Summary
With the tamoxifen-inducible CreERT2 system, genetic recombination can be temporally controlled in a cell-type-specific manner in intact animals, permitting dissection of the molecular underpinnings of mammalian physiology. Here we present a significant drawback to CreERT2 technology for analysis of intestinal stem cells. Using the intestine-specific Villin-CreERT2 mouse strain, we observed delayed intestinal regeneration post irradiation. Villin-CreERT2 activation was associated with DNA damage and cryptic loxP site cleavage. Analysis of stem cell-specific CreERT2 strains showed that the genome toxicity impairs function of crypt base columnar stem cells, resulting in loss of organoid initiating activity. Importantly, the stem cell impairment is short-lived, with return to normal by 7 days post tamoxifen treatment. Our findings demonstrate that mouse genetic experiments that utilize CreERT2 should consider the confounding effects of enhanced stem cell sensitivity to genome toxicity resulting from CreERT2 activation.
Keywords: intestinal stem cell, organoid, CreERT2, loxP, genotoxicity, tamoxifen, mouse, crypt regeneration
Graphical Abstract
Highlights
-
•
Intestinal stem cell (ISC) toxicity induced in mice by CreERT2 activation
-
•
Impaired organoid formation after activation of ISC-specific CreERT2 strains
-
•
Genotoxicity and impaired crypt regeneration in Villin-CreERT2 mice
-
•
Impaired ISC function and genotoxicity repaired by 7 days after activation
Samuelson and colleagues demonstrate that activation of CreERT2 in the mouse intestine leads to intestinal stem cell (ISC) toxicity. Impaired stem cell function was shown by reduced organoid-forming ability in ISC-CreERT2 strains. Also, Villin-CreERT2 mice exhibited impaired crypt regeneration after ISC injury induced by γ-irradiation. DNA damage due to cryptic loxP site cleavage suggested a mechanism of ISC genotoxicity.
Introduction
The Cre-loxP system is a powerful genome-editing tool that revolutionized in vivo genetic studies. The site-specific Cre recombinase catalyzes recombination between two 34-bp loxP DNA recognition sites to induce deletion or activation of target transgenes (Bouabe and Okkenhaug, 2013). The adaptation of this system from its bacteriophage origin requires that Cre and loxP be engineered into the mouse genome. Since the mouse genome does not contain loxP sites, recombination is designed to be specific to the engineered target construct.
One advance to the Cre-loxP system was the development of inducible Cre by fusion with a mutated ligand-binding domain of the estrogen receptor (Metzger et al., 1995). The CreER recombinases (e.g., CreERT2) are activated by the estrogen receptor antagonist tamoxifen (TX), which allows temporal control of target gene rearrangement. In the absence of TX, CreER is cytoplasmic. TX binding induces CreER transfer into the nucleus to catalyze recombination between loxP sites. The recombined allele is a permanent genetic change. Thus, this system has been a powerful tool to study adult stem cell function. In particular, there are numerous CreER mouse strains used to study intestinal stem cells (ISCs), including Villin-CreERT2 (el Marjou et al., 2004), which is expressed throughout the intestinal epithelium, including stem and progenitor cells, and ISC-specific Olfm4-CreERT2 (Schuijers et al., 2014) and Lgr5-CreERT2 (Barker et al., 2007).
Off-target recombination has been observed at cryptic loxP (cloxP) sites, which have DNA sequence similarity to loxP (Thyagarajan et al., 2000). The consequences of illegitimate Cre recombination vary from cellular toxicity to overt developmental and pathological defects. Cre expression in developing spermatids led to male sterility due to genomic rearrangements (Schmidt et al., 2000), and widespread developmental defects occurred after TX activation of CreERT2 during embryonic development (Naiche and Papaioannou, 2007). CreERT2 genotoxicity in proliferating adult tissues has also been described, with TX-activated CreERT2 causing epithelial atrophy and metaplasia in stomach (Huh et al., 2010), and chromosomal rearrangements in immature hematopoietic cells (Higashi et al., 2009). These reports suggest that proliferating stem and progenitor cells may be particularly sensitive to Cre-mediated genotoxicity, although this has not been tested in most adult stem cell populations.
One of the most proliferative adult tissues is the intestine, where adult stem cells fuel rapid epithelial cell turnover. Whether off-target DNA cleavage and genotoxicity are an issue for ISC Cre drivers has not been reported. In this study we observed functional ISC defects following TX induction of CreERT2 in the mouse intestine. Whole-body γ-irradiation subsequent to Villin-CreERT2 activation resulted in delayed intestinal regeneration. ISC defects were demonstrated by impaired organoid-forming efficiency. Our findings suggest that the flood of TX-activated CreERT2 into the nucleus leads to cleavage at cloxP sites and DNA double-stranded breaks (DSBs), which impair ISC function. Thus, this study holds significant implications for experiments studying intestinal homeostasis and regeneration in mouse genetic models to mitigate CreERT2 toxicity in ISCs.
