Abstract
Introduction:
Disruption of E-cadherin function and increased expression of vimentin and the transcriptional oncogene, SOX2, are thought to characterize epithelial to mesenchymal transition (EMT) in HNSCC that contributes to invasive and metastatic behavior. To determine if such changes relate to prognosis or host immune response, expression of these markers and correlations with clinical characteristics, histologic worst pattern of invasion (WPOI) and tumor infiltrating lymphocytes (TIL) and survival were assessed.
Methods:
Immunohistologic expression of markers was determined in tissue microarrays from 274 previously untreated HNSCC patients. Expression was correlated with levels of TILs in microcores and WPOI in biopsy specimens. Correlations were assessed by Kruskal-Wallis testing and Spearman correlation coefficients where appropriate. Overall and relapse-free survival were analyzed with Cox proportional hazards models. Median follow up was 60.0 months.
Results:
Loss of E-cadherin expression was significantly associated with low or absent SOX2 expression (R=0.433, p<0.0001). SOX2 expression and low grade WPOI were significantly associated with favorable overall (OS) and relapse free (RFS) survival in multivariable analysis. E-cadherin expression did not correlate with TILs, however WPOI score correlated indirectly with CD4, CD8, and FoxP3 levels. When grouped by primary treatment, lower grades (1,2) of WPOI predicted improved RFS and OS in patients treated with primary surgery but not for patients treated with chemoradiation.
Conclusion:
The findings suggest that SOX2 expression and WPOI are significant prognostic factors and that WPOI correlates with decreased T cell infiltration. The combination of markers and TILs might be useful in selecting patients for primary surgery.
Keywords: Head and neck cancer, E-cadherin, Vimentin, SOX2, Pattern of invasion, Survival
Introduction:
Of the various phenotypic changes that characterize carcinogenesis in head and neck squamous carcinoma (HNSCC), the transition from an epithelial to a mesenchymal phenotype (EMT) is a hallmark in the ability of individual cancer cells to migrate, invade and metastasize [1–5]. This transition is thought to be associated with cellular plasticity and loss of the epithelial adhesion marker, E-cadherin, and gain in expression of mesenchymal markers such as N-cadherin and vimentin [5,6–8]. These changes have been termed “cadherin switching” and have been associated with poor prognosis [4,7, 9–12]. EMT is also a characteristic of cancer initiating stem cells that may be responsible for tumor recurrence and treatment resistance [13,14]. The identification of cancer stem cells in HNSCC [15] that are associated with epithelial to mesenchymal transition and tumor invasiveness, metastasis and resistance to therapy prompted us to hypothesize that the degree of EMT in a tumor could reflect the growth characteristics manifest by histologic patterns of invasion and the microenvironment immune response and perhaps overall prognosis and response to therapy [16].
E-cadherin is an adhesion protein that plays a pivotal role in epithelial cell behavior in many cancers including HNSCC [11, 17–19]. The loss of E-cadherin expression occurring with EMT is thought to be primarily due to methylation of its promoter or upregulation of SNAIL, SLUG or ZEB1 genes that are markers of “stemness”. Similarly, stability of the stem cell population is thought to be regulated in part by the important regulatory oncogene, SOX2 [3, 20–22]. SOX2, is a transcription factor located on chromosome 3p26 and thought to be an oncogene in HNSCC that functions at various levels of tumor growth involving proliferation, cell survival and stem cell maintenance [20, 21, 23, 24]. Reports of correlations of SOX2 expression with clinical outcome have been conflicting with some suggesting high expression as favorable [21, 23,24] and others suggesting the opposite [22, 25, 26]. Gene expression is believed to induce cell motility via increased vimentin and to play a role in regulation of other transcription factors critical in cell proliferation, wound healing and cancer [20]. Knock down of SOX2 dramatically reduces tumor formation in HNSCC tumor models [27]. Silencing of SOX2 leads to upregulation of several genes including VIM (vimentin), CDH2 (N-cadherin), FN1, NLRP3, IL1R and functional pathways involved in programmed cell death, immune activation and cell motility suggesting that SOX2 could potentiate an immunosuppressive tumor microenvironment [21]. Indeed, we found that SOX2 promotes HNSCC development by suppressing host immunosurveillance [25].
Although EMT is a characteristic of increased motility and invasiveness, it remains unclear if EMT and tumor “stemness” are associated with the host immune response or immunosuppression in the tumor microenvironment [4,28,29]. The relationship of markers of EMT and potentially important prognostic factors such as pattern of invasion [30–33] and tumor infiltrating lymphocytes [34–38] remains largely unknown. We hypothesized that markers of EMT could be associated with levels of TILs and with aggressive patterns of invasion and could be predictive factors for therapy. To determine the potential prognostic importance of such tumor phenotypic changes and interactions with tumor immune response and clinical outcomes, immunohistologic staining densities for E-cadherin, vimentin, and the expression of SOX2, were assessed in biopsy specimens from a large cohort of patients with previously untreated HNSCC. Correlations with clinical tumor characteristics, histologic worst pattern of invasion (WPOI) and levels of TILs and patient survival were determined.
Methods:
Patient Population:
From November 2008 through June 2012, a total of 291 of subjects were enrolled in this longitudinal epidemiology study and signed a written, Human Subjects Institutional Review Board approved informed consent and had paraffin-embedded tissue biopsy blocks available with sufficient tissue to create a tissue microarray. Some subjects were excluded because of uncommon tumor sites (unknown primary, nasopharynx, salivary gland, paranasal sinus). The final count of cohort subjects was 280 patients. The cohort included 72% males and 28% females with median age 59 years. All patients were evaluated and discussed at our tumor board where standardized treatment recommendations were made. A total of 164 patients underwent definitive surgery, 82 underwent primary chemoradiation and 14 received radiation alone (Table 1). Twenty patients received palliative treatment, chemotherapy alone, or no treatment. Detailed epidemiologic and clinical data were collected at enrollment and annually until death or when patients were lost to follow-up. Median patient follow-up was 60.0 months.
