Abstract
A lithotripsy and percutaneous cystolithotomy (PCCL) were performed on a 5-year-old intact male English bulldog. The composition of the uroliths was 100% cystine. When a second PCCL was performed 2 months later, the nidus of the largest urolith was a segment of an optical fiber broken off during laser lithotripsy.
Résumé
Fibre laser formant le nidus d’une urolithiase à cystine récurrente chez un Bulldog Anglais mâle entier. Une lithotripsie et une cystolithotomie percutanée (PCCL) ont été réalisées sur un Bulldog Anglais mâle entier de 5 ans. L’urolithe était constitué à 100 % de cystine. Lors d’une seconde PCCL réalisée 2 mois plus tard, le nidus du plus grand calcul de cystine se révéla être un fragment de fibre optique brisée durant la lithotripsie au laser.
(Traduit par Dre Emmanuelle Butty)
Any foreign material in the bladder can act as a nidus for stone formation, especially in a patient with risk factors for stone formation and having had previous uroliths. In human medicine, foreign bodies are a major cause of large urolith formation and are mainly secondary to broken equipment during endourologic procedures, including broken laser fibers, forgotten material during open transvesicular surgery, retained catheter tips, or sutures from bladder surgery (1–4). In veterinary medicine, sutures are the most common cause of iatrogenic cystolith formation, being the cause of 0.6% of canine uroliths and 9.4% of recurrent canine uroliths (5). In 2 case reports, a sewing needle in 1 dog and a mouse barley awn (Hordeum murinum) in 2 dogs were found to be the nidus of struvite uroliths likely formed secondary to infection caused by the foreign material (6,7).
Case description
A 5-year-old male intact English bulldog was presented for episodes of hematuria and dysuria. Prior to his presentation, cystine crystals were observed in the urine sediment and radiographs had shown multiple poorly radiopaque bladder and urethral stones. Retrograde urohydropulsion failed to retropulse the urethral stones into the bladder. The dog was referred to our internal medicine service for further evaluation and treatment.
A lateral abdominal radiograph showed at least 11 poorly radiopaque bladder stones with a smooth contour varying in size from 1.4 to 6.4 mm and 10 urethral stones varying in size from 1.8 to 5.7 mm in diameter. It was decided that retrograde cystoscopy and lithotripsy would be attempted. If unsuccessful, a percutaneous cystolithotomy (PCCL) would be performed. Considering the probability of androgen-dependent cystinuria, castration was recommended but declined.
A flexible ureteroscope (FLEX-X 11278/VSU Flexible ureteroscope; Karl Storz Endoscopy, Culver City, California, USA) was advanced in a retrograde direction into the penile urethra under continuous saline irrigation. Multiple stones, partially obstructing the urethra and embedded within the mucosa were identified. A laser fiber [365-μm Holmium (H-30) YAG laser fiber and 30-W Hol:YAG lithotrite; Convergent, Alameda, California, USA] was inserted through the working channel of the ureteroscope until it came into contact with the stones. The stones were fragmented using a low powered holmium:yag (Ho:YAG) laser (Medical-Holmium Laser, Model HL-30; Cook Medical, Bloomington, Indiana, USA) at the following settings: 10 Hz and 0.8 J at a pulse width of 350 ms. Stone fragments were removed using a stone retrieval basket (NCompass Nitinol Stone extractor 2,4 Fr × 115 cm; Cook Medical). Following removal of half of the urethral stones, the urethral mucosa became increasingly edematous and visibility had decreased.
