Summary
Background
Vitamin B12 (cobalamin) deficiency is a prevalent worldwide health concern. Several factors are associated with vitamin B12 deficiency including lifestyle, genetic predisposition, and malfunctions in the absorption and transport of vitamin B12. In the current case-control study, we aimed at investigating the association between MTHFR polymorphisms and vitamin B12 deficiency in a Jordanian population.
Methods
Two polymorphic sites of the MTHFR gene (c.677C>T, rs1801133 and c.1286A>C, rs1801131) were analyzed using RFLP and DNA sequencing in a group of vitamin B12 deficient individuals (45 males and 55 females). As a control, 100 matching individuals (age and sex) with vitamin B12 levels > 200 ng/mL were also recruited for this study.
Results
The MTHFR c.677C>T variant was significantly associated with vitamin B12 deficiency in individuals from northern Jordan. The frequency of the homozygous MTHFR c.677C>T genotype was significantly higher in B12 deficient individuals in comparison with the control group (X2 = 8.397, p = 0.0150). The T allele frequency showed significant association with vitamin B12 deficiency in the study population (OR= 1.684, 95% CI: 1.116 to 2.542, p = 0.017). On the other hand, the MTHFR c.1286A>C variant did not show significant association with vitamin B12 deficiency in the selected population.
Conclusions
Our results showed a significant association between homozygous MTHFR c.677C>T variant and T allele frequencies and vitamin B12 deficiency in the Jordanian population.
Keywords: MTHFR polymorphisms, vitamin B12 deficiency, folate metabolism, homocysteine
Kratak sadržaj
Uvod
Nedostatak vitamina B12 (kobalamina) predstavlja svetski rasprostranjen zdravstveni problem. Sa deficijencijom vitamina B12 povezano je nekoliko faktora, kao što su način života, genetska predispozicija i poteškoće u apsorpciji i transportu vitamina B12. U ovoj anamnestičkoj studiji, cilj nam je bio da istražimo povezanost između polimorfizama MTHFR i deficijencije vitamina B12 u jednoj populaciji Jordanaca.
Metode
Dva polimorfna mesta na genu MTHFR (c.677C>T, rs1801166 i c.1286A>C, rs1801131) analizirana su pomoću metoda RFLP i DNK sekvenciranja u grupi sa deficijencijom vitamina B12 (45 muškaraca i 55 žena). Kao kontrolna grupa, u ovu studiju takođe je uključeno 100 osoba odgovarajuće starosti i pola sa nivoima vitamina B12 > 200 ng/mL.
Rezultati
Otkrivena je značajna povezanost između varijante MTHFR c.677C>T i deficijencije vitamina B12 kod osoba iz severnog Jordana. Učestalost homozigotnog genotipa MTHFR c.677C>T bila je značajno veća kod osoba sa nedostatkom B12 u poređenju s kontrolnom grupom (X2=8,397, p=0,0150). Učestalost T alela ukazala je na značajnu povezanost sa deficijencijom vitamina B12 u proučavanoj populaciji (OR = 1,684, 95% CI: 1,116 do 2,542, p = 0,017). S druge strane, varijanta MTHFR c.1286A>C nije bila u značajnoj asocijaciji s nedostatkom vitamina B12 u izabranoj populaciji.
Zaključak
Naši rezultati ukazuju na značajnu povezanost između učestalosti homozigotnih MTHFR c.677C>T i T alela i deficijencije vitamina B12 u ovoj populaciji Jordanaca.
Ključne reči: polimorfizmi MTHFR, nedostatak vitamina B12, metabolizam folata, homocistein
Introduction
Vitamin B12 (cobalamin) deficiency is a worldwide health problem with variable prevalence (1). In Jordan, vitamin B12 deficiency has been estimated at 16–50%, with higher prevalence in the elderly above 55 years (2, 3, 4). The most common symptoms of vitamin B12 deficiency are hematological and/or neurological disorders (5, 6). Therefore, some risk factors of vitamin B12 deficiency have been investigated including lifestyle, age, and ethnic origin (7, 8, 9, 10, 11, 12, 13). Moreover, genetic predisposition was demonstrated in vitamin B12 deficiency. For instance, mutations and polymorphisms in transport proteins such as transcobalamin TCN, gastric intrinsic factor GIF and metabolic enzymes such as methylenetetrahydrofolate reductase MTHFR have been associated with vitamin B12 deficiency. Other vitamin-B12 related abnormalities like homocysteinemia and neural tube defect (NTD) have also been associated with genetic variants in these genes (13, 14, 15, 16, 17, 18, 19, 20, 21, 22).