Table 1:
Patient population and clinical and treatment characteristics
| Variable | Level | N (%) |
|---|---|---|
| Gender | Male | 202 (72%) |
| Female | 78 (28%) | |
| Stage | 0/1 | 42 (15%) |
| 2 | 35 (13%) | |
| 3 | 43 (15%) | |
| 4 | 160 (57%) | |
| Disease Site | Larynx | 49 (18%) |
| Oral Cavity | 139 (50%) | |
| Oropharynx | 83 (30%) | |
| Hypopharynx | 9 (3%) | |
| ACE Comorbidities Score, n=279 | none | 82 (29%) |
| mild | 126 (45%) | |
| moderate | 48 (17%) | |
| severe | 23 (8%) | |
| HPVstat, n=257 | negative | 168 (65%) |
| positive | 89 (35%) | |
| Drinker | never | 26 (9%) |
| current | 186 (66%) | |
| former (quit >12 months) | 68 (24%) | |
| Smoker (cigarettes) | never | 62 (22%) |
| current | 132 (47%) | |
| former (quit >12 months) | 86 (31%) | |
| Initial Treatment | Surgery | 164 (59%) |
| Radiation | 14 (5%) | |
| Chemotherapy | 5 (2%) | |
| Chemoradiation | 82 (29%) | |
| Palliaffigtion/no treatment | 15 (5%) |
Tissue Microarray:
Tissue microarrays from the 280 patients consisted of 42 Stage 1, 35 Stage 2, 43 Stage 3, and 160 Stage 4 patients representing larynx (18%), oral cavity (50%), oropharynx (30%), or hypopharynx (3%). HPV testing [39,40] was available for 257 patients of which 168 were negative and 89 were positive. A total of 62 patients were never smokers, 132 were current smokers and 86 were former (quit>12 months) smokers. Representative hematoxylin and eosin stained slides from each block were reviewed by an expert head and neck pathologist (JBM) and a central area of non-necrotic tumor was marked for tissue microarray sampling. For tissue array construction, blocks were transferred to a central lab (DT) for creation of the tissue array and map where triplicate 0.7mm diameter cores for each sample were selectively punched/extracted from the most representative, non-necrotic tumor areas marked on the blocks, and transferred to a recipient tissue array block [41]. Worst pattern of invasion grading (Scale 1–5) was performed and recorded from the H and E slides according to published guidelines, [31–33] and summarized as Type 1=pushing border; Type 2=finger-like growth; Type 3=large separate islands, more than 15 cells per island; Type 4=small tumor islands, 15 cells or fewer which were discontiguous, and Type 5=tumor satellites, more than 1 mm from main tumor or next closest satellite in a dispersed, discontiguous growth pattern. There were only 3 patients with pattern 5 invasion and therefore patterns of invasion Type 4 and 5 were combined for analysis to be similar to the four-tiered system originally proposed for pattern of invasion by Anneroth et al. and modified by Bryne, et al [42,43]. Although the prognostic significance of invasion grading was established in early stage oral cancers, here we applied this methodology to a variety of tumor sites and stages which is a novel application.
Immunohistochemistry:
The TMA slides were incubated in hot-air oven at 65°C overnight, deparaffinized, rehydrated with xylene, graded alcohols, and buffer immersion steps. Antigen retrieval was carried out by HIER (heat-induced epitope retrieval) method. Slides were incubated in a preheated pressure cooker with Citrate buffer pH6 or Tris-EDTA buffer pH9 and blocked with horse serum (30 minutes at 25oC) followed by liquid strepavidin biotin (Dako Biotin detection kit) detection using antibodies specific for E-cadherin (Zymed #13–1700, clone HECD-1 at concentration 1:200) or vimentin (Dako MO725, clone V9 at concentration 1:500). Immunohistochemical staining was completed on the DAKO autostainer (DAKO, Carpinteria, CA) using LSAB+ and DBA (DAKO labeled avidin-biotin-peroxidase kits) as the chromogens. Appropriate negative (without primary antibodies) and positive (tonsillar tissue and various carcinomas) controls were stained concurrently on the same slides. HPV status was determined by an ultrasensitive method using real-time competitive polymerase chain reaction and matrix-assisted laser desorption/ionization time of flight mass spectroscopy with separation of products on a matrix loaded silicon chip array, as described previously [36]. The stained TMA slides were digitally imaged, scanned (200x magnification), and retrieved with Aperio ImageScope version 12 software and assessed by a technician naive to patient outcome. CD104 staining (beta-4 integrin) for each core was examined to locate and confirm the extent and location of the carcinoma within the tissue cores.
E-cadherin expression was scored semiquantitatively by intensity grading from 0 to 3+ according to 0= no staining, 1= weak patchy cytoplasmic staining with little membrane staining, 2= strong membrane staining with outlining of tumor nodules, and 3= intense uniform staining. Vimentin positive cells present in tumor parenchyma only were manually counted and averaged for 3 tissue micro-cores per patient. If the core was not completely present or did not consist entirely of tumor, the percentage of tumor was calculated and the vimentin positive cell counts were normalized to the number of cells that would be present if 100% of the core was tumor. Expression was correlated with prior data available on levels of TILs (CD4, CD8, FoxP3, CD68 cells) in each core that were previously shown to correlate with patient survival using counting methods described by Nguyen et al [36]. Based on extensive prior experience [35–37] only TILs infiltrating in tumor parenchyma were quantified and included in analysis.
