Abstract
Tumor-associated angiogenesis is postulated to be regulated by the balance between pro- and anti-angiogenic factors. We demonstrate here that the critical step in establishing the angiogenic capability of human tumor cells is the repression of a key secreted anti-angiogenic factor, thrombospondin-1 (Tsp-1). This repression is essential for tumor formation by mammary epithelial cells and kidney cells engineered to express SV40 early region proteins, hTERT, and H-RasV12. In transformed epithelial cells, a signaling pathway leading from Ras to Tsp-1 repression induces the sequential activation of PI3 kinase, Rho and ROCK, leading to activation of Myc through phosphorylation, thereby enabling Myc to repress Tsp-1 transcription. In transformed fibroblasts, however, the repression of Tsp-1 can be achieved by an alternative mechanism involving inactivation of both p53 and pRb. We thus describe novel mechanisms by which the activation of oncogenes in epithelial cells and the inactivation of tumor suppressors in fibroblasts permits angiogenesis and, in turn, tumor formation.
INTRODUCTION
The process of neoplastic transformation involves the sequential acquisition of a number of genetic and epigenetic alterations by the genomes of evolving, premalignant cell populations.1,2 These alterations culminate in a deregulation of the growth-controlling circuitry of cells. Among other biological changes, these alterations provide tumor cells with constitutive mitogenic signals, deregulate the control of the cell cycle and, as shown in several laboratories, enable the maintenance of telomeric DNA.3,4 In addition, the alterations that take place during tumor progression enable the tumor to interact with its stromal environment in ways that enhance its ability to proliferate in the primary site and, in malignant primary tumors, to metastasize to distant sites in the body.5,6
A key component of the tumor-associated stroma is the neovasculature, which supplies oxygen, nutrients and growth-promoting signals to the tumor cells and removes metabolic waste generated by the tumor.7 The newly acquired vasculature may also serve as a conduit through which tumors can disseminate to distant sites.8–10 These observations underscore the importance of elucidating the cancer cell-specific processes that enable tumors to interact with the pre-existing vasculature and to induce the formation of neovasculature.
Observations of tumor growth have indicated that small tumor masses of 1–2 mm diameter can persist in a tissue without acquiring any tumor-specific neovasculature.11–14 The limitations to further growth of such non-vascularized tumors have been attributed to the effects of hypoxia at the center of the tumor, because the diffusion of oxygen through living tissue is effectively limited to distances less than 200 μm.15 A substantial body of evidence indicates that tumors can emerge from this growth arrest by developing a neovasculature, a discrete change in phenotype that has been termed the ‘angiogenic switch’.12,16
Several growth factors act as positive regulators of angiogenesis. Foremost among these are vascular endothelial growth (VEGF-A),17 basic fibroblast growth factor,18 HGF,19,20 interleukin-621 and interleukin-8.22 Conversely, proteins such as thrombospondin-1 (Tsp-1),23 angiostatin,24 endostatin,25 tumstatin26 and placental growth factor27 function as inhibitors of angiogenesis.
Tsp-1 was the first naturally occurring inhibitor of angiogenesis to be identified.23 The Tsp-1 secreted by cells inhibits the activity of MMP-9,28 an extracellular matrix metalloproteinase that releases VEGF-A sequestered in the extracellular matrix.29 In addition, Tsp-1 can act directly to inhibit angiogenesis by binding to the CD36 receptor protein, which is present on the luminal surface of endothelial cells in mature blood vessels, as well as by binding to β1-integrins.30,31 In an effort to more closely re-create the signaling conditions that operate in spontaneously arising human tumors, we created transformed cell lines in which experimentally immortalized human kidney and mammary epithelial cells were constructed to express relatively low levels of the H-RasV12 oncoprotein, thereby mirroring its expression levels in such tumors.32 Having done so, we discovered that the mammary epithelial and human embryonic kidney cells expressing the SV40 early region proteins, hTERT and relatively low levels of H-RasV12 were either unable to form tumors when injected subcutaneously into nude mice or did so only with long latency. As described herein, we have discovered that the prime defect of these cells derived from their inability to effectively provoke neoangiogenesis. We therefore set out to determine how signaling by the Ras oncoprotein enables cells to emerge from a non-angiogenic, poorly tumorigenic state. We also investigate whether Ras signaling plays an equally important role in the regulation of angiogenesis in fibroblasts and epithelial cells.
RESULTS
Effect of Ras oncoprotein levels on Tsp-1 expression
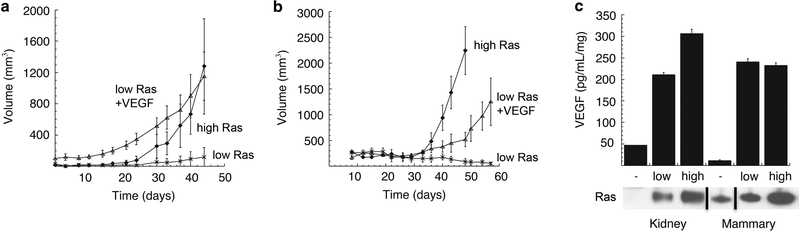
We found that human mammary epithelial- and kidney-derived cells that express the SV40 early region, hTERT and relatively low levels of oncogenic H-RasV12G (~3–7× above endogenous levels of wild-type Ras expression, Figure 1c) form small tumors of approximately 1–2 mm in diameter that never progress beyond this size (Figure 1a). This behavior contrasted with that of the corresponding cells expressing higher levels of the Ras oncoprotein (12–50× above endogenous levels), which succeeded in forming tumors of substantial size (1.5 cm diameter) within 3–7 weeks after implantation into host mice (Figure 1a). We speculated that the inability of the low Ras-expressing mammary and kidney epithelial cells to grow beyond the diameter of 1–2 mm was attributable to a deficiency in angiogenesis.
Figure 1.
Effects of VEGF-A on tumor formation Growth curve of tumors formed by kidney-derived cells (a) and breast-derived cells (b) expressing low levels of Ras, low levels of Ras+VEGF-A or high levels of Ras. (c) ELISA of secreted VEGF-A by kidney and mammary derived cells expressing no (−), low and high levels of oncogenic Ras grown in 0.1% O2. Black lines represent cases where lanes were digitally removed from the scanned image.
To test this hypothesis directly, we measured the amount of VEGF-A secreted by polyclonal populations of cells expressing hTERT, SV40 early region and various levels of oncogenic H-RasV12 by ELISA. We found that the ectopic expression of low levels of the Ras oncoprotein dramatically stimulated the production and secretion of VEGF-A in cells grown in 0.1% serum and 0.4% O2 compared with the parental immortalized cells expressing only hTERT and SV40 early region (Figure 1a). However, further increases in the expression levels of the Ras oncoprotein resulted in only a relatively modest 1.4-fold further increase in VEGF-A production and secretion by both epithelial cell types (Figure 1c). Hence, the great differences in tumor-forming ability of the high-Ras-versus low-Ras-expressing cells elevations could not be ascribed to significant differences in VEGF-A secretion.
Importantly, the cells expressing low levels of the Ras oncoprotein were not impaired in their ability to proliferate in vitro; for this reason, we continued to investigate whether other mechanisms regulating neoangiogenesis might be responsible for their observed behavior in vivo.32 Thus, we overexpressed murine VEGF-A 164 (a potently angiogenic isoform of this protein) in the low Ras-expressing kidney and mammary epithelial cells and injected them subcutaneously into nude mice. In both cases, these mice formed tumors with equivalent efficiency to the high Rasexpressing cells (Figures 1a and b). Taken together, these results indicated that the differences in tumorigenicity of the low and high Ras-expressing cells could be ascribed to differing abilities to stimulate neoangiogenesis. However, these differing angiogenic powers were not because of significant differences in VEGF-A secretion. Moreover, these limitations could be overridden by forced high expression of VEGF-A.
The balance of pro- and anti-angiogenic factors regulates angiogenesis
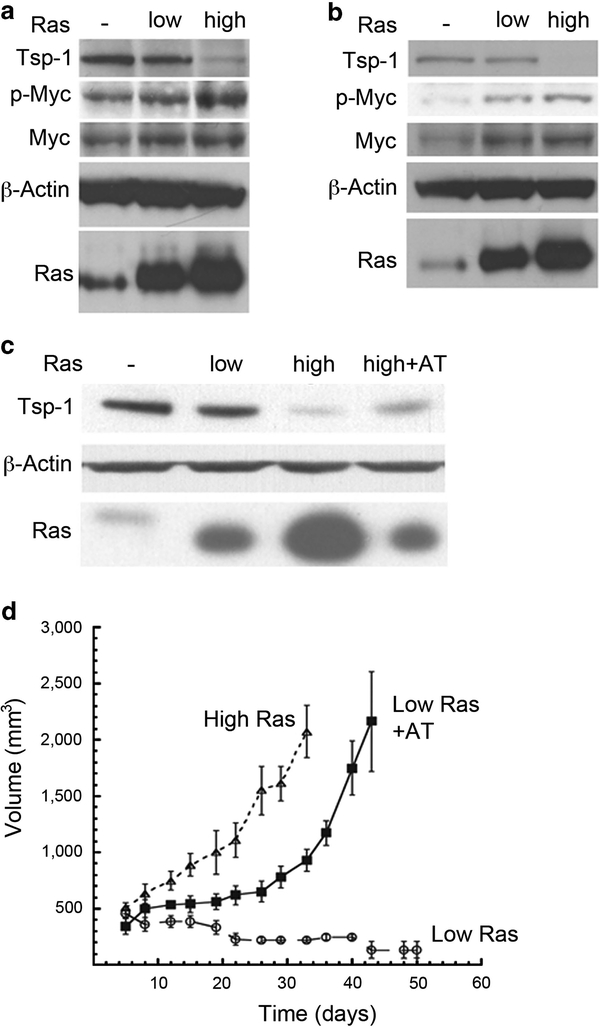
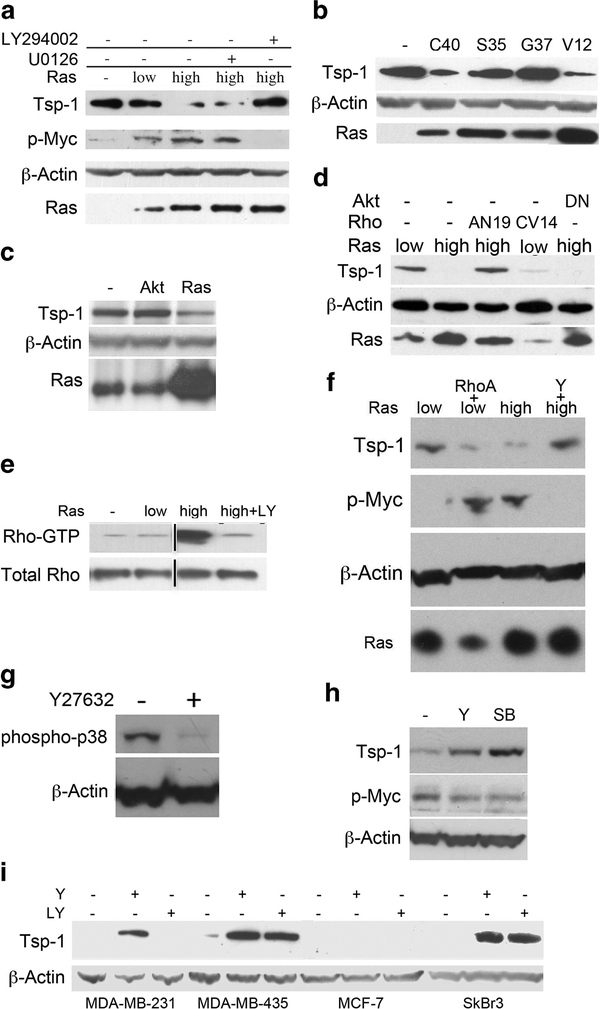
These various observations suggested that the observed differences in angiogenicity between low and high Ras-expressing cells might be traced to regulators of neovascularization other than VEGF-A. Accordingly, we turned our attention to the antiangiogenic factor, Tsp-1, and its expression, as it has been demonstrated that oncogenic Ras can inhibit the expression of Tsp-1.33–37 An immunoblot analysis of proteins extracted from both the mammary and kidney cells revealed that the levels of Tsp-1 expression were essentially unchanged between the parental immortalized cells not expressing introduced oncogenic Ras and those that expressed low levels of introduced Ras (Figure 2a). However, the mammary and kidney cells that were forced to express high levels of oncogenic Ras exhibited between six- and eightfold lower levels of Tsp-1 (Figures 2a and b) relative to those expressing low levels of the oncoprotein. These results confirmed that high levels of Ras oncoprotein can indeed suppress Tsp-1 expression and suggested, in turn, that the angiogenesis deficiency of the low Ras-expressing cells was because of high levels of Tsp-1 that they produced.