Results
Impaired Intestinal Regeneration in Villin-CreERT2 Mice
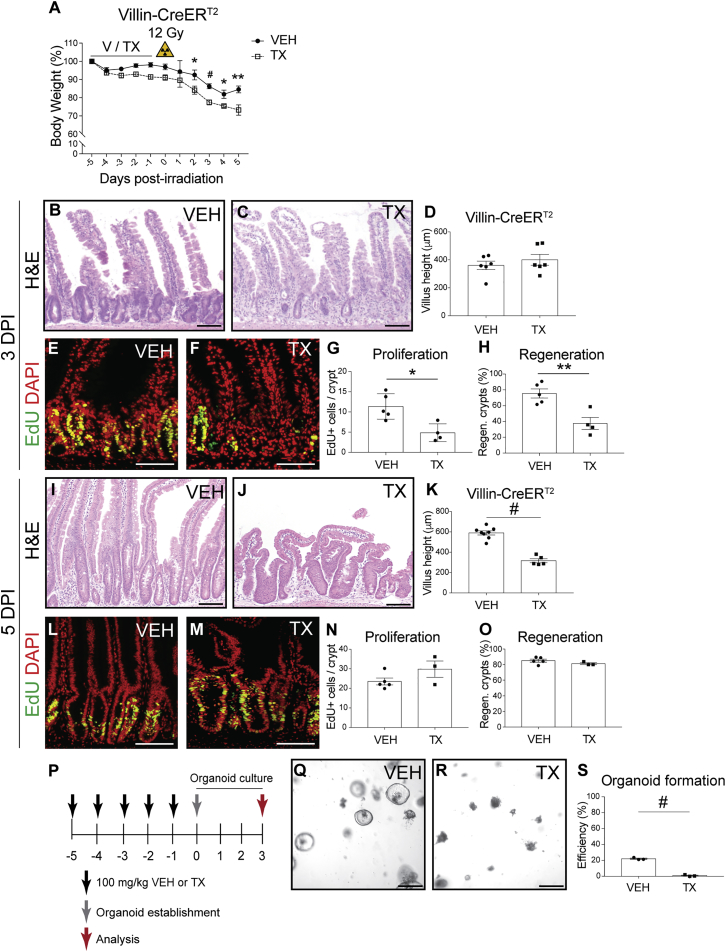
We tested the effect of Villin-CreERT2 on ISC function after treatment with TX (100 mg/kg) or vehicle (VEH). Histological analysis did not reveal any gross intestinal changes induced by TX treatment; tissue architecture and cellular proliferation did not differ from controls (Figure S1). However, marked differences were observed between TX- and VEH-treated Villin-CreERT2 mice after challenge with 12 Gy irradiation (Figure 1). TX-treated mice had a more pronounced post-irradiation weight loss compared with controls (Figure 1A), and histological analysis showed more extensive intestinal damage (Figures 1B–1O). Three days post irradiation (DPI), the intestines of VEH-treated mice began to recover with a typical regenerative response, characterized by expanded crypts and increased proliferation (Figures 1B and 1E). In contrast, TX-treated mice had extensive decellularized crypts and very few, small crypt structures (Figure 1C). We also observed decreased proliferation and fewer regenerating crypts in the TX group (Figures 1G and 1H). At 5 DPI, the villi of TX-treated Villin-CreERT2 mice were blunted, consistent with impaired regeneration at 3 DPI (Figures 1I–1K). However, crypts at this time point were undergoing robust regeneration, similar to control (Figures 1L–1O). Thus, TX activation of Villin-CreERT2 results in delayed intestinal regeneration, consistent with enhanced damage following 12 Gy irradiation.
Figure 1.
Impaired Intestinal Regeneration and Organoid Formation in TX-Treated Villin-CreERT2 Mice
(A–O) Villin-CreERT2 mice were treated with TX (100 mg/kg) or vehicle (V; also VEH) daily for 5 days, and 1 day later either (A–O) challenged with 12 Gy γ-irradiation or (P–S) tested for organoid-forming efficiency. (A) Mouse body weight relative to weight at the initiation of treatment (n = 7–15 mice/group). (B–O) Duodenal crypt regeneration was assessed at (B–H) 3 days post irradiation (DPI) and (I–O) 5 DPI by (B, C, I, and J) H&E staining, and (E, F, L, and M) EdU incorporation. (D and K) Villus height (n = 5–8 mice/group), (G and N) cellular proliferation, and (H and O) crypt regeneration were measured (n = 4–5 mice/group).
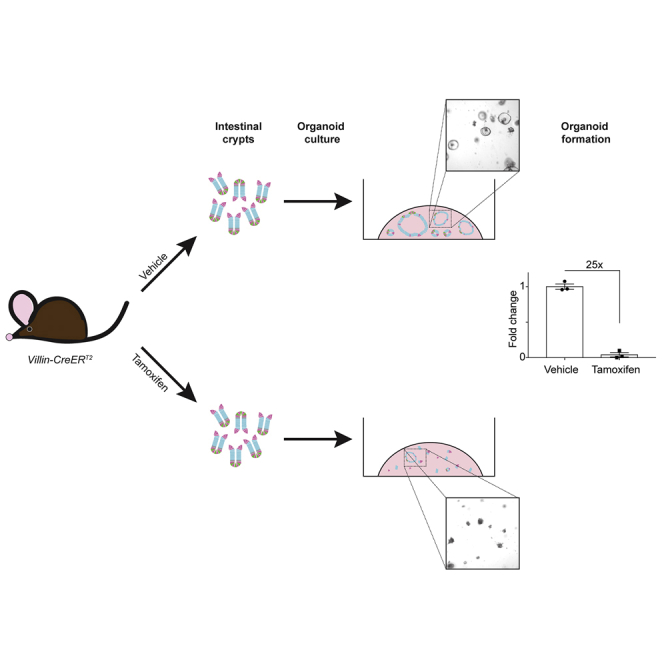
(P) Schematic of organoid formation assay to test stem cell activity in non-irradiated Villin-CreERT2 mice. Duodenal crypts were isolated from TX- or VEH-treated mice and plated in Matrigel to form organoids.