As there were also multiple bladder stones to be removed, it was deemed best to remove the remaining stones by PCCL. A 2-cm ventral midline skin incision and a 1.5-cm incision through the linea alba preceded a stab incision into the bladder lumen between 3 stay sutures. A 6-mm laparoscopic screw-tip trocar (Trocar, 5.5 mm inner diameter; Richard Wolf, Vernon Hills, Illinois, USA) was advanced into the bladder lumen and a rigid 10.5 Fr cystoscope (Rigid endoscope, 3.5 mm with a 10.5-Fr sheath, 30° lens; Richard Wolf ) was inserted. Bladder stones were removed with a stone retrieval basket (NCompass Nitinol Stone extractor 2,4 Fr × 115 cm; Cook Medical) passed through the working channel of the cystoscope. The urethra was evaluated with a flexible ureteroscope (FLEX-X 11278/VSU Flexible ureteroscope; Karl Storz Endoscopy) passed through the trocar. Large embedded stones in the penile urethra were fragmented using the laser passed through the channel of the ureteroscope and retrieved with a stone basket (NCircle Nitinol Tipless Stone Extractor, 2,2 Fr × 115 cm; Cook Medical) in an antegrade manner.
At the end of the procedure, the entire lower urinary tract was inspected and no stone fragments remained. The urethral mucosa showed focal erosions and edema at the site at which the stones were embedded. Quantitative bladder stone analysis was performed at the Canadian Veterinary Urolith Centre at the University of Guelph. Using light microscopy combined with crystallographic techniques and scanning electron microscopy with X-ray microanalysis, the uroliths were confirmed to be 100% cystine (stone and shell). Type III androgen-dependent cystinuria was suspected. The owner declined submission of urine samples to the School of Veterinary Medicine at University of Pennsylvania, Section of Medical Genetics for qualitative analysis of the urine amino acids cystine, ornithine, lysine, and arginine (COLA).
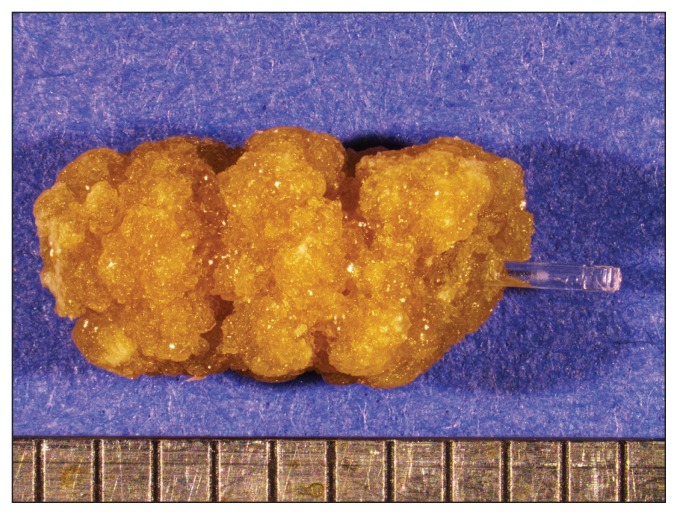
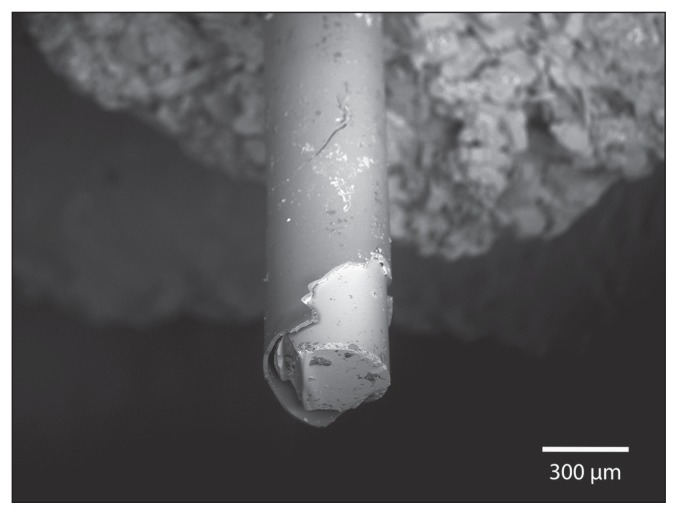
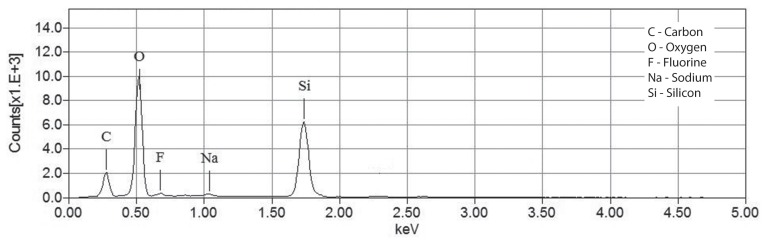
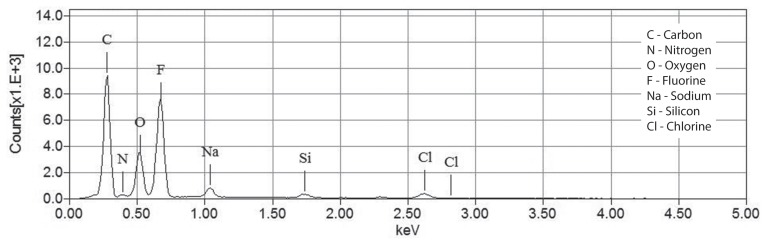
