG might cause OA and CPPD are not readily apparent. CPPD is typically considered a disease of articular cartilage, and excess accumulation of extracellular PPi in articular cartilage is necessary for CPP crystal formation9. PPi can be exported from the cytosol by transporters such as ANKH5 or produced from extracellular ATP (eATP) by the action of ectonucleoside triphosphatase 1 (ENPP1)10. Interestingly, neither OPG nor the active ligand, RANKL, have reproducible effects on articular cartilage or chondrocytes11–16. However, TNFSRF11B mRNA is upregulated in lesional cartilage of patients with OA17, and if and how the OPG/RANKL/RANK pathway contributes to joint disease remains uncertain.
In order to prove that TNFRSF11B is CCAL1, we characterized a new CPDD kindred from Long Island NY, and re-examined samples from an Israeli family with CPDD lacking an ANKH mutation. We sought to determine if mutations in TNFRSF11B were present in these two additional CPDD families, and explored potential mechanisms though which OPG might directly affect cartilage to promote CPP crystal formation. Our findings confirm the identity of TNFRSF11B as CCAL1, and suggest novel ANKH-independent pathways of CPP crystal formation that do not primarily target cartilage.
Patients and methods
Family A
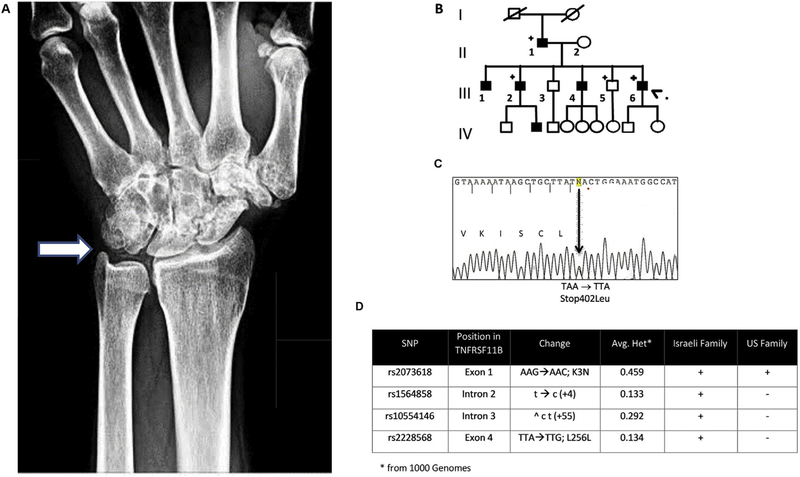
The proband, a 43 year-old male patient, was initially evaluated at age 31, when he presented with progressive polyarticular degenerative arthritis resulting in considerable pain and disability. Radiographs of his knees, wrists, elbows and ankles revealed severe OA characterized by subchondral sclerosis and marked joint space narrowing. There was extensive classic radiographic chondrocalcinosis strongly suggestive of CPDD [Fig. 1(A)]. Arthrocentesis and synovial fluid analysis were not performed, but acute inflammatory flares involving wrists, elbows, ankles and the cervical and thoracolumbar spine were described. Diagnostic work up to detect metabolic disorders associated with CPDD was negative. Similar presentations were noted in six out of ten members of the family affecting three consecutive generations [Fig. 1(B)]. One of the affected patients, Patient III:2, underwent bone mineral density testing at age 54, which demonstrated a T-Score of 1.7 at the left hip and femur.
Fig. 1. Typical patient radiograph and family tree of Long Island Family with the Stop402Leu mutation.
A. Wrist radiograph of an affected patient from the Long Island family. The arrow points to typical chondrocalcinosis seen in and around the triangular cartilage of the wrist. B. Pedigree of PGOA/CPDD US family. The proband (indicated by large arrowhead) is affected member III:6. Family members delineated with a (+) were subjected to DNA sequence analysis. C. Sequencing chromatograph shows Stop402Leu change in termination codon. D. SNP analyses of TNFRSF11B polymorphic variants in Israeli family vs US family demonstrate that the two families are not related.
Family B
The pedigree and clinical details of this family have been described previously18. Briefly, six members of this 5-generation Israeli family, of Ashkenazi Jewish origin, were shown to have CPDD and premature OA with an average age of onset of 25 years, and clear radiographic evidence of osteoarthritis and chondrocalcinosis by age 30. The presence of CPP crystals in synovial fluids was confirmed in affected family members using compensated polarized light microscopy. Since publication18, one additional family member in generation five was confirmed to be affected.
Linkage analyses
To determine the logarithm of the odds (LOD) that the mutation in TNFRSF11B was linked to the phenotype of the affected members of families A and B, two-point parametric linkage analyses were performed using the FASTLINK 2.2 version of the MLINK linkage program suite. The radiographically-confirmed disease in affected individuals was assumed to be autosomal dominant and 100% penetrant with a frequency of 0.001. Family members who were not clinically evaluated were considered unknown.
Studies of patient material
Patient material was obtained under guidance from the Institutional Review Board (IRB) at Jefferson University Medical Center, where the samples from family B were stored, as well as the IRB from John T Mather Memorial Hospital, where family A received medical care, and SUNY Stony Brook, sponsor of the GME programs at John T Mather Hospital. DNA isolated from peripheral blood samples from both families underwent PCR amplification and DNA sequencing at the Sidney Kimmel Cancer Center Genomics Shared Resource, Thomas Jefferson University, Philadelphia, PA. Fibroblasts were obtained from skin biopsies of two affected members of family B and four un-related healthy controls. Explant cultures were grown as previously described19. ANKH mRNA expression was quantified in the cell layer using quantitative real-time polymerase chain reaction (qRT-PCR).