(Q and R) Bright-field images of organoids 3 days post establishment from crypts isolated from (Q) VEH-treated or (R) TX-treated Villin-CreERT2 mice.
(S) Organoid-forming efficiency was determined by counting organoid number and presented as percent of the number plated (n = 3 mice/group with three technical replicates per mouse).
Quantitative data are presented as means ± SEM (∗p < 0.05, ∗∗p < 0.01, #p < 0.0001 TX versus VEH by Student's t test). Scale bars, 100 μm (duodenum) and 250 μm (organoids). See also Figures S1 and S2.
Impaired Organoid Formation after Villin-CreERT2 Activation
To understand the basis for the altered response of Villin-CreERT2 mice to irradiation, we tested if CreERT2 activation affects ISC function by measuring organoid-forming efficiency in unirradiated, treated mice. Duodenal crypts were isolated from TX- or VEH-treated mice 1 day post treatment, and cultured under conditions that support ISC growth (Figure 1P). While crypts isolated from VEH-treated mice grew into typical spheroids by 3 days in culture, crypts isolated from TX-treated Villin-CreERT2 mice exhibited very poor organoid growth (Figures 1Q and 1R). Quantification showed that 25-fold fewer organoids grew in cultures initiated from TX-treated mice than VEH-treated mice (Figure 1S). The extreme loss of organoid-forming activity in TX-activated Villin-CreERT2 mice suggests impaired ISC function.
Impaired ISC Function Is Not due to TX Toxicity
We tested whether the delayed regenerative response to irradiation and the impaired organoid-forming efficiency were due to TX toxicity, which has been observed in other studies (Huh et al., 2012, Zhu et al., 2013). Irradiated, nontransgenic C57BL/6 mice treated with TX or VEH had similar changes to body weight and intestinal histology, including villus height, proliferation rate, and crypt regeneration (Figures S2A–S2H). Further, TUNEL staining and organoid-forming efficiency did not differ between the two groups (Figures S2I–S2K). These data showed that toxicity caused by TX treatment of Villin-CreERT2 mice was not a direct effect of TX.
Next, we determined whether the TX effect on Cre recombinase was independent of CreER-mediated nuclear translocation. We treated Villin-Cre mice, which exhibit constitutive Cre expression in intestinal epithelial cells (Madison et al., 2002), with TX or VEH, followed by 12 Gy irradiation. In contrast to the response in Villin-CreERT2 mice, we saw no heightened sensitivity to irradiation in TX-treated Villin-Cre mice (Figures S2L–S2S). Further, there was no change in TUNEL staining or organoid-forming efficiency (Figures S2T–S2V). Notably, these transgenic strains express similar amounts of Cre protein, so the toxicity is not due to higher levels of Cre recombinase expression in Villin-CreERT2 mice (Figure S2W). Together these results support the conclusion that TX activation of Villin-CreERT2 mediates impaired intestinal regeneration and organoid formation, and not TX toxicity, or interactions between TX and constitutively active Cre recombinase.
Impaired Organoid Formation after CreERT2 Activation in ISCs
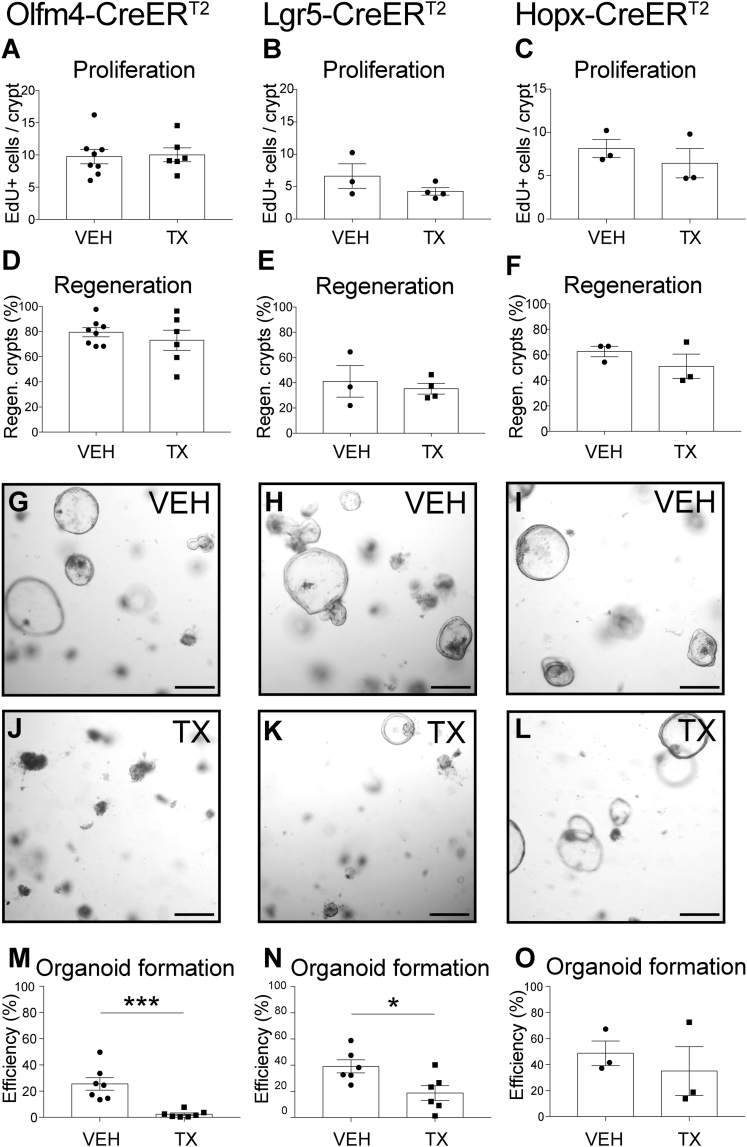
