Abstract
The clinical, histological, and immunophenotypic findings are presented for 4 horses affected by different types of lymphoma. Diagnoses of a monomorphic epitheliotropic intestinal T-cell lymphoma, a diffuse splenic large B-cell lymphoma, a peripheral T-cell lymphoma, and a T-cell rich large B-cell lymphoma of the third eyelid were made.
Résumé
Constatations cliniques et immunophénotypiques pour quatre formes de lymphomes équins. Les constatations cliniques, histologiques et immunophénotypiques sont présentées pour quatre chevaux affectés par différents types de lymphome. Des diagnostics d’un lymphome intestinal épithéliotrope et monomorphe à cellules T, d’un lymphome splénique diffus à grandes cellules B, d’un lymphome périphérique à cellules T et d’un lymphome à grandes cellules B riche en cellules T de la troisième paupière ont été posés.
(Traduit par Isabelle Vallières)
Case descriptions
Horse 1
A 26-year-old Italian Saddle horse gelding was treated for colic at the Veterinary Teaching Hospital of Perugia (OVUD-UNIPG). The gelding had a 7-day history of diarrhea and lack of appetite. Fluid and antispasmodic drugs had previously been administered by the referring veterinarian, but after 3 d, the symptoms resumed.
On presentation, the horse appeared depressed, with a body condition score (BCS) of 8/9. The vital parameters were outside the normal limits: heart rate: 88 beats/min [reference interval (RI): 25 to 40 beats/min]; respiratory rate: 40 breaths/min (RI: 8 to 16 breaths/min); and serum lactate 4.1 mmol/L [reference value (RV) < 2 mmol/L]. The feces contained stones.
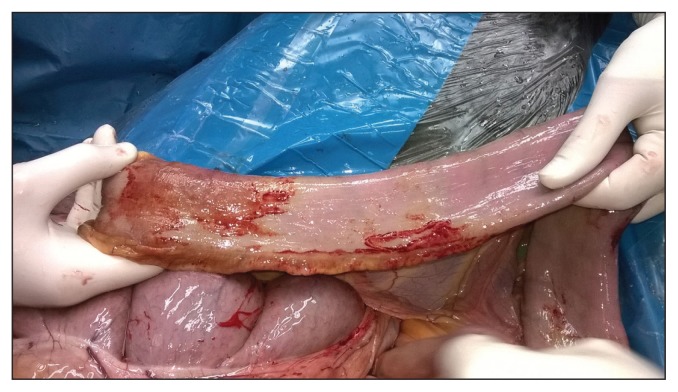
Abdominal ultrasonography (LOGIQ C5 Premium; GE Healthcare, Jakarta Selatan, Indonesia) was performed using a 7.5 MHz probe: a large amount of anechoic peritoneal fluid was found especially in the caudal aspect of the abdomen. The stomach was distended until the 15th intercostal space, and the walls of both the small and large intestine were thickened (colonic vessels were seen). An exploratory laparotomy was carried out under general anesthesia. Thickness of the ileum, pelvic flexure, and enlargement of mesenteric lymph nodes were found during surgery. Full thickness biopsy samples of the pelvic flexure and lymph nodes were collected (Figure 1).
Figure 1.
Horse 1 — thickness of the ileum found during the surgery.
The gelding received supportive care with constant rate infusion of lidocaine 2% (Lidocaina 2%; A.T.I. s.r.l, Ozzano dell’Emilia Bologna, Italy), 0.05 mL/kg BW/min, and Ringer’s lactate solution IV (Ringer’s Lactate S.A.L.F; S.A.L.F S.p.a, Cenate Sotto, Bergamo, Italy), 0.5 mL/kg BW/h, for 48 h after surgery. Benzylpenicillin procaine (Depocillina; MSD Animal Health srl, Happyfarma, Vicenza, Italy), 4 mg/kg body weight (BW), IM, and flunixin meglumine (Flogend; Intervet Productions Srl, Happyfarma, Vicenza, Italy), 1.1 mg/kg BW, IV, were administered for 7 d after surgery.
During hospitalization a nasogastric tube was inserted many times because of gastric reflux due to post-operative paralytic ileum. Hot and painful edema of the forelimbs appeared. Complete blood (cell) count (CBC) (Sysmex-XT 1800 vet; DASIT S.P.A. Cornaredo Milano, Italy), serum biochemistry (Hitachi 904 Automatic Analyzer; Roche Diagnostics, S.P.A, Monza, Italy) and concentrations of serum amyloid-A (SAA) and fibrinogen (FIB) (Equinostic EVA1; Sandhold ID, Copenhagen, Denmark) were determined. Biochemical examination revealed an increase in lactate dehydrogenase (LDH), glutamic oxaloacetic transaminase (GOT), and creatine kinase (CK). In addition, SAA and FIB concentrations were elevated (Table 1).
Table 1.
Hematological and biochemical findings in 4 horses with lymphoma.
| Parameters | Horse | Reference interval | |||
|---|---|---|---|---|---|
|
| |||||
| 1 | 2 | 3 | 4 | ||
| RBC (106/uL) | 9.05 | 6.57 | 8 | 8.94 | 5.5 to 7.9 |
| HGB (g/L) | 171 | 126 | 130 | 134 | 120 to 180 |
| HCT (%) | 44.6 | 31.5 | 33.6 | 33.9 | 37 to 55 |
| MCV (fL) | 49.3 | 47.9 | 42 | 37.9 | 34 to 58 |
| MCH (pg) | 18.9 | 19.2 | 16 | 15 | 10 to 18 |
| MCHC (g/L) | 383 | 400 | 380 | 395 | 320 to 380 |
| RDW (%) | 22.4 | 22.7 | 23.4 | 26.4 | 12 to 16 |
| PLT (109/L) | 114 | 251 | 220 | 131 | 100 to 400 |
| MPV (fL) | 8.1 | 7.8 | 6.6 | NA | 6 to 11 |
| PCT (109/L) | 0.09 | 0.2 | 0.14 | NA | 0.05 to 0.22 |
| WBC (109/L) | 5,74 | 13,52 | 9,96 | 9,93 | 5.5 to 16 |
| NEU (%) | 52.5 | 73.7 | 77.5 | 72 | 35 to 75 |
| LYMPH (%) | 41.5 | 18.8 | 17.3 | 23 | 25 to 45 |
| MONO (%) | 4.9 | 6.5 | 4.2 | 5 | < 4 |
| EOS (%) | 0.9 | 0.6 | 0.6 | 1.1 | < 4 |
| BAS (%) | 0.2 | 0.4 | 0.4 | 0.4 | < 1 |
| TP (g/L) | 66 | 96 | 75 | 62 | 55 to 75 |
| TOT BIL (μmol/L) | 82.08 | 24,282 | 42,75 | 48,393 | 8.55 to 47.88 |
| LDH (U/L) | 1386 | 580 | 850 | 1545 | < 400 |
| GOT (U/L) | 433 | 194 | 200 | 261 | < 240 |
| ALP (U/L) | 923 | 971 | 180 | 301 | < 240 |
| CK (U/L) | 429.7 | 129 | 44 | 241.7 | < 55 |
| FIB (g/L) | 18.7 | 32 | 10.4 | 9.52 | 1 to 4 |
| SAA (mg/L) | 492.07 | 10.3 | 32 | 27.86 | 0.5 to 20 |
| A/G (g/L) | NA | 5.3 | 0.66 | NA | 9.3 to 16.5 |
| α-1globulin (g/L) | NA | 2 | 1.2 | NA | 2 to 3 |
| α-2globulin (g/L) | NA | 15.2 | 8.3 | NA | 5 to 9 |
| β-globulin (g/L) | NA | 28.7 | 12.2 | NA | 5 to 13 |
| γ-globulin (g/L) | NA | 17 | 23.5 | NA | 6 to 13 |
| Ca2+ (mmol/L) | NA | 2.92 | NA | NA | 2–3 to 24 |
RBC — red blood cell; HGB — hemoglobin; HCT — hematocrit; MCV — mean corpuscular volume; MCH — mean corpuscular hemoglobin; MCHC — mean corpuscular hemoglobin concentration; RDW — red cell distribution width; PLT — platelet; MPV — mean platelet volume; PCT — plateletcrit; WBC — white blood cell; NEU — neutrophil; LYMPH — lymphocytes; MONO — monocytes; EOS — eosinophils; BAS — basophil; TP — total protein; TOT BIL — total bilirubin; LDH — lactate dehydrogenase; GOT — glutamate oxaloacetate transaminase; ALP — alkaline phosphatase; CK — creatine kinase; FIB — fibrinogen; SAA — serum amyloid-A; A/G — albumin/globulin ratio; NA — not available.
