Summary
Background
We investigated the sensitivity of neutrophil to lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), as well as a combination of NLR and PLR to predict endoscopic disease severity based on mucosal assessment in ulcerative colitis (UC).
Methods
The study group consisted 104 patients with active UC, 104 patients in remission, and 105 healthy individuals. Disease activity was described with Rachmilewitz endoscopic activity index (EAI). Curve analysis was used to determine the optimal cutoff values of NLR and PLR for obtaining remission. The patients with both PLR and NLR values higher than the cutoff values were coded as »high risk,« those with one parameter higher were coded as »moderate risk«, those with both parameters lower than the cutoff values were coded as »low-risk« patients.
Results
The mean NLR and PLR values in the endoscopically active disease group were higher than the others, with higher values in the endoscopic remission group compared with the control group (p<0.001). Rachmilewitz EAI in high-risk patients was significantly higher than that in others (p<0.001). In Cox regression analyses, moderate and high risk, high erythrocyte sedimentation rate and high EAI were found as independent predictors of endoscopic active disease.
Conclusions
This is the first study that investigated the use of NLR and PLR combination to assess endoscopic disease severity in UC. Either high NLR or PLR levels can predict active endoscopic disease. However, the use of these parameters in combination is more accurate in evaluating mucosal disease and inflammation in UC.
Keywords: mucosal disease, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, ulcerative colitis
Kratak sadržaj
Uvod
Izučavana je osetljivost odnosa neutrofili prema limfocitima (NLR) i trombociti prema limfocitima (PLR), kao i kombinacija NLR i PLR za predviđanje težine endoskopskog oboljenja na osnovu procene oštećenja mukoze u ulcerativnom kolitisu (UC).
Metode
Izučavanje je obuhvatilo 104 pacijenta sa aktivnin UC, 104 pacijenta u remisiji i 105 zdravih osoba. Težina oboljenja opisivana je sa Rachmilewitz-ovim endoskopskim indeksom aktivnosti (EAI). Određene su optimalne »cutoff« vrednosti NLR i PLR za dobijenu remisiju. Pacijenti sa obe vrednosti PLR i NLR više od predviđenih »cuoff« vrednosti označene su kao »visoki rizik«, one sa jednim višim parametrom označene su kao »umereni ritzik«, a one sa oba niža paramatera od »cutoff« vrednosti kao pacijenti sa »niskim rizikom«.
Rezultati
Srednje vrednosti NLR i PLR u grupi endoskopski aktivih oboljenja bile su više nego kod drugih sa višim vrednostima u grupi sa endskopskom remisijom u poređenju sa kontrolnom grupom (p>0,001). Rachmilewitz EAI u pacijenata sa visokimm rizikom bile su značajno više nego kod drugih pacijenata (p<0,001). Primenom Cox regresione analize, umereni i visoki rizik, visoka sedimentacija eritrocita i visoki EAI su nađeni kao nezavisni prediktori endoskopski aktivnog oboljenja.
Zaključak
Ovo je prvo izučavanje koje je koristilo NLR i PLR kombinaciju za procenu težine endoskopskog oboljenja u UC, kao i da li visoki nivoi NLR i PLR mogu da predvide aktivno endoskopsko oboljenje. Međutim, primena ovih parametara u kombinaciji mnogo je korisnija za priocenu mukoznog oboljenja i inflamacije u UC.
Ključne reči: mukozno oboljenje, odnos neutrofili prema limfocitima, odnos trombociti prema limfocitima, ulcerativni kolitis
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) that diffusely involves various parts of the colon (1, 2). The disease may be limited to the rectum, or it may involve the entire colon. The main complaints of the patients are bloody diarrhea and abdominal pain (3). Mucosal examination via colonoscopy is the basic method in diagnosis; it monitors the activity of the disease and is used in the follow-up of patients (4).
The exacerbations of UC may appear in varying frequency and severity. Bleeding, abdominal pain, and fever are frequent during exacerbations (5). Exacerbation is mild in most patients, and 15% of the patients need hospitalization (6). Colonoscopy is performed after excluding infectious causes, and determining the disease severity is beneficial and guides the clinician for treatment and prognosis of the disease. Ulcers, exudates, fragile mucosa, and bleeding are frequent in cases of active disease (7). Early diagnosis and appropriate treatment of disease exacerbation is important in the course of the disease. Some indirect methods have been recently used to determine the disease activity, and their sensitivity has been studied. The neutrophil-to-lymphocyte ratio (NLR) is one of them (8).