Two months later, the dog was presented with a new episode of dysuria. Radiographs showed multiple radiopaque bladder and urethral stones. The dog had been exclusively fed a cystine and methionine-restricted diet (Veterinary Diet Urinary UC Low Purine Dry Dog Food; Royal Canin, Puslinch, Ontario). A second PCCL with a rigid cystoscope (Rigid endoscope, 3.5 mm with a 10.5-Fr sheath, 30° lens; Richard Wolf ) and stone basket (NCircle Nitinol Tipless Stone Extractor, 2,2 Fr × 115 cm; Cook Medical) was performed as previously described and the dog was neutered. Stones in the urethra were partially obstructive and embedded within the mucosa. Proliferative polypoid-like structures reducing the urethral lumen were visible in the distal urethra, at the site of the previous urethroliths. The stones were sent to the Canadian Veterinary Urolith Centre and the nidus of the largest urolith appeared to be a clear cylindrical rod. An image of the urolith containing the rod and a higher magnification image of the rod are shown in Figures 1 and 2. The material from the core and the coating of the rod was analyzed in a scanning electron microscope equipped with an X-ray microanalysis system [JEOL JSM-6010LA scanning electron microscope with attached energy dispersive spectrometer (EDS); Jeol USA, Peabody, Massachusetts, USA] to determine elemental compositions. The X-ray spectra for the core and the outer coating of the rod are shown in Figures 3 and 4.
Figure 1.
Dissecting microscope image of the urolith with the clear cylindrical rod composing the nidus. Scale in mm.
Figure 2.
Scanning electron microscope image of the rod and its coating.
Figure 3.
X-ray spectrum for the core of the rod protruding from the urolith.
Figure 4.
X-ray spectrum of the coating on the rod protruding from the urolith.
The core was primarily composed of silicon and oxygen, suggesting Quartz (silica). The coating was found to be primarily composed of carbon and fluorine, typical of a number of fluoropolymers. It was determined to most likely be a segment of an optical fiber broken off in the urinary tract during lithotripsy. The remainder of the urolith (stone and surface) was confirmed to be composed of 100% cystine.
One month after the dog had been neutered, a colorimetric cyanide-nitroprusside test on urine sent to the University of Pennsylvania, Section of Medical Genetics, was negative. The restricted diet (Veterinary Diet Urinary UC Low Purine Dry Dog Food; Royal Canin) was discontinued. One year later, the dog had not shown any lower urinary tract signs. He is fed a low caloric diet (Veterinary Diet Weight Control Dry Dog Food; Royal Canin) and the colorimetric cyanide-nitroprusside test on urine remains negative.