Chondrocytes
Weekly, four pig hind legs were collected from two to four 3–5 year-old pigs from the local abattoir (Perryway Foods, Watertown, WI). Hyaline articular cartilage was removed from the tibial and femoral surfaces of knee joints within 6 h of slaughter and random quantities of cartilage from four legs were combined and divided into three separate flasks (Supplementary Fig. 2). Chondrocytes were enzymatically isolated from surrounding matrix using trypsin and collagenase as previously described20. A combination of primary cells from four bones were then plated at high density (4 × 105 cells/cm2) in Dulbecco’s modified Eagle’s media (DMEM) with 10 % fetal calf serum and 1% antibiotics (10-1-D) for 24 h. Organ cultures were established by mincing cartilage and placing ~100 mg aliquots into with 10-1-D adjusted to a constant weight/ volume ratio. All experiments were performed in serum-free DMEM with 0.35 mg/ml bovine serum albumin and 1% antibiotics with or without additives. All experiments were completed within 7 days of establishing cell cultures. A single data point was collected from each experimental well. Each well contained a unique combination of cells and wells were assumed independent although we could not totally eliminate the possibility that two knees were from a single animal.
Site directed mutagenesis, transformation and transfection
The Stop402Leu mutant was prepared from the TNFRSF11B wild type plasmid (InvivoGen, San Diego, CA) by site-directed mutagenesis using a commercially available kit (QuikChange Lightning Fast; Agilent, Santa Clara, CA) according to manufacturer’s protocol. The TNFRSF11B Stop402Leu mutation-positive colonies were selected on the basis of antibiotic resistance, purified (MaxiPrep Plasmid Preparation; Qiagen, Germantown, MD), and subjected to dideoxy nucleotide sequencing to confirm successful mutagenesis.
For production of recombinant OPG, HEK293T cells were plated at a concentration of 7.5 × 104 cells per ml in 6-well plates and transfected the following day with either TNFRSF11B wild type plasmid or the Stop402Leu mutant plasmid (1 ug per ml media) using Fugene HD reagent (Promega, Madison, WI; plasmid to Fugene HD ratio equal to 3:1). After 48 h, media were collected, centrifuged to remove cells and debris, and OPG expression was quantified via ELISA and western blotting using a rabbit polyclonal antibody to human OPG (ab189580; Abcam, Cambridge, MA).
PPi levels
Levels of extracellular PPi were measured in porcine chondrocyte conditioned media after 48 h of exposure to no additives, 10–100 ng/ml OPG (human recombinant, R&D System, Minneapolis, MN), 100 ng/ml RANKL (human recombinant, Abcam, Cambridge, UK), 50 ng/ml TNF-related apoptosis-inducing ligand (TRAIL) (human recombinant, R&D Systems), 100 ng/ml OPG-Fc (human recombinant, Sigma-Aldrich, St. Louis, MO) or 10 ng/ml Interleukin-1β (IL-1β, R&D Systems). 100 ng/ml of either mutant- or WT-OPG was added to chondrocytes for 48 h and extracellular PPi was measured. An equivalent volume of HEK293T conditioned media was added as an additional control. In some experiments, endogenous OPG was silenced using a species-specific small interfering RNA or scramble control (Silencer Select, Invitrogen, Carlsbad, CA) as previously described21. PPi levels in conditioned media were determined using a standard radiometric assay based on the UDPG pyrophosphorylase method of Lust and Seegmiller20,22.
ATP levels
Levels of eATP were quantified in porcine chondrocyte conditioned media with and without 100 ng/ml OPG or 100 ng/ml RANKL using a bioluminescent assay (FLAAM, Sigma-Aldrich) as previously described7.
Calcification assay
Formation of CPP crystals can be modeled in porcine cartilage organ culture and chondrocyte monolayers by using an assay based on 45Ca uptake by cells or tissues in the presence and absence of exogenously added ATP23. Minced cartilage or chondrocyte monolayers were incubated with media trace labeled with 45Ca and containing 1 mM ATP, 1 mM β-glycerophosphate (as a phosphate donor control), or no additives. Experiments were performed with 100 ng/ml OPG, 100 ng/ml RANKL or no additives. After 96 h, media were removed, cell layers or cartilage pieces were washed and dissolved in 1 N NaOH or 6 M HCl respectively, and retained radioactivity was quantified using liquid scintigraphy.
Protein assays
For Western blotting of OPG protein, the cytosol, membrane and matrix fractions were loaded onto 4–12% Bis–Tris gels and electrophoresed. Proteins were then transferred to nitrocellulose membranes, and after blocking with 5% milk were incubated overnight with primary antibodies to OPG (Abcam, 1:1000) and then with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:10,000, Zymed, South San Francisco, CA). Blots were developed with a chemiluminescent substrate (Thermo Scientific Super Signal West Femto Substrate, Thermo Fisher, Waltham, MA). ANKH levels in chondrocyte cell layers and cell fractions were measured using a commercial ELISA according to manufacturer’s directions (My BioSource, San Diego, CA). Levels of total protein in cell layers were determined using the Lowry assay.
Immunocytochemistry
The presence of RANK on porcine chondrocytes was confirmed by incubating cells with anti-RANK antibody (1:100, Abcam) for 2 h. After washing, goat anti-mouse antibodies labeled with Texas Red (1:1000 Invitrogen) were added for 45 min. Slides were washed with phosphate buffered saline and counterstained with a drop of 4′ 6-diamidino-2-phenylindole (DAPI) stain. Images were obtained using a Leica TCS SP8 confocal microscope with VIS laser.