Inflammatory markers, including leukocyte count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) have been well known to increase in case of active diseases. However, the data on the sensitivity of some inflammation markers, including NLR and platelet-to-lymphocyte ratio (PLR) on identifying endoscopic active disease, and their correlation with mucosal injury are scarce. In this study, we investigated the sensitivity of new inflammation markers, NLR and PLR, as well as conventional inflammation markers to determine endoscopically active disease, and their correlation with mucosal injury. In addition, we investigated the sensitivity of NLR and PLR combination to predict disease severity according to mucosal disease.
Materials and Methods
Study setting and population
This is a single-center retrospective cross-sectional study. This study included 208 patients with UC. All these patients had been consecutively followed up in Türkiye Yüksek htisas Training and Research Hospital, Department of Inflammatory Bowel Disease between January 2016 and July 2016. The diagnosis of UC was established based on previous endoscopic, clinical, and pathological features. Furthermore, 105 healthy individuals who had no IBD based on their previous colonoscopy reports were included in the study. Colonoscopy was performed for various indications except IBD in the healthy population. Patients and/or healthy individuals having active infections, detected via chest X-ray, urine sample analysis, and stool test, were excluded from the study.
Endoscopic procedure
Endoscopic procedures were performed in the endoscopy unit of our Gastroenterology Department with various experienced gastroenterology specialists. After optimal bowel preparation with Sennozid A+B Calcium solution, a colonoscope (EVIS LUCERA ELITE CLV-290-SL; Olympus Medical Systems, Tokyo, Japan) was used in each colonoscopic procedure. Colonoscopy was performed either regularly or as indicated to evaluate mucosal disease activity based on the patient’s complaints. Rachmilewitz endoscopic activity index (EAI) is used routinely to describe endoscopic findings in patients with UC in our department. Therefore, we evaluated the colonoscopic findings of patients based on Rachmilewitz EAI. There are four components in this endoscopy based scoring system: granulation of the mucosa, vascular pattern of the mucosa, mucosal vulnerability and mucosal damage. Maximum point is 12. Remission and active disease were defined according to the mucosal disease. Due to Rachmilewitz EAI, patients with 4 points were considered to have an active disease, remission was defined with Rachmilewitz EAI<4 Rachmilewitz EAI is a real-time scoring system, which is calculated during colonoscopy.
Laboratory investigation
Laboratory tests, including complete blood count, blood chemistry including ALT, AST, GGT, ALP, total protein, albumin, urea and creatinine, erythrocyte sedimentation rate, and fibrinogen were analyzed on the day of the colonoscopic procedure. Hemoanalyzer Cell-Dyn 3700 (Abbott, Illinois, USA) was used. The laboratory findings that were examined on the colonoscopic examination day were obtained from the patient’s medical records.
Statistical analysis
Statistical analysis of the data was performed using the Statistical Program for Social Sciences version 15.0 for Windows package program. The Youden index method with receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff values of NLR and PLR for obtaining remission. The normality of the distribution of data was analyzed with Kolmogorov-Smirov test. Normally distributed numerical variables were presented as mean ± standart deviation and the parameters that did not show a normal distribution were presented as median-interquartile range. Categorical variables were presented as numbers and percentages. Student T test was used for two group comparison of normally distributed numerical variables, ANOVA test was used to comparison three or more groups. Mann-Whitney U test was used for two group comparison of numerical variables which did not showed normally distribution, Kruskal Wallis test was used to comparison three or more groups. Sperman correlation analysis test was used for determining correlation between EAI and other numerical variables. Chi-square and Fisher exact chi-square tests were used to compare categorical variables. Risk factors, including age, sex, hemoglobin, CRP, ESR, fibrinogen, EAI, PLR, NLR, PLR and NLR combination, white blood cell (WBC) counts, neutrophils, platelets, and lymphocyte counts were inserted into stepwise Cox regression analysis to determine the independent predictors of remission. The time to remission was analyzed with Kaplan–Meier graph for the risk classification with PLR-NLR combination. P<0.05 was considered as statistically significant.
The study was conducted in accordance with the Decleration of Helsinki and was approved by the Local Ethics Research Comitte.
Results
Table I summarizes the characteristics and laboratory findings of the study groups. The study group
Table I.