SOX2 Expression:
Five micron sections were stained with anti-SOX2 antibody (Cell Signaling Technologies #23064) at a dilution of 1:300. Biotinylated goat anti-rabbit antibody (diluted 1:100), was applied and incubated for 30 minutes. Slides were incubated with Avidin-Biotinylated HRP Complex (ABC reagent) for 30 minutes, followed by a 5-minute incubation with the HRP substrate, DAB (3,3’-diamnobenzidine) from the Vectastain ABC HPR kit (Cat. PK-4001, Vector Laboratories, Burlingame, CA). SOX2 staining densities within cancer cores were quantified using Aperio ImageScope by an experimenter naïve to clinical information. The immunohistochemical score of a given specimen was averaged from all optimally stained cores. Cores with inadequate representation of tumor tissue were omitted from further analysis. H-Scores were determined using the nuclear quantification algorithm according to the following formula: H score = [(3×strong%)+(2×moderate%)+(weak%)]. Continuous variable scoring was used to evaluate correlations with other markers but we also looked at the dichotomous versions for SOX2, but not e-cadherin or vimentin. Low expression of SOX2 was defined as scores of 0 or 1. SOX2 expression scores were available on 195 patients.
Statistical Analysis:
Correlations with clinical characteristics were tested with the Kruskal-Wallis test and correlations among markers were assessed with Spearman correlation coefficients. Overall and relapse-free survival from date of diagnosis were analyzed with the Kaplan-Meier method, log-rank test and multivariable Cox proportional hazards models. Multivariable models included marker(s) plus a set of variables known to be associated with outcome (age, tumor stage, tumor site, comorbidity, HPV status and smoking status). Because of potential confounding of the analysis by HPV status and tumor site, interaction models were also considered for specific markers by HPV status interaction.
Results:
EMT Markers and Survival in HNSCC:
Loss of E-cadherin expression was significantly associated with low or absent expression of SOX2 (R=0.43, p<0.01). Increased vimentin expression was inversely associated with loss of E-cadherin (R=−0.22; p<0.01), but was not significantly associated with survival outcomes (correlation of markers is shown in Supplementary Table 1). A total of 82% (152/185) of patient tumors showed SOX2 expression. The percentage of patients showing SOX2 expression score >1 was 65% (120/185). Interestingly, SOX2 expression (expression score >1) was significantly associated with favorable OS and RFS in univariable analysis and in multivariable analysis even after controlling for age, tumor stage, tumor site, comorbidity, HPV status and smoking status. (Figure 1 and Table 2, HR for RFS=0.40; 95% C.I.= 0.21, 0.76; p=0.005). Further, when the known prognostic variable of CD4 T cell infiltration was also accounted for, SOX2 expression remained a significant prognostic factor in multivariable analysis for RFS (HR 0.40; 95% C.I. = 0.21, 0.76; p=0.005) and a trend for OS (HR 0.59; 95% C.I.=0.32, 1.06; p=0.07). Because of the importance of HPV status and prognosis and the association of tumor site with HPV status, we tested for interaction between markers and HPV status in all of our multivariable analysis to determine if HPV status was an effect modifier. We found no significant interactions (data not shown) and left the interaction terms out of the reported models because the term was essentially null.
Figure 1.
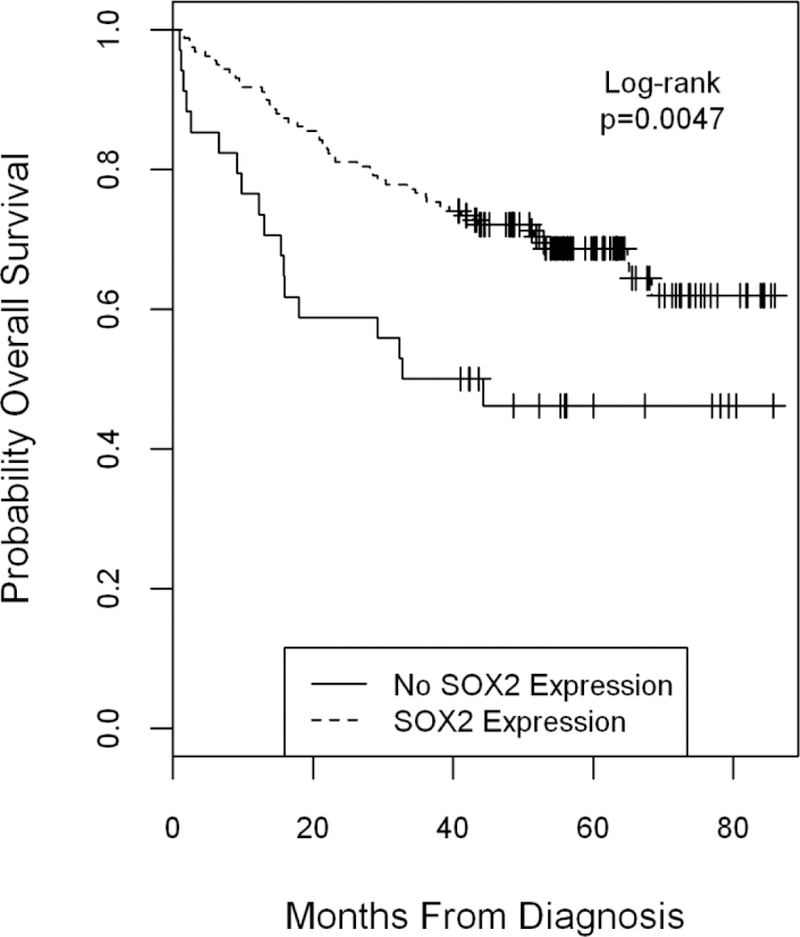
Overall survival by SOX2 expression score comparing any expression to no expression, Logrank p=0.0047. By multivariable Cox regression analysis, the Hazard Ratio was 0.43; 95% CI = 0.25, 0.74; p= 0.002, Supplementary Table 2.