Heightened sensitivity to radiation and impaired organoid-forming capacity of Villin-CreERT2 mice after TX treatment suggested that CreER activation induced stem cell damage. We tested ISC-specific CreERT2 mouse strains that target crypt base columnar (CBC) ISCs, including Olfm4-CreERT2 and Lgr5-CreERT2. Similar to our findings with Villin-CreERT2 mice, TX-treated Olfm4-CreERT2 mice had normal intestinal histology and proliferation under basal conditions (Figures S1F–S1J). In contrast to the delayed regenerative response in TX-treated Villin-CreERT2 mice, we observed normal responses to irradiation in TX-treated Olfm4-CreERT2 and Lgr5-CreERT2 mice, with cellular proliferation and crypt regeneration at 3 DPI similar to VEH-treated controls (Figures 2A, 2B, 2D, 2E, and S3A–S3F). However, organoid-forming activity was reduced in both strains after TX treatment, similar to Villin-CreERT2 (Figures 2G, 2H, 2J, and 2K). TX treatment resulted in 10-fold fewer organoids in Olfm4-CreERT2 and 2-fold fewer organoids in Lgr5-CreERT2 (Figures 2M and 2N). The results suggest that actively cycling, CBC ISCs are sensitive to CreERT2 activation, leading to impaired ISC function.
Figure 2.
Reduced Organoid-Forming Efficiency after CreERT2 Activation in Intestinal Stem Cells
Mouse strains with TX-inducible CreERT2 drivers specific for CBC (Olfm4-CreERT2 and Lgr5-CreERT2) or facultative (Hopx-CreERT2) ISCs were tested for (A–C) proliferation and (D–F) crypt regeneration after irradiation, or for (G–O) organoid-forming efficiency in non-irradiated mice. (A–F) Mice were treated with TX or VEH daily for 5 days, irradiated a day later, and tissue was collected 3 DPI. (A–C) Cellular proliferation and (D–F) crypt regeneration were quantified (n = 3–8 mice/group). (G–O) Organoids were established from duodenal crypts 1 day after TX- or VEH-treatment, imaged and counted at 3 days post establishment (n = 3–7 mice/group with three technical replicates per mouse).
Quantitative data are presented as means ± SEM (∗p < 0.05, ∗∗∗p < 0.001 TX versus VEH by Student's t test). See also Figures S1 and S3.
We also tested one CreER strain that targets a facultative stem cell (FSC) population, HopX-CreERT2 (Takeda et al., 2011). The expression of this Cre driver is limited to very few cells in the crypt, which can participate in crypt regeneration after γ-irradiation (Li et al., 2016). In contrast to toxicity observed after TX activation of CreERT2 in CBCs, the response to radiation, and organoid-forming ability were unchanged in HopX-CreERT2 mice (Figures 2C, 2F, 2I, 2L, 2O, and S3G–S3I).
CreERT2 Activates DNA Cleavage at cloxP Sites
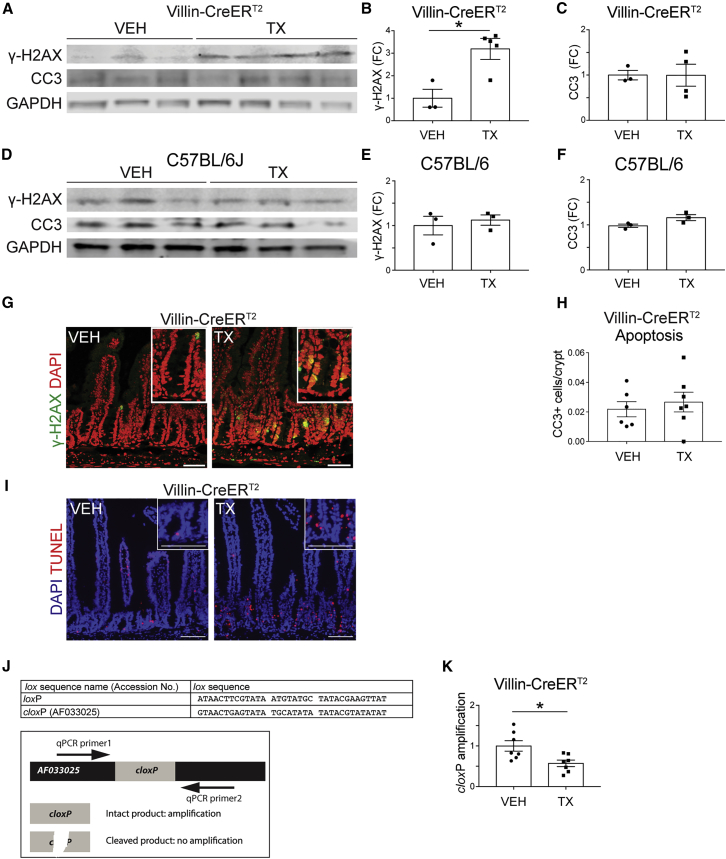
We next considered the mechanism by which CreERT2 activation leads to impaired ISC function. We posited that TX-mediated CreERT2 nuclear translocation induces DNA cleavage. To test this, we performed western blotting for γ-H2AX, which marks DNA DSBs (Kuo and Yang, 2008) and observed a 3-fold increase in the crypts of TX-treated Villin-CreERT2 mice compared with controls (Figures 3A and 3B). Analysis of C57BL/6 mice showed no differences in γ-H2AX levels between TX- and VEH-treated mice, again demonstrating that the effect is due to activation of CreERT2 and not to TX toxicity (Figures 3D and 3E). We also saw increased γ-H2AX staining in cells at the crypt base (Figure 3G). In agreement, TUNEL staining mirrored the γ-H2AX results, demonstrating increased DNA damage (Figure 3I).