Real time RT-PCR
Total RNA was extracted from human fibroblasts or porcine chondrocytes with PureLink Mini RNA kit (Invitrogen). cDNA was synthesized from 0.5 to 1 μg total RNA using QuantiTect Reverse Transcription kit (Qiagen, Valencia, CA). Reference genes were empirically tested for stability. A melting curve was performed for each PCR cycling reaction to ensure recovery of a single SYBR green fluorescing species in the reaction product. Normalized relative mRNA expression was determined by the formula 2dCt, where dCt Ct(target) Ct(reference). All assays were performed at least in triplicate.
Statistics
All experiments were repeated 3–5 times starting with cartilage from four pig legs and using 3–6 biological replicates per group. For qRT-PCR statistical significance was determined with either an unpaired two-tailed Student’s t-test for normalized OPG levels or one-way ANOVA for normalized ANKH levels. The remaining data were not normally distributed, therefore a Kruskal-Wallis with Dunn’s multiple comparison test was used to determine statistical significance from samples collected from a single batch of pig bones. Because of random allocation of cells and tissue pieces from multiple animals to a single well, each experiment was considered independent despite sharing some biologic material. Therefore, N refers to the number of wells used. A univariate general linear model (GLM) with Tukey’s-b post hoc was used to test statistical significance from samples collected from more than one batch of bones to control for batch effects. Statistical analyses were conducted with IBM SPSS v. 22 and GraphPad PRISM v.7 software. Statistical significance was set at P ≤0.01. Note the box plots display the median and data quartiles.
Results
Exon sequencing of TNFRSF11B demonstrates mutations in both affected families
Blood samples from two CPPD families were examined for mutations in ANKH and TNFRSF11B. While no mutations were observed in ANKH in either family, both families displayed the same read-through mutation of the termination codon [Stop402Leu; Fig. 1(C)] of the TNFRSF11B gene as observed in the Dutch family8. Genotyping of SNPs in and around the TNFRSF11B gene in affected members of each family demonstrated that the families appear to be unrelated [Fig. 1(D)]. Additional polymorphic variants around the TNFRSF11B gene were genotyped in family members available for study (n=14) and submitted to linkage analysis. Significant evidence for linkage between the disease phenotype and the TNFRSF11B read-through mutation was observed (LOD score = 3.31; see Supplementary Table 1).
Levels of ANKH mRNA are unchanged in skin fibroblasts from affected family members compared to controls
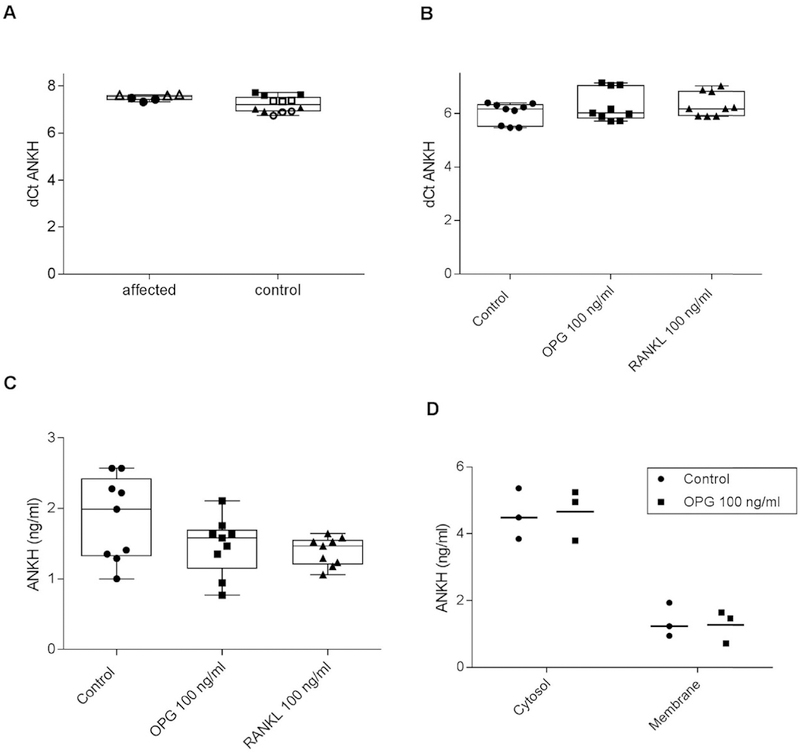
Although the phenotypic differences in the OPG families and the ANKH families suggest that they do not fully share disease mechanisms, we first investigated the possibility that the OPG mutation caused CPDD by increasing ANKH levels. Fibroblasts from affected members of the Israeli family demonstrated expression levels of ANKH transcript which seemed similar to those from unaffected controls [Fig. 2(A)], suggesting that the Stop402Leu mutation in TNFRSF11B does not impact the expression of ANKH in patient fibroblasts. Statistics were not performed due to the small sample size.
Fig. 2. OPG and RANKL do not increase ANKH levels in human fibroblasts or porcine chondrocytes.
A. ANKH mRNA levels were measured in cultured skin fibroblasts from affected Israeli family members and healthy controls using qRT-PCR. Controls were derived from unrelated individuals. dCt (sample) is defined as mean Ct (target) e mean Ct (reference; where reference = β-actin). Each individual is represented by a different shape. There were no apparent differences between affected and un-affected individuals (n = 6). ANKH mRNA (B.) and protein (C.) levels were measured by qRT-PCR and ELISA respectively in articular chondrocytes after 48 h of exposure to no additives (Control), 100 ng/ml OPG, or 100 ng/ml RANKL. There were no significant differences between treatment and controls (n = 9 per group, P = 0.235) and (n = 9 per group, P = 0.254) respectively. D. ANKH levels in membrane and cytosol fractions of chondrocytes were measured by ELISA after 48 h of exposure to no additives (Control) or 100 ng/ml OPG. No differences were noted between groups (n = 3 per group).