Table 2:
Multivariable outcome models with clinical factors, HPV status, Worst Pattern of Invasion (WPOI) and SOX2 score
| OS | RFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | HR | 95% Hazard Ratio Confidence | p-value | HR | 95% Hazard Ratio Confidence | p-value | |||
| Age | 10 years | 1.15 | 0.9 | 1.48 | 0.27 | 0.95 | 0.72 | 1.27 | 0.75 |
| Stagea | 1 (ref) | ||||||||
| 2 | 1.31 | 0.3 | 5.8 | 0.72 | 0.67 | 0.09 | 4.93 | 0.7 | |
| 3 | 3.32 | 0.86 | 12.87 | 0.08 | 4.63 | 0.86 | 24.77 | 0.07 | |
| 4 | 2.45 | 0.7 | 8.58 | 0.16 | 3.1 | 0.64 | 14.87 | 0.16 | |
| Disease Site | Larynx (ref) | ||||||||
| Oral Cavity | 1.72 | 0.79 | 3.78 | 0.17 | 1.4 | 0.54 | 3.64 | 0.49 | |
| Oropharynx | 3.29 | 1.15 | 9.4 | 0.03 | 5.16 | 1.61 | 16.59 | 0.006 | |
| Hypopharynx | 9.96 | 2.53 | 39.3 | 0.001 | 11.3 | 2.76 | 46.32 | 0.0008 | |
| HPV status | Negative (ref) | ||||||||
| Positive | 0.3 | 0.11 | 0.78 | 0.01 | 0.14 | 0.04 | 0.42 | 0.0005 | |
| invalid/missing | 0.79 | 0.32 | 1.98 | 0.62 | 0.81 | 0.27 | 2.48 | 0.72 | |
| Comorbidities | none (ref)_ | ||||||||
| mild | 2.27 | 1.05 | 4.92 | 0.04 | 1.56 | 0.71 | 3.44 | 0.27 | |
| moderate | 1.3 | 0.47 | 3.61 | 0.61 | 0.88 | 0.29 | 2.68 | 0.83 | |
| severe | 4.57 | 1.61 | 12.96 | 0.004 | 3.39 | 1.13 | 10.16 | 0.03 | |
| Smoking | never (ref) | ||||||||
| current in past 12 mos | 2.87 | 1.09 | 7.54 | 0.03 | 0.95 | 0.36 | 2.49 | 0.91 | |
| former smoker > 12 mos | 1.26 | 0.47 | 3.38 | 0.65 | 0.52 | 0.2 | 1.38 | 0.19 | |
| WPOI | 1 (ref) | ||||||||
| 2 | 1.41 | 0.38 | 5.3 | 0.61 | 0.76 | 0.23 | 2.54 | 0.66 | |
| 3 | 2.1 | 0.55 | 8.03 | 0.28 | 0.8 | 0.23 | 2.83 | 0.73 | |
| 4 | 1.5 | 0.36 | 6.31 | 0.58 | 0.73 | 0.2 | 2.7 | 0.64 | |
| SOX2 >1 | 0.48 | 0.26 | 0.89 | 0.02 | 0.4 | 0.21 | 0.76 | 0.005 | |
American Joint Committee on Cancer - 7.0 Edition; OS = Overall Survival, RFS = Recurrence Free Survival, HR = Hazard Ratio
EMT Markers, Histologic Pattern of Invasion (WPOI) and Tumor Infiltrating Lymphocytes:
Vimentin expression was the only EMT marker that directly correlated with TIL tumor infiltration by CD4, CD8, and FoxP3 positive cells (p<0.01, for all; rho: 0.33 to 0.43; Supplementary Table 1). E-cadherin expression and SOX2 scores did not correlate with levels of any of the TILs. TIL infiltration by CD4, CD8, and FoxP3 positive cells correlated indirectly with worst pattern of invasion score (p=0.02, p<0.01, and p<0.01, respectively; rho: −0.15 to −0.24). Tumors with higher grades of invasion had lower levels of T cell infiltrates. Consistent with this finding, WPOI was also found to be a significant prognostic factor for overall and RFS survival (Logrank p=0.0025 and p=0.0157, respectively, Figure 2) and in univariable Cox regression analysis, (Supplementary Table 2). In multivariable analysis (Supplementary Table 3) including all biomarkers and adjusting for age, tumor stage, tumor site, HPV status, comorbidity and smoking, WPOI remained a significant predictor of OS (Grade 4 vs. 1: HR =3.3; 95% CI 1.1, 9.9, p=0.04). SOX2 expression was not directly related to WPOI even though both variables predicted survival outcomes. When considered together, SOX2 score remained significant for OS and RFS while WPOI lost prognostic significance in multivariable analysis (SOX2 OS: HR=0.48; 95% CI 0.26, 0.89, p=0.02; RFS: HR=0.40; 95% C.I.= 0.21, 0.76, p=0.005 Table 2 and Supplementary Table 3).
Figure 2.
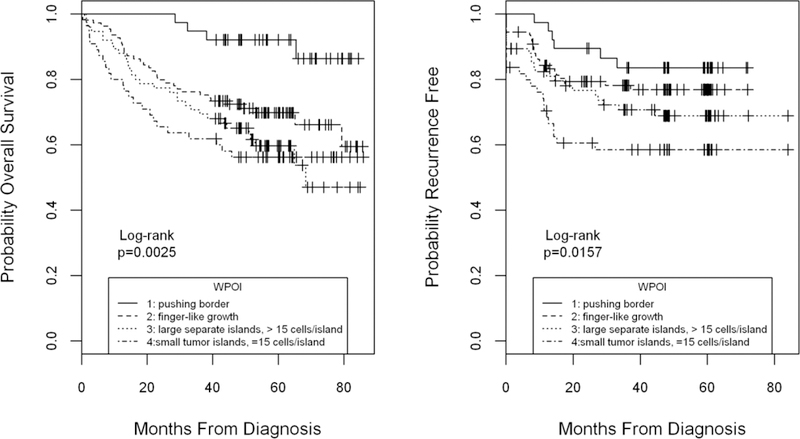
Overall and recurrence free survival by worst pattern of invasion grade (WPOI). Grading of histologic WPOI was a highly significant prognostic factor for overall (left panel) and recurrence free survival (right panel) by univariable analysis (Kaplan Meier Logrank p=0.0025 and p=0.0157 respectively). In multivariable analysis adjusting for age, tumor stage, tumor site and comorbidity, WPOI remained a significant predictor of OS.