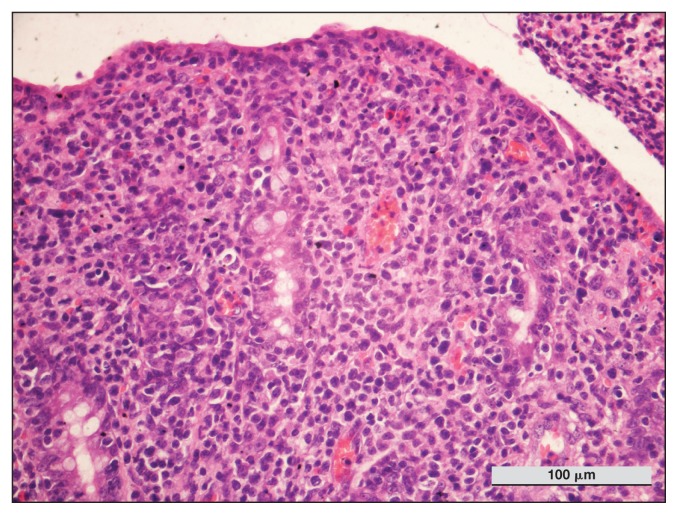
Histologic examination of intestinal biopsy samples showed a severe and diffuse infiltration of monomorphic large and atypical lymphocytes, with round or slightly irregular nuclei, distributed within the epithelium and with involvement of submucosal layers. The results indicated a necrotic-hemorrhagic inflammatory process involving the mucosa, lamina propria, and muscular layers, as evidenced by a high number of neutrophils and a low number of eosinophils, which were admixed with fibrin and associated with thrombi of small arterioles, surrounded by lymphocytes, plasma cells and macrophages. Anisocytosis and anisokaryosis were moderate. Mitotic count (MC), determined by counting mitotic figures in 10 consecutive high-power fields (HPF), was 6/10HPF (Figure 2).
Figure 2.
Horse 1 — intestinal biopsy. Diffuse infiltration of lamina propria by homogeneous population of lymphoid cells. Note the increase in intraepithelial lymphocytes. Hematoxylin and eosin (H&E) stain, × 100 magnification.
Histologic examination of mesenteric lymph nodes indicated chronic necrotic-hemorrhagic lymphadenitis with evidence of infiltration by a population of homogeneous lymphoid cells associated with increased mitotic activity; rare plasma cells were also observed. Immunohistochemistry (IHC) of the intestine and lymph node tissue with antibodies against CD3 (Dako, 1:200; Agilent, Santa Clara, California, USA) and CD20 (Thermo Scientific, 1:200; Thermo Fisher Scientific, Rodano Milano, Italy) antigens was performed (Figure 2). Most of the lymphoid cells present were CD3-positive (T-cells).
DNA was extracted from paraffin-embedded intestinal biopsy samples according to the manufacturer’s instructions (QIAamp DNA FFPE Tissue Kit; Qiagen, PrecisionMed. Solana Beach California, USA). A semi-nested polymerase chain reaction (PCR) protocol targeting a conserved fragment of glycoprotein B (gB) of Equid Herpesvirus type 5 (EHV-5) was performed (1), and the results from the intestine were positive.
Based on histology and IHC, a monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) was diagnosed according to the revised World Health Organization (WHO) criteria (2), and the lymph nodes were considered metastatic. The MC indicated indolent lymphoma.
The horse’s condition worsened because of paralytic ileum and the gelding was euthanized. The owner did not allow necropsy.
Horse 2
A 13-year-old Hirsh gelding horse was referred to the OVUD-UNIPG with a 3-month history of weight loss, depression, and polyuria/polydipsia. Serologic tests performed by the RV for Babesia caballi, Theileria equi, Leptospira bratislava, L. canicola, L. copenhageni, L. Grippothyphosa, L. hardjo, L. icterohaemorrhagiae, L. pomona, L. Saxkoebing, and L. Tarassovi were negative. A blood test for adrenocorticotropic hormone (ACTH) revealed a high level of this hormone in the month of June (212 pg/mL between November and July, RV: < 29 pg/mL) and in the month of October (66 pg/mL between August and October, RV: < 47 pg/mL); thus a diagnosis of Cushing syndrome was made, and pergolide mesylate (Prascend; Boehringer Ingelheim Pty, Animal Health Division, Australia), 1 mg/kg BW, PO, was prescribed.
On presentation, the horse was lethargic, with a BCS of 4/9 and slightly hyperemic mucous membranes; the vital parameters were within normal limits. Abdominal ultrasonography detected a large well-defined splenic mass with heterogeneous echogenicity. Palpation per rectum revealed an abnormal location of the spleen in the middle of the abdominal cavity; the mass could not be palpated. No abnormalities were detected during ultrasonography of the thorax.