High levels of OPG and its target ligand, RANKL do not alter ANKH levels in healthy articular chondrocytes
To ensure that we were not missing a cartilage-specific effect of OPG over-expression on ANKH, we next investigated the action of exogenous OPG and RANKL on ANKH levels in adult articular porcine chondrocytes. As shown in Fig. 2, neither 100 ng/ml OPG nor 100 ng/ml RANKL increased levels of mRNA (2B) or ANKH protein (2C) in chondrocytes at 48 h. To investigate the possibility that OPG or RANKL increased membrane levels of ANKH without altering total levels of ANKH, both cytoplasmic and membrane compartments were measured. There were no apparent differences in ANKH levels in the membrane after treatment with OPG [Fig. 2(D)].
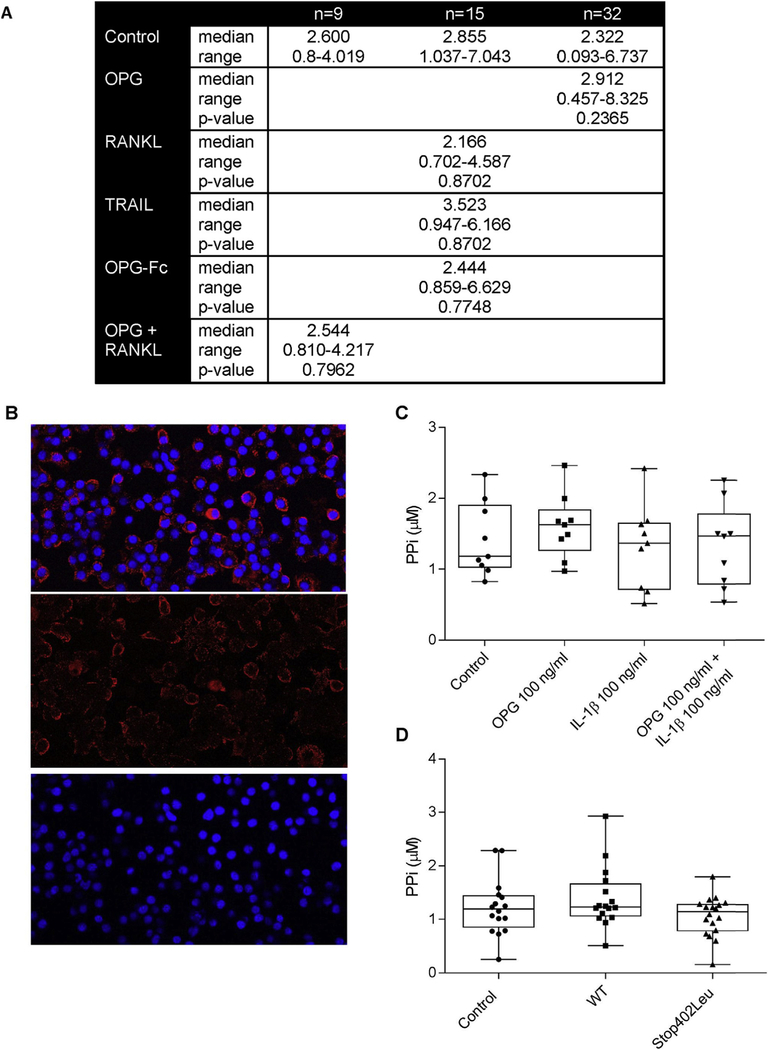
Neither OPG nor its target ligand, RANKL, affect PPi levels in chondrocyte media
Elevated levels of PPi in cartilage are necessary for CPP crystal formation. While ANKH levels are important in PPi production, other factors also regulate PPi levels in cartilage9. We explored the effects of 100 ng/ml OPG and/or RANKL on PPi levels in chondrocyte conditioned media after 48 h of exposure. As shown in the table in Fig. 3(A) neither OPG nor RANKL changed PPi levels compared to controls. Data analysis with the GLM accounts for batch effect as well as treatment group variability. The overall model showed P-values ranging from 0.001 to 0.995, but none of these values were explained by differences between control and treatment group. Time points from 24 to 96 h, as well as concentrations from 10 to 100 ng/ml, were also tested with no effects on [PPi] (Data not shown). Ectoenzymes which regulate PPi levels, including nucleoside triphosphate pyrophosphohydrolase, 5ʹ nucleotidase, and alkaline phosphatase were easily measurable on chondrocytes and were unchanged in the presence of OPG or RANKL (Supplementary data Fig. 1).
Fig. 3. OPG and RANKL do not increase levels of extracellular PPi in porcine chondrocyte conditioned media.
A. PPi levels were measured in chondrocyte conditioned media after 48 h of exposure to no additives (Control), 100 ng/ml OPG, 100 ng/ml RANKL, 50 ng/ml TRAIL, 100 ng/ml OPG-Fc or 100 ng/ml RANKL and 100 ng/ml OPG. Statistical differences between control and treatment were assessed with GLM to account for batch effects. There were no significant differences between control and any treatment. B. RANK was detected on fixed cultured chondrocytes using RANK antibody binding as detected with a Texas red tagged secondary antibody. DAPI was used as nuclear stain. RANK is present on a population of chondrocytes. The top panel shows the overlay of Texas red and DAPI. The middle panel is Texas red staining alone. The bottom panel is DAPI staining alone. C. Chondrocytes were incubated with no additives (Control), or 100 ng/ml OPG with or without 10 ng/ml IL-1b. PPi was measured in conditioned media after 48 h. There were nosignificant differences between control and any group (n = 9 per group, P = 0.242) using GLM. D. PPi levels were measured in chondrocyte conditioned media after 48 h of exposure to conditioned media from HEK293T cells (Control), 100 ng/ml WT-OPG or Stop402Leu mutant-OPG. There were no significant differences between groups (n = 18 per group, P = 0.2876) using Kruskal Wallis.