EMT Markers and Response to Treatment:
When patients were grouped by primary treatment modality (surgery or radiation/chemoradiation) and analyzed for survival by WPOI grade, significant differences in predicting outcome were found. The distribution of treatment assignment (Table 1) is shown according to clinical characteristics and biomarkers in Supplementary Table 4. Lower patterns of invasion (grade 1,2) were predictive of improved recurrence free and overall survival in patients treated with primary surgery compared to patients with more aggressive WPOI (grade 3,4) (Figure 3). This contrasted with patients treated with radiation or chemoradiation, where WPOI was not significantly associated with predicted outcomes. This lack of predictive difference appeared due in part to better than expected outcomes among patients with aggressive patterns of infiltration (grades 3,4) and worse outcomes for patients with the least invasive patterns (grades 1,2). We performed a similar analysis of treatment outcomes by primary therapy for SOX2 expression and also found that SOX2 expression tended to predict favorable OS (p=0.04) and RFS (p=0.006) for patients treated with surgery. However, we had only one patient with SOX expression score <1 who was treated with chemoradiation and could not evaluate whether SOX2 expression would be prognostic in this group.
Figure 3.
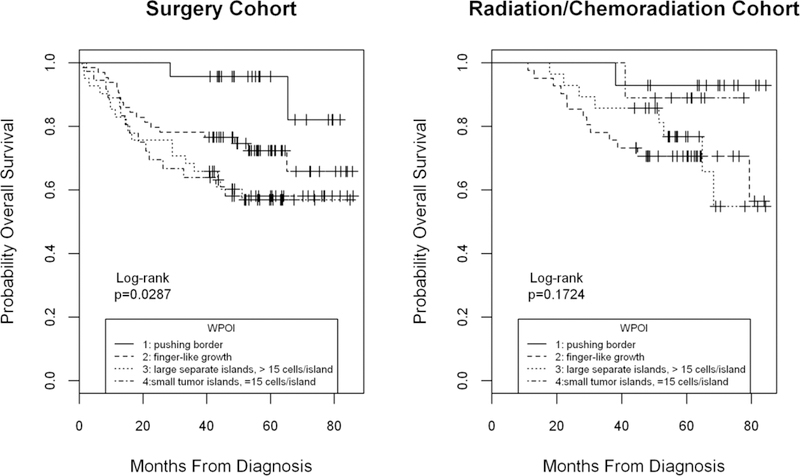
Overall survival by worst pattern of invasion grade (WPOI) in surgery vs radiation/chemoradiation cohorts. Grading of histologic WPOI was a significant prognostic factor for OS in the surgical cohort (left panel) but not in the radiation/chemoradiation cohort (right panel) by univariable analysis (Kaplan Meier Logrank p=0.0287 and p=0.1724 respectively). Markedly better than expected survival was evident for higher grades WPOI among radiation/chemoradiation patients.
Correlations of EMT Markers with Clinical Characteristics:
E-cadherin and vimentin scores varied by disease site and HPV status. E-cadherin expression was significantly lower in patients with oral cavity and hypopharyngeal cancers (p<0.0001) compared to larynx or oropharynx while vimentin scores were higher in patients with oropharyngeal cancers (p=0.0007). Both E-cadherin expression (p=0.0006) and vimentin scores (p=0.002) were higher in patients with HPV+ cancers. SOX2 scores were also significantly higher in patients with HPV+ cancers (p =0.0001). Vimentin scores were lower in patients with advanced T stage (p=0.009), higher in patients with advanced N stage (p=0.07) and in never smoking patients (p=0.01). E-cadherin expression was higher among Stage 4 patients (p=0.02) and did not differ by smoking status. Vimentin scores correlated directly and significantly with levels of CD4, CD8, FoxP3 and CD68 TIL levels in tumor and stroma (Supplementary Table 1). These correlations may have been related to higher levels of infiltrating T lymphocytes in patients with oropharyngeal cancers previously reported [34,36].
Discussion:
As we try to better individualize treatment approaches for patients with HNSCC, we continue to search for appropriate phenotypic markers that can guide therapy selection and avoid unnecessary morbidity. Classically, clinical measures of tumor extent (size, volume) and biologic growth properties (proliferation, pattern of invasion, extranodal extension, lymph node metastases, etc.) have been used to guide the selection and aggressiveness of treatment. Difficulties in reproducibility and uniform application of a variety of other phenotypic biomarkers and lack of validated correlations with treatment response and outcomes have limited the clinical usefulness of such biomarkers. A recent exception has been the rapid acceptance of Human Papilloma Virus (HPV) testing as a marker of less aggressive oropharyngeal cancers which has led to exploration of treatment intentsity de-escalation for selected HPV+ patients. Thus, the identification of other new markers reflecting individual tumor biology for the majority of patients remains a high priority and holds clinical promise.
Epithelial to mesenchymal transition (EMT) has been associated with tumor heterogeneity and with the acquisition of properties characteristic of cancer initiating cells (stem cells) including increased motility, invasiveness and resistance to therapy [13]. Studies in HNSCC have implicated cancer stem cells with prognosis [1,5,17, 21,22], but considerable plasticity of the stem cell population and heterogeneity in expression of stem cell markers within tumors has limited any practical usefulness in therapy selection. Genetic analyses have also implicated a mesenchymal tumor phenotype with poor prognosis [44,45]. Our current findings of a significant association of SOX2 expression with clinical outcomes extend and confirm prior tumor marker observations and support a role for SOX2 assessment as a potentially useful and independent marker compared to other established EMT markers such as E-cadherin or vimentin. As expected, expression of E-cadherin and vimentin were inversely related and uniquely, vimentin positive cells were directly related to tumor infiltration by multiple T cell subsets. This is the first time vimentin positive tumor infiltrating cells (mesenchymal phenotype) have been associated with T cell infiltration and could reflect a population of vimentin positive immune reactive cells. Despite this finding, vimentin expression was not a significant prognostic factor and was not associated with SOX2 expression or histologic pattern of invasion. Neither vimentin score nor E-cadherin expression were significantly associated with overall or relapse free survival after controlling for age, stage, site comorbidities, HPV and smoking status. This contrasts with other studies that have suggested a relationship of these markers with patient prognosis [11,12,17]. One limitation of interpretation of our findings is potential heterogeneity in expression of EMT markers within the tumor microenvironment. Since a TMA samples only a small representative portion of the central tumor, differing expression in other areas, such as the invasive front could have prognostic significance that would not be detected with this methodology. A similar concern could be raised for determining TIL levels in TMA cores since most studies have also characterized TILs at the invasive front. It is important to note that it appears that abundant TILs in central tumor tissue cores is a strong enough signal of favorable prognosis to overcome potential shortcomings of sampling heterogeneity.