Figure 2.
Tsp-1 suppression stimulates tumor growth immunoblot analysis of Tsp-1, Myc, phosphorylated Myc (p-Myc), β-Actin and Ras proteins expressed in kidney (a) and breast-derived (b) cells. (c) Immunoblot analysis of Tsp-1, β-Actin and Ras expressed by kidney-derived cells expressing no (−), low or high levels of Ras or of cells expressing low Ras plus anti-sense Tsp-1 (AT). (d) Growth curves of tumors formed by kidney-derived cells expressing low or high levels of Ras and cells expressing low Ras plus anti-sense Tsp-1 (AT).
Responding to these various findings, we hypothesized that an unfavorable ratio of VEGF-A to Tsp-1 might preclude neoangiogenesis in the tumors formed by the low Ras-expressing cells. Such a model predicted, among other things, that we could confer a tumorigenic phenotype on the low Ras-expressing cells simply by reducing the amount of Tsp-1 that they expressed. To test this notion, we introduced a retroviral vector specifying anti-sense THBS1, the Tsp-1 gene, into the low Ras-expressing cells. We found that this anti-sense construct reduced the levels of Tsp-1 protein expression roughly fourfold compared with the levels produced by low Ras-expressing cells (Figure 2c).38 Importantly, these altered cells subsequently formed subcutaneous tumors in Nude mice with a latency and kinetics that were comparable to those of the high Ras-expressing cells, reaching the diameter of ~ 1.5 cm within 7 days of the tumors formed by the aggressively tumorigenic high-Ras cells (i.e., 34 vs 41 days) (Figure 2d). As anticipated, the parental low-Ras cells expressing only the drug resistance marker (zeocin) were unable to form tumors during this period (Figure 2d). This observation confirmed that the level of Tsp-1 expression strongly influences the tumorigenicity of these tumor cells, and that this tumorigenicity can be achieved either by increasing the expression of the pro-angiogenic protein VEGF-A or by reducing the expression of the anti-angiogenic protein Tsp-1.
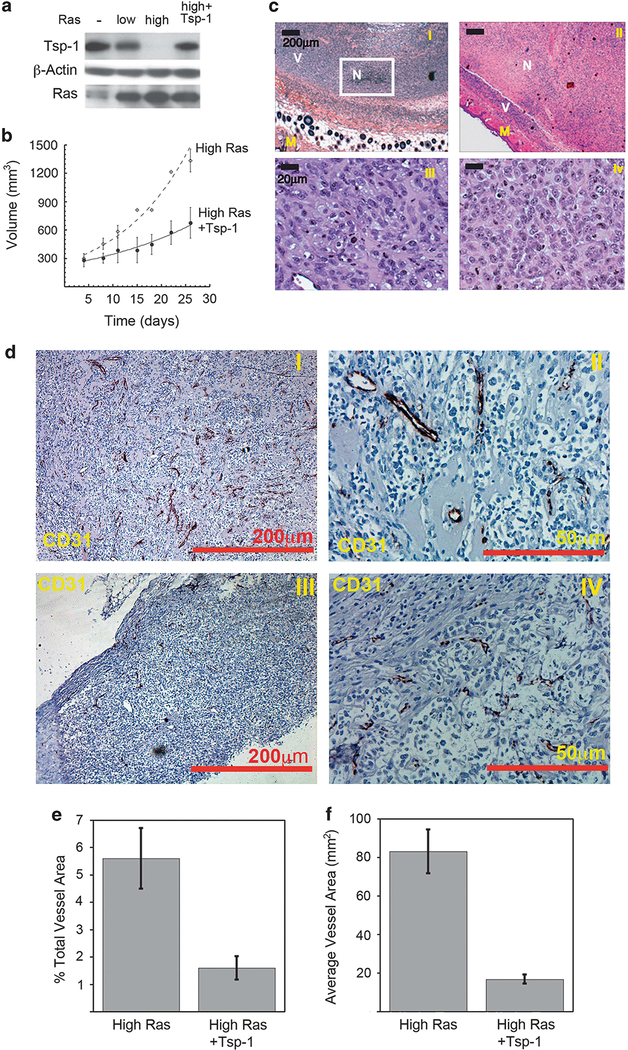
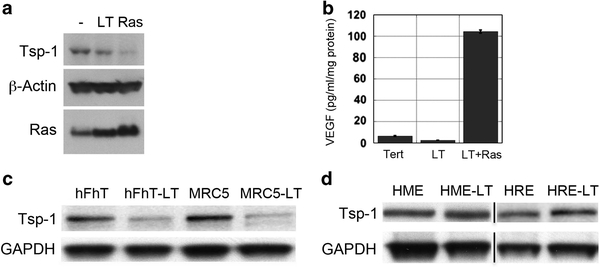
To further substantiate the role of Tsp-1 in tumor formation, we transduced the high Ras-expressing kidney cells with a construct specifying Tsp-1. The resulting cells expressed Tsp-1 levels similar to those of the low Ras-expressing cells (Figure 3a). When these modified cells were injected subcutaneously into nude mice, the Tsp-1-expressing cells formed tumors ~60% smaller than the high Ras-expressing cells transduced with a control empty vector (Figure 3b). However, histological examination revealed that the centers of the Tsp-1-expressing tumors were completely necrotic (comprising 60–90% of the total tumor volume) with viable cells present in only the periphery of the tumor (Figure 3c, panels I and II). In contrast, tumors formed by the cells expressing high Ras, but not Tsp-1, exhibited only sparse patches of necrosis comprising 5–10% of these tumors (Figure 3c, panel I). When tumor size and viability were taken into account, the viable tumor burden of the mice bearing high Ras-expressing cells was more than eightfold greater than that of the derived tumors formed by cells forced to overexpress Tsp-1. Consistent with an impairment in angiogenesis, the rims of viable cells in tumors formed by Tsp-1-expressing cells were no more than 200 μm in thickness at any given point (Figure 3c, panel II). Moreover, CD31 immunohistochemistry revealed that tumors formed by cells forced to express high levels of Tsp-1 had decreased microvessel density (Figures 3d and f). In addition, the vessels that were observed in the tumors were smaller and appeared to be non-functional based on vessel diameter (Figure 3d).
Figure 3.
Tsp-1 overexpression inhibits tumor growth by inhibiting angiogenesis. (a) Immunoblot analysis of Tsp-1, β-Actin and Ras expressed by kidney-derived cells expressing no (−), low or high levels of Ras or of cells expressing high Ras plus Tsp-1. (b) Growth curves of tumors formed by kidney-derived cells expressing high levels of Ras and cells expressing high Ras plus Tsp-1. (c) H&E staining of tumors formed by cells expressing high levels of Ras plus control vector (I and III) and high levels of Ras plus Tsp-1 (II and IV). Panels I and III are × 4 magnification and panels II and IV are × 40 magnification. M denotes normal mouse tissue, V denotes areas of viable tumor cells, N denotes areas of necrosis. (d) CD31 staining of tumors formed by kidney-derived cells expressing high levels of Ras plus control vector (I and II) and tumors formed by cells expressing high levels of Ras plus Tsp-1 (III and IV). Panels I and III are ×5 magnification and panels II and IV are × 20 magnification scale bar = 200 μm in × 5 panels and 50 μm in × 20 panels. (e) Microvessel density quantification of tumors expressing high Ras plus vector control or high Ras plus Tsp-1 as percent vessel area per field. (f) Plot of average area of vessels in tumors expressing high Ras plus vector control or high Ras plus Tsp-1.
It has been reported that Tsp-1 can inhibit tumor growth in an angiogenesis-independent manner. Specifically, Tsp-1 has been found to stimulate macrophage recruitment and phagocytic activity.39–41 Accordingly, we analyzed the control tumors and those forced to express high levels of Tsp-1 for macrophage infiltration. We observed that, irrespective of Tsp-1 expression, there was little to no inflammation in the tumors as measured by macrophage infiltration as judged using Mac-3 immunohistochemistry (Supplementary Figure S1). These findings suggest that in this model system, Tsp-1 inhibits tumor growth predominantly by inhibiting angiogenesis.
The role of Myc in Tsp-1 regulation
Ras signaling has been demonstrated to affect the stability of the Myc protein.42 Furthermore, previous work had suggested a role for Myc in the repression of Tsp-1 expression.43,44 Indeed, Myc has been shown to be able to activate and directly repress the expression of a number of genes in addition to the THBS1 gene of interest here.45,46 For this reason, we decided to examine the effect of Ras signaling on the levels of Myc protein expression in both the cultured mammary cells and kidney cells, doing so at 0.1% serum in order minimize signals deriving from mitogenic sources other than oncogenic Ras, which was expressed experimentally at either low or high levels. We observed that Myc protein levels were unaffected by the expression levels of the Ras oncoprotein (Figures 2a and b). This ruled out the possibility that high levels of Ras were inducing the accumulation of Myc protein, thereby mimicking the overexpression of Myc observed in a variety of human tumors.47–49 Hence, if the Myc protein were responsible for repression of the transcription of the Tsp-1 gene in the high Ras cells, this action depended on a mechanism distinct from an increase in the steady-state levels of this protein.