Fine-needle aspiration (FNA) and biopsies of the splenic mass and bone marrow were performed with the horse in standing sedation with detomidine hydrochloride (Domidine; Fatro Spa, Ozzano dell’Emilia Bologna, Italy), 10 μg/kg BW, IV. During hospitalization, the horse experienced colic and weight loss despite a good diet.
Serum biochemistry and CBC indicated normocytic normochromic anemia, and elevated LDH, alkaline phosphatase (ALP), and total protein (TP). A blood smear revealed reactive/atypical lymphocytes (1%) and rare erythroblasts. Electrophoresis of serum proteins showed a decreased albumin/globulin ratio and increased α-2, β- and γ-globulins (Table 1).
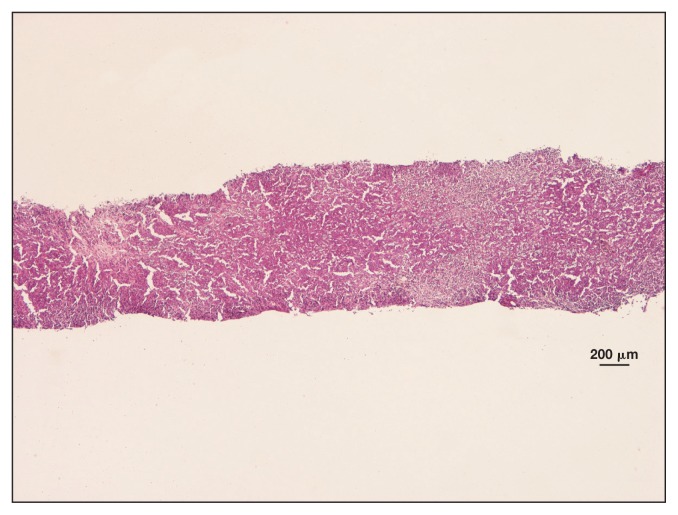
The bone marrow cytology was normal, but cytological evaluation of the spleen revealed blood contamination and marked cellularity characterized by medium-sized lymphoblasts, small lymphocytes, and macrophages with erythrophagocytosis. Histological evaluation of the spleen revealed a population of large round cells with a low nuclei/cytoplasm ratio, and ovoid-round nuclei with irregularly thickened chromatin and 1 visible nucleolus. Anisocytosis and anisokaryosis were evident, and the MC was 8/10 HPF (Figure 3).
Figure 3.
Horse 2 — spleen biopsy showing areas of marked and diffuse cell proliferation alternating to areas of necrosis. H&E stain, × 1.25 magnification.
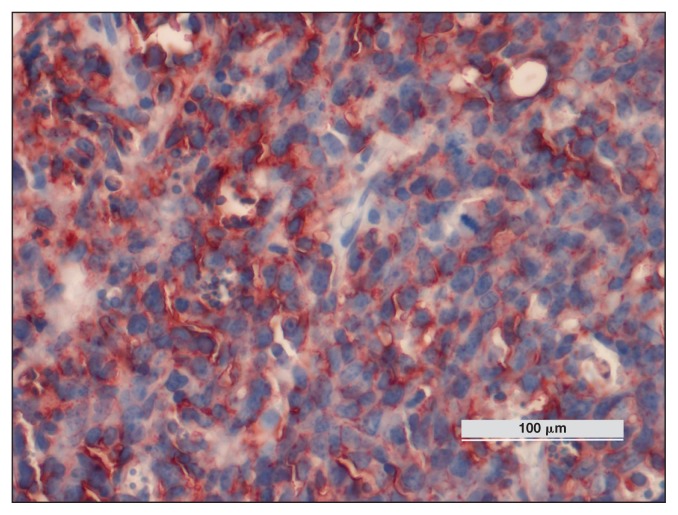
Immunohistochemistry of the splenic tissue with antibodies against CD3 and CD20 antigens was performed. Most of the lymphoid cells were CD20+ (B-cells) (Figure 4). Flow cytometry (FC) (BD Accuri C6; BD Bioscienses, Milan, Italy) of the splenic mass, peripheral blood, and bone marrow was conducted for the following: CD4 (T-cell; CVS4 BioRad, Clinical Diagnostics BioRad, Milan, Italy), CD21 (B-cell; CA2.1D6 BioRad), cyCD3 (T-cell; CD3–12 BioRad), and cyCD79a (B-cell; HM57 BioRad). The spleen sample was cyCD79+ (64%) and CD21+ (41%), whereas the peripheral blood and the bone marrow blood were CD21+ (0.1% and 0.2%, respectively). The PCR for EHV-5 from paraffin-embedded spleen samples was positive.
Figure 4.
Horse 2 — diffuse expression of CD20 protein on lymphoid cells detected by immunohistochemistry. DAB chromogen, Carazzi’s counterstain, × 100 magnification.
The cytological, histological, IHC, and FC results suggested a diagnosis of diffuse large B-cell lymphoma (DLBCL) of the spleen according to the revised WHO (2016) classification (2). The MC indicated mid-grade lymphoma. At the request of the owner, the horse was discharged, but died several days later. The owner did not allow necropsy.
Horse 3
A 12-year-old pony mare was presented to the OVUD-UNIPG with a 5-month history of tear duct obstruction as well as third eyelid prominence and a 1-month history of mandibular lymph node enlargement. On thoracic auscultation, crackles were heard. Acetylcysteine, sodic cefazolin, and flunixin meglumine therapy was started by the referring veterinarian (dosage unknown). No improvement was seen. Edema of the nasal meatuses and laryngeal collapse were found by the referring veterinarian during an endoscopic examination of the primary airways. One month before the hospitalization dexamethasone was administered to the mare (dosage unknown) for 3 d; this resulted in improvement in the respiratory signs but not the ophthalmic signs.
On presentation, the mare had pale mucous membranes, right serous ocular discharge, enlarged mandibular lymph nodes, and a BCS of 7/9. Respiratory tract evaluation indicated hyperemia of the nasal mucosa and muco-purulent discharge from the left nostril; traction on the trachea elicited a dry and deep cough. A respiratory bag test indicated the auscultation of a reinforced breath sound over the cranial-ventral parts of the thorax.
Thoracic ultrasonography showed comet tails in the ventral quadrant, but the findings were considered unremarkable. Thoracic latero-lateral X-rays revealed a round radiopaque zone in the throat region. Fine-needle aspiration of the mandibular lymph nodes was carried out. Endoscopic examination of the primary airways showed enlargement of the sampled pharyngeal lymphoid tissue. Third eyelid resection was carried out on the horse in standing sedation with detomidine hydrochloride (Domidine), 0.04 mg/kg BW, IV, and the tissue was sampled. Benzylpenicillin procaine (Depocillina), 4 mg/kg BW, IM and flunixin meglumine (Flogend), 1.1 mg/kg BW, IV treatments were administered for 7 d after the surgery. Chloramphenicol, colistimethate sodium rolitetracycline (Colbiocin; SIFI S.P.A., Bologna, Italy) and chloramphenicol and betamethasone (Betabioptal; Farmila Theà, Milan, Italy) eye drops were administered.