MTHFR catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate; the latter is required for the conversion of homocysteine to methionine by methionine synthase (MS). MS catalytic function is dependent on vitamin B12 (23). More than 40 mutations have been identified in the MTHFR gene in individuals diagnosed with homocystinuria, and several polymorphisms in the MTHFR gene have been associated with an increased risk of neural tube defects (NTD) and anencephaly (16). Moreover, the MTHFR gene polymorphisms have also been linked to cardiovascular diseases, stroke, psychiatric disorders, and certain types of cancer (24, 25, 26, 27, 28). In particular, the c.677C>T variant (NM_005957.4: c.677C>T, rs1801133) is the most common genetic variant in homocystinuria (24, 29). The c.677C>T variant encodes a thermolabile form of the MTHFR which is less active at high temperatures (30). Another MTHFR variant, 1298A>C (NM_005957.4: c.1286A>C, rs1801131), does not cause increased homocysteine levels in heterozygous or homozygous individuals, but combined heterozygous genotypes of c.1298A>C and c.677C>T result in an outcome similar to c.677C>T homozygous individuals (31, 32, 33). Recent studies have associated the c.677C>T variant with vitamin B12 deficiency and homocystinuria (13, 14, 15). In the present case-control study, we investigated the association of the c.677C>T and c.1298A>C variants in the MTHFR gene with vitamin B12 deficiency in a subset of the Jordanian population living in the northern part of the country.
Patients and Methods
One hundred individuals (45 males and 55 females) diagnosed with B12 deficiency (B12 < 200 mg/mL) were enrolled in this study from hospitals in northern Jordan, with an average age of 37 years. The control group included 100 individuals (53 males and 47 females) with an average age of 32 years. Blood samples (3 mL) were collected from participants in EDTA tubes and stored at 4 °C until the time of DNA extraction. Demographics and clinical information were obtained from hospital clinical records and questionnaires filled in by participants. All participants were aware of the scope of the study and informed consents were obtained according to the guidelines of the Research Ethics Committee at Yarmouk University.
Genomic DNA extraction
The phenol-chloroform extraction method was used for genomic DNA extraction. Briefly, 200 μL of blood was mixed with 500 μL of extraction buffer (0.01 mol/L Tris-HCl, 0.1 mol/L NaCl, 0.01 mol/L EDTA, 2% SDS and 0.39 mol/L DTT) and 20 μL of 20 mg/mL proteinase K, followed by incubation for 2 hours at 56 °C, then 500 μL of phenol: chloroform: isoamyl alcohol were mixed vigorously and spun at 14,000 g for 5 minutes. The aqueous layer was transferred to an Eppendorf tube, mixed with phenol: chloroform: isoamyl alcohol and centrifuged at 14,000 g for 5 minutes. The last aqueous portion was mixed with one mL of ice-cold absolute ethanol and centrifuged at 14,000 g for 5 minutes. The DNA pellet was washed twice with 70% ethanol and eluted with 100 μL of TE buffer and stored at –20 °C until further use.