Because high levels of Ras were not inducing increased levels of Myc, we reasoned that Ras might be affecting the functional state of the Myc protein as governed by its phosphorylation. Specifically, phosphorylation of Myc at its residues T58 and S62 is known to alter its ability to transactivate gene expression50,51 as well as its metabolic stability.42 To pursue this possibility, we examined the phosphorylation state of Myc in these various cell populations. When cells were grown in 0.1% serum, we observed a modest increase of phosphorylated Myc in low Ras cells compared with the levels in immortalized cells not expressing oncogenic Ras (Figures 2a and b). However, the amount of phosphorylated Myc was dramatically increased in the cells expressing high levels of oncogenic Ras when compared with the level seen in low Ras cells, as determined by immunoblot analysis with an antibody that recognized Myc protein either singly phosphorylated at its residue T58 or doubly phosphorylated at residues T58 and S62 (Figures 2a and b). These results demonstrated that the Myc phosphorylation modulated by Ras was positively correlated with the repression of Tsp-1 expression.
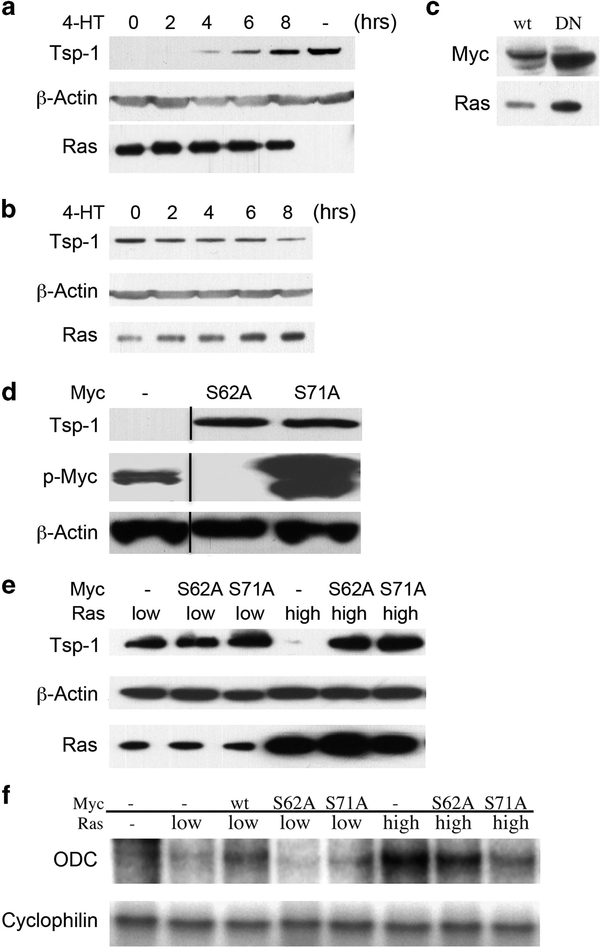
We then sought to determine whether the differences in Myc phosphorylation in the low and high Ras-expressing cells were functionally relevant to the repression of Tsp-1. To do so, we introduced an inducible dominant-negative version of Myc (DNMycER), in which it is fused to a mutant version of the estrogen receptor (ER), into the high Ras-expressing kidney cells.52,53 In the absence of the ER ligand, 4-hydroxy-tamoxifen (4-HT), the fusion protein is functionally inactive; following 4-HT addition, however, it is rapidly activated and migrates to the nucleus. This fusion protein therefore made it possible to induce DN Myc activity through the addition of 4-HT to the growth medium.
In high Ras-expressing kidney cells that also expressed DNMycER, treatment with 4-HT caused the level of Tsp-1 protein to increase within 4h, eventually reaching that of the cells expressing no oncogenic Ras by 8 h after 4-HT addition. In contrast, mock-treated and control cells were unchanged in their expression of Tsp-1 protein (Figure 4a). Together, these results demonstrated that active Myc was indeed responsible for the repression of Tsp-1 expression. As a control, we transduced a construct specifying wild-type Myc fused to the same modified version of the estrogen receptor (MycER) into low Ras-expressing kidney cells52 (Figure 4c). Upon treatment with 4-HT, these cells exhibited reduced expression of Tsp-1 protein within 4h, decreasing to the Tsp-1 levels made by high Ras-expressing kidney cells within 8h after 4-HT addition (Figure 4b). Furthermore, mock-treated cells were unchanged in their expression of Tsp-1 (Figure 4b). We concluded from these results that Myc activity is required for the repression of Tsp-1 by Ras, and that interference with endogenous Myc activity is sufficient to abolish this repression.
Figure 4.
Effects of Myc activity on Tsp-1 expression. (a) Immunoblot analysis of Tsp-1, β-Actin and Ras proteins expressed by kidney- derived cells expressing high levels of oncogenic Ras plus dominant-negative MycER after various treatment times with 4-HT. (b) Immunoblot analysis of Tsp-1, β-Actin and Ras proteins expressed by kidney-derived cells expressing low levels of oncogenic Ras plus MycER after various treatment times with 4-HT. (c) Immunoblot analysis of Myc, β-Actin and Ras proteins in low Ras cells expressing MycER (wt) and high Ras cells expressing DNMycER (DN). (d) Immunoblot analysis of Tsp-1, phospho Myc, β-Actin and Ras proteins expressed by kidney-derived cells expressing high levels of oncogenic Ras transfected with S62AMyc (62) or S71AMyc (71) genes. (e) Immunoblot analysis of Tsp-1, β-Actin and Ras proteins expressed by kidney-derived cells expressing low levels of oncogenic Ras, or high levels of oncogenic Ras that were mock transfected or transfected with S62AMyc (62) and S71AMyc (71) genes. (f) Ribonuclease protection assay of ODC and cyclophilin expressed in kidney-derived cells expressing no oncogenic Ras (−), low levels of oncogenic Ras, or high levels of oncogenic Ras that were mock transfected or transfected with wtMyc (58), S62AMyc (62) or S71AMyc (71) genes. Black lines represent cases where lanes were digitally removed from the scanned image.
In order to assess the role of Myc phosphorylation in greater detail, we utilized two phosphorylation-defective mutants of Myc containing alanine substitutions at two known sites of phosphorylation—S62A and S71A. In particular, the phosphorylation of S62 has been shown to increase the stability of c-Myc, whereas the phosphorylation of S71 has not been linked to any specific activity or property of the protein.54 We then transiently transfected the human Ras-expressing kidney cells with constructs expressing each of the phosphorylation-defective mutant Myc proteins; unlike the mammary epithelial cells, these kidney cells could be readily transfected. Strikingly, transient transfection of high Ras-expressing cells with MycS62A resulted in the loss of Tsp- 1 repression, that is, the reappearance of significant levels of Tsp-1 (Figure 4d). This suggested that the ectopically expressed MycS62A protein was acting in a dominant-negative manner, thereby interfering with the ability of endogenously expressed Myc to repress Tsp-1 expression. Furthermore, by immunoblot analysis with an anti-phosphoMyc antibody, which recognizes Myc that is singly phosphorylated at T58 or doubly phosphorylated at T58 and S62, we determined that phosphorylation at residue 58 was also abolished by expression of the S62A mutant (Figure 4d). This could be attributed to either of two previous observations: (i) the preferential degradation of Myc protein singly phosphorylated at T58; or (ii) the process of processive phosphorylation—that is, initial phosphorylation at residue 62 being a prerequisite for subsequent phosphorylation at residue 58.55
Although no role for phosphorylation at residue S71 has previously been identified, we found that transient expression of a mutant Myc carrying a single amino acid substitution at residue 71 (that is, S71A) also acted in dominant-negative manner to relieve the repression of Tsp-1 (Figures 4d and e). All the while, transient transfection of low Ras-expressing kidney cells with Myc bearing two substitutions (S62A and S71A) had no effect on the level of Tsp-1 protein (Figures 4d and e). In addition, expression of MycS71A had no effect on the phosphorylation of Myc at T58 and S62 in the high Ras-expressing cells (Figure 4d). This result extended observations of others that Myc phosphorylation is processive, by indicating that phosphorylation of Myc at S71 depends on prior phosphorylation of residues T58 and S62.55
To further control for the effects of alterations of Myc on its function, the effects of the phosphorylation-defective mutants were then assayed on an established target of Myc transcriptional activation—the gene encoding ornithine decarboxylase (ODC)—using a ribonuclease protection assay. Transient transfection of the low Ras-expressing kidney cells with wild-type Myc resulted in the upregulation of ODC mRNA as anticipated, whereas S62A and S71A had no effect on its levels (Figure 4f). At the same time, transient transfection of the high Ras-expressing kidney cells with either S62AMyc or S71AMyc resulted in the downregulation of ODC mRNA (Figure 4f). These results confirmed that phosphorylation at S62 and S71 is required for Myc to function both as a transactivator of ODC and a repressor of Tsp-1.
Mechanism of repression of Tsp-1 expression by Ras
The above experiments indicated that signaling downstream from Ras was regulating the repression of THBS1, and that this repression was dependent upon the activity of Myc. We therefore decided to further characterize the functional interactions between these two oncoproteins and the THBS1 gene. To begin, we sought to determine which of the effector pathways down-stream of Ras was responsible for suppressing Tsp-1 expression. The three major Ras effector pathways enumerated to date involve the Raf-MAPK cascade, the phosphatidyl inositol-3 kinase (PI3K) enzyme and the Ral guanine nucleotide dissociation stimulator (RalGDS) protein.56
In order to dissect the respective contributions of these three Ras effector pathways to Myc-mediated Tsp-1 repression, we used chemical inhibitors of the Raf and PI3K pathways. Treatment of the high Ras-expressing kidney cells with U0126, a specific inhibitor of MEK1/257 that blocks ERK1/2 phosphorylation in the Raf-MAPK pathway, had no effect on the level of Tsp-1 protein (Figure 5a). In contrast, treatment of the high Ras-expressing cells with the PI3K inhibitor LY29400258 completely abrogated Tsp-1 repression and restored Tsp-1 protein levels to those seen in cells not expressing oncogenic Ras (Figure 5a). Consistently, Myc phosphorylation was strongly inhibited in high Ras-expressing cells treated with LY294002, whereas treatment with the U0126 MEK1/2 inhibitor had no effect on Myc phosphorylation (Figure 5a). These results allowed the tentative conclusion that the PI3K effector pathway plays a major role in the Ras-mediated repression of Tsp-1 and that this pathway acts via Myc phosphorylation to achieve this repression.
Figure 5.