The serum biochemistry and CBC revealed normochromic normocytic anemia and elevated concentrations of LDH, FIB, and SAA. Electrophoresis of serum proteins showed a decreased albumin/globulin ratio due to an increase in γ-globulins and a decrease in α-1globulins (Table 1). Cytological evaluation of the lymph nodes revealed a mixed population of small and medium lymphocytes, numerous macrophages, and giant multinucleated cells, which contained rod-shaped bacteria.
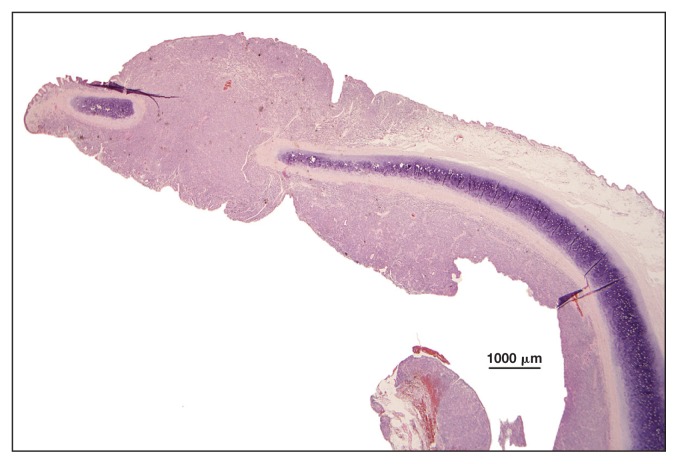
Histological examination of the eyelid tissue showed severe infiltration by a mixed population of cells composed predominantly of numerous small lymphocytes and fewer large round cells (2 or 3 times the size of red blood cells) with little eosinophilic cytoplasm, ovoid nuclei with irregularly coarse chromatin and 1 or more nucleoli, which were referred to as lymphoid cells. These lymphocytes widely infiltrated the epithelium of the eyelid and the surgical margins. Anisocytosis and anisokaryosis were moderate. The MC was 11/10 HPF (Figure 5). The IHC with anti-CD3, and anti-CD20 antibodies indicated CD3 positivity of atypical cells (Figure 6). Histological examination of the pharyngeal sample was characterized by hyperplasia of the associated lymphoid tissue. Bacterial culture of the lymph nodes was negative. The PCR for EHV-5 from paraffin-embedded biopsy samples of the pharynx and eyelid tissues was positive.
Figure 5.
Horse 3 — marked thickening of the lamina propria of the third eyelid by small lymphocytes. H&E stain, × 1.5 magnification.
Figure 6.
Horse 3 — diffuse expression of CD3 protein on lymphoid cells detected by immunohistochemistry. DAB chromogen, Carazzi’s counterstain, × 20 magnification.
The revised WHO classification (2), the histological findings, and the IHC results supported a diagnosis of peripheral T-cell lymphoma (PTCL) of the third eyelid associated with granulomatous lymphadenitis of the mandibular lymph nodes (2). The MC indicated high grade lymphoma. The animal was discharged upon the owner’s request but died after 2 wk. The owner did not allow necropsy.
Horse 4
A 7-year-old Italian Saddle mare was presented to the OVUD-UNIPG with a 6-month history of right ocular discharge, hyperemia, and prominence of the third eyelids. On presentation, the mare was in good body condition (BCS 8/9). The vital parameters were within normal limits. The third eyelids, however, were hyperemic, edematous, and protruding, and the mandibular lymph nodes were enlarged. Ophthalmological ultrasonography was performed using orbital, frontal, and palpebral nerve blocks with 5 mL of lidocaine 2% (Lidocaina 2%) for each eye, but no abnormalities were found. Thoracic ultrasonography showed comet tails, but the data were considered unremarkable.
Both third eyelids were removed from the horse in standing sedation with detomidine hydrochloride (Domidine), 10 μg/kg BW, IV, and butorphanol (Dolorex; MSD Animal Health srl), 0.04 mg/kg BW, IV. Histological analysis and FNA of the mandibular lymph node were performed. Benzylpenicillin procaine (Depocillina), 4 mg/kg BW, IM, and flunixin meglumine (Flogend), 1.1 mg/kg BW, IV, treatments were administered for 7 d after the surgery.
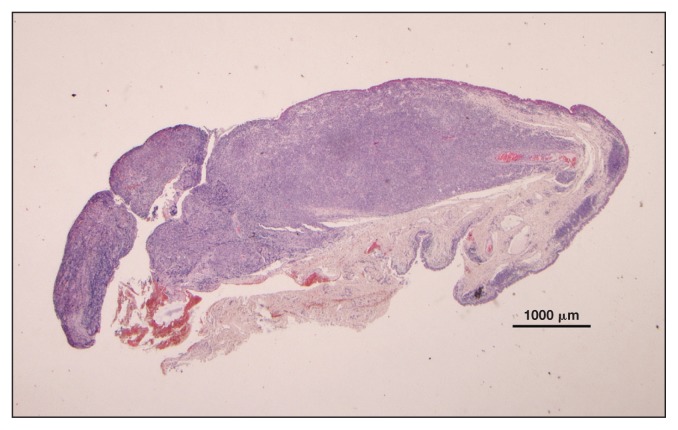
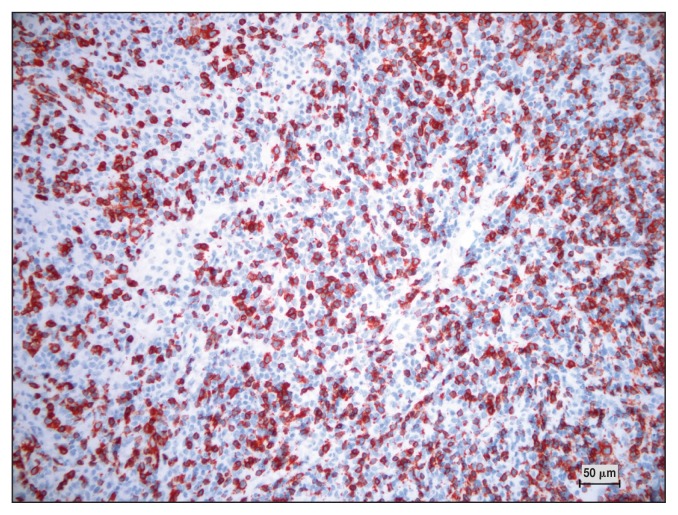
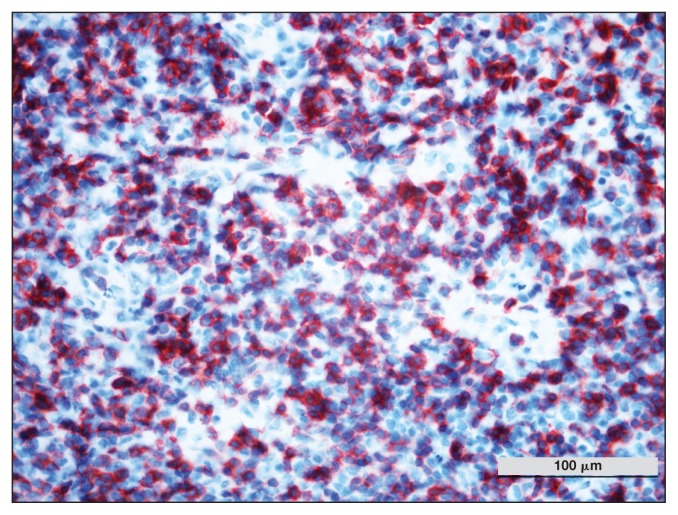
Serum biochemistry showed an increase in LDH, SAA, and FIB (Table 1). Microscopic evaluation of a peripheral blood smear indicated atypical/reactive lymphocytes (2%). Histological evaluation of the third eyelid showed a hypercellular, poorly delineated, encapsulated proliferation of round cells that infiltrated the tear glands and the fat tissue. These cells were characterized by little basophilic cytoplasm, round indented nuclei with coarse chromatin and poorly defined nucleoli, and numerous small cells surrounding the large cell. Anisocytosis and anisokaryosis were moderate. The MC was 7/10 HPF; rare macrophages, plasma cells and giant multinucleated cells were also seen. The IHC results for CD3 (T-cell) and CD20 (B-cell) were positive for both antigens (Figures 7, 8). The results of FNA of the mandibular lymph nodes were characterized by small lymphocytes (75%), macrophages (15%), giant multinucleated cells (7%), and plasma cells (3%).