The SOX family of transcription factors are important regulators of stem cell fate [20]. Some authors have found improved prognosis for patients with tumors with high SOX2 expression [21,23,24] while others reported worse prognosis for patients with tumors showing amplification or overexpression [26]. SOX2, in particular, is thought to have bifunctional properties where too little or too much may inhibit proliferation [22]. In the current cohort, we found SOX2 expression to be a strong prognostic factor. Using a continuous scale, SOX2 expression scores >1 predicted improved survival compared to low or no expression, however, if patients were grouped by median score, survival curves were similar. Thus, it remains unclear what very high expression may reflect. Our recent study using a syngeneic HNSCC mouse model found that Sox2 potently promoted tumor growth in immunocompetent hosts. As a mechanism, we found that high Sox2 inhibited Sting-mediated type I interferon signaling and potentiated immune escape. Through robust TIL deconvolution using 519 HNSCC specimens in the TCGA [44], we recently confirmed that tumors with high levels of SOX2 expression contained significantly higher frequencies of inhibitory regulatory T cells and lower M1-like macrophages, both of which suggest a phenotype of immune suppression [25]. In agreement, other investigators have shown that CD44 overexpression is associated with worse prognosis [49] and that CD44 positive HNSCC stem cells could induce immunosuppression via PDL-1 expression and loss of interferon-γ production [30] which suggests that regulation of stem cell phenotype via SOX2 or other stem cell regulators such as Twist, BMI1, ZEB1 or SNAIL could have immunologic sequelae [29,47]. The possible reasons underpinning the phenotypic discrepancy between SOX2-driven immunosuppression in the tumor microenvironment and patient survival are multifactorial. SOX2 is thought to stabilize stem cell phenotype and prevent EMT [1]. However, besides a role potentiating immunosuppression in the microenvironment and modulating cancer stemness, SOX2 could promote mesenchymal-epithelial transition (MET) which is consistent with its correlation with E-cadherin in this study and would attenuate the invasive phenotype [48]. In addition, the mechanisms regulating tumor development and host response to treatment do not always overlap. Thus, expanded analyses of SOX2 function in controlled disease models and clinical trials would be very useful to better dissect its role in regulating the immune microenvironment and tumor response to treatments. In our multivariable analysis that included TIL levels, SOX2 expression remained significant supporting its role as an independent prognostic factor that could be useful in prognostication in addition to measures of TILs. Thus, levels of TILs do not appear to be influenced directly by changes in EMT or transcription factors regulating EMT, but since our investigations did not determine the functional status of TILs, it is still possible that EMT changes could affect microenvironment cytokines, PD1 and PD-L1 expression and the function of TILs.
A second major finding was confirmation that histologic pattern of invasion is an important prognostic factor. Higher grades of invasion also correlated with less tumor infiltration by CD4, CD8 and FoxP3 positive T cells indicating a correlation of invasion and immune response and further supporting the negative prognostic association of WPOI with overall and relapse free survival. Although WPOI has previously been reported to be prognostically significant in patients with cancers of the oral cavity, [30–33] we reported that WPOI was a significant prognostic factor in our prior work in patients with laryngeal cancer [49] and extended and confirmed these findings in the current study that included patients with multiple sites of cancer including the oropharynx and hypopharynx. Interestingly, WPOI was found to be independent of SOX2 expression even though both were associated with survival. Also, WPOI did not correlate with expression of E-cadherin or vimentin although there was a trend for lower E-cadherin expression in tumors with invasive pattern 4 and 5 grades. This is likely due to loss of adhesion proteins among those most invasive cells. Importantly, SOX2 remained prognostically significant after adjusting for WPOI. Further, when patients were group by primary treatment modality, WPOI was prognostically significant for patients undergoing primary surgery and not significant for patients treated with primary chemoradiation. This differential in predicting outcome according to primary treatment is consistent with our findings in the VA Laryngeal Cancer randomized trial where we analyzed outcomes according to pattern of invasion for each of the treatment arms (laryngectomy vs. chemoradiation) [49]. Our current confirmation of WPOI as a predictive factor for type of treatment modality success lends support to the contention that histologic pattern of invasion could be a useful marker for selection of primary therapy. In prior work analyzing TILs and prognosis, we also found that low levels of CD8+ cells tumor infiltrates predicted poor prognosis regardless of initial therapy, however, a low level of CD4+ infiltrating cells was a negative prognostic factor only for patients treated with chemoradiation but not for primary surgery [36]. Because of the interaction of tumor site and treatment selection, we should caution that it remains unclear if the associations of these markers with treatment response are due primarily to the effectiveness of the treatment and tumor phenotype or to biologic differences due to tumor site. Large studies of patients with tumors of a single site may be needed to better address this issue. It is noteworthy that we have consistently found that markers of less aggressive tumor growth seem predictive of good outcomes with primary surgical treatment and more aggressive markers predict better response to radiation or chemoradiation [50].