Effects of Ras signaling pathways on Myc phosphorylation and Tsp-1 expression. (a) Immunoblot analysis of Tsp-1, phospho Myc, phospho ERK1/2, β-Actin and Ras proteins expressed by kidney-derived cells expressing no oncogenic Ras (−), low levels of oncogenic Ras or high levels of oncogenic Ras that were otherwise untreated or treated with either UO126 or LY294002. (b) Immunoblot analysis of Tsp-1, β-Actin and Ras proteins expressed by kidney-derived cells expressing no oncogenic Ras (−), RasV12C40 (C40), RasV12G37 (G37) and RasV12S35 (S35), or high levels of oncogenic Ras (V12). (c) Immunoblot analysis of Tsp-1, β-Actin and Ras proteins expressed by kidney-derived cells expressing no oncogenic Ras (−), no oncogenic Ras plus myristoylated Akt (Akt) or high levels of oncogenic Ras. (d) Immunoblot analysis of Tsp-1, β-Actin and Ras proteins expressed by kidney-derived cells expressing low levels of oncogenic Ras, low levels of oncogenic Ras plus RhoCV14 (C14), high levels of oncogenic Ras, high levels of oncogenic Ras plus RhoAN19 (AN19) and high Ras plus dominant-negative Akt (DNAkt). (e) Immunoblot analysis of GTP-bound Rho and total Rho in kidney- derived cells expressing no oncogenic Ras (−), low levels of oncogenic Ras, high levels of oncogenic Ras or high levels of oncogenic Ras plus LY294002. (f) Immunoblot analysis of Tsp-1, phospho Myc and Ras proteins expressed by kidney-derived cells expressing low levels of oncogenic Ras, low levels of oncogenic Ras plus RhoAV14 (AV14), high levels of oncogenic Ras or high levels of oncogenic Ras plus Y27632. (g) Immunoblot analysis of phospho p38MAPK and β-Actin levels in human mammary epithelial cells expressing high levels of oncogenic Ras that were untreated (−) or treated with Y27632. (h) Immunoblot analysis of Tsp-1, phospho Myc and β-Actin expressed by human mammary epithelial cells expressing high levels of oncogenic Ras that were untreated (−) or treated with Y27632 (Y) or SB203580 (SB). (i) Immunoblot analysis of Tsp-1 and β-actin expressed in human breast cancer cell lines treated with Y27632, LY294002. Black lines represent cases where lanes were electronically removed from the scanned image.
These results were confirmed and extended by analyzing Tsp-1 expression in cells ectopically expressing effector loop mutants of Ras that signal primarily through only one of the three major downstream effector pathways.59 Only the PI3-kinase effector loop mutant (C40), which retains PI3K-activating ability but lacks the other two effector functions, was able to downregulate Tsp-1 expression. In contrast, the Ras mutant that retained the ability to activate the RalGDS (G37) pathway had no effect on Tsp-1 levels, and selective activation of the Raf pathway by the third mutant (S35) actually increased Tsp-1 expression (Figure 5b). Taken together, these results indicated that the ability of Ras to downregulate Tsp-1 expression is due largely if not entirely to its ability to upregulate PI3K signaling.
PI3 kinase repression of Tsp-1
We next sought to determine the downstream effectors of PI3K involved in the repression of Tsp-1. In fact, PI3K, acting via its ability to generate PIP3, can affect multiple downstream signaling pathways. The best-studied effect of PI3K involves its actions on the Akt/PKB kinase.60 Accordingly, we attempted to mimic the actions of PI3K by expressing a constitutively active mutant of Akt that contains a myristoylation sequence at its carboxyl terminus.61 We found, however, that cells not expressing oncogenic Ras but expressing the constitutively active version of Akt failed to downregulate Tsp-1 protein levels (Figure 5c). An essential role of Akt/PKB in Tsp-1 repression could be further excluded by retroviral transduction of a dominant-negative mutant of Akt62 into the high Ras-expressing kidney cells (Figure 5d). As this mutant had no effect on the expression of Tsp-1, we concluded that Akt signaling was neither necessary nor sufficient for the Ras-mediated repression of Tsp-1.
Having excluded a role for Akt in Tsp-1 repression, we turned our attention to other molecules activated by PIP3, the product of the PI3K enzyme. In fact, several guanine exchange factors for the Rho family of GTPases have been identified that contain the PH domains that are known to bind PIP3.63 To determine whether Rho proteins were likely to be involved in mediating the PI3K effects on Myc and Tsp-1, we assessed the level of Rho activation by measuring the levels of GTP-bound Rho using a GST-Rhotekin (which only binds the GTP-bound form of Rho) pull-down assay.64 The pull-down assay indicated that the amount of GTP-bound Rho was significantly higher in high-Ras expressing cells than in low Ras-expressing cells (Figure 5e). Furthermore, treatment of the high Ras cells with the LY294002 PI3K inhibitor reduced the level of GTP-bound Rho compared with that seen in the cells expressing no introduced Ras oncoprotein (Figure 5e).
These observations indicated a correlation between the level of Myc phosphorylation, Tsp-1 repression and Rho activation. This suggested, in turn, the possibility that PI3K was acting through Rho proteins to achieve Myc phosphorylation and Tsp-1 repression. To assess the possible role of Rho as an intermediary in this signalling cascade, we introduced a dominant-negative mutant allele of the RhoA gene (RhoAN19)65 into the high Ras-expressing kidney cells. Ectopic expression of RhoAN19 relieved the repression of Tsp-1 in high Ras-expressing cells, restoring the level to that observed in the low Ras-expressing cells (Figure 5d). We further explored the possible involvement of Rho by ectopically expressing mutant, constitutively active versions of RhoA and RhoC in the low Ras cells. Indeed, when these cells were infected with a retroviral vector expressing RhoCV14 or transiently transfected with a vector expressing RhoAV14, Tsp-1 expression was suppressed66 (Figures 5d and f). Taken together, these observations provided a strong indication that a Rho protein serves as a conduit through which the Ras protein releases signals that lead to Tsp-1 repression.
We next sought to identify the particular downstream effector of Rho involved in the repression of Tsp-1. Two of the major effectors of both RhoA and Rho C are p160ROCKI and ROCKII (Rho-associated coiled-coil-containing protein kinase).67,68 Inhibition of ROCKs with the specific kinase inhibitor, Y27 6 32,69 is known to inhibit Ras-induced focus formation and transformation.70 In our hands, treatment of the high Ras-expressing cells with Y27632, which has a specificity for both ROCK I and II that is more than 3 orders of magnitude greater than for other kinases, relieved the repression of Tsp-1 expression, restoring the level to that of low Ras-expressing cells and simultaneously abolishing the phosphorylation of Myc (Figure 5f). Together, these lines of evidence indicate that Rho signaling is necessary and sufficient for Tsp-1 repression, and that this repression is achieved through the actions of a Rho-associated kinase, either ROCK-I or II.
These experiments did not, on their own, reveal whether ROCK I or II was directly responsible for phosphorylating Myc or, alternatively, whether they acted on Myc via a kinase cascade involving an intermediary kinase or kinases. As such we investigated whether p38MAPK, which has been shown to be downstream of Rho and ROCK,71 was involved in the signal transduction cascade. Of note, it has been demonstrated that a dominant-negative mutant of p38 is able to block EGF-induced phosphorylation of c-Myc.72 Accordingly, we treated cells with the ROCK inhibitor Y27632 and observed that phosphorylation of p38 was virtually abolished (Figure 5g). We then treated cells with a p38MAPK inhibitor, SB203580,73 and observed that Myc phosphorylation was decreased and Tsp-1 expression levels increased to virtually the same extent as achieved by the ROCK inhibitor Y27632 (Figure 5h). These results strongly suggest that p38MAPK lies downstream of Rho-Kinase and upstream of c-Myc in the Rasmediated repression of Tsp-1.
We then examined whether human breast cancer cell lines also utilized the PI3K-Rho pathway to repress Tsp-1. Thus, we treated MDA-MB-231, MCF7, SkBr3 and MDA-MB-435 cells with either LY294002 or Y27632 and measured the resulting Tsp-1 levels by immunoblot. We observed that either LY294002 or Y27632 relieved the repression of Tsp-1 in MDA-MB-231, SkBr3 and MDA-MB-435 cells, while having no effect on MCF7 cells (Figure 5i). Notably, these cell lines activate the PI3K/Rho pathway via distinct mechanisms, either through mutation in K-Ras (MDA-MB-231), overexpression of HER2 (SkBr3) or alteration of the PI3 kinase pathway (MDA-MB-435).74–76 At the same time, MCF7 cells, in which the myc locus on chromosome 8 has been amplified and myc expression is driven by ER, may only require minimal signaling from Ras or PI3 kinase to repress Tsp-1, potentially explaining why these cells were insensitive to chemical inhibitors that affect this pathway.77,78 These results confirm that this pathway is active in a subset of human breast cancer cell lines, in which it ostensibly plays a role in repressing the expression of the anti-angiogenic protein Tsp-1.
Tsp-1 repression in fibroblasts does not require oncogenic Ras activity The results described above trace a novel signal transduction pathway leading from Ras via PI3K to Rho and thereafter to Myc, resulting in Tsp-1 repression in experimentally transformed human epithelial cells and human breast cancer cell lines. Because both human epithelial cells and fibroblasts can be transformed via the introduction of hTERT, SV40 early region and HRasV12,32,79 we sought to determine whether the repression of Tsp-1, which we predicted would be required for tumor formation in both cell types, was achieved via the same pathway in epithelial cells and fibroblasts. Accordingly, we measured the expression levels of Tsp-1 protein in human dermal fibroblasts expressing either hTERT alone, hTERT and SV40 early region, or hTERT, SV40 early region and oncogenic Ras. In stark contrast to what we observed previously in various types of transformed epithelial cells, we observed that Tsp-1 levels were dramatically reduced upon the introduction into the dermal fibroblasts of hTERT and the SV40 early region with only modest additional reduction of expression conferred by oncogenic Ras expression (Figure 6a). Hence, in these fibroblasts Ras oncoprotein function was not required for repression of Tsp-1 expression.
Figure 6.
Tsp-1 is differentially regulated in fibroblasts and epithelial cells. Western blot analysis of (a) immunoblot analysis of Tsp-1, β-Actin and Ras proteins expressed by human dermal fibroblasts expressing hTERT (−), hTERT and SV40 early region (LT) and hTERT, SV40 early region and Ras (Ras). (b) ELISA of secreted VEGF-A by human dermal fibroblasts expressing hTERT (−), hTERT and SV40 early region (LT) and hTERT, SV40 early region and Ras (Ras). (c) Tsp-1 and GAPDH expression in human dermal fibroblasts (hFhT) or lung fibroblasts (MRC5) expressing hTERT alone or with SV40 LTC. (d) Tsp-1 and GAPDH expression in mammary and renal epithelial cells expressing hTERT alone (HME, HRE) or with SV40 LTC (HM-LT, HR-LT). Black lines represent cases where lanes were electronically removed from the scanned image.
We also examined whether oncogenic Ras had any effect on the expression of the pro-angiogenic factor VEGF-A in these fibroblasts. We found that SV40 early region had no effect on the secretion of VEGF-A by human fibroblasts but that the level of VEGF-A secretion was stimulated ~ 20-fold following the introduction of oncogenic H-RasV12 into these cells (Figure 6b). Hence, fibroblasts responded to introduced oncogenes in one way that was very different from the previously examined epithelial cells: the expression of Tsp-1 was reduced in fibroblasts by expression of the early region of SV40, whereas Tsp-1 failed to respond in this manner in epithelial cells. However, consistent with previously published results,80 the expression of high levels of the Ras oncoprotein strongly induced VEGF-A secretion in both epithelial cells and fibroblasts.