PCR amplification of the MTHFR target sequence
Polymerase chain reaction was performed by using specific primers that target the sequences of interest in the MTHFR gene. For the c.677C>T genotypes, the following pairs of primers were used: (F-5’CCT TGA ACA GGT GGA GGC CAG-3’ and R-5’GCG GTG AGA GTG GGG TGG AG-3’). For the 1298A>C genotyping, the following pairs of primers were used: (5’-CTT TGG GGA GCT GAA GGA CTA CTA-3’ and 5’-CAC TTT GTG ACC ATT CCG GTT TG-3’). The amplification was performed in 25 μL final volume by using Taq 2X Master Mix (New England BioLabs, USA) according to the manufacturer’s instructions. Briefly, 3 μL of DNA were added to 12.5 μL of 2X master mix with a final concentration of primers 0.2 μmol/L and completed to 25 μL by adding dd.H2O. PCR conditions were as follows: initial denaturation at 95 °C for 5 minutes, 35 cycles of denaturation at 95 °C for 1 minute, annealing (65 °C for the c.677C>T and 72 °C for the 1298A>C) for 30 seconds and extension at 72 °C for 1 minute, followed by 3 minutes of final extension at 72 °C.
Restriction fragment length polymorphism (RFLP)
For the MTHFR c.677C>T genotyping, restriction enzyme digestion was performed by using Hinf I restriction enzyme (New England BioLabs, USA). Restriction enzyme digestion tube contained 8 μL of PCR product, 4 units of Hinf I, 1 X Hinf I buffer, 0.1 mg/mL of BSA and H2O up to a final volume of 20 μL, followed by incubation at 37 °C for 90 minutes. Inactivation step was performed by heating the mixture at 65 °C for 15 minutes. Products of the RFLP digestion were resolved by 3% agarose gel at 10 V/cm current for 1.5 hours (Figure 1).
Figure 1.
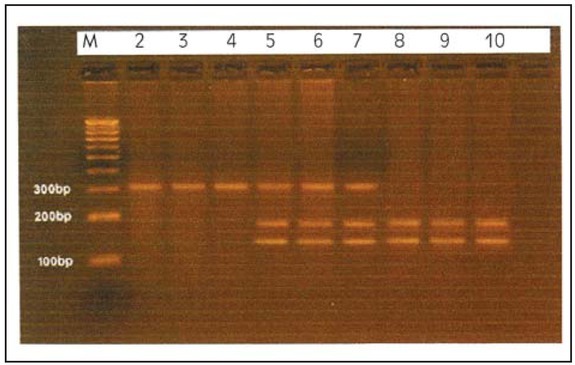
RFLP-PCR products of the MTHFR-c.677C>T genotypes by Hinf1. Lane 1: 100 bp DNA ladder, lanes 2-4: homozygous CC genotype, lanes 5-7: heterozygous CT genotype and lanes 8-10: recessive genotype TT.
DNA sequencing of the 1298A>C genotype
Sanger DNA sequencing of the PCR products of the amplified target of the MTHFR 1298A>C genotype was performed at GENWIZ, USA.
Statistical Analysis
Chi-square and Fisher’s exact test analyses were used for the calculation of p value, odds ratio (OR), and 95% confidence interval (CI) and Hardy–Weinberg Equilibrium (HWE) evaluation. GraphPad Prism-6 software was used for statistical analysis, p < 0.05 cut off was considered significant.
Results
MTHFR c.677C>T and c. 1298A>C genotypic frequencies
In the samples analyzed, DNA sequencing for the c.677C>T polymorphic site identified the genotypic frequencies of CC (41%), CT (45%) and TT (14%). In addition, frequencies of homozygous AA, heterozygous AT and homozygous TT genotypes for the 1298A>C variants were 34%, 59% and 7%, respectively (Table I). There was no digression from the Hardy-Weinberg equilibrium (HWE) for the c.677C> T variants (Table II). On the other hand, frequencies of the 1298A>C variants showed deviation from the HWE (p < 0.05) (Table III).
Table I.