Demographic characteristics, and laboratory findings of the study populations.
| Variables | Control (n=105) | Endoscopically active disease (n=104) | Remission (n=104) | p |
|---|---|---|---|---|
| Age (years) | 46.5±13.8 | 47.6±14.0 | 48.6±13.7 | 0.510 |
| Gender, n (%) | ||||
| Male | 47(44.8) | 45(43.3) | 51(59.0) | 0.327 |
| Hemoglobin (g/L) | 138±16 | 132±19 | 145±16 | <0.001* |
| CRP (mg/L) | 1.8(3.8) | 5.0(9.2) | 3.6(4.1) | <0.001* |
| ESR (mm/hr) | 6.0(6.0) | 15.0(19.0) | 9.0(10.0) | <0.001* |
| WBC (x 109/L) | 6500±1204 | 9376.9±2580.6 | 7870.2±2123.4 | <0.001* |
| Neutrophil(x 109/L) | 3925.7±1066.0 | 5792.3±2532.7 | 4891.3±1662.9 | <0.001* |
| Platelet (x 109/L) | 226828.6±62300.9 | 311692.3±92082.5 | 269894.2±53402.5 | <0.001* |
| Lymphocyte (×109/L) | 2.42±0.35 | 3.26±0.81 | 3.00±0.54 | <0.001* |
| NLR | 1.8±0.6 | 2.9±0.8 | 2.2±0.9 | <0.001* |
| PLR | 104.1±30.4 | 153.7±72.1 | 122.1±38.3 | <0.001* |
| Fibrinogen (g/L) | 2.5±0.3 | 3.3±0.8 | 3.0±0.5 | <0.001* |
| EAI | - | 8.0(2.0) | 2.0(2.0) | <0.001* |
| UC Type | ||||
| Proctitis | - | 22(21.2) | 41(39.4) | |
| Left-sided colitis | - | 72(69.2) | 55(52.9) | 0.016* |
| Pan-ulcerative colitis | - | 10(9.6) | 8(7.7) |
*p<0.05 indicates statistical significance.Abbreviations: CRP: C-reactive protein, ESR: Erythrocyte sedimentation rate, WBC: White blood cell, NLR: Neutrophil to lymphocyte ratio, PLR: Platelet to lymphocyte ratio, EAI: Endoscopic activity index, UC: Ulcerative colitis.
consisted of 313 patients, including 104 patients with endoscopically active ulcerative colitis, 104 patients in remission, and 105 controls. The sex and mean ages of the groups were similar (P>0.05).
The hemoglobin level was higher in patients with endoscopic remission, and hemoglobin levels were comparable between the controls and patients with active endoscopic disease (14.5 ± 1.6 vs. 13.8 ± 1.6 vs. 13.2 ± 1.9; P<0.001).
The highest median CRP and ESR levels were found in patients with endoscopically active ulcerative colitis, followed by endoscopic remission and control groups (5.0 vs. 3.6 vs. 1.8; P<0.001 and 15.0 vs. 9.0 vs. 6.0; P<0.001, respectively). The mean WBC, neutrophil, and platelet counts in the active endoscopic disease group were higher compared with those in the other groups, and these were higher in the patients in remission compared with the controls (P<0.001). The mean NLR and PLR values in the active endoscopic disease group were higher compared with those in the remission and control groups, and these were higher in the endoscopic remission group compared with the control group (2.9 ± 0.8 vs. 2.2 ± 0.9 vs. 1.8 ± 0.6; P<0.001 and 153.7 ± 72.1 vs. 122.1 ± 38.3 vs. 104.1 ± 30.4; P<0.001, respectively). The mean fibrinogen level was the highest in the active group, lower in the remission group, and lowest in the control group (3.3 ± 0.8 vs. 3.0 ± 0.5 vs. 2.5 ± 0.3; P<0.001, respectively). The median EAI in the active group was higher compared with that in the remission group (8 vs. 2; P<0.001). The rate of patients with proctitis was lower (21.2% vs. 39.4%), the rate of patients with left-sided ulcerative colitis was higher (69.2% vs. 52.9%), and the rate of patients with pancolitis was higher (9.6% vs. 7.7%) in the active group compared with the remission group (P=0.016).
The median CRP and ESR levels in the active group were the highest, followed by the remission and control groups, in rank order (5.0 vs. 3.6 vs. 1.8; P<0.001 and 15.0 vs. 9.0 vs. 6.0; P<0.001, respectively). The mean leukocyte, neutrophil, and platelet counts in the active group were significantly higher than those in the other groups, and significantly higher compared to that of the control group (P<0.001). The NLR and PLR values were also significantly higher in the active group than in the other groups.