In conclusion, we believe this is the first study to directly evaluate TILs and expression of EMT-MET markers along with histologic patterns of tumor invasion in patients with HNSCC. SOX2 expression and WPOI were clearly significant prognostic factors and WPOI correlated inversely with decreased T cell infiltration. Additional confirmatory studies will be necessary to determine if the combination of SOX2 expression, low WPOI grade and low CD4 TIL counts might be useful in selecting patients for primary surgical resection.
Supplementary Material
Highlights:
E-cadherin expression correlated inversely with increased vimentin expression.
Vimentin positive cells correlated with levels of tumor infiltrating lymphocytes.
Loss of E-cadherin was associated with low or absent SOX2 expression.
SOX2 expression and pattern of invasion predicted favorable overall and relapse free.
Pattern of invasion correlated inversely with tumor infiltrating lymphocytes.
Low grade invasion predicted improved survival for primary surgery but not chemoradiation.
Acknowledgements:
This work was supported by NIH/NCI grants P50CA097248, P50CA192977 and P30 CA046592, NIH/NIDCR grants R00 DE024173, RO1 DE026728 and the Diane and Sinabaldo Tozzi Research Fund. The authors thank the many investigators in the University of Michigan Head and Neck Specialized Program of Research Excellence for their contributions to patient recruitment, assistance in data collection and encouragement including Carol R. Bradford, MD, Thomas E. Carey, PhD, Douglas B. Chepeha, MD, Sonia Duffy, PhD, Avraham Eisbruch, MD, Joseph Helman, DDS, Kelly M. Malloy, MD, Jonathan McHugh, MD, Scott A. McLean, MD, Tamara H. Miller, RN, Jeff Moyer, MD, Mark E. Prince, MD, Nancy Rogers, RN, Matthew E. Spector, MD, Nancy E. Wallace, RN, Heather Walline, PhD, Brent Ward, DDS, and Francis Worden, MD. We greatly thank patients and their families who tirelessly participated in survey and specimen collections in the University of Michigan Head and Neck Specialized Program of Research Excellence.
Footnotes
Conflict of Interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Statement:
The data used in this analysis includes scoring of tissue microarrays by independent observers and is available on spreadsheets and can be requested through the University of Michigan Head and Neck SPORE Program data release approval system at: https://uhintwebspr1.mcit.med.umich.edu/cancerctr/hnspore/consent.cfm
References
- 1.Thierauf J, Veit JA, Hess J. Epithelial-to-Mesenchymal transition in the pathogenesis and therapy of head and neck cancer. Cancers 2017; 9:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev. Mol. Cell Biol 2014; 15:178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canel M, Serrels A, Frame M, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastases. J Cell Science 2013; 126:393–401. [DOI] [PubMed] [Google Scholar]
- 4.Mezi S, Chiappetta C, Carletti R, Nardini A, et al. Clinical significance of epithelial-to-mesenchymal transition in laryngeal carcinoma: its role in the different subsites. Head Neck 2017; 1–13. [DOI] [PubMed]
- 5.Linge A, Lock S, Gudziol V, Nowak A, et al. Low cancer stem cell marker expression and low hypoxia identify good prognosis subgroups in HPV(−) HNSCC after postoperative radiochemotherapy: a multicenter study of the DKTK-ROG. Clin Cancer Res 2016; 22:2639–49. [DOI] [PubMed] [Google Scholar]
- 6.Heerboth S, Housman G, Leary M, et al. EMT and tumor metastasis. Clin Transl Med 2015; 26:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J. Cell Biol 1966: 135:1643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res 2007. December 1;13(23):7003–11. [DOI] [PubMed] [Google Scholar]
- 9.Satelli A, Li S. Vimentim as a potential molecular target in cancer therapy. Cell Mol Life Sci 2011; 68:3033–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci 2008. March 15;121(Pt 6):727–35. [DOI] [PubMed] [Google Scholar]
- 11.Liu LK, Jiang XY, Zhou XX, Wang DM, Song XL, Jiang HB. Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcome. Mod Pathol. 2010. February;23(2):213–24. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z, Ge J, Sun Y, Tian L, Lu J, Liu M, Zhao Y. Is E-cadherin immunoexpression a prognostic factor for head and neck squamous cell carcinoma (HNSCC)? A systematic review and meta-analysis. Oral Oncol 2012. September;48(9):761–7. [DOI] [PubMed] [Google Scholar]
- 13.Brabletz T, Kalluri R, Nieto MA, Wienberg RA. EMT in cancer. Nat Rev Cancer 2018; 18:128–134. [DOI] [PubMed] [Google Scholar]
- 14.Chu PY, Hu FW, Y CC, et al. Epithelial-mesenchymal transition transcription factor ZEB1/ZEB2 co-expression predicts poor prognosis and maintains tumor-initiating properties in head and neck cancer. Oral Oncol 2013; 49:34–41. [DOI] [PubMed] [Google Scholar]
- 15.Prince ME, Siranardon R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE: Identification of subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 2007; 104:973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf GT. Surgical margins in the genomic era: The Hayes Martin Lecture, 2012. Arch Otolaryngol Head Neck Surg 2012; 138:1001–13. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Pedrero JM, Garcia-Cabo P, Villaronga MA, et al. Prognostic significance of E-cadherin and Beta-catenin expression in HPV-negative oropharyngeal squamous cell carcinomas. Head Neck 2017; 11:2293–2300.. [DOI] [PubMed] [Google Scholar]