MTHFR Genotypes frequencies according to the level of vitamin B12.
| B12 Deficient | Control | p Value | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| B12 (mg/mL) | 142.31±24.09 | 441.76±179.06 | 2.84E-39* | |||
| Age (years) | 38.04±11.69 | 32.71±15.04 | ||||
| Male n (%) | 53 (53 %) | 45 (45 %) | 0.258 | 1.378 | 0.79–2.4 | |
| Female n (%) | 47 (47 %) | 55 (55 %) | ||||
| MTHFR 677C>T | TT | 21 | 7 | 0.015* | ||
| CT | 43 | 47 | ||||
| CC | 36 | 46 | ||||
| C | 115 | 139 | 0.017* | 1.684 | 1.116–2.542 | |
| T | 85 | 61 | ||||
| MTHFR 1298A>C | AA | 36 | 32 | 0.662 | ||
| AC | 56 | 62 | ||||
| CC | 8 | 6 | ||||
| A | 128 | 126 | 1.000 | 1.016 | 0.677–1.524 | |
| C | 72 | 74 | ||||
| MTHFR 677C>T And 1298A>C | CC/AA | 9 | 13 | 0.173 | ||
| CC/AC | 23 | 29 | ||||
| CC/CC | 4 | 4 | ||||
| CT/AA | 19 | 18 | ||||
| CT/AC | 23 | 27 | ||||
| CT/CC | 1 | 2 | ||||
| TT/AA | 8 | 1 | ||||
| TT/AC | 10 | 6 | ||||
| TT/CC | 3 | 0 | ||||
* p value < 0.05
Table II.
MTHFR c.677C>T Genotypes frequencies in association with B12 level and age.
| Total | TT | CT | CC | p value | |
|---|---|---|---|---|---|
| MTHFR genotype 677C>T | n=200 | n = 28 | n = 90 | n = 82 | 0.68 (HWE)* |
| Age (year) | 35.4±13.7 | 43.7±10.9 | 36.4±14.5 | 31.4±11.9 | 0.007** |
| Males | 98 (49%) | 15 (54 %) | 35 (39%) | 48 (59%) | 0.6 |
| B12 (< 200 mg/mL) | 100 (50%) | 21 | 43 | 36 | 0.015** |
| B12 (≥ 200 mg/mL) | 100 (50%) | 7 | 47 | 46 |
*Hardy-Weinberg equilibrium, **p value < 0.05
Table III.
MTHFR c.1298A>C Genotypes of frequencies in association with B12 level and age.
| Total | AA | AC | CC | p value | |
|---|---|---|---|---|---|
| MTHFR 1298A>C genotype | n = 200 | n = 68 | n = 118 | n = 14 | 0.0001 (HWE)* |
| Age (year) | 35.4 ± 13.7 | 35.1 ± 13.9 | 34.9 ± 13.2 | 40.9 ± 16.8 | 0.243 |
| Males | 99 (50%) | 29 (43 %) | 66 (56%) | 4 (29%) | 0.06 |
| B12 (< 200 mg/mL) | 100 (50%) | 36 | 56 | 8 | 0.662 |
| B12 (≥ 200 mg/mL) | 100 (50%) | 32 | 62 | 6 |
*Hardy-Weinberg equilibrium
MTHFR c.677C>T and c. 1298A>C genotypes distribution in B12 deficient individuals
DNA sequencing and RFLP analysis for the c.677C>T variants showed that the frequency of homozygous CC genotype was 36/100 (36%) in the B12 deficient individuals. In addition, frequencies of heterozygous CT and homozygous TT genotypes were 43/100 (43%) and 21/100 (21%), respectively. On the other hand, analysis of the control group showed the following frequencies for the c.677C> T variants: homozygous CC genotype 46/100 (46%), heterozygous CT and homozygous TT genotypes 47/100 (47%) and 7/100 (7%), respectively. Hence, the c.677C>T genotypes frequencies distribution revealed a significant difference in individuals with vitamin B12 compared to controls. Particularly, the frequency of the homozygous TT genotype is significantly higher in B12 deficient individuals in comparison with the control group (X2 = 8.397, p = 0.0150). Moreover, T allele frequency showed significant association with vitamin B12 deficiency in the study population (OR = 1.684, 95% CI: 1.116 to 2.542, p = 0.017). The results are shown in Table I.