The parameters that were found to be correlated with EAI in patients with ulcerative colitis on correlation analysis are presented in Table II. In the patient group, EAI showed a negative correlation with hemoglobin level (r=−0.318; P<0.001), and positive correlations with CRP (r=0.289; P=0.006), ESR (r=0.268; P<0.001), WBC count (r=0.316, P<0.001), neutrophil count (r=0.281; P=0.009), platelet count (r=0.324; P=0.001), NLR (r=0.321; P=0.001), PLR(r=0.340, P<0.001), and fibrinogen level (r=0.266; P=0.016).
Table II.
The findings correlated with EAI in the patient group on correlation analysis.
| Variables | EAI | |
|---|---|---|
| r | p | |
| Age | -0.093 | 0.183 |
| Hemoglobin | -0.318 | <0.001* |
| CRP | 0.289 | 0.006* |
| ESR | 0.268 | <0.001* |
| WBC | 0.316 | <0.001* |
| Neutrophil | 0.281 | 0.009* |
| Platelet | 0.324 | 0.001* |
| Lymphocyte | -0.082 | 0.237 |
| NLR | 0.321 | 0.001* |
| PLR | 0.340 | <0.001* |
| Fibrinogen | 0.266 | 0.016* |
Abbreviations: EAI: Endoscopic activity index, CRP: C-reactive protein, ESR: Erythrocyte sedimentation rate, WBC: White blood cell, NLR: Neutrophil to lymphocyte ratio, PLR: Platelet to lymphocyte ratio.
The optimal cutoff point for remission for PLR was 138.6 with sensitivity and specificity of 77.9% and 74%, respectively (AUC ± SE: 0.748 ± 0.038; P<0.001). The optimal cutoff point for NLR was found to be 2.42 with a sensitivity and specificity of 76.0% and 70.2%, respectively (AUC ± SE: 0.718 ± 0.039; P=0.003). Considering the cutoff points of PLR and NLR, the patients with both PLR and NLR values higher than the cutoff values were coded as »high risk« those with one parameter higher than the cutoff value were coded as »moderate risk,« and those with both parameters lower than the cutoff values were coded as »low-risk« patients.
The distributions of demographic characteristics, and clinical and laboratory findings in the risk groups based on the NLR and PLR values are presented in Table III. The risk groups were similar for the mean age and sex. Hemoglobin levels were comparable between the moderate-and high-risk groups, but were lower than those in the low-risk patients. The median CRP and ESR levels were higher in the high-risk patients than in the other groups; CRP levels were similar in the moderate- and low-risk patients, whereas ESR levels were higher in the moderate-risk patients than in the low-risk patients.
Table III.
The distribution of the demographic characteristics, and clinical and laboratory findings in relation with the risk groups.
| Variables | Low-risk (n=110) | Moderate-risk (n=49) | High-risk (n=49) | p |
|---|---|---|---|---|
| Age ( years) | 49.4±13.9 | 49.1±13.5 | 44.7±13.8 | 0.122 |
| Gender, n (%) | ||||
| Male | 50(45.5) | 24(49.0) | 22(44.9) | 0.529 |
| Hemoglobin (g/L) | 14.4±1.7 | 13.3±1.7 | 13.1±2.1 | <0.001* |
| CRP (mg/L) | 3.3(4.5) | 3.7(6.5) | 6.2(8.9) | <0.001* |
| ESR (mm/hr) | 8.5(10.0) | 15.0(12.4) | 19.0(20.0) | <0.001* |
| WBC (× 109/L) | 8323.6±2107.1 | 8320.4±2420.7 | 9600.0±3034.8 | 0.006* |
| Neutrophil (× 109/L) | 4744.5±1627.8 | 5259.2±2131.9 | 6765.3±2668.9 | <0.001* |
| Platelet (× 109/L) | 271290.9±53536.9 | 297020.4±73344.1 | 328346.9±09399.4 | <0.001* |
| Lymphocyte (× 109/L) | 2719.1±666.1 | 2108.2±536.5 | 1632.7±510.5 | <0.001* |
| NLR | 1.7±0.4 | 2.5±0.6 | 4.4±2.0 | <0.001* |
| PLR | 102.7±19.9 | 144.1±29.5 | 210.8±72.8 | <0.001* |
| Fibrinogen (g/L) | 2.9±.5 | 3.2±0.8 | 3.5±0.8 | <0.001* |
| EAI | 3.0(4.0) | 6.0(6.0) | 8.0(5.0) | <0.001* |
| Disease activity | ||||
| Active | 40(36.4) | 29(59.2) | 35(71.4) | <0.001* |
| Remission | 70(63.6) | 20(40.8) | 14(28.6) | |
| UC Type | ||||
| Proctitis | 35(31.8) | 16(32.7) | 12(24.5) | 0.156 |
| Left-sided colitis | 66(60.0) | 29(59.2) | 32(65.3) | |
| Pan-ulcerative colitis | 9(8.2) | 4(8.2) | 0.8915(10.2) | |
* p<0.05 indicates statistical significance.Abbreviations: CRP: C-reactive protein, ESR: Erythrocyte sedimentation rate, WBC: White blood cell, NLR: Neutrophil to lymphocyte ratio, PLR: platelet to lymphocyte ratio, EAI: Endoscopic activity index, UC: ulcerative colitis.