The absence of an OPG effect is not due to differences between the mutant and normal proteins, lack of co-factors or rapid OPG turnover
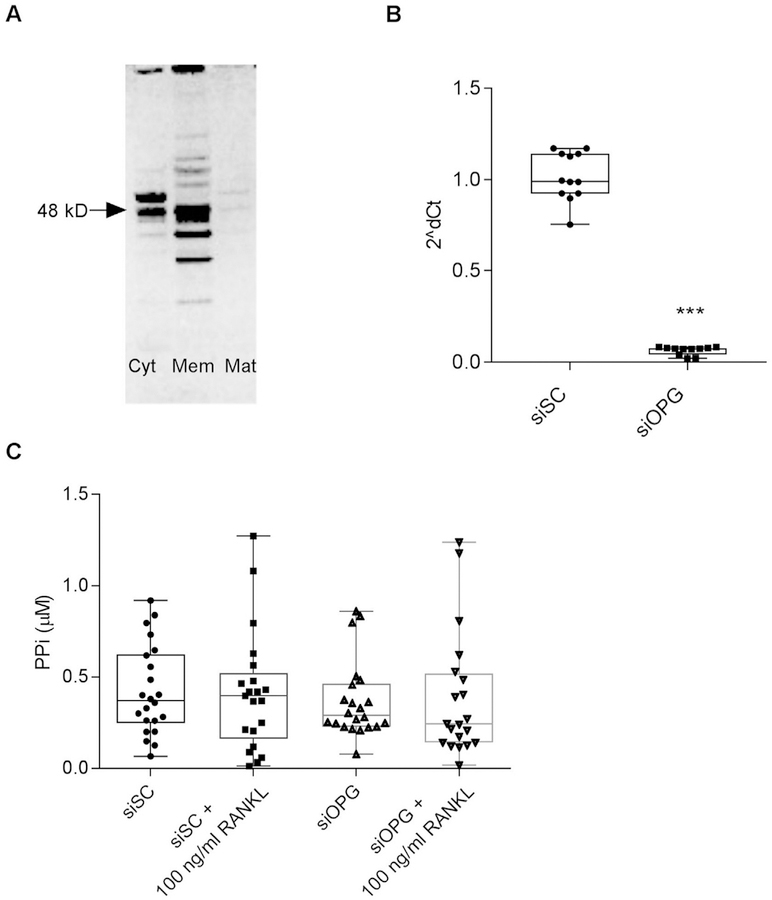
As few factors to date have been identified which contribute to CPPD, independently of PPi, we next investigated multiple explanations for the absence of an effect. For example, some prior work suggests that normal articular chondrocytes lack RANK16. However, our chondrocytes demonstrate measurable levels of RANK [Fig. 3(B)]. Others have shown that OPG actions on chondrocytes may require the presence of IL-1b16, but we found no significant effect of OPG on PPi levels with or without IL-1β [Fig. 3(C)]. Kwan Tat et al. hypothesized that OPG is rapidly degraded or inactivated in culture and this is in part mediated by the heparan sulfate binding domain24. To investigate the half-life issue, we added OPG daily which had no effect on [PPi] (Data not shown). OPG lacking its heparan sulfate binding C-terminal domain (OPG-Fc) also demonstrated no consistent effects on PPi levels in chondrocyte culture media [Fig. 3(A)] compared to controls. As OPG also acts as a decoy receptor for TRAIL25, we investigated the effect of TRAIL on PPi levels. No significant effects were observed [Fig. 3(A)] compared to controls. We also determined whether mutant OPG protein might behave differently from normal OPG. As shown in Fig. 3(D), neither mutated OPG protein nor its normal control significantly altered PPi levels. Lastly, as chondrocytes generate endogenous OPG [Fig. 4(A)], we also investigated the hypothesis that RANKL was unable to affect [PPi] because its actions were blocked by endogenous OPG. Effectively reducing endogenous OPG levels using siRNA [Fig. 4(B)] did not significantly alter the actions of RANKL on PPi levels [Fig. 4(C)].
Fig. 4. OPG is generated by porcine chondrocytes, but reducing endogenous levels does not alter the effects of RANKL on PPi.
A. OPG was identified using Western blotting in fractions of untreated articular chondrocytes grown in serum-free media. Lanes are from left to right, cytosol (CYT), membrane (MEM), and matrix (MAT). Arrow connotes the 48 k Dalton band. B. Chondrocytes were treated with siRNA for OPG or a scramble control for 144 hours. Silencing OPG effectively reduced levels of OPG mRNA (n = 12 per group, P = 0.0001). C. At 96 h post transfection, cells were treated with 100 ng/ml RANKL or no additives for an additional 48 h. PPi levels were then measured in conditioned media. While mRNA was significantly suppressed, there were no significant differences in PPi levels between groups (n = 24 per group, P = 0.377) using GLM.