Discussion
To our knowledge, this is the first report of a laser fiber, likely broken off in the urinary tract during previous lithotripsy, to be in the nidus of a urolith in a veterinary patient. In this report, the dog had risk factors for stone formation and stone recurrence was primarily due to persistent cystinuria and not solely secondary to the fiber foreign body as multiple stones recurred and the laser fiber was only identified in one of the stones retrieved. Cystinuria is an inherited transport dysfunction of the amino acids cystine, ornithine, lysine, and arginine (8). While cystinuria has been described in more than 70 dog breeds (9), recent studies have described the genetics of cystine transport dysfunction. More recently, a new classification has been published (10) with autosomal recessive (type I), dominant (type II), and a type III or androgen dependent cystinuria. While the exact gene mutation has yet to be established, type III affects mature males. Castration is known to result in correction of the underlying tubular defect in mastiffs, Scottish deerhounds, and Irish terriers (8). The dog in our report had stone recurrence despite exclusively eating a cystine and methionine restricted diet. Type III cystinuria is highly suspected since 4 wk (while on a restricted cystine diet) and 1 year (while on a regular maintenance diet) after neutering, the nitroprusside test remained negative.
Laser fiber fracture has been reported in the human literature (1–3,11–13). In our patient, a short pulse width was used for fragmentation to allow removal of stone fragments and avoid excess stone dust. Despite the low energy and low frequency used to decrease the risk of fiber fracture as previously reported (12), the authors believe that this patient was at increased risk of fiber fracture during lithotripsy because of 2 factors. First, the bending of the scope and therefore the fiber to navigate the penile and pelvic urethra (smaller bending radius) likely contributed to an increased fracture risk (11–13). Second, lithotripsy was done in the urethra and despite continuous saline flush, this restricted liquid environment may also have increased the risk of fiber damage, as the Ho:YAG laser is a pulsed type of laser that emits energy absorbed by the water (14). Given the increasing awareness and recommendations for alternative, non-invasive methodologies for urolith removal (15), this complication may occur more frequently in the future. To avoid this type of complication, the authors recommend a thorough examination of the laser fiber (entire length and tip) before and after each procedure in the following manner. The laser aiming beam (visible light) should be first activated to inspect the entire length of the fiber. The working beam is coincident with the aiming beam and breaks will appear as bright as the laser aiming beam. With a minimum magnification (loupe or video system), the user should inspect the output tip for pits, cracks, and chips. The fiber is finally checked using the aiming beam output (optical fiber spot check) about 5 cm from a light surface. The tip spot shape is acceptable if the spot appears as a perfect uniform circle with a dark center and sometimes a light halo. A circle with a thick halo, a spot with comet tail extending from one edge or any other changes are deemed unacceptable (16). Finally, a copious flush of the entire urinary tract system and a final inspection with the endoscope is recommended at the end of each procedure. Laser fiber bending occurs less with a percutaneous cystolithotomy approach and this approach may be associated with a lower fracture rate.
In conclusion, a segment of laser fiber, likely broken off in the urinary tract during cystoscopy and lithotripsy was found to be the nidus of a cystine urolith. To the authors’ knowledge, this is the first reported case of a laser fiber as the nidus of a stone in a veterinary patient. As lithotripsy is being performed more and more in companion animals, it is imperative to take measures to ensure laser fiber integrity and early recognition when a fiber breaks.