The mean WBC counts were similar in the low-and moderate-risk patients, and lower than the high-risk group. The mean neutrophil and platelet counts were higher in the high-risk group compared with the other groups, and they were higher in the moderate-risk group when compared to the low-risk group. The mean lymphocyte counts were found to be the lowest in the low-risk patients, followed by moderate- and
high-risk patients in rank order. The mean fibrinogen level was higher in the high-risk group compared with the other risk groups, and it was higher in the moderate-risk group compared with the low-risk group. The risk groups created in accordance with PLR and NLR combination showed higher remission rates as the severity decreased; however, the disease activity rate increased as the risk increased. The sites of involvement did not show any difference in accordance with the risk groups.
The risk factors inhibiting remission were analyzed with stepwise Cox regression analysis. The high-risk group with 49 patients and a median follow-up of 20±3 days, moderate-risk group with 49 patients and a median follow-up of 28±6 days, and low-risk group with 110 patients and a median follow-up of 36±5 days were investigated in this analysis. Moderate and high risk, high ESR level, and high EAI were found as independent predictors of endoscopic active disease. Compared with patients in low risk group, being in moderate risk group increases the risk of endoscopic active disease by 2.386 fold and being in high risk group increases the risk of endoscopic active disease by 2.941 fold in the high-risk patients, respectively. A one-unit increase in the ESR level increased the risk for endoscopic active disease by 1.055-fold, and a one-point increase in EAI increased the risk for endoscopic active disease 1.808-fold (Table IV). The Kaplan–Meier graph showed that high-risk patients had endoscopic active disease in a shorter time (Figure 1).
Table IV.
Independent predictors of endoscopic active disease.
| Variables | HR | 95% C.I | P | |
|---|---|---|---|---|
| lower | upper | |||
| Combination Risk | ||||
| Low risk | ref | ref | ref | |
| Moderate risk | 2.387 | 1.426 | 4.000 | 0.001* |
| High risk | 2.941 | 1.623 | 5.319 | <0.001* |
| ESR | 1,055 | 1.032 | 1.081 | 0.001* |
| EAI | 1.808 | 1,595 | 2.053 | <0.001* |
| -2 Log Likelihood= 830.838; 2 = 166.791; p<0.001* | ||||
| Age, gender, hemoglobin, CRP, ESR, WBC, neutrophil, platelet, lymphocyte, fibrinogen, EAI, PLR, NLR and Combination PLR+NLR were analyzed with stepwise regression model | ||||
Abbreviations: HR: Hazard Ratio, 95% CI: 95% Confidence Intervals, CRP: C-reactive protein, ESR: Erythrocyte sedimentation rate, WBC: White blood cell, NLR: Neutrophil to lymphocyte ratio, PLR: Platelet to lymphocyte ratio, EAI: Endoscopic activity index.
Figure 1.
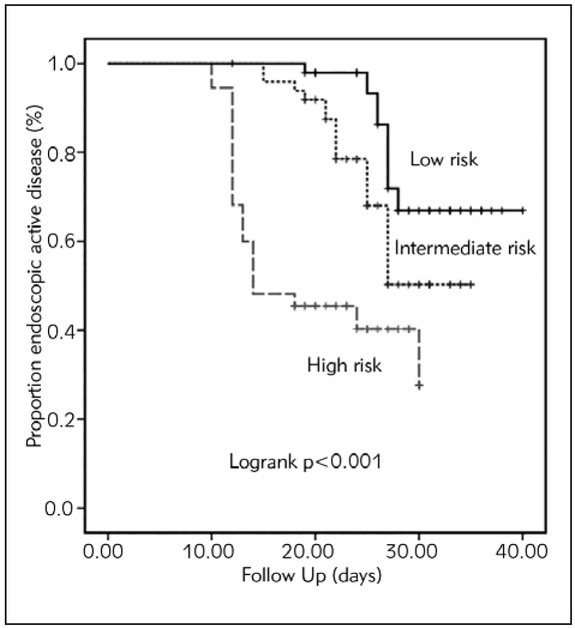
Status of endoscopic active disease in relation to the risk levels.