Figure 3.
Villin-CreERT2 Activation Induces DNA Cleavage at cloxP Sites
Villin-CreERT2 or C57BL/6 mice were treated with VEH or TX daily for 5 days and intestinal crypts were collected 1 day following the last injection.
(A and D) Western blots probing for γ-H2AX, cleaved caspase-3 (CC3), and loading control GAPDH were generated from duodenal crypt lysates prepared from (A) Villin-CreERT2 or (D) C57BL/6 mice treated with VEH or TX.
(B, C, E, and F) γ-H2AX (B and E) and CC3 (C and F) band signals were quantified and are displayed as means ± SEM (n = 3–4 mice/group; ∗p < 0.05 by Student's t test).
(G and I) Immunofluorescent images of (G) γ-H2AX- and (I) TUNEL-stained VEH- or TX-treated Villin-CreERT2 duodenum at 1 day post treatment.
(H) Quantified CC3-positive cells per crypt from Villin-CreERT2 mice 1 day post treatment.
(J) Known loxP sequence compared with the reported cloxPAF033025 site (GenBank). Schematic of the qPCR assay designed to measure the amount of intact cloxP genomic DNA.
(K) qPCR results from cloxP assay normalized to Gapdh (n = 3–6 mice/group; ∗p < 0.05, by Student's t test).
Scale bars, 50 μm. See also Figure S4.
To determine whether TX-treated Villin-CreERT2 mice exhibited DNA damage-induced programmed cell death, we immunoblotted for the apoptotic marker cleaved caspase-3 and found levels to be unchanged in both TX-treated Villin-CreERT2 and C57BL/6 mice (Figures 3A, 3C, 3D, and 3F). We also confirmed these results by quantifying the number of cleaved caspase-3-positive cells per crypt in tissue sections, showing that induction of DSBs did not induce apoptosis (Figure 3H). The findings suggest that Villin-CreERT2 activation results in increased DNA cleavage without inducing apoptosis. This agrees with our results showing no obvious histological changes to the duodenum following TX activation under basal conditions (Figures S1A–S1E).
We tested whether activated CreERT2 might induce DNA damage by inappropriately targeting regions in the mouse genome with sequence similarity to loxP. We designed real-time qPCR primers around the locus of a cloxP site (accession number AF033025) previously reported to serve as an active site for Cre recombinase (Thyagarajan et al., 2000). We assessed the integrity of this genomic region following CreERT2 activation by comparing amplification from crypt cell DNA isolated from TX- and VEH-treated Villin-CreERT2 mice (Figure 3J). Real-time qPCR analysis revealed that TX-treated duodenal Villin-CreERT2 crypt DNA had reduced amplification of this genomic region compared with VEH-treated controls, indicating reduced concentration of this cloxP site in the genome (Figure 3K). These results demonstrate that TX-mediated translocation of CreERT2 to the nucleus is associated with illegitimate DNA cleavage at a cloxP site.