To follow up these contrasting findings in more detail, we sought to determine whether the repression of Tsp-1 expression induced by SV40 early region was because of its expression of the LT antigen or to one of the other two proteins expressed from the SV40 early region, small T (ST) and 17kT.81–87 Accordingly, we transduced human dermal and lung fibroblasts expressing hTERT with the cDNA of LT (LTc), which does not encode ST or 17kT. Western blot analysis revealed that LTc, on its own, was able to markedly repress Tsp-1 expression in both human dermal and lung fibroblasts (Figure 6c). These findings contrasted with the previous observations that LT expression resulted in no reduction, and perhaps modest stimulation, of Tsp-1 expression in epithelial cells (Figure 6d). Together, these observations indicated that the regulation of Tsp-1 in fibroblasts is mediated by fundamentally different mechanisms in human fibroblasts and epithelial cells.
p53, pRb and E2F1 involvement in Tsp-1 repression
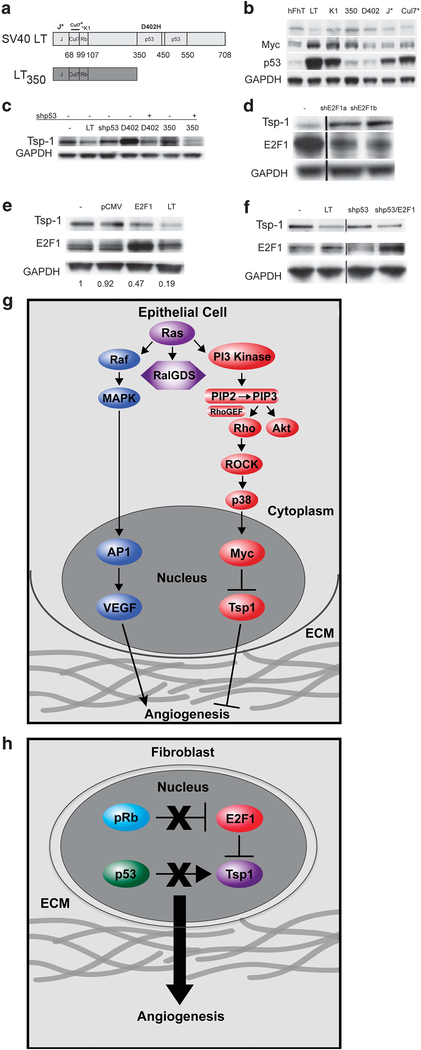
Given these results, we sought to determine the mechanism by which LT expression led to the inhibition of Tsp-1 expression. To do so, we transduced human dermal fibroblasts with retroviral constructs specifying mutant forms of LT, in order to determine which region of LT was responsible for the loss of Tsp-1 expression (Figure 7a). We observed that the LT mutant LT-K1, which lacks pRb-binding activity but retains p53 binding, had lost the ability to affect Tsp-1 expression in fibroblasts, although it did modestly stimulate Myc expression (Figure 7b). Moreover, the LT mutant, LT350, which lacks p53-binding activity, also had no effect on Tsp-1 expression (Figure 7b). Indeed, only LT mutants that retained both p53- and pRb-binding activity were able to repress Tsp-1 expression (Figure 5f). Hence, repression of Tsp-1 in fibroblasts is achieved via the concomitant inactivation of p53 and pRb without the need for supraphysiologic levels of oncogenic Ras.
Figure 7.
Regulation of Tsp-1 expression in fibroblasts. (a) Schematic depiction of the domain structure of the SV40 LTC (LT) protein and the various mutants: LTK1-point mutation in the Rb binding motif, LT350-deletion mutation that only contains the first 350 amino acids of LT, LTD402H- point mutation in the p53 binding motif, J*-point mutation in the J domain and Cul7*-deletion mutation in the Cul7 binding motif; Immunoblot analysis of (b) Tsp-1, Myc, p53, and GAPDH expression in immortalized human dermal fibroblasts expressing the Large T mutants depicted in Figure 4a. (c) Tsp-1 and GAPDH expression in human dermal fibroblasts expressing hTERT (−) alone or in combination with: SV40 LTC (LT), shp53 (shp53), LTD402H (402), shp53 and LTD402H, LT350 (350), and shp53 and LT350. (d) Tsp-1, E2F1 and GAPDH expression in human dermal fibroblasts expressing hTERT alone (−) or in combination with shRNA specific for E2F1 (shE2F1a and shE2F1b). (e) Tsp-1, E2F1 and GAPDH expression in human dermal fibroblasts expressing hTERT alone (−), or in combination with pCMV, pCMVE2F1 (E2F1) or Large T (LT), numbers represent relative intensity of Tsp-1 in each lane normalized to GAPDH. (f) Tsp-1, E2F1 and GAPDH expression in by human dermal fibroblasts expressing hTERT alone (−), or in combination with SV40 LTC (LT), shp53, and shp53 plus pCMVE2F1 (E2F1). (g) Schematic diagram of Tsp-1 regulation in epithelial cells. (h) Schematic diagram of Tsp-1 regulation in fibroblasts. Black lines in western blots represent cases where lanes were digitally removed from the scanned image.
To confirm these requirements, we performed complementation studies to determine whether p53 inhibition collaborates with pRB inactivation to repress THBSi expression. We observed that Tsp-1 expression is repressed in fibroblasts that express both shp53 and LT-D402H, a LT mutant that binds and inactivates pRB but not p53, but not in fibroblasts expressing either shp53 or LT-D402H alone88 (Figure 7c). Similarly, Tsp-1 is repressed in cells expressing LT350, a mutant of LT that is truncated after amino acid residue 350 and thus lacks the p53- binding domain, when expressed in combination with shRNA-mediated silencing of p5389 (Figure 7c). These results confirmed that Tsp-1 repression in human fibroblasts requires the inactivation of both the p53 and the pRb pathways, in contrast to epithelial cells, in which LT, while sequestering both p53 and pRb, has no effect on Tsp-1 expression (Figure 6c and d). While it has demonstrated that p53 can stimulate Tsp-1 expression in fibroblasts,90 this is the first report that Tsp-1 repression in fibroblasts requires the inactivation of both p53 and pRb.
We then sought to determine the mechanism by which pRb was involved in the regulation of Tsp-1 expression in fibroblasts. To that end, we examined the role of the E2F1 transcription factor—a direct target of pRB inhibition.91–93 Here we found that silencing of E2F1 expression via shRNA resulted in the stimulation of Tsp-1 expression in dermal fibroblasts (Figure 7d). Additionally, transient ectopic expression of E2F1 resulted in ~ 2-fold repression of Tsp-1 though not to the same level as induced by SV40 LT (Figure 7e). However, consistent with the complementation studies depicted in Figure 7c, ectopic expression of E2F1 in combination with shRNA-mediated silencing of p53 resulted in the repression of Tsp-1 comparable to that induced by LT (Figure 7f). Therefore, repression of Tsp-1 in human fibroblasts requires not only inhibition of p53 activity, but also liberation of the E2F1 transcription factor from the inhibitory effects of pRB. These results demonstrate a novel synergy between p53, pRb and E2F1 in the regulation of angiogenesis.
DISCUSSION
In previous work, we demonstrated the genetic requirements for the experimental transformation of human cells.32,79 This work shed no light, however, on the mechanisms whereby these cells acquired an essential attribute of tumorigenicity, specifically angiogenicity. In the course of the present work we have uncovered a novel signaling pathway that leads in epithelial cells from the Ras oncoprotein via Myc to the repression of expression of the potent anti-angiogenic protein, Tsp-1. Critical intermediates between Ras and Myc are, in order, PI3K, a Rho GEF, a Rho, a ROCK and p38MAPK. Strikingly, p38MAPK has been demonstrated to stimulate Tsp-1 in human mesangial cells, a specialized smooth muscle cell of mesenchymal lineage, in response to angiotensin II,94 further underscoring the differences between epithelial and mesenchymal cells described in this manuscript.
The cooperative actions of myc and ras oncogenes in transforming rodent cells have been known for three decades.95 We demonstrate here that activation via phosphorylation of Myc via a Ras-mediated signaling pathway is sufficient to confer an angiogenic phenotype by enabling Myc to repress the expression of Tsp-1. Moreover, this observation suggests that the various cell-physiologic contributions of the myc gene to tumorigenesis have not yet been fully enumerated. Although others have demonstrated links between Rho and Myc and between Rho and Tsp-1,96–99 we report here for the first time that a signal transduction cascade leading from Ras to PI3K to Rho to ROCK to Myc to Tsp-1 repression.
In a murine model of melanoma that utilizes a doxycyclin-inducible transgene specifying H-RasV12, Ras signaling is required for the maintenance of the tumor vasculature.100 Interestingly, in this model, withdrawal of doxycyclin and subsequent loss of Ras expression led to blood vessel regression. This blood vessel regression could not be rescued by the ectopic expression of VEGF-A, suggesting that Ras was doing more than merely inducing VEGF-A expression. Such observations could be explained if Ras signaling resulted in the repression of Tsp-1, a protein known to induce apoptosis of endothelial cells,30,101 doing so in melanoma cells in a manner similar to what we describe here in breast cancer cells.
The present results suggest that in transformed human epithelial cells, repression of Tsp-1 expression is a critical process in the acquisition of angiogenicity and thus tumorigenicity. One signaling strategy for achieving Tsp-1 repression is via a PI3K-mediated activation of Myc, followed by repression of the THBS1 gene by Myc. Once sufficient levels of PI3K activity are achieved, they act, as demonstrated here, via a signal transduction cascade that leads via Rho and ROCK to the activation of Myc.
Although previous work has shown that supraphysiologic levels of oncogenic Ras are capable of downregulating Tsp-1 expression,35 and that loss of PTEN can lead to a decrease in Tsp-1 expression,102 the signaling pathway(s) that regulate this repression have remained unclear. The present results indicate that oncogenic Ras, expressed at near-physiologic levels in epithelial cells, is not sufficient to repress Tsp-1. These observations indicate that in spontaneously arising human tumors of epithelial origin carrying RAS oncogenes, the tumor cells must devise additional means to down-modulate Tsp-1 expression if they are to succeed in becoming angiogenic. One mechanism would seem to involve the acquisition of increased levels of Ras signaling via amplification of the RAS oncogenes—a change that has indeed been documented in a subset of human carcinomas.103–106 Another mechanism might involve the deregulation of PI3K signaling, which has been documented in a large number of human cancers.107–113 Alternatively, increased expression of Myc could also be capable of achieving this result. For example, increased Myc activity can be achieved by gene amplification or overexpression, as is seen in many human tumors,47,114,115 thereby potentially bypassing the requirement of PI3 kinase hyperactivation altogether. This hypothesis is supported by our observation that introduction of the inducible MycER fusion protein in low Rasexpressing cells is sufficient to repress Tsp-1 expression, presumably by increasing the amount of available substrate for the Rho-dependent signaling pathway operating downstream of Ras.
As demonstrated here, the Rho signaling pathway that is activated by PI3 kinase is required for the repression of Tsp-1 in epithelial cells. In fact, such activation of Rho may contribute in other ways to tumor progression. For instance, it has been demonstrated that overexpression of RhoC increases both the metastatic potential and motility of human B16F0 melanoma cells and A375P amelanotic cells.116 Hence, the ability of RhoC to increase the metastatic potential of tumor cells could be accomplished not only via its ability to increase the motility and migration of the cells at the primary tumor but also to increase their angiogenicity at their metastatic destination via down-regulation of Tsp-1.