Figure 7.
Horse 4 — third eyelid biopsy showing diffuse infiltration of lamina propria by relatively homogeneous population of lymphoid cells. H&E stain, × 1.25 magnification.
Figure 8.
Horse 4 — multifocal expression of CD20 protein on lymphoid cells detected by immunohistochemistry. DAB chromogen, Carazzi’s counterstain, × 100 magnification.
Flow cytometry was performed on the third eyelid and on the mandibular lymph node with a restricted panel of antibodies to the following: CD18 (granulocytes; CVS9 BioRad, Clinical Diagnostics BioRad, Milan, Italy), CD4 (T-cell), CD21 (B-cell) and cyCD3 (T-cell). The third eyelid was characterized by a population of large CD18+ cells (65%). Lymphocytes represented 25% of the cell population; the CD3+and CD4+ cells accounted for 16.9% and 12.2%, respectively. The lymph node sample showed a mixed population with a majority of small cells CD18+ (91%). The cells were mostly cyCD3+ (78%), and fewer were CD4+ (24%) and CD4 (54%). The remaining population (9%) was composed of large CD18+ cells that were partly cyCD3 (9%), partly CD4+ (4%) and CD4− (5%). The PCR results for EHV-5 from paraffin-embedded biopsy samples of the third eyelid tissue were positive.
The histological and IHC results of the third eyelid were suggestive of TCRLBCL according to the revised WHO classification (2). Flow cytometry of the third eyelid indicated a mixed inflammatory infiltrate. The mean mitotic index indicated mid-grade lymphoma. The FNA results for the lymph nodes showed chronic (macrophage) inflammation, whereas FC indicated a reactive lymph node owing to the presence of different cell populations. The use of a restricted panel of antibodies did not allow for complete analysis. After the surgery, the mare was affected by viral keratitis, which was treated with ganciclovir (Virgan; Farmila Theà, Milan, Italy) and tobramycin (Tobral; Alcon S.P.A, Milan, Italy) eye drops.
After 2 y, the mare is still alive, and there is no evidence of recurrence.
Discussion
Lymphoma is the most common hematopoietic neoplasia in horses (3). Clinical findings such as weight loss, fever, inappetence, lymphadenopathy, ventral edema, swelling of the limbs, and colic are nonspecific and vary according to the organ involved. No gender, breed, or age predispositions have been found, and there are no known etiological agents (4).
Based on the anatomic site, lymphoma can be divided into multicentric (generalized), alimentary (intestinal), mediastinal (thymic), cutaneous, and solitary/atypical (i.e., spleen, palate, nasopharynx, and extraocular) forms. The multicentric form is the most frequent, whereas the alimentary form is the second most frequent and is the most common intestinal neoplasia in horses (5–8).
In contrast, in human and veterinary medicine, the World Health Organization (WHO) classification system (2) integrates knowledge of not only topography but also immunophenotype, cell morphology, genetic features, clinical presentation and course (9–11). In a recent study, use of the WHO classification in equine lymphoma indicated that T-cell rich large B-cell lymphoma-TCRLBCL is the most frequent phenotype found in multicentric, cutaneous and gastrointestinal forms (9). Additionally, the MC in histology is related to the grade of lymphoma: indolent (0 to 1 mitosis/HPF), low grade (2 to 5 mitoses/HPF), mid-grade (6 to 10 mitoses/HPF), and high grade (> 10 mitoses/HPF) lymphoma (9).
This retrospective study describes 4 cases of equine lymphoma: 1 MEITL, 1 splenic DLBCL, 1 PTCL, and 1 TCRLBCL of the third eyelid. No multicentric form was diagnosed.
All horses had nonspecific clinical findings, thus necessitating differential diagnosis from other pathologies (e.g., systemic Lyme borreliosis, infection, and gastrointestinal disease) (3,6,11,12). Anemia was identified in 2 horses (Horse 2, Horse 3) probably because of the chronic inflammation caused by the neoplasm (6,13). The hyperfibrinogenemia, found in all cases, may have derived from cytokine interleukin-6 production (3,6). All animals had elevated LDH, as has been reported in lymphoproliferative disorders in horses probably because of the increase in anaerobic glycolysis due to the neoplastic cells (14). Over the course of lymphoma in humans and dogs, elevated LDH has been shown to be due to specific isoenzymes. Particularly, reports have indicated an increase in LDH-5 in human patients and an increase in LDH-2 and LDH-3 in dogs. Monitoring the concentration of these isoenzymes over time has a prognostic value in humans (15,16). Although LDH concentrations seem to increase in horses with lymphoma no study has demonstrated the involvement of specific isoenzymes and, unfortunately, in our cases they were not isolated (17).