OPG and RANKL have no effect on eATP levels
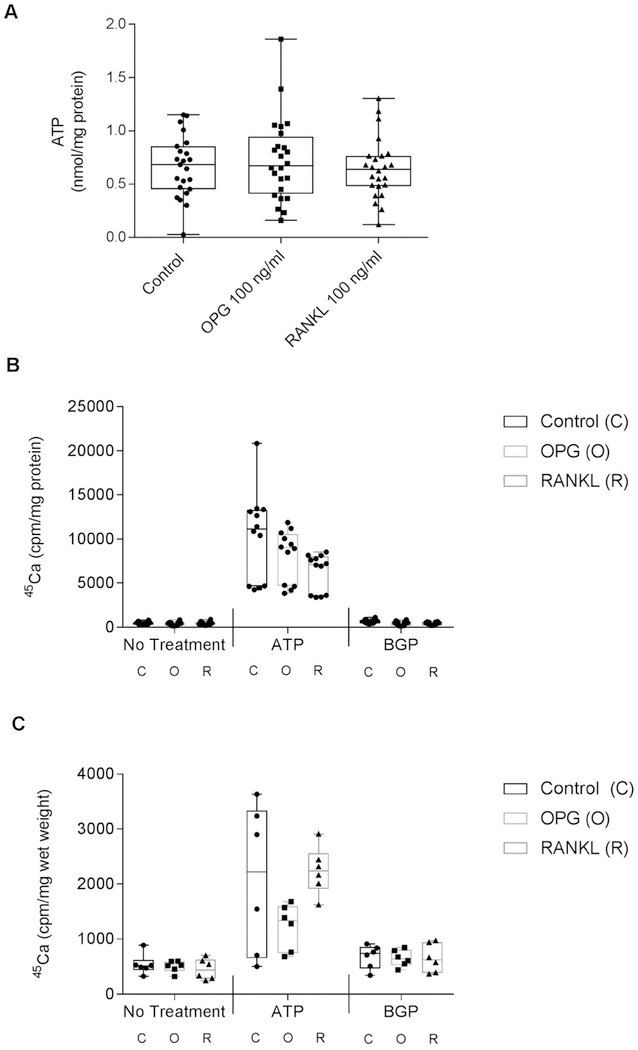
In general, PPi and eATP are coordinately regulated in chon-drocytes26. However, to fully explore the actions of OPG and RANKL on chondrocyte factors important in CPDD, we measured the effect of these proteins on eATP levels. In Fig. 5(A), levels of eATP in conditioned media from OPG- and RANKL-treated chondrocytes were unchanged from controls after 24 h of exposure to these agents. Time points from 30 min to 48 h were tested with no effects on eATP (Data not shown).
Fig. 5. OPG and RANKL do not increase levels of eATP or calcification.
A. Porcine chondrocytes were incubated with no additives (Control), 100 ng/ml OPG, or 100 ng/ml RANKL for 48 h. Extracellular ATP levels were measured in the conditioned media. No significant differences were noted between groups (n = 24 per group, P = 0.8234). Chondrocytes in monolayer cultures (B.) or organ cultures (C.) were incubated with media trace-labeled with 45Ca, in the presence of no additives (Control), 100 ng/ml OPG or 100 ng/ml RANKL with no additional treatment, 1 mM ATP, or 1 mM β glycerophosphate (BGP). 45Ca was measured in the cell layer or cartilage pieces after 96 h. Neither OPG nor RANKL significantly altered ATP-induced calcification of chondrocytes (n = 12 per group, P = 0.0169) or cartilage (n = 6 per group, P = 0.0859).
OPG and RANKL do not affect cartilage or chondrocyte mineralization
CPP mineral formation requires excess PPi, but can be modulated by other factors such as extracellular matrix components27. To investigate the possibility that OPG was acting through factors other than PPi, we added 100 ng/ml OPG or 100 ng/ml RANKL to cartilage or chondrocytes and measured ATP-induced 45Ca retention in the cell layer or cartilage pieces. This correlates to CPP crystal formation. In the presence of ATP, neither OPG nor RANKL significantly altered levels of 45Ca uptake compared to controls, suggesting that these factors do not directly affect CPP crystal formation in cartilage [Fig. 5(B) and (C)].
Discussion
We report here two additional families with the PGOA/CPDD phenotype exhibiting the Stop402Leu OPG mutation8; this confirms that TNFRSF11B is CCAL1. Although genotyping of polymorphic markers around the mutation site were not reported for the Dutch family, genetic studies clearly illustrate that the Stop402Leu mutation arose on distinct genetic backgrounds in our two families. In the Israeli family, the OPG mutation segregated with the deposition of microscopically-verified CPP crystals in affected family members18, and typical radiographic chondrocalcinosis was observed in the Long Island and Dutch families. The resemblance of PGOA/CPDD patients harboring the Stop402Leu OPG mutation to patients with sporadic (non-familial) CPDD increases the significance of this finding. Both phenotypes may share an increased prevalence of osteopenia28; as well as clinically important OA29.
Because the only other gene to show linkage to familial CPP deposition is ANKH, we explored the effect of the OPG Stop402Leu mutant protein on ANKH expression. We demonstrate that the OPG mutation does not affect ANKH mRNA levels in patient fibroblasts, nor does excess OPG affect location or levels of ANKH in normal chondrocytes. The absence of a role for altered ANKH activity in the PGOA/CPDD families is further substantiated by the differences in clinical presentation between families with OPG mutations and those with ANKH mutations. While ANKH-associated families have onset of chondrocalcinosis well before the development of significant degenerative arthritis, families with OPG mutations have simultaneous onset of PGOA and chondrocalcinosis. These findings support the existence of ANKH-independent mechanisms that promote CPPD.