Conversely, B12 deficient individuals did not show any significant difference in the genotype frequencies distribution for the 1298A>C variant in comparison with the control group (X2 = 0.83, p = 0.662). The frequency of homozygous AA genotype for the 1298A>C variants was 36/100 (36%) in the B12 deficient individuals. In addition, heterozygous AC and homozygous CC genotypes were 56/100 (56%) and 8/100 (8%), respectively. In the control group, frequencies of homozygous AA genotype were 32/100 (32%), heterozygous AC and homozygous CC genotypes 62/100 (62%) and 6/100 (6%), respectively, as shown in Table I and Table III.
Interestingly, our results showed a significant association between the c.677C>T genotypes and the age at onset of vitamin B12 deficiency (p = 0.007). In particular, the homozygous TT genotype or T allele is more frequent in older individuals with vitamin B12 deficiency (Table II).
Discussion
Vitamin B12 deficiency is a diet-related and slowly developing disorder. Consequently, low levels of vitamin B12 are associated with neurological and hematological disorders such as neural tube defects, cardiovascular diseases, dementia, as well as some types of cancer (8, 16, 28, 34). However, genetic predisposition to vitamin B12 deficiency has been demonstrated in various studies (14, 15, 16, 17, 18, 19, 20, 21, 22). For in stance, vitamin B12 deficiency has been demonstrated by ABCD4 mutations and LMBRD1 mutations (35, 36). However, a few mutations in the susceptibility genes underscored the importance of more investigations for other influencing polymorphisms in the transportation and metabolic complexes of vitamin B12 and maybe folate pathways. In this case-control study, the results showed a significant association between c.677C>T variants of the MTHFR gene and vitamin B12 deficiency in the studied population. Specifically, the TT genotype and T allele of the MTHFR-c.677C>T variant were found to be significantly associated with low levels of vitamin B12, which is consistent with the recent report by Thuesen et al. (13). Similarly, Shiran et al. (15) and Zittan et al. (15) have shown vitamin B12 deficient individuals homozygous for the TT genotype of MTHFR-c.677C>T variant suffer from endothelial dysfunction and homocysteinemia. In the case of MTHFR 1298A>C variants, our results showed a lack of association with vitamin B12 deficiency. Moreover, the results showed that c.677C>T and c.1298A>C variants exhibited linkage disequilibrium for allelic distribution.
Hypothetically, c.677C>T and c.1298A>C variants of the MTHFR gene result in a decreased MTHFR activity, however, thermolabile protein results only from the c.677C>T variant. The difference in the functional properties of these variants could be explained by their respective positions in the protein, such as the c.677C>T is located in the catalytic domain, whereas the 1298A>C is located in the regulatory domain (31).
In the current study, individuals with TT genotype of the MTHFR-c.677C>T variant showed a simultaneous vitamin B12 deficiency. According to our results and previously reported findings (13, 15), we propose that vitamin B12 deficiency might be due to mutations and/or polymorphisms in the MTHFR gene. The metabolic explanation of the association between B12 deficiency and TT homozygosity is yet to be resolved. However, two explanations have been suggested for this relationship; firstly, abnormal homocysteine metabolism due to the T allele of the MTHFR-c.677C>T variant may cause consumption of vitamin B12; secondly, intestinal absorption of vitamin B12 is genetically associated with the MTHFR-c.677C>T variant. Nevertheless, lifestyle might be another factor that influences the level of vitamin B12 in Jordan. Therefore, more studies are recommended to elucidate the influence of genotypes of the susceptibility genes and lifestyle on the status of vitamin B12 in Jordan. In an unpublished large-scale study (> 2500 individuals), we demonstrated different prevalence of vitamin B12 level in different geographical locations.
Furthermore, our results showed a significant difference in age between B12 deficient individuals and those of the control group (p = 0.006). In addition, the TT genotype of the MTHFR-c.677C>T variant is more frequent in older individuals with vitamin B12 deficiency (p = 0.007), which suggests an expected role of this variant in age-onset of vitamin B12 deficiency. This is consistent with the fact that vitamin B12 deficiency is an age-related, slow-developing disorder (8, 37).