Resolution of CreERT2-Induced ISC Genotoxicity
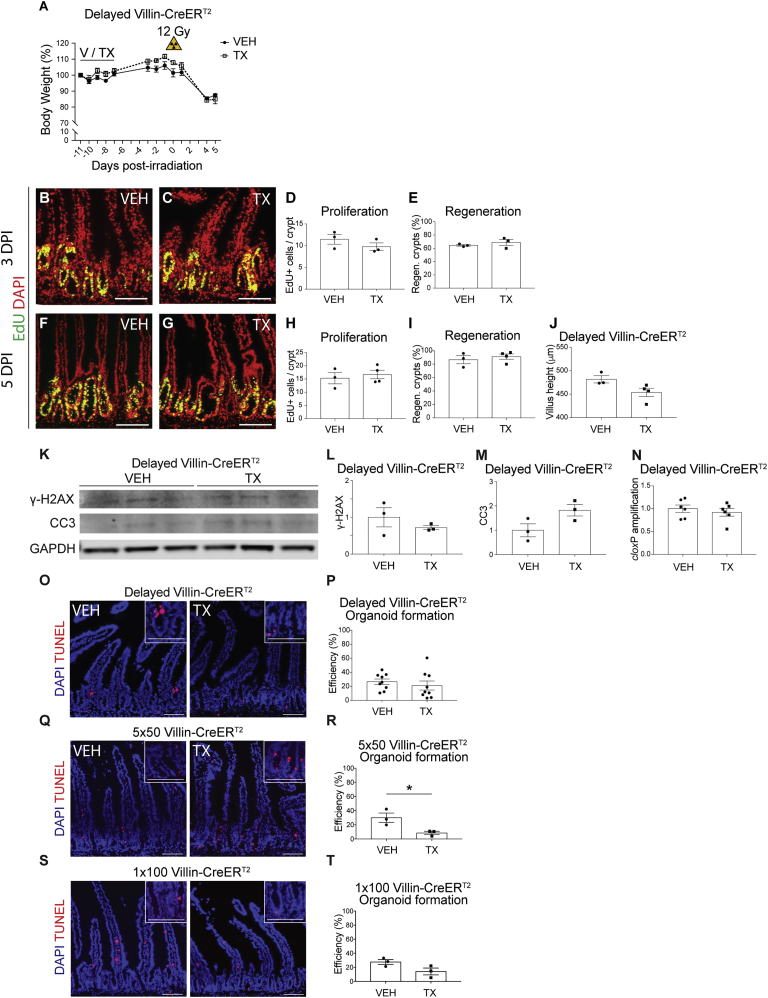
Understanding the value of the inducible Villin-CreERT2 mouse strain for genetic analysis of mammalian ISC function, we investigated three methods to minimize ISC toxicity. The first, termed “delayed,” involved postponing intestinal challenge for 1 week after the final TX injection (Figures 4A–4J). Analysis of body weight after irradiation showed similar profiles in TX- and VEH-treated mice (Figure 4A). Analysis of intestinal regeneration at 3 and 5 DPI revealed no changes to intestinal histology, including cellular proliferation, crypt regeneration, and villus height (Figures 4B–4J).
Figure 4.
Villin-CreERT2 Toxicity Is Mitigated by Delay and Reduced TX Dose
(A) Body weight data from Villin-CreERT2 mice treated with VEH or TX daily for 5 days, followed by γ-irradiation after a 7-day delay (n = 3–4 mice/group).
(B–J) EdU-stained duodenal tissue sections (B, C, F, and G) at 3DPI (B and C) and 5 DPI (F and G). (D and H) Proliferation, (E and I) regenerating crypts, and (J) villus height were quantified.
(K) Western blot analysis probing for γ-H2AX, CC3, and GAPDH, using duodenal crypt lysates from Villin-CreERT2 mice 7 days post treatment.
(L and M) γ-H2AX (L) and CC3 (M) band signals were quantified and are displayed as means ± SEM (n = 3 mice/group).
(N–T) qPCR gene amplification of cloxP normalized to Gapdh (N) (n = 6 mice/group). TUNEL staining of duodenum of non-irradiated (O) “delayed” VEH- or TX-treated Villin-CreERT2 mice, (Q) Villin-CreERT2 mice administered five daily doses of 50 mg/kg TX and analyzed 1 day later (5 × 50), and (S) Villin-CreERT2 mice administered a single dose of 100 mg/kg TX and analyzed 1 day later (1 × 100). Organoid-forming efficiency was also determined for (P) delayed, (R) 5 × 50, and (T) 1 × 100 VEH- and TX-treated Villin-CreERT2 mice (n = 3–9 mice/group with three technical replicates per mouse.
∗p < 0.05 by Student's t test). Scale bars, 100 μm. See also Figure S4.
Further evidence in support of a delay resolving the Villin-CreERT2 genotoxicity was shown by normal levels of γ-H2AX and cleaved caspase-3 in the duodenal crypts of Villin-CreERT2 mice isolated 7 days following the final TX or VEH injection (Figures 4K–4M). Similarly, crypt DNA isolated from TX-treated Villin-CreERT2 mice 7 days following the final injection had normal cloxP amplification (Figure 4N). Further, TUNEL labeling was similar between VEH- and TX-treated Villin-CreERT2 animals with delay (Figure 4O). Finally, duodenal crypts isolated 7 days following treatment showed normal organoid-forming efficiency (Figure 4P). Similar findings were observed for the CBC-specific Olfm4-CreERT2 mouse (Figures S4E–S4H; compare Figure S4D with Figure 2M).
We investigated two additional methods of administering TX: daily administration of a lower TX dose (50 mg/kg) over 5 days (5 × 50; Figures 4Q and 4R), and administration of a single 100-mg/kg dose of TX (1 × 100; Figures 4S and 4T), with tissue harvest 1 day later. The results revealed a modest increase in DSBs, as observed by TUNEL staining, in the 5 × 50 experimental paradigm (Figure 4Q) together with a significant decrease in organoid-forming efficiency (Figure 4R). In contrast, we did not observe TX-mediated CreERT2 toxicity in the 1 × 100 experiment (Figures 4S and 4T). Thus, we have shown that genotoxicity is dose and time dependent, and identified two methods that minimize damage by reducing the TX dose (1 × 100) or building in a delay after TX treatment.