As CPPD is considered a cartilage disease, we sought to identify an effect of normal or mutant OPG or its target ligand, RANKL, on factors known to affect CPP crystal formation in cartilage. We observed no effects of OPG or RANKL on processes involved in CPP crystal formation despite the presence of RANK in chondrocytes. Before we abandoned the idea that OPG directly targets cartilage to produce this phenotype, we exhaustively explored other possibilities. For example, OPG also acts as a decoy receptor for other ligands including TRAIL25,30. We found no evidence of a role for OPG in modulating the actions of TRAIL in cartilage. We also investigated the possibility that the absence of observed effects was due to the short half-life of OPG, but this was not the case. Endogenous levels of OPG were easily measurable in chondrocytes at baseline, but reducing endogenous OPG levels did not alter the actions of RANKL. Indeed, few reproducible effects of OPG or RANKL have been described to date in cartilage or chondrocytes. Komuro et al. found no effect of OPG or RANKL on levels of pro-inflammatory mediators or collagenase in human chondrocytes11. Kwan Tat et al. found that RANKL had no effects on catabolic markers, but a modified form of OPG, missing its heparan binding domain (OPG-Fc), produced increased levels of MMP-13 and PAR-2 in OA chondrocytes through uncertain mechanisms16. We noted no effect of this modified protein. We did not directly investigate the effect of OPG on OA chondrocytes. However, the simultaneous onset of OA and CPDD in these families is not consistent with a mechanism in which OA develops first and then CPDD is a secondary phenomenon.
Ramos et al. suggested that the TNFRSF11B mutation in these families produces a gain of function in OPG. This was based on the ability of mutant OPG to inhibit osteoclastogenesis mediated by soluble RANKL more effectively than wild type OPG. The clinical phenotype in these patients is difficult to reconcile with a gain-of-function in OPG. High levels of OPG should reduce osteoclasto-genesis and would logically result in increased bone density as shown in transgenic rats8,31. While only one affected family member had an available bone density examination, none of our patients had clinically apparent high bone densities on conventional radiographs.
Our findings suggest that cartilage is not the primary target for OPG in CPDD. It is certainly possible that this phenotype is explained by a synovial target. There is increased support however, for a role for altered osteoclastogenesis in subchondral bone as a major contributor to OA32. We believe that taken together with information about the clinical phenotype, that this may be the first example of a primary bone abnormality producing CPDD. Certainly, further work to investigate the regulation and function of normal and mutant OPG in articular tissues will be necessary.
In summary, we have confirmed the identity of CCAL1 as TNFRSF11B (OPG) by finding the identical mutation in two additional kindreds with PGOA/CPDD. Excitingly, these findings implicate a target tissue other than articular cartilage in CPDD for the first time. This phenotype closely resembles that of sporadic CPDD in its association with joint degeneration and osteopenia. Further studies to delineate mechanisms through which mutant OPG affects joint tissues may eventually result in the identification of druggable targets for CPDD.
Supplementary Material
Acknowledgements
We greatly appreciate the generosity of the affected family members of both kindreds. We would like to thank Katherine Sherman, MS for her invaluable biostatistical advice and insight. This work was supported by the VA Research Service (I01 CX001143, AKR and CW).
Financial support
CJ Williams and A Ortiz, Dean’s Fund for Research Development, CMSRU; Veterans Affairs Merit Review Grant, I01 CX001143 (AKR and CJW).
Footnotes
Conflicts of interest
None.
References
- 1.Rosenthal AK, Ryan LM. Calcium pyrophosphate deposition disease. N Engl J Med 2016;374(26):2575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitnan D, Sit’Aj S. Chondrocalcinosis articularis Section L Clinical and radiological study. Ann Rheum Dis 1963;22: 142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes AE, McGibbon D, Woodward E, Dixey J, Doherty M. Localisation of a gene for chondrocalcinosis to chromosome 5p. Hum Mol Genet 1995;4(7):1225–8. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin CT, Farrer LA, Adair R, Dharmavaram R, Jimenez S, Anderson L. Linkage of early-onset osteoarthritis and chondrocalcinosis to human chromosome 8q. Am J Hum Genet 1995;56(3):692–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science 2000;289(5477):265–70. [DOI] [PubMed] [Google Scholar]
- 6.Pendleton A, Johnson MD, Hughes A, Gurley KA, Ho AM, Doherty M, et al. Mutations in ANKH cause chondrocalcinosis. Am J Hum Genet 2002;71(4):933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Lutz MK, Dubyak GR, Ryan LM. The progressive ankylosis gene product ANK regulates extracellular ATP levels in primary articular chondrocytes. Arthritis Res Ther 2013;15(5):R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos YF, Bos SD, van der Breggen R, Kloppenburg M, Ye K, Lameijer EW, et al. A gain of function mutation in TNFRSF11B encoding osteoprotegerin causes osteoarthritis with chondrocalcinosis. Ann Rheum Dis 2015;74:1756–62. [DOI] [PubMed] [Google Scholar]
- 9.Costello JC, Ryan LM. Modulation of chondrocyte production of extracellular inorganic pyrophosphate. Curr Opin Rheumatol 2004;16(3):268–72. [DOI] [PubMed] [Google Scholar]