Alimentary lymphoma is the most frequent intestinal neoplasia in horses (3,6). As in our case (Horse 1, 26-year-old), elderly animals are generally affected by this form of lymphoma (13,18–20). The horse showed diarrhea and increased peritoneal fluid, which are commonly seen in horses with alimentary lymphoma; however, the horse did not show protein loss enteropathy, as has been frequently reported in the course of intestinal lymphoma (19–23). Most cases of alimentary lymphoma in horses have a postmortem diagnosis; in our case, an explorative laparotomy provided an antemortem diagnosis. Thus, the surgical approach seems to be the best procedure for diagnosis of this kind of lymphoma (13,17,18–21). Histologically, there was a diffuse infiltration of the mucosa, as typically reported in young horses; in contrast, the presence of a well-defined intestinal mass is more frequently reported in older horses (17,18). Histological and IHC investigation supported a diagnosis of MEITL, based on the revised WHO classification (2). Previously, MEITL was known as a variant (type II) of the enteropathy-associated T-cell lymphoma-EATCL. The EATCL is an intestinal neoplasia characterized by T-epitheliotropic cells, diffuse infiltration, and diffuse ulceration of the mucosa, owing to TIA-1 cytotoxic granules contained in cytotoxic T-lymphocytes. This disease is divided into type I, which shows a polymorphic cellular composition and is closely linked to celiac disease (now simply designated as enteropathy associated T-cell lymphoma/EATCL), and type II, which is not associated with celiac disease (now formally designated as monomorphic epitheliotropic intestinal T-cell lymphoma/MEITL). To our knowledge this is the first case of MEITL described in horses (19,22,24,25). This anatomical location has been found to have poor outcomes and the T phenotype is more aggressive; in fact, the horse’s state rapidly worsened. Exploratory laparotomy confirmed that both the small and large intestine were affected by the disease, as described in other cases, although the small intestine has been reported to be involved more frequently than the large intestine (6,13,23). The surgery allowed us to sample a mesenteric lymph node, which was characterized by the presence of neoplastic lymphocytes. The infiltration of only mesenteric lymph nodes is commonly seen in alimentary lymphoma and is considered a metastatic infiltration (21,23).
Horse 2 was affected by primary splenic diffuse large B-cell lymphoma which is rarely reported in human or veterinary medicine (25–27). To our knowledge, there is only 1 case that has been well-described in the horse. Generally, splenic involvement is linked to a multicentric form, and the spleen is characterized by multifocal/diffuse nodules (28,29). In our case, the spleen was affected by a well-defined solitary mass, and other organs did not show evidence of neoplasia; therefore, a solitary/atypical form of DLBCL was diagnosed. As in other cases (28,29), no significant clinical abnormalities were identified in our case, despite the large solitary splenic mass. Unfortunately, we could not define the size of the splenic mass because necropsy was not allowed. Histologically, the splenic mass was characterized by large lymphoid cells in both the present case and a previously reported case (25). In human medicine, DLBCL is a rare neoplasm (2% of non-Hodgkin lymphomas) known as Primary Malignant Lymphoma of the Spleen (PMLS) (27). It is usually characterized by small lymphocytes and large cells or mixed cell type, and mostly of the B phenotype. In contrast, large cells were identified in our case and a previously described case (25). Both in our case and in the case described by Tanimoto et al (25), the phenotype is B, even though in the latter, the cell type was confirmed based on the results from cytochemical staining. To our knowledge, this is the first case of DLBCL of the spleen in a horse investigated with the combined use of FC (CD21+ and cyCd79+) and immunohistochemistry (CD20+). Moreover, the FC showed the presence of a small population of CD21+ cells in the peripheral blood (0.1%) and in the bone marrow (0.2%). These data may suggest a dissemination of the neoplasm; nevertheless, there are no studies regarding cut off of infiltration by neoplastic cells of the blood and bone marrow in horses. In dogs a cutoff of 3% infiltration is used to discriminate dogs having different prognoses (30). Thus, the very low percentage of cells CD21+ in this case seems to be inconsistent with tumor dissemination. In fact, despite the high mitotic index of the lymphoma cells, no invasive growth or metastasis was observed in a previous case (25). In humans, PMLS has been described to metastasize to the splenic lymph node and rarely to other organs, although metastasis to the bone marrow, liver, and other lymph nodes has been reported. Human patients with only splenic involvement have a favorable prognosis after splenectomy (31). The prognosis in horses is not defined. In our case, the owner did not allow surgery, and the animal died after several days; in the other case reported in the literature the animal was euthanized for another cause, and the mass was found during necropsy (25).
Horses 3 and 4 had lymphoma of the third eyelid; adnexal lymphoma is an uncommon neoplasm, and there are limited reports of solitary forms in the literature. Lymphoid tissue of the ocular adnexa is sometimes an affected site in the course of the multicentric form (7,8,32,33). In both animals, the absence of involvement of tissue other than the third eyelid indicated a solitary/atypical form of the neoplasm. Unfortunately, in Horse 3 the lack of necropsy prevented us from excluding a multicentric form. In contrast, in Horse 4, the long follow-up (2 y) and the good health status suggest a solitary form of lymphoma.
Equine conjunctival, corneoscleral, and extraocular lymphoma appears to be present in 2 varieties: nodular and diffuse (8). In both horses the histological evaluation of both the third eyelids showed a diffuse infiltration of lymphocytes. The diffuse infiltration seems to be more malignant in nature than the nodular form and to be related to a poor outcome, as seen in Horse 3 (8). Notably, in that horse, histology showed a high mitotic index and infiltration extended beyond the margins of the tissue removed. In fact, surgical excision followed by evaluation of surgical margins is the treatment of choice for all periorbital neoplasia. Moreover, in cases of diffuse infiltration lacking clean margins, enucleation of the eyes should be performed to avoid dissemination of the neoplasm and more aggressive behavior of the tumor (8). In Horse 3, a subsequent enucleation was not possible, and the animal later died. In contrast, Horse 4 had a good outcome despite the diffuse architecture of the neoplastic tissue; this outcome may be associated with the radical removal of the tumor, which was made possible by the absence of neoplastic cells into the margins. As described in another horse with third eyelid lymphoma (34), a good surgical treatment allows for a long survival.
In both horses, the mandibular lymph nodes were enlarged because of granulomatous lymphadenitis in Horse 3 and chronic (macrophage) lymphadenitis in Horse 4. Granulomatous inflammation is observed in the course of infections of verminous, bacterial, fungal, or foreign body origin (35,36); nevertheless, in the course of lymphoma reports have indicated increased production of interferon-γ by the neoplastic T-lymphocytes, thus increasing the number of multinucleated cells surrounding the neoplastic lesion (14,37). The cytological results and the negative bacterial culture support this hypothesis.
The phenotype of third eyelid lymphoma is rarely reported; in this report, the use of IHC allowed us to make a diagnosis of T-cell lymphoma in Horse 3 and TCRLBCL in Horse 4. The T phenotype of the neoplasia in Horse 3 was linked to a poor prognosis; to our knowledge, Horse 4 is the first described case of equine TCRLBCL of the third eyelid (8,33).