The regulation of angiogenesis is an essential, rate-limiting process in tumor formation. Much work has gone into the elucidation of the signaling pathways that regulate the expression of VEGF-A, one of the most potent angiogenic factors. However, to date, relatively little has been learned about the mechanisms governing the expression of Tsp-1, which antagonizes the proangiogenic effects of VEGF-A. The results presented here support the hypothesis that neo-angiogenesis and thus tumor progression is governed by the relative levels of conflicting pro-and antiangiogenic factors, specifically VEGF-A and Tsp-1.13 Strikingly, changes in the balance between these factors can be achieved very differently in different cell types. Thus, in human fibroblasts, the repression of Tsp-1 appears to be regulated by a process that does not rely on supraphysiologic levels of Ras, but rather can be achieved by inactivating the tumor suppressors p53 and pRb. In contrast, fibroblasts still require oncogenic Ras activity to stimulate the secretion of VEGF-A and therefore achieve a balance of pro- and anti-angiogenic factors that favors angiogenesis. The distinct modes of regulation of Tsp-1 in fibroblasts and epithelial cells warrant additional investigation and may ultimately provide useful insights into the processes leading to the pathogenesis of sarcomas versus carcinomas (Figures 7g and h).
Significantly, we demonstrate that the regulation of angiogenesis and hence tumorigenicity may be as dependent or even more dependent on the ability to repress Tsp-1 expression than the stimulation of VEGF-A expression. Such findings raise the hope that chemical inhibitors that disrupt the pathway(s) leading to Tsp-1 repression may prove to be effective in diminishing the angiogenicity of certain human tumors and, in turn, slow or even halt their further progression.
MATERIALS AND METHODS
Cell lines and constructs
The retroviral constructs expressing mVEGF were created by digesting the vector pmVEGF164 (a gift from Bruce Spiegelman, Dana Farber Cancer Institute, Boston, MA, USA) with BamH1 and EcoR1, and ligating it to similarly digested pBabeZeo or pWZLBlast to create pBabeZeo-VEGF and pWZLBlast-VEGF. The retroviral vector pWZLBlast-Tsp1 was created by digesting the vector pCDNATsp-1 (a gift from Michael Detmar, Harvard Medical School) with EcoRI and 5a/I, and ligating the tsp-1 DNA to similarly digested pWZLBlast. The retroviral vector pWZLBlast-DNmycER was created by digesting pBabePuro-DNMycER (a generous gift from Gerard Evan, UCSF) with EcoRI, and ligating the DNA to pWZLBlast and orientation confirmed by restriction digest with BamHI. The retroviral vector pBabeZeo-AT was created by digesting pWZLBlast-Tsp1 with EcoRI and 5a/I, blunting the ends using the Klenow fragment of DNA polymerase (Roche, Indianapolis, IN, USA), and ligating it into pBabeZeo digested with 5naB1, the anti-sense orientation was confirmed by restriction digest. The constructs pMIG-DNRhoA and pMIG-RhoCV14 were gifts from Richard O. Hynes (MIT Center for Cancer Research, Cambridge, MA, USA). The construct pEXV-RhoAV14 was a gift from Alan Hall (MRC, London). The V12H-Ras mutants (a gift of Dr Julian Downward, London Research Institute, London, UK) were subcloned from pSG5 into the EcoRI site of the pWZL-Blast retroviral vector and directionality was confirmed by immuno- blot analysis using a Ras-specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
The generation of the human embryonic kidney cells HA1E, HA1EhR, HA1EpR and the human mammary epithelial cells HMLE, HMLEhR, HMLEpR were described previously.32,79 HA1EhRV and HA1EpRV were generated by retroviral transduction of the parental cells with pBabeZeo-VEGF, whereas HMLEhRV and HMLEpRV were transduced with pWZLBlast-VEGF. HA1Eh- RAT was generated by transducing HA1EhR with pBabeZeo-AT. HA1ER- asC40, G37 and S35 cell lines were created by transducing the parental HA1E cells with pWZL-RasC40, pWZL-Ras G37 and pWZL-RasS35. HA1EpR- RhoAN19 and HA1EhR-RhoCV14 were created by transducing the parental cell lines with pMIG-RhoAN19 and pMIG-RhoCV12. Retroviruses were produced as previously described.32
Both HME and HRE cell lines were cultured in a 1:1 mixture of F-12 Nutrient Mixture and Dulbecco’s Modified Eagle Medium (DMEM) (GIBCO, Carlsbad, CA, USA) and supplemented with 5% fetal bovine serum (GIBCO) 1 μg/ml hydrocortisone, 10ng/ml EGF and 10μg/ml insulin (Sigma Chemicals). Dermal fibroblasts were cultured in DMEM supplemented with 10% FBS. Lung fibroblasts were cultured in Minimum Essential Medium (GIBCO) supplemented with 10% FBS.
The retroviral constructs pBabeZeo LTc and pBabePuro LTK1 and pBabeHygrohTERT and the lentiviral shRNA vector pLK0.1, the retroviral SV40 large T antigen mutant constructs, pBabePuro LT-A69–83, pBabeNeo LT350, pBabeNeo LT-D402H, and pBabeNeo LT-H42Q were a generous gift from Dr James DeCaprio, Dana Farber Cancer Institute. The two lenitviral shRNA vectors pLK0.1 E2F1a and E2F1b were purchased from Sigma-Aldrich (St Louis, MO, USA) and contained the following sequences: shE2F1a: CCGGCAGGATGGATATGAGATGGGACT CG-AGTCCCATCTCATATCCATCCTGTTTTTG and shE2F1b: CCGGCGCTAT GAGACCTCACT-GAAT TCGAGATTCAGTGAGGTCTCATAGCGTTTTTG. The pCMV and pCMV-E2F1 vectors were a generous gift from Jacqueline Lees, MIT Center for Cancer Research. The pCMV-p53 plasmid was a generous gift from Meredith Irwin, Hospital for Sick Kids, Toronto, ON, Canada.
Human renal and lung (bronchial) epithelial cells (Lonza, Walkersville, MD, USA) were immortalized via transduction of the retroviral vector pBabeHygro hTERT. Human lung fibroblasts (WI38 and MRC5) were obtained from ATCC and were immortalized via retroviral transduction with pBabeHygro hTERT to yield hFhT and MRC5-hT. HME, HRE, hFhT and MRC5-hT cell lines expressing the SV40 large T antigen and LT mutants were generated by retroviral transduction with the pBabe vectors described above.
Human dermal fibroblasts expressing pCMV and pCMV-E2F1 were produced through transient transfections as previously described117 Human fibroblasts and epithelial cells expressing the lentriviral shRNA constructs described above were generated via lentiviral transduction as previously described.118,119
Tumor formation assays
Tumorigenicity of the cell lines created above was assessed by injecting 2×106 cells subcutaneously, either with or without Matrigel (Becton Dickinson, Palo Alto, CA, USA) into nude mice that had been irradiated with 4 grays 24 h prior to injectio. The tumor diameter was measured using calipers and the diameter converted to volume using the formula 4/3πr3
ELISA assays
The kidney cells were grown in MEMα +10%IFS in either 0.1% oxygen or 20% oxygen for 48 h. The mammary cells were grown in a 1:1 ratio of DMEM and F12 media with 5% fetal calf serum and 10 μg/ml insulin, 10ng/ml hEGF and 1 μg/ml hydrocortisone (Sigma Chemicals). The conditioned media was filtered through 0.45 μm syringe filters and the levels of VEGF were measured using an ELISA kit from R&D (Minneapolis, MN, USA). VEGF levels were normalized against total protein from the cells used in the assay.
Western blotting
For western blot analysis, the human embryonic kidney-derived cells were grown in MEMα containing 10%IFS and then switched to MEMα containing 0.1% IFS for 24 h.The mammary epithelial derived cells were grown in a 1:1 mixture of DMEM and F12+5% fetal calf serum with 10 μg/ml insulin, 10ng/ml hEGF and 1 μg/ml hydrocortisone (Sigma Chemicals) and then switched to DMEM containing 2.5% of the standard growth media for 24 h. For experiments involving kidney cells, cells expressing DNMycER were switched to MEMα containing 0.1% IFS for 6h followed by addition of 100 nM 4-OH tamoxifen for 18 h (Sigma Chemicals). For experiments utilizing the chemical inhibitors (Calbiochem, San Diego, CA, USA) cells were grown in MEMα containing 0.1% IFS for 14 h followed by addition of 10 μM LY294002, 5 μM UO126 or 10 μM Y27632 for 10 h.
Cells were lysed in 50 mM Tris-Cl pH 7.4, 150 mM NaCl, 1% NP40, 1 mM sodium orthovanadate, 5 mM NaF, 20 mM β-glycerophosphate and Complete protease inhibitor (Roche). Fifty micrograms of protein, as determined by the BioRad protein assay (BioRad, Hercules, CA, USA) was loaded per well onto a 4–12% pre-cast polyacrylamide gradient gel (Invitrogen, Carlsbad, CA, USA). The extracts were electrophoresed and transferred to an Immobilon-P membrane (Millipore, Bedford, MA, USA). The membranes were blocked in 5% non-fat milk and incubated in primary antibody to Ras (c-20, Santa Cruz Biotechnology), Tsp-1 (Ab11, Lab Vision, Fremont, CA, USA), β-actin (Abcam, Cambridge, UK), c-myc (hybridoma 9E10), phospho-c-myc, phospho-Akt, phospho-p44/42 ERK, (Cell Signal Transduction,Beverly, MA, USA).The membranes were then washed in PBS +0.1%Tween-20 and incubated with either HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA) followed by another wash. The membranes were then developed with Supersignal Dura extended (Pierce Chemicals, Rockford, IL, USA) and exposed to film.
Transient transfections
Kidney-derived cells expressing either low or high levels of oncogenic H-RasV12 were transiently transfected with 5 μg pCMV2-Flag or pCMV2-FLAG expressing wtMyc, S62AMyc, S71AMyc (gifts from Yoshiyuki Kuchino, National Cancer Center Research Institute, Tokyo, Japan), pBabepuroMycER (a gift from Gerard Evan, UCSF) or pEXVRhoAV14 using FuGENE 6 transfection reagent. The media was changed 12 h post-transfection, and 12 h later the cells were switched to media containing 0.1%IFS. Cells transfected with pBabepuroMycER were grown in 0.1% serum for 6h followed by addition of 4-HT for 18h. Cells were harvested and lysed after an additional 24 h and analyzed by western blot as described above.
Ribonuclease protection assays
Human embryonic kidney-derived cells, described above, were transfected with 5 μg of pCMV2-Flag or pCMV2-Flag expressing wtMyc, S62AMyc or S71AMyc. Following serum deprivation, RNA was prepared from transfected cells using the Trizol protocol (Invitrogen). The probe specific for Cyclophilin was prepared via T7 in vitro transcription from linearized pTRIPLEscript-cyclophylin (Ambion, Austin, TX, USA) incorporating [α−32P] UTP (NEN, Boston, MA, USA) using MaxiScript T7 kit (Ambion). The probe specific for ODC was prepared via T7 in vitro transcription from linearized pDP18-ODC incorporating [α−32P] UTP using MaxiScript T7 kit (Ambion). Ribonuclease protection assays were then performed using the ribonu-clease protection assay III kit (Ambion). The protected fragments were run on a Criterion 5% TBE-Urea gel (BioRad), dried on 3 mm filter paper and visualized by autoradiography.