Our results could further help explain higher rates of vitamin B12 deficiency in Jordan. The current cohort is under investigation for other genotypes of transportation complexes with demographic data including folate and homocysteine levels. Collectively, this promising experimental design could initiate the system biology approach that might add to the knowledge of general practitioners and health policy makers to evaluate proper methods of supplementation. Clinically, the MTHFR genotyping in B12 deficient individuals could be one of the potential markers for the development of vitamin B12 deficiency. Therefore, deficient or high-risk individuals could have a special protocol of assessment, diet and treatment regimen. It has been reported that supplemental vitamin B12 during pregnancy significantly improved the size-at-birth for children of maternal carriers of the 677TT genotype (38). Moreover, early genotyping will help in the risk assessment of a certain subset of the population, especially the elderly.
In conclusion, our results showed a significant association between MTHFR-c.677C>T genotypes and vitamin B12 deficiency in a subset of the Jordanian population. On the other hand, the c.1298A>C variants did not show an association with the deficiency of B12. Further studies are required to elucidate the role of MTHFR variants in the metabolism of vitamin B12 and other associated metabolites like folate and homocysteine.
Acknowledgments
We are very thankful to the Deanship of Scientific Research and Graduate Study/Yarmouk University (Grant # 19/2008) and The Higher Council For Science And Technology.
Footnotes
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Gupta AK, Damji A, Uppaluri A. Vitamin B12 deficiency. Prevalence among South Asians at a Toronto clinic. Canadian family physician Medecin de Famille Canadien. 2004;50:743. –. [PMC free article] [PubMed] [Google Scholar]
- 2.Alomari MA, Khabour OF, Gharaibeh MY, Qhatan RA. Effect of physical activity on levels of homocysteine, folate, and vitamin B12 in the elderly. The Physician and Sportsmedicine. 2016;44(1):68. doi: 10.1080/00913847.2016.1135037. –. [DOI] [PubMed] [Google Scholar]
- 3.Barghouti FF, Younes NA, Halaseh LJ, Said TT, Ghraiz SM. High frequency of low serum levels of vitamin 12 among patients attending Jordan University Hospital. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2009;15(4):853. –. [PubMed] [Google Scholar]
- 4.Fora MA, Mohammad MA. High frequency of suboptimal serum vitamin B12 level in adults in Jordan. Saudi Medical Journal. 2005;26(10):1591. –. [PubMed] [Google Scholar]
- 5.Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC) 1998. [PubMed]
- 6.Baik HW, Russell RM. Vitamin B12 deficiency in the elderly. Annual Review of Nutrition. 1999;19:357. doi: 10.1146/annurev.nutr.19.1.357. –. [DOI] [PubMed] [Google Scholar]
- 7.Yuksel M, Ates I, Kaplan M, Arikan MF, Ozin YO, Kilic YMY, Topcuoglu C, Kayacetin E. Is oxidative stress associated with activation and pathogenesis of inflammatory bowel disease? J Med Biochem. 2017;36:341. doi: 10.1515/jomb-2017-0013. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan-Harshman M, Aldoori W. Vitamin B12 and health. Canadian family physician Medecin de Famille Canadien. 2008;54(4):536. –. [PMC free article] [PubMed] [Google Scholar]
- 9.Matteini AM, Walston JD, Fallin MD, Bandeen-Roche K, Kao WH, Semba RD. Markers of B-vitamin deficiency and frailty in older women. The Journal of Nutrition, Health & Aging. 2008;12(5):303. doi: 10.1007/BF02982659. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson MA, Hawthorne NA, Brackett WR, Fischer JG, Gunter EW, Allen RH. Hyperhomocysteinemia and vitamin B-12 deficiency in elderly using Title IIIc nutrition services. The American Journal of Clinical Nutrition. 2003;77(1):211. doi: 10.1093/ajcn/77.1.211. et al. –. [DOI] [PubMed] [Google Scholar]