Discussion
We found that NLR increased in cases of endoscopically active disease, and increased in correlation with the mucosal injury. Due to limited data concerning the diagnostic accuracy of NLR for UC. NLR has been documented to increase in clinically active colitis, and this increase was correlated with an increase in fecal calprotectin (9). Beyond its chronic nature with lypmhoplasmocytic infiltration, flares or activations of ulcerative colitis is associated with neutrophil mediated epithelial injury. Crypt abscesses and cryptitis are characterized by neutrophil infiltration into the mucosa and/or crypts. We consider that neutrophil dominant infiltration of the bowel in active disease is a reflection of increased peripheral neutrophil count. Recently, in two independent studies, the sensitivity of NLR was 61.2% and 81.8%, respectively, to predict clinically active UC disease (10, 11). We detected an accurate sensitivity (76.0%, AUC ± SE: 0.718 ± 0.039; P=0.003) of NLR to predict endoscopically active disease. Demir et al. showed that although NLR was higher in clinically active disease, CRP was the only independent predictor for clinically active UC disease using multivariate logistic regression analysis (12). In our study, we have shown that higher levels NLR and PLR combination were the independent predictors of endoscopically active UC disease. In addition, ESR and not CRP was an independent predictor of endoscopically active disease in our study. NLR did not differ between patients with extensive and non-extensive disease in the study of Çelikbilek et al (13). Similarly, we determined that NLR was not able to predict the extension of the disease.
We demonstrated, for the first time, that PLR increased significantly in endoscopically active UC. Similar to NLR, PLR is a new parameter of systemic inflammation. Platelet count can be affected by cytokines released in acute inflammation and thrombocytosis is a common feature of acute inflammation. So we consider that increased PLR ratio may be a secondary effect of increased NLR ratio. This parameter was widely investigated in some cancer types to predict prognosis. Moreover, PLR had been studied in different inflammatory conditions (14). However, this is the first study evaluating PLR in ulcerative colitis. We demonstrated that EAI showed a significant positive correlation with NLR, PLR, and other inflammatory markers. Although correlations between endoscopic activity and these indices were mild, Cox regression analysis showed that higher NLR and PLR values were independent predictors of endoscopically active disease. We also investigated the use of NLR and PLR combination to assess disease severity in UC for the first time. Based on our study, NLR and PLR combination can predict mucosal disease more accurately than either NLR or PLR alone.
Colonoscopy is the basic diagnostic and therapeutic modality in ulcerative colitis (4, 15). However, colonoscopy may not be always feasible in these patients. Oversensitivity to air insufflation in patients with active ulcerative colitis during the procedure, or unavailability of colonoscopy necessitates the use of different parameters to evaluate the patients. In our study, we showed that NLR and PLR were effective markers to show active mucosal disease in ulcerative colitis. More importantly, the use of the combination of these methods can also predict mucosal injury. Therefore, NLR and PLR can predict endoscopically active disease before colonoscopy is performed, and can serve as an indirect method to evaluate endoscopic disease activity when colonoscopy cannot be performed.
The main goal of treatment is mucosal healing in patients with ulcerative colitis, similar to Crohn's disease (16). Although no validated, definitive diagnosis of mucosal healing in ulcerative colitis is found, absence of ulcers, bleeding, erosions, and friability define mucosal healing (17). Mucosal healing is important for efficacy of treatment and long-term prognosis of the disease (18). In ulcerative colitis, clinical improvement may not always accompany mucosal healing, and endoscopic examination may show mucosal disease in clinically stable patients. Clinical studies reported that mucosal biopsies obtained during clinical remission might be associated with an increased recurrence of the disease activity in the long term. Mucosal evaluation is important in ulcerative colitis independent of the clinical evaluation, as indicated by the aforementioned data. Except from colonoscopy, fecal immunochemical tests may be used to evaluate mucosa (19). However, they are not practical in routine practice. The usefulness of noninvasive markers for the detection of mucosal disease in UC was evaluated in several cohort studies (20, 22). Solem et al. investigated CRP and leukocytes for the detection of clinical, endoscopic, and radiographic activity in IBD, and they found that elevated CRP levels were associated with higher endoscopic disease activity in IBD (21). In a prospective cohort study, Rosenberg et al. showed that leukocytes and CRP have an ability to predict the probability of ongoing endoscopic activity in UC, and these parameters can identify patients who require treatment for active mucosal disease (22). In our study, we showed that routinely used parameters, including hemoglobin, leukocyte, ESR, fibrinogen NLR, and PLR identified endoscopically active disease. In addition, the levels of these parameters were associated with mucosal injury. To the best of our knowledge, this is the first study that shows higher levels of NLR and PLR values as independent predictors of endoscopically active disease. Furthermore, it is the first study that analyzed the predictive value of PLR for endoscopically active UC disease.