The differential diagnosis for third eyelid lymphoma includes extraocular pseudotumors. Histologically, they are characterized by nodules or lymphoid follicles and by different types of cells (lymphocytes, macrophages, plasma cells), and generally there are no systemic signs (38). The diagnosis of lymphoma was based on the histological architecture which indicated a diffuse infiltration of lymphoid cells; furthermore, the use of IHC showed an infiltration of only T-cells in Horse 3, and T- and B-cells in Horse 4. Extraocular pseudotumors were therefore not considered, and lymphoma of the third eyelid was diagnosed.
Likewise, the increases in serum SAA and fibrinogen could also indicate that there was inflammation, maybe linked to the neoplasia. In fact, no laboratory test is 100% sensitive and 100% specific for any disease including neoplasia and the combined use of different diagnostic tools is necessary.
Interestingly, in all cases described herein neoplastic samples showed EHV-5 positivity in biomolecular investigation. Recently EHV-5 has been associated with some forms of equine lymphoma (multicentric, cutaneous, and solitary), with a TCRLBCL or T phenotype (3,39). Notably, in one of these cases (solitary TCRLBCL of mandibular lymph nodes) recurrence was observed 7 mo after surgery and chemotherapy. Therefore, the animal patient was treated with acyclovir, and complete remission was reported (39). However, the correlation between EHV-5 and lymphoma in horses has not been established, although gamma herpesviruses have been associated with malignant and non-malignant lymphoproliferative disorders in humans, mice, and poultry (40). Owing to this recent information, viral involvement in equine lymphoma should be analyzed more carefully to improve diagnosis and therapy.
In conclusion, cytological, histological, and immunophenotypic techniques can aid in achieving a complete antemortem diagnosis and defining a precise prognosis and treatment course for equine lymphoma. The description of these solitary cases and the results of thorough diagnostic investigations improve understanding of this neoplasia. Furthermore, future investigations into, LDH isoenzyme concentrations in equine lymphoma should be performed to evaluate their possible prognostic value. Finally, studies on the possible involvement of EHV-5 in the initiation or development of lymphoproliferative neoplasia should be performed.
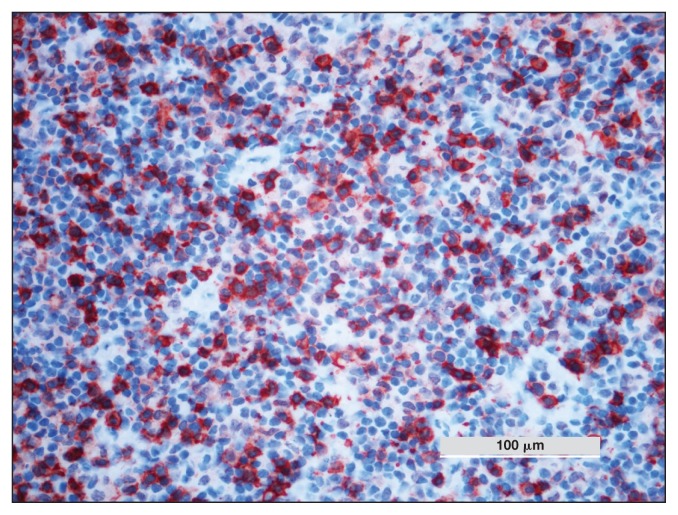
Figure 9.
Horse 4 — multifocal CD3 protein expression of inflammatory lymphoid cells detected by immunohistochemistry. DAB chromogen, Carazzi’s counterstain, × 100 magnification.
Acknowledgments
The authors are grateful to the staff of the OVUD-UNIPG for help in the management of the cases. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Marenzoni ML, Coppola G, Maranesi M, et al. Age-dependent prevalence of equid herpesvirus 5 infection. Vet Res Commun. 2010;34:703–708. doi: 10.1007/s11259-010-9443-9. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SS, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz A, Riber C, Trigo P, Castejon F. Hematopoietic neoplasias in horses: Myeloproliferative and lymphoproliferative disorders. J Equine Sci. 2009;20:59–70. doi: 10.1294/jes.20.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vander Werf KA, Davis E, Janardhan K, Bawa B, Bolin S, Almes K. Identification of equine herpesvirus 5 in horses with lymphoma. J Equine Vet Sci. 2014;34:738–741. [Google Scholar]
- 5.Meyer J, DeLay J, Bienzle D. Clinical, laboratory, and histopathologic features of equine lymphoma. Vet Pathol. 2006;43:914–924. doi: 10.1354/vp.43-6-914. [DOI] [PubMed] [Google Scholar]
- 6.Schleis S. Equine lymphoma. Equine Vet Educ. 2011;23:205–213. [Google Scholar]
- 7.Rendle DI, Hughes KJ, Farish C, Kessell A. Multicentric T-cell lymphoma presenting as inferior palpebral swelling in a Standardbred mare. Aust Vet J. 2012;90:485–489. doi: 10.1111/j.1751-0813.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 8.Schnoke AT, Brooks DE, Wilkie DA, et al. Extraocular lymphoma in the horse. Vet Ophthalmol. 2013;16:35–42. doi: 10.1111/j.1463-5224.2012.01016.x. [DOI] [PubMed] [Google Scholar]
- 9.Durham AC, Pillitteri CA, San Myint M, Valli VE. Two hundred three cases of equine lymphoma classified according to the World Health Organization (WHO) classification criteria. Vet Pathol. 2013;50:86–93. doi: 10.1177/0300985812451603. [DOI] [PubMed] [Google Scholar]
- 10.Valli VE. Veterinary Comparative Hematopathology. 1st ed. Ames, Iowa: Blackwell Publishing; 2007. B-cell neoplasm; pp. 260–262. [Google Scholar]
- 11.Passamonti F, Veronesi F, Cappelli K, et al. Polysynovitis in a horse due to Borrelia burgdorferi sensu lato infection — Case study. Ann Agric Environ Med. 2015;22:247–250. doi: 10.5604/12321966.1152074. [DOI] [PubMed] [Google Scholar]
- 12.Foreman JH. Changes in body weight. In: Reed SM, Bayly WM, Sellon DC, editors. Equine Internal Medicine. 3rd ed. St Louis, Missouri: Saunders Elsevier; 2010. pp. 96–99. [Google Scholar]
- 13.Taylor SD, Pusterla N, Vaughan B, Whitcomb MB, Wilson WD. Intestinal neoplasia in horses. J Vet Intern Med. 2006;20:1429–1436. doi: 10.1892/0891-6640(2006)20[1429:inih]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Bernard WV, Sweeney CR, Morris F. Primary lymphocytic leukemia in a horse. Vet Clin North Am Equine Pract. 1988;10:24–26. [Google Scholar]