Immunohistochemistry
Samples were fixed in 10% paraformaldyhide and subsequently paraffin-embedded for sectioning. Four micrometer sections were deparaffinized with xylene and rehydrated in decreasing concentrations of ethanol to water. Tyramide Signal Amplification kit (PerkinElmer, Boston, MA, USA) was used to enhance sensitivity of the staining and used according to the manufacturer’s instructions. Briefly, antigen retrieval was performed using proteinase K (Roche Diagnostics, Indianapolis, IN, USA) at the final concentration of 20 μg/ml in 0.2M Tris PH 7.2 at 37°C for 25min. Slides where then incubated for 30min with blocking buffer Tris-NaCl with 0.5% (w/v) blocking reagent (TNB) provided in the Tyramide Signal Amplification kit.
Slides were incubated overnight at 4 °C with primary antibodies rat anti-mouse CD-31 (clone: MEC 13.3, BD Biosciences Pharmingen, San Jose, CA, USA) 1:250 or rat anti-mouse MAC3 (clone:M3/84, BD Biosciences) 1:100 in TNB buffer. The next day slides were washed, re-blocked and incubated with Biotinylated Rabbit Anti-Rat IgG secondary Antibody (VECTOR laboratories, Burlingame, CA, USA) 1:200 in TNB at room temperature for 30min. Sections were then incubated twice with streptavidin-HRP (provided in the Tyramide Signal Amplification kit) 1:100 in TNB for 30 min at room temperature before using peroxidase substrate kit Vector Novared (VECTOR laboratories) for 10–15min. Sections were counterstained with hematoxylin (Sigma-Aldrich).
Image analysis
To quantify the vessel diameter, histological sections were examined with an inverted microscope (Zeiss, Oberkochen, Germany). Photos were taken, and using the image-processing program ImagJ, the outlines of CD31-positive vessels in five fields of view of a section were encircled manually at X 200 magnification. Graphs shown represent the average vessel density and vessel area from five independent fields of view.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr Richard O Hynes, Dr Alan Hall, Dr Michael Detmar, Dr Yoshiyuki Kuchino and Dr James DiCaprio for reagents. We would like to thank Drs Ittai Ben-Porath, Scott Dessain, Lisa Spirio, Sendurai Mani and Sheila Stewart for useful discussions. This work was supported by NIH/NCI grant 5 R01CA78461 and Merck/MIT to RAW. We also thank the Damon Runyon Cancer Research Foundation for post-doctoral support to RSW.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
This paper has been corrected since online publication and a corrigendum appears in this issue
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 3.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279: 349–352. [DOI] [PubMed] [Google Scholar]
- 4.Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/ p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 1998; 396: 84–88. [DOI] [PubMed] [Google Scholar]
- 5.Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela Arispe ML, Yeo TK et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 1999; 5: 1041–1056. [PubMed] [Google Scholar]
- 6.MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev 1995; 14: 351–362. [DOI] [PubMed] [Google Scholar]
- 7.Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 1992; 3: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ou YC, Chen JT, Yang CR, Horng YY, Kao YL, Cheng CL. Tumor angiogenesis and metastasis: correlation in invasive renal cell carcinoma. Zhonghua Yi Xue Za Zhi (Taipei) 1998; 61: 441–447. [PubMed] [Google Scholar]
- 9.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metas-tasis--correlation in invasive breast carcinoma. New Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 10.Xiangming C, Hokita S, Natsugoe S, Tanabe G, Baba M, Takao S et al. Angiogenesis as an unfavorable factor related to lymph node metastasis in early gastric cancer. Ann Surg Oncol 1998; 5: 585–589. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 1999; 13: 1382–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Christofori G, Naik P, Arbeit J. Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur J Cancer 1996; 32A: 2386–2393. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–364. [DOI] [PubMed] [Google Scholar]
- 14.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression [see comments]. Nat Med 1995; 1: 149–153. [DOI] [PubMed] [Google Scholar]
- 15.Olive PL,Vikse C, Trotter MJ. Measurement of oxygen diffusion distance in tumor cubes using a fluorescent hypoxia probe. Int J Radiat Oncol Biol Phys 1992; 22: 397–402. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J, Hanahan D. Switch to the angiogenic phenotype during tumor- igenesis. Princess Takamatsu Symp 1991; 22: 339–347. [PubMed] [Google Scholar]
- 17.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246: 1306–1309. [DOI] [PubMed] [Google Scholar]
- 18.Rogelj S, Weinberg RA, Fanning P, Klagsbrun M. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature 1988; 331: 173–175. [DOI] [PubMed] [Google Scholar]
- 19.Wojta J, Kaun C, Breuss JM, Koshelnick Y, Beckmann R, Hattey E et al. Hepatocyte growth factor increases expression of vascular endothelial growth factor and plasminogen activator inhibitor-1 in human keratinocytes and the vascular endothelial growth factor receptor flk-1 in human endothelial cells. Lab Invest 1999; 79: 427–438. [PubMed] [Google Scholar]
- 20.Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci USA 2003; 100: 12718–12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci USA 1990; 87: 3092–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992; 258: 1798–1801. [DOI] [PubMed] [Google Scholar]
- 23.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA 1990; 87: 6624–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 1996; 2: 689–692. [DOI] [PubMed] [Google Scholar]
- 25.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88: 277–285. [DOI] [PubMed] [Google Scholar]
- 26.Maeshima Y, Sudhakar A, Lively JC, Ueki K, Kharbanda S, Kahn CR et al. Tum-statin, an endothelial cell-specific inhibitor of protein synthesis. Science 2002; 295: 140–143. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson A, Cao R, Pawliuk R, Berg SM, Tsang M, Zhou D et al. Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell 2002; 1: 99–108. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA 2001; 98: 12485–12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribatti D, Alessandri G, Vacca A, Iurlaro M, Ponzoni M. Human neuroblastoma cells produce extracellular matrix-degrading enzymes, induce endothelial cell proliferation and are angiogenic in vivo. Int J Cancer 1998; 77: 449–454. [DOI] [PubMed] [Google Scholar]
- 30.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol 1997; 138: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Short SM, Derrien A, Narsimhan RP, Lawler J, Ingber DE, Zetter BR. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol 2005; 168: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev 2001; 15: 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet 2006; 38: 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming JB, Shen GL, Holloway SE, Davis M, Brekken RA. Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: justification for K-ras-directed therapy. Mol Cancer Res 2005; 3: 413–423. [DOI] [PubMed] [Google Scholar]
- 35.Rak J, Mitsuhashi Y, Sheehan C, Tamir A, Viloria_Petit A, Filmus J et al. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res 2000; 60: 490–498. [PubMed] [Google Scholar]
- 36.Udagawa T, Fernandez A, Achilles EG, Folkman J, D_Amato RJ. Persistence of microscopic human cancers in mice: alterations in the angiogenic balance accompanies loss of tumor dormancy. FASEB J 2002; 16: 1361–1370. [DOI] [PubMed] [Google Scholar]
- 37.Venkatesha S, Hanai J, Seth P, Karumanchi SA, Sukhatme VP. Lipocalin 2 antagonizes the proangiogenic action of ras in transformed cells. Mol Cancer Res 2006; 4: 821–829. [DOI] [PubMed] [Google Scholar]
- 38.Castle V, Varani J, Fligiel S, Prochownik EV, Dixit V. Antisense-mediated reduction in thrombospondin reverses the malignant phenotype of a human squamous carcinoma. J Clin Invest 1991; 87: 1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 2012; 119: 5512–5521. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res 2008; 68: 7090–7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Qi X, Tong X, Wang S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol Immunol 2013; 10: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 2000; 14: 2501–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngo CV, Gee M, Akhtar N, Yu D, Volpert O, Auerbach R et al. An in vivo function for the transforming Myc protein: elicitation of the angiogenic phenotype. Cell Growth Differ 2000; 11:4. [PMC free article] [PubMed] [Google Scholar]
- 44.Tikhonenko AT, Black DJ, Linial ML. Viral Myc oncoproteins in infected fibroblasts down-modulate thrombospondin-1, a possible tumor suppressor gene. J Biol Chem 1996; 271: 30741–30747. [DOI] [PubMed] [Google Scholar]
- 45.Adhikary S, Peukert K, Karsunky H, Beuger V, Lutz W, Elsasser HP et al. Miz1 is required for early embryonic development during gastrulation. Mol Cell Biol 2003; 23: 7648–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li LH, Nerlov C, Prendergast G, MacGregor D, Ziff EB. c-Myc represses transcription in vivo by a novel mechanism dependent on the initiator element and Myc box II. EMBO J 1994; 13: 4070–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Little CD, Nau MM, Carney DN, Gazdar AF, Minna JD. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature 1983; 306: 194–196. [DOI] [PubMed] [Google Scholar]
- 48.Seshadri R, Matthews C, Dobrovic A, Horsfall DJ. The significance of oncogene amplification in primary breast cancer. Int J Cancer 1989; 43: 270–272. [DOI] [PubMed] [Google Scholar]
- 49.Trent J, Meltzer P, Rosenblum M, Harsh G, Kinzler K, Mashal R et al. Evidence for rearrangement, amplification, and expression of c-myc in a human glioblastoma. Proc Natl Acad Sci USA 1986; 83: 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S, Seth A, Davis RJ. Transactivation of gene expression by Myc is inhibited by mutation at the phosphorylation sites Thr-58 and Ser-62. Proc Natl Acad Sci USA 1993; 90: 3216–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seth A, Alvarez E, Gupta S, Davis RJ. A phosphorylation site located in the NH2- terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem 1991; 266: 23521–23524. [PubMed] [Google Scholar]
- 52.Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res 1995; 23: 1686–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacGregor D, Li LH, Ziff EB. Dominant negative mutants of Myc inhibit cooperation of both Myc and adenovirus serotype-5 E1a with Ras. J Cell Physiol 1996; 167: 95–105. [DOI] [PubMed] [Google Scholar]
- 54.Noguchi K, Kitanaka C, Yamana H, Kokubu A, Mochizuki T, Kuchino Y. Regulation of c-Myc through phosphorylation at Ser-62 and Ser-71 by c-Jun N-terminal kinase. J Biol Chem 1999; 274: 32580–32587. [DOI] [PubMed] [Google Scholar]
- 55.Lutterbach B, Hann SR. Hierarchical phosphorylation at N-terminal transformation-sensitive sites in c-Myc protein is regulated by mitogens and in mitosis. Mol Cell Biol 1994; 14: 5510–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White MA, Nicolette C, Minden A, Polverino A, Van_Aelst L, Karin M et al. Multiple Ras functions can contribute to mammalian cell transformation. Cell 1995; 80: 533–541. [DOI] [PubMed] [Google Scholar]
- 57.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 1998; 273: 18623–18632. [DOI] [PubMed] [Google Scholar]