Discussion
Our study shows that intestine-specific CreERT2 drivers promote illegitimate DNA cleavage events at cloxP sites and markedly diminish CBC ISC function. TX activation of the widely used Villin-CreERT2 resulted in delayed crypt regeneration after epithelial cell damage induced by γ-irradiation. The intestine normally has a remarkable regenerative capacity, with ISC replacement and crypt repair completed within a week after almost complete elimination of the proliferating crypt compartment with 12 Gy whole-body γ-irradiation (Kim et al., 2017). TX-treated Villin-CreERT2 mice exhibited enhanced weight loss and a delay in crypt regeneration after irradiation, in comparison with VEH-treated Villin-CreERT2 controls. The regenerative defect suggested a mechanism of ISC toxicity, which was confirmed by loss of organoid-forming activity in TX-treated CreERT2 mouse strains. Impaired organoid formation was observed in Villin-CreERT2 mice, a strain with broad CreERT2 expression in all intestinal epithelial cells, as well as two strains with expression limited to CBC ISCs, Olfm4-CreERT2 and Lgr5-CreERT2. These CreERT2 driver strains have been extensively used for studies of ISC function, including analysis of mechanisms regulating crypt regeneration after irradiation injury, and ISC activity by measurement of organoid-forming potential.
While we observed changes to both crypt regeneration and organoid-forming efficiency in TX-treated Villin-CreERT2 mice, we were surprised that TX-treated Olfm4-CreERT2 and Lgr5-CreERT2 mice had impaired organoid-forming efficiency but normal regenerative responses. Administration of γ-irradiation doses above 10 Gy has been shown to induce loss of CBC ISCs through apoptosis (Potten, 2004, Wang et al., 2015). Normal regeneration in Olfm4-CreERT2 and Lgr5-CreERT2 mice suggests that the effect observed in Villin-CreERT2 animals may not be solely caused by CreERT2 activation in CBCs. Rather, the delayed regenerative response could be a result of CreERT2-induced damage to FSCs, which are mobilized to repair the crypts following CBC loss (Roche et al., 2015, Takeda et al., 2011, Tian et al., 2011, Yan et al., 2012). FSCs are also thought to contribute to organoid formation. This led to our analysis of the HopX-CreERT2 mouse strain, which activates CreERT2 in a small subset of FSCs (Takeda et al., 2011). This strain showed no effect on intestinal regeneration or organoid formation following TX administration. This may reflect the small number of crypt cells targeted by HopX-CreERT2. A rigorous interrogation of CreERT2 mouse strains with different coverage of FSCs may be warranted (e.g., Bmi1-CreERT2 and Sox9-CreERT2). An additional possibility for our HopX-CreERT2 results may be the different sensitivities of CreERT2 activation in FSCs versus CBC stem cell populations. The susceptibility of various crypt cell populations to CreERT2-induced genotoxicity warrants further study.
The Villin-CreERT2 and Lgr5-CreERT2 mouse strains are commonly used, with hundreds of published studies employing these Cre drivers to manipulate genes for analysis of intestinal development, physiology, and pathophysiology. In particular, these strains have been important to study ISC function. The genotoxicity and ISC defects uncovered in our study are a serious consideration for studies that employ these, or other Cre drivers, expressed in the intestinal crypt.
Mouse studies using Cre recombinase have become a mainstay for analysis of gene function in vivo. It is commonly assumed that Cre activation per se does not induce adverse events. However, Cre-mediated cellular toxicity resulting from illegitimate DNA cleavage at cloxP sites has been previously observed in cultured cells and mouse tissues (Loonstra et al., 2001, Schmidt et al., 2000, Silver and Livingston, 2001, Thyagarajan et al., 2000). Cre-mediated genotoxicity appears to be dosage dependent, and proliferating cells seemingly exhibit enhanced sensitivity (Higashi et al., 2009, Loonstra et al., 2001, Naiche and Papaioannou, 2007, Schmidt et al., 2000), which would predict that proliferating stem and progenitor cells would be particularly sensitive to Cre-mediated toxicity. However, few studies have examined adult stem cell toxicity after CreERT2 activation in vivo. Our finding of CreERT2-induced ISC toxicity would prompt stem cell biologists studying other adult stem cell populations to be cautious when activating CreERT2 alleles. Careful experimental design must include the proper controls to rule out Cre-mediated genotoxicity as a potential cause of stem cell phenotypes induced in studies using CreER mouse strains.
Experimental Procedures
A detailed description of all methods is included in Supplemental Information.
Animal Treatment
Mouse use was approved by the Institutional Animal Care & Use Committee at the University of Michigan. To activate CreERT2-mediated recombination, mice were injected intraperitoneally with TX (50 or 100 mg/kg; 10 mg/mL in 5% ethanol and 95% corn oil; Sigma) or VEH (5% ethanol, 95% corn oil) once per day for 1 or 5 days, and tissue was collected as indicated. To induce intestinal injury, mice were exposed to one dose of 12 Gy whole-body irradiation from a 137Cs source. Animals were injected intraperitoneally with 5-ethynyl-2′-deoxyuridine (EdU) (25 mg/kg; Life Technologies) 2 hr prior to tissue collection.