- 18.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol 2013. April;49(4):287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtz KA, Hoffman HT, Zimmerman MB, Robinson RA. Decreased E-cadherin but not beta-catenin expression is associated with vascular invasion and decreased survival in head and neck squamous carcinomas. Otolaryngol Head Neck Surg 2006. January;134(1):142–6. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar A, Hochedlinger KI. The Sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013; 12:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayo P, Jou A, Stnezinger A, et al. Loss of SOX2 expression induces cell motility via mimentin up-regulation and is an unfavorable risk factor for survival of head and neck squamous cell carcinoma. Mol. Oncol; 2015; 9: 1704–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weubben EL, Rizzino A. The dark side of SOX2: cancer-a comprehensive overview. Oncotarget 2017; 8:44917–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung JH, Jung HR, Jung AR et al. SOX2 activation predicts prognosis in patients with head and neck squamous cell carcinoma. Sci Rep 2018. January 26;8(1):1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brochen F, Adisurya H, Wemmert S, et al. Effect of 3q oncogenes SEC62 and SOX2 on lymphatic metastasis and clinical outcome of head and neck squamous cell carcinomas. Oncotarget 2017; 8:4922–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan YS, Sansanaphongpricha K, Xie Y, Donnelly CR et al. A Type I interferon-inducing nanosatellite vaccine mitigates immune suppression in head and neck squamous cell carcinoma. 2018. (in press) Clin Cancer Res [DOI] [PMC free article] [PubMed]
- 26.Schrock A, Bode M, Johanna F, et al. Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis 2014; 35: 1636–42. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Oh SY, Do SI, Lee HJ, Kang HJ, Rho YS, Bae WJ, Lim YC. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br J Cancer 2014. November 25;111(11):2122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastatsis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 2009; 15:195–206. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y, Shin JH, Longmire M, Wang H, et al. CD44+ cells in head and neck squamous cell carcinoma suppress T cell-mediated immunity by selective constitutive and inducible expression of PD-L1. Clin Cancer Res 2016; 22:3571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandwein–Gensler M, Smith RV, Wang B, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol 2010; 34:676–688. [DOI] [PubMed] [Google Scholar]
- 31.Brandwein–Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol 2005; 29:167–1 [DOI] [PubMed] [Google Scholar]
- 32.Carillo JF, Carrillo LC, Cano A, et al. Retrospective cohort study of prognostic factors in patients with oral cavity and oropharyngeal squamous cell carcinoma. Head Neck, 2016; 38:536–541.. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Bai S, Carrroll W, et al. Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol 2013; 7:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wansom D, Light E, Thomas D, Worden F, Prince M, Urba S, Dchepeha D, Kumar B, Eisbruch A, Taylor J, Moyer J, Bradford C, D”silva N, Carey T, McHugh J, Wolf GT, The UM Head and Neck SPORE Program. Infiltrating lymphocytes and human papillomavirus-16 associated oropharynx cancer. Laryngoscope 122:121–7, 2012PMID: 22183632.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J; The University of Michigan Head and Neck SPORE Program. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol 2015. ;51:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, Peterson L, Carey TE, Walline H, Moyer J, Spector M, Perim D, Prince M, McLean S, Bradford CR, Taylor JM, Wolf GT; Head and Neck SPORE Program Investigators. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous carcinoma. Head Neck 2016. July;38(7):1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf GT, Hudson JL, Peterson KA, Miller HL, McClatchey KD: Lymphocyte subpopulations infiltrating head and neck squamous carcinomas: Correlations with tumor extent and prognosis. Otolaryngol Head Neck Surg 95:142–152, 1986 [DOI] [PubMed] [Google Scholar]
- 38.Hoesli R, Birkeland AC, Rosko AJ, Issa M, et al. Proportion of CD4 and CD8 tumor infiltrating lymphocytes predicts survival in persistent/recurrent laryngeal squamous cell carcinoma. Oral Oncol 2018; 77:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang H, Yang K, Khafagi A, et al. Sensitive detection of human papillomavirus in cervical, head/neck, and schistosomiasis-associated bladder malignancies. Proc Natl Acad Sci U S A 2005;102:7683–7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol 2008;26:3128–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nocito A, Kononen J, Kallioniemi OP, Sauter G. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer 2001;94:1–5. [DOI] [PubMed] [Google Scholar]
- 42.Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E.J New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. Oral Pathol Med 1989. September;18(8):432–7. [DOI] [PubMed] [Google Scholar]
- 43.Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res 1987. June;95(3):229–49. [DOI] [PubMed] [Google Scholar]
- 44.Keck MK, Zuo Z, Khattri A, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin. Cancer Res 2015; 21:870–881. [DOI] [PubMed] [Google Scholar]
- 45.DeCecco L, Nicolau M, Giannoccaro M, et al. Head and neck cancer subtypes with biological and clinical relevance: Meta-analysis of gene-expression data. Oncotartet 2015; 6:9627–9642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayes D, Walter V, Harris T, Prystowsky M, et al. , The Cancer Genome Atlas Network et al: Comprehensive genomic characterization of head and neck squamous cell carcinomas, Nature 2015; 517:578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodrigues MFSD, Xavier FL, Andrade NP, Lopes C et al. Prognostic implications of CD44, NANOG, OCT4 and BMI1 expression tongue squamous cell carcinoma. Head Neck 2018; 1–15. [DOI] [PubMed]
- 48.He S, Chen J, Zhang Y, Zhang M, et al. Sequential EMT-MET induces neuronal conversion through Sox2. Cell Discovery 2017; 3:17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Truelson JM, Fisher S, Beals TF, McClatchey KD, Wolf GT: DNA content and histologic growth pattern correlate with prognosis in patients with advanced squamous cell carcinoma of the larynx. Cancer 1992; 70:56–62. [DOI] [PubMed] [Google Scholar]
- 50.Wolf GT: Tradition, Teamwork and Tailored Treatment: Surgical Oncology in the Genomic Era. Arch Otolaryngol Head Neck Surg 135(4):337–41, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Wolf GT, Winter W, Bellile E, Nguyen A, Donnelly C, McHugh JB, Thomas D, Amlani L, Rozek L, Lei YL, the Head and Neck SPORE Program, HISTOLOGIC PATTERN OF INVASION AND EPITHELIAL-MESENCHYMAL PHENOTYPE PREDICT PROGNOSIS IN SQUAMOUS CARCINOMA OF THE HEAD AND NECK, Oral Oncology, 10.1016/j.oraloncology.2018.10.010 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