- 10.Terkeltaub R, Rosenbach M, Fong F, Goding J. Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein-1. Relevance to calcium pyrophosphate dihydrate deposition disease. Arthritis Rheum 1994;37(6):934–41. [DOI] [PubMed] [Google Scholar]
- 11.Komuro H, Olee T, Kuhn K, Quach J, Brinson DC, Shikhman A, et al. The osteoprotegerin/receptor activator of nuclear factor kappaB/receptor activator of nuclear factor kappaB ligand system in cartilage. Arthritis Rheum 2001;44(12):2768–76. [DOI] [PubMed] [Google Scholar]
- 12.Feng ZY, He ZN, Zhang B, Li YQ, Guo J, Xu YL, et al. Adenovirus-mediated osteoprotegerin ameliorates cartilage destruction by inhibiting proteoglycan loss and chondrocyte apoptosis in rats with collagen-induced arthritis. Cell Tissue Res 2015;362(1): 187–99. [DOI] [PubMed] [Google Scholar]
- 13.Feng ZY, He ZN, Zhang B, Chen Z. Osteoprotegerin promotes the proliferation of chondrocytes and affects the expression of ADAMTS-5 and TIMP-4 through MEK/ERK signaling. Mol Med Rep 2013;8(6):1669–79. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Rubio J, Herrero-Beaumont G, Tardio L, Alvarez-Soria MA, Largo R. Nonsteroidal antiinflammatory drugs and prostaglandin E(2) modulate the synthesis of osteoprotegerin and RANKL in the cartilage of patients with severe knee osteoarthritis. Arthritis Rheum 2010;62(2):478–88. [DOI] [PubMed] [Google Scholar]
- 15.Marzaioli V, McMorrow JP, Angerer H, Gilmore A, Crean D, Zocco D, et al. Histamine contributes to increased RANKL to osteoprotegerin ratio through altered nuclear receptor 4A activity in human chondrocytes. Arthritis Rheum 2012;64(10): 3290–301. [DOI] [PubMed] [Google Scholar]
- 16.Kwan Tat S, Amiable N, Pelletier JP, Boileau C, Lajeunesse D, Duval N, et al. Modulation of OPG, RANK and RANKL by human chondrocytes and their implication during osteoarthritis. Rheumatology (Oxford) 2009;48(12):1482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos YF, den Hollander W, Bovee JV, Bomer N, van der Breggen R, Lakenberg N, et al. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PLoS One 2014;9(7):−103056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eshel G, Gulik A, Halperin N, Avrahami E, Schumacher HR, McCarty DJ, et al. Hereditary chondrocalcinosis in an Ashkenazi Jewish family. Ann Rheum Dis 1990;49(7):528–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takashima A Establishment of fibroblast cultures. In: Bonifacino J, Harford J, Lippincott-Schwartz J, Yamada K, Eds. Current Protocols in Cell Biology Wiley Online; 2001. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal AK, Cheung HS, Ryan LM. Transforming growth factor beta 1 stimulates inorganic pyrophosphate elaboration by porcine cartilage. Arthritis Rheum 1991;34(7):904–11. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Grewal R, Ninomiya J, Coyne CB, et al. Autophagy modulates articular cartilage vesicle formation in primary articular chondrocytes. J Biol Chem 2015;290:13028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lust G, Faure G, Netter P, Seegmiller JE. Increased pyrophosphate in fibroblasts and lymphoblasts from patients with hereditary diffuse articular chondrocalcinosis. Science 1981;214(4522):809–10. [DOI] [PubMed] [Google Scholar]
- 23.Ryan LM, Kurup IV, Derfus BA, Kushnaryov VM. ATP-induced chondrocalcinosis. Arthritis Rheum 1992;35(12):1520–5. [DOI] [PubMed] [Google Scholar]
- 24.Standal T, Seidel C, Hjertner O, Plesner T, Sanderson RD, Waage A, et al. Osteoprotegerin is bound, internalized, and degraded by multiple myeloma cells. Blood 2002;100(8): 3002–7. [DOI] [PubMed] [Google Scholar]
- 25.Sandra F, Hendarmin L, Nakamura S. Osteoprotegerin (OPG) binds with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL): suppression of TRAIL-induced apoptosis in ameloblastomas. Oral Oncol 2006;42(4):415–20. [DOI] [PubMed] [Google Scholar]
- 26.Costello JC, Rosenthal AK, Kurup IV, Masuda I, Medhora M, Ryan LM. Parallel regulation of extracellular ATP and inorganic pyrophosphate: roles of growth factors, transduction modulators, and ANK. Connect Tissue Res 2011;52(2):139–46. [DOI] [PubMed] [Google Scholar]
- 27.Jubeck B, Gohr C, Fahey M, Muth E, Matthews M, Mattson E, et al. Promotion of articular cartilage matrix vesicle mineralization by type I collagen. Arthritis Rheum 2008;58(9):2809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abhishek A, Doherty S, Maciewicz R, Muir K, Zhang W, Doherty M. Association between low cortical bone mineral density, soft-tissue calcification, vascular calcification and chondrocalcinosis: a case-control study. Ann Rheum Dis 2014;73(11):1997–2002. [DOI] [PubMed] [Google Scholar]
- 29.Kleiber Balderrama C, Rosenthal AK, Lans D, Singh JA, Bartels CM. Calcium pyrophosphate deposition disease and associated medical comorbidities: a national cross-sectional study of us veterans. Arthritis Care Res (Hoboken) 2016;69(9):1400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zannettino AC, Holding CA, Diamond P, Atkins GJ, Kostakis P, Farrugia A, et al. Osteoprotegerin (OPG) is localized to the Weibel-Palade bodies of human vascular endothelial cells and is physically associated with von Willebrand factor. J Cell Physiol 2005;204(2):714–23. [DOI] [PubMed] [Google Scholar]
- 31.Stolina M, Dwyer D, Ominsky MS, Corbin T, Van G, Bolon B, et al. Continuous RANKL inhibition in osteoprotegerin transgenic mice and rats suppresses bone resorption without impairing lymphorganogenesis or functional immune responses. J Immunol 2007;179(11):7497e505. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Yang J, Liu D, Li J, Niu K, Feng S, et al. Knee loading inhibits osteoclast lineage in a mouse model of osteoarthritis. Sci Rep 2016;6:24668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.