Acknowledgments
The authors thank Dr. Anne-Marie Germain, Dr. Richard Ringuette, and Mrs. Brigitte Bouchard for their assistance with this manuscript. Special thanks to the Canadian Veterinary Urolith Centre team for the photographs and spectral analysis. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Alkan E, Basar MM. Endourological treatment of foreign bodies in the urinary system. JSLS. 2014;18 doi: 10.4293/JSLS.2014.00271. e2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBroom, Schenkman NS, Stoller ML. Retained laser fiber ureteral calculus. Urology. 2001;58:277–278. doi: 10.1016/s0090-4295(01)01135-9. [DOI] [PubMed] [Google Scholar]
- 3.Restrepo NC, Belis JA. Ureteral foreign body after laser lithotripsy. J Endourol. 1994;8:29–31. doi: 10.1089/end.1994.8.29. [DOI] [PubMed] [Google Scholar]
- 4.Thatte A, Rajendran S, Murphy L, Allen M. Intravesical foreign body: Clinical features and diagnostic clues. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-204828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel SL, Lefebvre SL, Houston DM, et al. Evaluation of risk factors associated with suture-nidus cystoliths in dogs and cats: 176 cases (1999–2006) J Am Vet Med Assoc. 2008;233:1889–1895. doi: 10.2460/javma.233.12.1889. [DOI] [PubMed] [Google Scholar]
- 6.Del Angel-Caraza J, Pérez-García CC, Bende B, Diez-Prieto I, García-Rodríguez B. Mouse barley awn (Hordeum murinum) migration induced cystolithiasis in 2 male dogs. Can Vet J. 2011;52:67–69. [PMC free article] [PubMed] [Google Scholar]
- 7.Houston DM, Eaglesome H. Unusual case of foreign body-induced struvite urolithiasis in a dog. Can Vet J. 1999;40:125–126. [PMC free article] [PubMed] [Google Scholar]
- 8.Giger U, Brons A, Mitzukami K, et al. Update on Fanconi Syndrome and Cystinuria. Proceedings of the WSAVA World Congress; Bangkok, Thailand. May 15–18, 2015. [Google Scholar]
- 9.Osborne CA, Lulich JP, Kruger JM, Ulrich LK, Koehler LA. Analysis of 451,891 canine uroliths, feline uroliths, and feline urethral plugs from 1981 to 2007: Perspectives from the Minnesota Urolith Center. Vet Clin North Am Small Anim Pract. 2009;39:183–197. doi: 10.1016/j.cvsm.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Brons AK, Henthorn PS, Raj K, et al. SLC3A1 and SLC7A9 mutations in autosomal recessive or dominant canine cystinuria: A new classification system. J Vet Intern Med. 2013;27:1400–1408. doi: 10.1111/jvim.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudsen BE, Glickman RD, Stallman KJ, et al. Performance and safety of holmium: YAG laser optical fibers. J Endourol. 2005;19:1092–1097. doi: 10.1089/end.2005.19.1092. [DOI] [PubMed] [Google Scholar]
- 12.Lusch A, Heidari E, Okhunov Z, Osann K, Landman J. Evaluation of contemporary holmium laser fibers for performance characteristics. J Endourol. 2016;30:567–573. doi: 10.1089/end.2015.0600. [DOI] [PubMed] [Google Scholar]
- 13.Haddad M, Emilani E, Rouchausse Y, et al. Impact of the curve diameter and laser settings on laser fiber fracture. J Endourol. 2017;31:918–921. doi: 10.1089/end.2017.0006. [DOI] [PubMed] [Google Scholar]
- 14.Dołowy Ł, Krajewski W, Dembowski J, Zdrojowy R, Kołodziej A. The role of lasers in modern urology. Cent European J Urol. 2015;68:175–182. doi: 10.5173/ceju.2015.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lulich JP, Berent AC, Adams LG, Westropp JL, Bartges JW, Osborne CA. ACVIM Small Animal Consensus Recommendations on the treatment and prevention of uroliths in dogs and cats. J Vet Intern Med. 2016;30:1564–1574. doi: 10.1111/jvim.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duchamp JG. Evaluation of Holmium:YAG Laser Optical Fibers for Flexible Ureteroscopy using a Relevant Benchtop Model. EndoBeam. 2015. [Last accessed November 5, 2018]. Available from: https://www.urotoday.com/images/stories/documents/pdf_files/EndoBeamWhitePaper.pdf.