- 15.Yadav C, Ahmad A, D’Souza B, et al. Serum dehydrogenase in non-Hodgkin’s lymphoma: A prognostic indicator. Ind J Clin Biochem. 2016;31:240–242. doi: 10.1007/s12291-015-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanatta R, Abate O, D’Angelo A, Miniscalco B, Mannelli A. Diagnostic and prognostic value of serum lactate dehydrogenase (LDH) and LDH isoenzymes in canine lymphoma. Vet Res Commun. 2003;27:449–445. doi: 10.1023/b:verc.0000014201.82393.67. [DOI] [PubMed] [Google Scholar]
- 17.Dabareiner RM, Keneth ES, Goodrich LR. Large colon resection for treatment of lymphosarcoma in two horses. J Am Vet Med Assoc. 1996;208:895–897. [PubMed] [Google Scholar]
- 18.Schnabel LV, Bradley LN, Gold JR, Meseck EK. Primary alimentary lymphoma with metastasis to the liver causing encephalopathy in a horse. J Vet Intern Med. 2006;20:204–206. doi: 10.1892/0891-6640(2006)20[204:palwmt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Sanz MG, Sellon DC, Potter KA. Primary epitheliotropic intestinal T-cell lymphoma as cause of diarrhea in a horse. Can Vet J. 2010;55:522–524. [PMC free article] [PubMed] [Google Scholar]
- 20.Smith KM, Clarck CK, Dark MM, Kiupel M, Gary J. T-cell rich B-cell lymphoma in the small colon of a yearling horse. Equine Vet Educ. 2012;25:74–78. [Google Scholar]
- 21.Herraez P, Berridge B, Marsh P, Weeks B, Ramiro-Ibanez F. Small intestine large granular lymphoma in a horse. Vet Pathol. 2001;28:223–226. doi: 10.1354/vp.38-2-223. [DOI] [PubMed] [Google Scholar]
- 22.Pinkerton ME, Bailey KL, Thomas KK, Goetz TE, Valli VE. Primary epitheliotropic intestinal T-cell lymphoma in a horse. J Vet Diagn Invest. 2002;14:150–152. doi: 10.1177/104063870201400209. [DOI] [PubMed] [Google Scholar]
- 23.Mitsui I, Jackson LP, Couetil LL, Lin TL, Ramos-Vara JA. Hypertrichosis in a horse with alimentary T-cell lymphoma and pituitary involvement. J Vet Diagn Invest. 2007;19:128–132. doi: 10.1177/104063870701900125. [DOI] [PubMed] [Google Scholar]
- 24.Seeling DM, Avery PR, Kisseberth WC, Modiano JF. Hematological neoplasia: T-cell lymphoproliferative disease. In: Weiss DJ, Wardrop JK, editors. Schalm’s Veterinary Hematology. 6th ed. Vol. 2010. Ames, Iowa: Wiley-Blackwell; p. 53. [Google Scholar]
- 25.Tanimoto T, Yamasaki S, Ohtsuki Y. Primary splenic lymphoma in a horse. J Vet Med Sci. 1994;56:767–769. doi: 10.1292/jvms.56.767. [DOI] [PubMed] [Google Scholar]
- 26.Eberle N, von Babo V, Nolte I, Baumgartner W, Bertz D. Splenic masses in the dog. Tierarztl Prax K. 2012;40:250–260. [PubMed] [Google Scholar]
- 27.Kattepur AK, Rohith S, Shivaswamy BS, Babu R, Santhosh CS. Primary splenic lymphoma: A case report. Indian J Surg Oncol. 2013;4:287–290. doi: 10.1007/s13193-013-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaffin KM, Schmitz DG, Brumbaugh GW, Hall DG. Ultrasonographic characteristics of splenic and hepatic lymphosarcoma in three horses. J Am Vet Med Assoc. 1992;201:743–747. [PubMed] [Google Scholar]
- 29.Roccabianca P, Paltrinieri S, Gallo E, Giuliani A. Hepatosplenic T-cell lymphoma in a mare. Vet Pathol. 2002;39:508–511. doi: 10.1354/vp.39-4-508. [DOI] [PubMed] [Google Scholar]
- 30.Riondato F, Miniscalco B, Poggi A, et al. Analytical and diagnostic validation of flow cytometry strategy to quantify blood and marow infiltration in dogs with large B-cell lymphoma. Cytometry B Clin Cytom. 2016;90:525–530. doi: 10.1002/cyto.b.21353. [DOI] [PubMed] [Google Scholar]
- 31.Ayturk S, Celik M, Mert O, et al. Primary splenic diffuse large B-cell lymphoma after splenectomy: A rare case with literature review. Am J Med Case Rep. 2015;3:265–268. [Google Scholar]
- 32.German SE, Richter M, Schwarzwald C, Wimmershoff J, Spiess BM. Ocular and multicentric lymphoma in a young racehorse. Vet Ophthalmol. 2008;11:51–56. doi: 10.1111/j.1463-5224.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 33.Martabano BB, Brooks DE, Whitley RD, et al. Recurrent adnexal lymphoma in horse. Equine Vet Educ. 2017 doi: 10.1111/eve.12850. [DOI] [Google Scholar]
- 34.D’agostino J, Brightman H. Bilateral lymphosarcoma of the nictating membrane in a Quarter Horse. J Equine Vet Sci. 2004;24:489–491. [Google Scholar]
- 35.Ackermann MR. Infiammazione cronica e guarigione delle ferite. In: McGavin MD, Zachary JF, editors. Patologia generale veterinaria. 4th ed. Milan, Italy: Elsevier Masson; 2007. pp. 155–156. [Google Scholar]
- 36.Lepri E, Beccati F, Miglio A, Passamonti F, Veronesi F, Mandara MT. Pathology in practice. J Am Vet Med Assoc. 2017;251:1153–1156. doi: 10.2460/javma.251.10.1153. [DOI] [PubMed] [Google Scholar]
- 37.Tornquist SJ. Bone marrow and lymph node evaluation. Vet Clin Equine. 2008;24:261–283. doi: 10.1016/j.cveq.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Moore CP, Grevan VL, Champagne ES, Collins BK, Collier LL. Equine conjunctival pseudotumors. Vet Ophthalmol. 2000;3:57–63. doi: 10.1046/j.1463-5224.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- 39.Vander Werf KA, Davis E. Disease remission in a horse with EHV-5 associated lymphoma. J Vet Intern Med. 2013;27:387–389. doi: 10.1111/jvim.12050. [DOI] [PubMed] [Google Scholar]
- 40.Bawa B, Vander Werf KV, Beard L, Davis E, Andrews G, Almes K. Equine multinodular pulmonary fibrosis and lymphoma in a horse associated with equine herpesvirus-5. J Equine Vet Sci. 2014;34:694–700. [Google Scholar]