This study has some limitations. First, it is a retrospective cohort study. However, our clinic is a tertiary referral center for UC, and all patients with UC are regularly followed up, and the medical records of all these patients are available. In addition, colonoscopic examinations are performed by experienced gastroenterologists concerning IBD. Second, the follow-up periods of the patients are relatively short. Because UC is a chronic disease with variable alternate periods of mucosal disease with activity or mucosal disease with remission, longer follow-up periods needs to confirm our findings. The Rachmilewitz EAI that we used to determine severity of endoscopic activity of patients was not validated, and it was another limitation of our study. With regard to this issue, a number of different scoring systems have been used to evaluate patients with ulcerative colitis (23). To date, none of the endoscopic scoring systems has been accepted as a standard system (4). The scoring systems may include clinical parameters, endoscopic parameters, or both (24, 25). All scoring systems share the characteristic of evaluating the mucosa. The presence of ulcer, exudate, and bleeding are associated with higher scores. Rachmilewitz EAI and Mayo endoscopic score have been used in most clinical trials (26). We used Rachmilewitz EAI in our clinical practice because it allows detailed mucosal assessment with four different parameters. Although it is not a validated scoring system, Rachmilewitz EAI has been documented to highly correlate with clinical activity indices in several studies (20, 26, 27). Kucharski et al. showed that Rachmilewitz EAI had the highest correlation coefficient with most of the clinical activity indices, and one of these clinical activity indices was Truelove–Witt’s (correlation coefficient, 0.710), which has been preferred by European Crohn’s and Colitis Organization guideline for UC (27).
In conclusion, we have shown for the first time in the literature that these easy-to-calculate and less-invasive parameters are accurate in predicting endoscopically active disease in UC, and it may alert the clinician for the presence of an active disease before colonoscopy. Mucosal evaluation together with clinical evaluation is essential in cases of disease activation. NLR and PLR may identify endoscopic active disease. The use of both can also predict endoscopic disease severity. Being aware of the levels of those parameters before colonoscopy may lead the clinician to a more careful examination during colonoscopy. NLR and PLR may provide information regarding disease activation and the degree of mucosal injury, if for any reason, colonoscopy cannot be performed.
Funding: There was no funding for this study, and the data collection was part of hospital procedure.
List of abbreviations
- CRP,
C-reactive protein
- EAI,
Endoscopic activity index
- ESR,
Erythrocyte sedimentation rate
- IBD,
Inflammatory bowel disease
- NLR,
Neutrophil-to-lymphocyte ratio
- PLR,
Platelet-to-lymphocyte ratio
- ROC,
Receiver operating characteristic
- UC,
Ulcerative colitis
- WBC,
White blood cell
Footnotes
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19:5. doi: 10.1155/2005/269076. et al. –. [DOI] [PubMed] [Google Scholar]
- 2.Singh UP, Singh NP, Murphy EA, Price RL, Fayad R, Nagarkatti M. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44. doi: 10.1016/j.cyto.2015.10.008. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langholz E, Munkholm P, Nielsen OH, Kreiner S, Binder V. Incidence and prevalence of ulcerative colitis in Copenhagen county from 1962 to 1987. Scand J Gastroenterol 1991; 26:1247. doi: 10.3109/00365529108998621. –. [DOI] [PubMed] [Google Scholar]
- 4.Leighton JA, Shen B, Baron TH, Adler DG, Davila R, Egan JV. Standards of Practice Committee, American Society for Gastrointestinal Endoscopy: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558. doi: 10.1016/j.gie.2006.02.005. et al. –. [DOI] [PubMed] [Google Scholar]
- 5.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641. doi: 10.1016/S0140-6736(07)60751-X. –. [DOI] [PubMed] [Google Scholar]
- 6.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci 1993; 38:1137. doi: 10.1007/BF01295733. –. [DOI] [PubMed] [Google Scholar]