- 58.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidyli-nositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 1994; 269: 5241–5248. [PubMed] [Google Scholar]
- 59.White E The pims and outs of survival signaling: role for the Pim-2 protein kinase in the suppression of apoptosis by cytokines. Genes Dev 2003; 17: 1813–1816. [DOI] [PubMed] [Google Scholar]
- 60.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 1995; 81: 727–736. [DOI] [PubMed] [Google Scholar]
- 61.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM et al. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA 1999; 96: 2110–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoover RR, Gerlach MJ, Koh EY, Daley GQ. Cooperative and redundant effects of STAT5 and Ras signaling in BCR/ABL transformed hematopoietic cells. Oncogene 2001; 20: 5826–5835. [DOI] [PubMed] [Google Scholar]
- 63.Holsinger LJ, Spencer DM, Austin DJ, Schreiber SL, Crabtree GR. Signal transduction in T lymphocytes using a conditional allele of Sos. Proc Natl Acad Sci USA 1995; 92: 9810–9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ren XD, Schwartz MA. Determination of GTP loading on Rho. Methods Enzymol 2000; 325: 264–272. [DOI] [PubMed] [Google Scholar]
- 65.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science 1995; 269: 1270–1272. [DOI] [PubMed] [Google Scholar]
- 66.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992; 70: 401–410. [DOI] [PubMed] [Google Scholar]
- 67.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 1996; 16: 5313–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 1996; 15: 2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 69.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997; 389: 990–994. [DOI] [PubMed] [Google Scholar]
- 70.Sahai E, Ishizaki T, Narumiya S, Treisman R. Transformation mediated by RhoA requires activity of ROCK kinases. Curr Biol 1999; 9: 136–145. [DOI] [PubMed] [Google Scholar]
- 71.Cardone RA, Bagorda A, Bellizzi A, Busco G, Guerra L, Paradiso A et al. Protein kinase A gating of a pseudopodial-located RhoA/ROCK/p38/NHE1 signal module regulates invasion in breast cancer cell lines. Mol Biol Cell 2005; 16: 3117–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He Z, Cho YY, Liu G, Ma WY, Bode AM, Dong Z. p38 Mitogen-activated protein kinase regulation of JB6 Cl41 cell transformation promoted by epidermal growth factor. J Biol Chem 2003; 278: 26435–26442. [DOI] [PubMed] [Google Scholar]
- 73.Gould GW, Cuenda A, Thomson FJ, Cohen P. The activation of distinct mitogen-activated protein kinase cascades is required for the stimulation of 2-deoxyglucose uptake by interleukin-1 and insulin-like growth factor-1 in KB cells. Biochem J 1995; 311(Pt 3): 735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jallal B, Schlessinger J, Ullrich A. Tyrosine phosphatase inhibition permits analysis of signal transduction complexes in p185HER2/neu-overexpressing human tumor cells. J Biol Chem 1992; 267: 4357–4363. [PubMed] [Google Scholar]
- 75.Kozma SC, Bogaard ME, Buser K, Saurer SM, Bos JL, Groner B et al. The human c-Kirsten ras gene is activated by a novel mutation in codon 13 in the breast carcinoma cell line MDA-MB231. Nucleic Acids Res 1987; 15: 5963–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singhal RL, Yeh YA, Look KY, Sledge GW, Weber G. Coordinated increase in activities of the signal transduction enzymes PI kinase and PIP kinase in human cancer cells. Life Sci 1994; 55: 1487–1492. [DOI] [PubMed] [Google Scholar]
- 77.Dubik D, Dembinski TC, Shiu RP. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res 1987; 47(24 Pt 1): 6517–6521. [PubMed] [Google Scholar]
- 78.Rummukainen J, Kytola S, Karhu R, Farnebo F, Larsson C, Isola JJ. Aberrations of chromosome 8 in 16 breast cancer cell lines by comparative genomic hybridization, fluorescence in situ hybridization, and spectral karyotyping. Cancer Genet Cytogenet 2001; 126: 1–7. [DOI] [PubMed] [Google Scholar]
- 79.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature 1999; 400: 464–468. [DOI] [PubMed] [Google Scholar]
- 80.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 1995; 55: 4575–4580. [PubMed] [Google Scholar]
- 81.Pipas JM, Levine AJ. Role of T antigen interactions with p53 in tumorigenesis. Semin Cancer Biol 2001; 11: 23–30. [DOI] [PubMed] [Google Scholar]
- 82.Prives C, Beck Y, Gidoni D, Oren M, Shure H. DNA binding and sedimentation properties of SV40 T antigens synthesized in vivo and in vitro. Cold Spring Harb Symp Quant Biol 1980; 44(Pt 1): 123–130. [DOI] [PubMed] [Google Scholar]
- 83.Rundell K, Parakati R. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin Cancer Biol 2001; 11: 5–13. [DOI] [PubMed] [Google Scholar]
- 84.Shenk TE, Berg P. Isolation and propagation of a segment of the simian virus 40 genome containing the origin of DNA replication. Proc Natl Acad Sci USA 1976; 73: 1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shenk TE, Carbon J, Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol 1976; 18: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Volckaert G, Feunteun J, Crawford LV, Berg P, Fiers W. Nucleotide sequence deletions within the coding region for small-t antigen of simian virus 40. J Virol 1979; 30: 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zerrahn J, Knippschild U, Winkler T, Deppert W. Independent expression of the transforming amino-terminal domain of SV40 large I antigen from an alternatively spliced third SV40 early mRNA. EMBO J 1993; 12: 4739–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beachy TM, Cole SL, Cavender JF, Tevethia MJ. Regions and activities of simian virus 40 T antigen that cooperate with an activated ras oncogene in transforming primary rat embryo fibroblasts. J Virol 2002; 76: 3145–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chao HH, Buchmann AM, DeCaprio JA. Loss of p19(ARF) eliminates the requirement for the pRB-binding motif in simian virus 40 large T antigenmediated transformation. Mol Cell Biol 2000; 20: 7624–7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 1994; 265: 1582–1584. [DOI] [PubMed] [Google Scholar]
- 91.Bagchi S, Weinmann R, Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell 1991; 65: 1063–1072. [DOI] [PubMed] [Google Scholar]
- 92.Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell 1991; 65: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 93.Chittenden T, Livingston DM, Kaelin WG Jr. The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell 1991; 65: 1073–1082. [DOI] [PubMed] [Google Scholar]
- 94.Naito T, Masaki T, Nikolic-Paterson DJ, Tanji C, Yorioka N, Kohno N. Angiotensin II induces thrombospondin-1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-beta1. Am J Physiol Renal Physiol 2004; 286: F278–F287. [DOI] [PubMed] [Google Scholar]
- 95.Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 1983; 304: 596–602. [DOI] [PubMed] [Google Scholar]
- 96.Li Z, Wang C, Jiao X, Lu Y, Fu M, Quong AA et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol 2006; 26: 4240–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sauzeau V, Berenjeno IM, Citterio C, Bustelo XR. A transcriptional cross-talk between RhoA and c-Myc inhibits the RhoA/Rock-dependent cytoskeleton. Oncogene 2010; 29: 3781–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res 2009; 69: 8742–8751. [DOI] [PubMed] [Google Scholar]
- 99.Mizukami Y, Fujiki K, Duerr EM, Gala M, Jo WS, Zhang X et al. Hypoxic regulation of vascular endothelial growth factor through the induction of phosphatidyli-nositol 3-kinase/Rho/ROCK and c-Myc. J Biol Chem 2006; 281: 13957–13963. [DOI] [PubMed] [Google Scholar]
- 100.Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N et al. Essential role for oncogenic Ras in tumour maintenance. Nature 1999; 400: 468–472. [DOI] [PubMed] [Google Scholar]
- 101.Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res 1997; 57: 1735–1742. [PubMed] [Google Scholar]
- 102.Wen S, Stolarov J, Myers MP, Su JD, Wigler MH, Tonks NK et al. PTEN controls tumor-induced angiogenesis. Proc Natl Acad Sci USA 2001; 98: 4622–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heighway J, Betticher DC, Hoban PR, Altermatt HJ, Cowen R. Coamplification in tumors of KRAS2, type 2 inositol 1,4,5 triphosphate receptor gene, and a novel human gene, KRAG. Genomics 1996; 35: 207–214. [DOI] [PubMed] [Google Scholar]
- 104.Janocko LE, Lucke JF, Groft DW, Brown KA, Smith CA, Pollice AA et al. Assessing sequential oncogene amplification in human breast cancer. Cytometry 1995; 21: 18–22. [DOI] [PubMed] [Google Scholar]
- 105.Galiana C, Lozano JC, Bancel B, Nakazawa H, Yamasaki H. High frequency of Kiras amplification and p53 gene mutations in adenocarcinomas of the human esophagus. Mol Carcinog 1995; 14: 286–293. [DOI] [PubMed] [Google Scholar]
- 106.Nagy A, Kozma L, Kiss I, Ember I, Takacs I, Hajdu J et al. Copy number of cancer genes predict tumor grade and survival of pancreatic cancer patients. Anticancer Res 2001; 21: 1321–1325. [PubMed] [Google Scholar]
- 107.Varticovski L, Daley GQ, Jackson P, Baltimore D, Cantley LC. Activation of phosphatidylinositol 3-kinase in cells expressing abl oncogene variants. Mol Cell Biol 1991; 11: 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoino-sitide 3-kinase/Akt pathway. Proc Natl Acad Sci USA 1998; 95: 15587–15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sepp-Lorenzino L, Rosen N, Lebwohl DE. Insulin and insulin-like growth factor signaling are defective in the MDA MB-468 human breast cancer cell line. Cell Growth Differ 1994; 5: 1077–1083. [PubMed] [Google Scholar]
- 110.Lebwohl DE, Muise-Helmericks R, Sepp-Lorenzino L, Serve S, Timaul M, Bol R et al. A truncated cyclin D1 gene encodes a stable mRNA in a human breast cancer cell line. Oncogene 1994; 9: 1925–1929. [PubMed] [Google Scholar]
- 111.Weng LP, Smith WM, Dahia PL, Ziebold U, Gil E, Lees JA et al. PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death. Cancer Res 1999; 59: 5808–5814. [PubMed] [Google Scholar]
- 112.Perren A, Weng LP, Boag AH, Ziebold U, Thakore K, Dahia PL et al. Immuno-histochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol 1999; 155: 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dahia PL, Aguiar RC, Alberta J, Kum JB, Caron S, Sill H et al. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanismsin haematological malignancies. Hum Mol Genet 1999; 8: 185–193. [DOI] [PubMed] [Google Scholar]
- 114.Escot C, Theillet C, Lidereau R, Spyratos F, Champeme MH, Gest J et al. Genetic alteration of the c-myc protooncogene (MYC) in human primary breast carcinomas. Proc Natl Acad Sci USA 1986; 83: 4834–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nau MM, Carney DN, Battey J, Johnson B, Little C, Gazdar A et al. Amplification, expression and rearrangement of c-myc and N-myc oncogenes in human lung cancer. Curr Top Microbiol Immunol 1984; 113: 172–177. [DOI] [PubMed] [Google Scholar]
- 116.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000; 406: 532–535. [DOI] [PubMed] [Google Scholar]
- 117.Helin K, Wu CL, Fattaey AR, Lees JA, Dynlacht BD, Ngwu C et al. Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative trans-activation. Genes Dev 1993; 7: 1850–1861. [DOI] [PubMed] [Google Scholar]
- 118.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science 2002; 296: 550–553. [DOI] [PubMed] [Google Scholar]
- 119.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 2003; 33: 401–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.