- 11.Loikas S, Koskinen P, Irjala K, Lopponen M, Isoaho R, Kivela SL. Vitamin B12 deficiency in the aged: a population-based study. Age and Ageing. 2007;36(2):177. doi: 10.1093/ageing/afl150. et al. –. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MA, Hausman DB, Davey A, Poon LW, Allen RH, Stabler SP. Vitamin B12 deficiency in African American and white octogenarians and centenarians in Georgia. The Journal of Nutrition, Health & Aging. 2010;14(5):339. doi: 10.1007/s12603-010-0077-y. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sladoje DP, Kisić B, Mirić D. The monitoring of protein markers of inflammation and serum lipid concentration in obese subjects with metabolic syndrome. J Med Biochem. 2017;36:366. doi: 10.1515/jomb-2017-0009. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiran A, Remer E, Asmer I, Karkabi B, Zittan E, Cassel A. Association of Vitamin B12 Deficiency with Homozygosity of the TT MTHFR C677T Genotype, Hyperhomocysteinemia, and Endothelial Cell Dysfunction. The Israel Medical Association Journal: IMAJ. 2015;17(5):288. et al. –. [PubMed] [Google Scholar]
- 15.Zittan E, Preis M, Asmir I, Cassel A, Lindenfeld N, Alroy S. High frequency of vitamin B12 deficiency in asymptomatic individuals homozygous to MTHFR C677T mutation is associated with endothelial dysfunction and homocysteinemia. American Journal of Physiology Heart and Circulatory Physiology. 2007;293(1):H860. doi: 10.1152/ajpheart.01189.2006. et al. –. [DOI] [PubMed] [Google Scholar]
- 16.Cunha AL, Hirata MH, Kim CA, Guerra-Shinohara EM, Nonoyama K, Hirata RD. Metabolic effects of C677T and A1298C mutations at the MTHFR gene in Brazilian children with neural tube defects. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2002;318(1–2):139. doi: 10.1016/s0009-8981(01)00764-1. –. [DOI] [PubMed] [Google Scholar]
- 17.Zheng S, Yang W, Wu C, Sun L, Lin D, Lin X. Association of ulcerative colitis with transcobalamin II gene polymorphisms and serum homocysteine, vitamin B12, and folate levels in Chinese patients. Immuno-genetics. 2017;69(7):421. doi: 10.1007/s00251-017-0998-2. et al. –. [DOI] [PubMed] [Google Scholar]
- 18.Nashabat M, Maegawa G, Nissen PH, Nexo E, Al-Shamrani H, Al-Owain M. Long-term Outcome of 4 Patients With Transcobalamin Deficiency Caused by 2 Novel TCN2 Mutations. Journal of Pediatric Hematology/Oncology. 2017 doi: 10.1097/MPH.0000000000000857. et al. [DOI] [PubMed] [Google Scholar]
- 19.Gueant-Rodriguez RM, Chery C, Fofou-Caillierez BM, Voirin J, Foliguet B, Josse T. Association of combined GIF290T>C heterozygous mutation/FUT2 secretor variant with neural tube defects. Clinical Genetics. 2017 doi: 10.1111/cge.13104. et al. [DOI] [PubMed] [Google Scholar]
- 20.Unal S, Rupar T, Yetgin S, Yarali N, Dursun A, Gursel T. Transcobalamin II Deficiency in Four Cases with Novel Mutations. Turkish Journal of Haematology: official journal of the Turkish Society of Haematology. 2015;32(4):317. doi: 10.4274/tjh.2014.0154. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palladino M, Chiusolo P, Reddiconto G, Marietti S, De Ritis D, Leone G. MTHFR polymorphisms involved in vitamin B12 deficiency associated with atrophic gastritis. Biochemical Genetics. 2009;47(9–10):645. doi: 10.1007/s10528-009-9256-0. et al. –. [DOI] [PubMed] [Google Scholar]
- 22.Sam RC, Burns PJ, Hobbs SD, Marshall T, Wilmink AB, Silverman SH. The prevalence of hyperhomocysteinemia, methylene tetrahydrofolate reductase C677T mutation, and vitamin B12 and folate deficiency in patients with chronic venous insufficiency. Journal of Vascular Surgery. 2003;38(5):904. doi: 10.1016/s0741-5214(03)00923-6. et al. –. [DOI] [PubMed] [Google Scholar]