- 7.Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44:653. doi: 10.1097/MPG.0b013e31805563f3. et al. –. [DOI] [PubMed] [Google Scholar]
- 8.Celikbilek M, Dogan S, Ozbakır O, Zararsız G, Kücük H, Gürsoy S. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. 2013;27:72. doi: 10.1002/jcla.21564. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851. doi: 10.1002/ibd.20986. –. [DOI] [PubMed] [Google Scholar]
- 10.Posul E, Yilmaz B, Aktas G, Kurt M. Does neutrophil-to-lymphocyte ratio predict active ulcerative colitis? Wiev Klin Wochenschr. 2015;127:262. doi: 10.1007/s00508-014-0683-5. –. [DOI] [PubMed] [Google Scholar]
- 11.Torun S, Tunc BD, Suvak B, Yildiz H, Tas A, Sayilir A. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. 2012;36:491. doi: 10.1016/j.clinre.2012.06.004. et al. –. [DOI] [PubMed] [Google Scholar]
- 12.Demir AK, Demirtas A, Kaya SU, Tastan I, Butun I, Sagcan M. The relationship between the neutrophil-lymphocyte ratio and disease activity in patients with ulcerative colitis. Kaohsiung J Med Sci. 2015;31(11):585. doi: 10.1016/j.kjms.2015.10.001. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celikbilek M, Dogan S, Ozbakır O, Zararsız G, Kücük H, Gürsoy S. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. 2013;27(1):72. doi: 10.1002/jcla.21564. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura Y, Takeshita S, Kanai T, Yoshida Y, Nonoyama S. The combined usefulness of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in predicting intravenous immunoglobulin resistance with Kawasaki disease. J Pediatr. 2016;178:281. doi: 10.1016/j.jpeds.2016.07.035. –. [DOI] [PubMed] [Google Scholar]
- 15.Makkar R, Bo S. Colonoscopic perforation in inflammatory bowel disease. Gastroenterol Hepatol. 2013;9:573. –. [PMC free article] [PubMed] [Google Scholar]
- 16.Rutka M, Milassin Á, Szepes Z, Sz cs M, Nyári T, Bálint A. Is mucosal healing more common than clinical remission in ulcerative colitis? Is it the truth or only a myth coming from the studies? Scand J Gastroenterol. 2015;50:985. doi: 10.3109/00365521.2015.1018313. et al. –. [DOI] [PubMed] [Google Scholar]
- 17.Dave M, Loftus EV. Mucosal healing in inflammatory bowel disease-a true paradigm of success? Gastroenterol Hepatol. 2012;8:29. Jr. –. [PMC free article] [PubMed] [Google Scholar]
- 18.El-Kheshen G, Moeini M, Saadat M. Susceptibility to ulcerative colitis and genetic polymorphisms of A251G SOD1 and C-262T CAT. J Med Biochem. 2016;35:333. doi: 10.1515/jomb-2016-0002. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakarai A, Kato J, Hiraoka S, Nakarai A, Takei D, Inokuchi T. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol. 2013;108:83. doi: 10.1038/ajg.2012.315. et al. –. [DOI] [PubMed] [Google Scholar]
- 20.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15(12):1851. doi: 10.1002/ibd.20986. –. [DOI] [PubMed] [Google Scholar]
- 21.Solem CA, Loftus EV. Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707. doi: 10.1097/01.mib.0000173271.18319.53. Jr. –. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg L, Lawlor GO, Zenlea T, Goldsmith JD, Gifford A, Falchuk KR. Predictors of endoscopic onflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013;19(4):779. doi: 10.1097/MIB.0b013e3182802b0e. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Haens Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763. doi: 10.1053/j.gastro.2006.12.038. et al. –. [DOI] [PubMed] [Google Scholar]
- 24.Lichtiger S, Present DH. Preliminary report: cyclosporin in treatment of severe active ulcerative colitis. Lancet. 1990;336:16. doi: 10.1016/0140-6736(90)91521-b. –. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625. doi: 10.1056/NEJM198712243172603. –. [DOI] [PubMed] [Google Scholar]
- 26.Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Correlations of C-reactive protein levels and erythrocyte se dimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci. 2014;59(4):829. doi: 10.1007/s10620-013-2907-3. –. [DOI] [PubMed] [Google Scholar]
- 27.Kucharski M, Karczewski J, Mańkowska-Wierzbicka D, Karmelita-Katulska K, Grzymisławski M, Kaczmarek E. Applicability of endoscopic indices in the determination of disease activity in patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 2016;28(6):722. doi: 10.1097/MEG.0000000000000601. et al. –. [DOI] [PubMed] [Google Scholar]