- 23.Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Molecular Genetics and Metabolism. 2000;71(1–2):121. doi: 10.1006/mgme.2000.3027. –. [DOI] [PubMed] [Google Scholar]
- 24.Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE. Biological and clinical implications of the MTHFR C677T polymorphism. Trends in Pharmacological Sciences. 2001;22(4):195. doi: 10.1016/s0165-6147(00)01675-8. –. [DOI] [PubMed] [Google Scholar]
- 25.Kelly PJ, Rosand J, Kistler JP, Shih VE, Silveira S, Plomaritoglou A. Homocysteine, MTHFR 677C-->T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology. 2002;59(4):529. doi: 10.1212/wnl.59.4.529. et al. –. [DOI] [PubMed] [Google Scholar]
- 26.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. Jama. 2002;288(16):2023. doi: 10.1001/jama.288.16.2023. et al. –. [DOI] [PubMed] [Google Scholar]
- 27.van Winkel R, Rutten BP, Peerbooms O, Peuskens J, van Os J, De Hert M. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophrenia Research. 2010;121(1–3):193. doi: 10.1016/j.schres.2010.05.030. –. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, Matsuo K, Hirose K, Hiraki A, Kawase T, Watanabe M. One-carbon metabolism-related gene polymorphisms and risk of breast cancer. Carcinogenesis. 2008;29(2):356. doi: 10.1093/carcin/bgm295. et al. –. [DOI] [PubMed] [Google Scholar]
- 29.Sibani S, Christensen B, O’Ferrall E, Saadi I, Hiou-Tim F, Rosenblatt DS. Characterization of six novel mutations in the methylenetetrahydrofolate reductase (MTHFR) gene in patients with homocystinuria. Human Mutation. 2000;15(3):280. doi: 10.1002/(SICI)1098-1004(200003)15:3<280::AID-HUMU9>3.0.CO;2-I. et al. –. [DOI] [PubMed] [Google Scholar]
- 30.Kang SS, Wong PW, Susmano A, Sora J, Norusis M, Ruggie N. Thermolabile methylenetetrahydrofolate reductase: an inherited risk factor for coronary artery disease. American Journal of Human Genetics. 1991;48(3):536. –. [PMC free article] [PubMed] [Google Scholar]
- 31.van der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? American Journal of Human Genetics. 1998;62(5):1044. doi: 10.1086/301825. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Renlund M. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas worldwide. Journal of Medical Genetics. 2003;40(8):619. doi: 10.1136/jmg.40.8.619. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. American Journal of Human Genetics. 1998;62(5):1258. doi: 10.1086/301836. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar P, Yadav U, Rai V. Methylenetetrahydrofolate reductase gene C677T polymorphism and breast cancer risk: Evidence for genetic susceptibility. Meta Gene. 2015;6:72. doi: 10.1016/j.mgene.2015.08.008. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coelho D, Kim JC, Miousse IR, Fung S, du Moulin M, Buers I. Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nature Genetics. 2012;44(10):1152. doi: 10.1038/ng.2386. et al. –. [DOI] [PubMed] [Google Scholar]
- 36.Gailus S, Suormala T, Malerczyk-Aktas AG, Toliat MR, Wittkampf T, Stucki M. A novel mutation in LMBRD1 causes the cblF defect of vitamin B(12) metabolism in a Turkish patient. Journal of Inherited Metabolic Disease. 2010;33(1):17. doi: 10.1007/s10545-009-9032-7. et al. –. [DOI] [PubMed] [Google Scholar]
- 37.van Wijngaarden JP, Doets EL, Szczecinska A, Souverein OW, Duffy ME, Dullemeijer C. Vitamin B12, folate, homocysteine, and bone health in adults and elderly people: a systematic review with meta-analyses. Journal of Nutrition and Metabolism. 2013;2013:486186. doi: 10.1155/2013/486186. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres-Sanchez L, Lopez-Carrillo L, Blanco-Munoz J, Chen J. Maternal dietary intake of folate, vitamin B12 and MTHFR 677C>T genotype: their impact on newborn’s anthropometric parameters. Genes & Nutrition. 2014;9(5):429. doi: 10.1007/s12263-014-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