Immunohistochemistry
Duodenal paraffin sections (5 μm) were stained with H&E to analyze intestinal morphology. Villus height was determined by measuring from the tip of intact villi to the top shoulder of adjacent crypts using ImageJ software (1.52a; Wayne Rasband, NIH). The number of EdU-positive cells was counted from well-oriented crypts, identified from images obtained from adjacent H&E-stained sections. Regeneration was assessed using the adapted crypt microcolony survival assay method (Cohn et al., 1997). Regenerating crypts were measured as the number of well-oriented crypts with four or more EdU-positive cells divided by the total number of well-oriented crypts.
Gene Integrity Analysis
For quantification of cloxP amplification, DNA from duodenal crypts was extracted using the Easy-DNA kit (Invitrogen, K1800-01). qPCR assays for cloxP were run in triplicate, and normalized to Gapdh as an internal control.
Author Contributions
N.B. and L.C.S. designed the project. N.B. and E.A.C. performed the experiments. N.B. and L.C.S. interpreted the data and wrote the manuscript. E.A.C. provided critical feedback.
Acknowledgments
We thank Dr. Ivan Maillard, whose insightful questions led to the conception of the project, Yasmine Abushukur and Theresa Keeley for technical help, Erin Collin for maintaining the mouse colony, and the Nusrat/Parkos lab for the gift of the Villin-Cre mice and for supplementing our Villin-CreERT2 mouse stores. N.B. was supported by the Cellular and Molecular Biology program, a Rackham research grant, and the Benard L. Maas Fellowship. The research was funded by NIH R01-DK096972 (to L.C.S.), and Core support from the Michigan Gastrointestinal Research Center Grant NIH P30-DK34933.
Published: November 15, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.10.014.
Supplemental Information
References
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bouabe H., Okkenhaug K. Gene targeting in mice: a review. Methods Mol. Biol. 2013;1064:315–336. doi: 10.1007/978-1-62703-601-6_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn S.M., Schloemann S., Tessner T., Seibert K., Stenson W.F. Crypt stem cell survival in the mouse intestinal epithelium is regulated by prostaglandins synthesized through cyclooxygenase-1. J. Clin. Invest. 1997;99:1367–1379. doi: 10.1172/JCI119296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi A.Y., Ikawa T., Muramatsu M., Economides A.N., Niwa A., Okuda T., Murphy A.J., Rojas J., Heike T., Nakahata T. Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. J. Immunol. 2009;182:5633–5640. doi: 10.4049/jimmunol.0802413. [DOI] [PubMed] [Google Scholar]
- Huh W.J., Mysorekar I.U., Mills J.C. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of “floxed” alleles. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G368–G380. doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W.J., Khurana S.S., Geahlen J.H., Kohli K., Waller R.A., Mills J.C. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24.e7. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.-K., Yang V.W., Bialkowska A.B. The role of intestinal stem cells in epithelial regeneration following radiation-induced gut injury. Curr. Stem Cell Reports. 2017;3:320–332. doi: 10.1007/s40778-017-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L.J., Yang L.-X. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In Vivo. 2008;22:305–309. [PubMed] [Google Scholar]
- Li N., Nakauka-Ddamba A., Tobias J., Jensen S.T., Lengner C.J. Mouse label-retaining cells are molecularly and functionally distinct from reserve intestinal stem cells. Gastroenterology. 2016;151:298–310.e7. doi: 10.1053/j.gastro.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonstra A., Vooijs M., Beverloo H.B., Allak B.A., van Drunen E., Kanaar R., Berns A., Jonkers J. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc. Natl. Acad. Sci. U S A. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison B.B., Dunbar L., Qiao X.T., Braunstein K., Braunstein E., Gumucio D.L. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- el Marjou F., Janssen K.-P., Chang B.H.-J., Li M., Hindie V., Chan L., Louvard D., Chambon P., Metzger D., Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- Metzger D., Clifford J., Chiba H., Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc. Natl. Acad. Sci. U S A. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiche L.A., Papaioannou V.E. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis. 2007;45:768–775. doi: 10.1002/dvg.20353. [DOI] [PubMed] [Google Scholar]
- Potten C.S. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat. Res. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- Roche K.C., Gracz A.D., Liu X.F., Newton V., Akiyama H., Magness S.T. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology. 2015;149:1553–1563.e10. doi: 10.1053/j.gastro.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E.E., Taylor D.S., Prigge J.R., Barnett S., Capecchi M.R. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc. Natl. Acad. Sci. U S A. 2000;97:13702–13707. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijers J., van der Flier L.G., van Es J., Clevers H. Robust cre-mediated recombination in small intestinal stem cells utilizing the olfm4 locus. Stem Cell Reports. 2014;3:234–241. doi: 10.1016/j.stemcr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D.P., Livingston D.M. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol. Cell. 2001;8:233–243. doi: 10.1016/s1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- Takeda N., Jain R., LeBoeuf M.R., Wang Q., Lu M.M., Epstein J.A. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan B., Guimarães M.J., Groth A.C., Calos M.P. Mammalian genomes contain active recombinase recognition sites. Gene. 2000;244:47–54. doi: 10.1016/s0378-1119(00)00008-1. [DOI] [PubMed] [Google Scholar]
- Tian H., Biehs B., Warming S., Leong K.G., Rangell L., Klein O.D., de Sauvage F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wei L., Cramer J.M., Leibowitz B.J., Judge C., Epperly M., Greenberger J., Wang F., Li L., Stelzner M.G. Pharmacologically blocking p53-dependent apoptosis protects intestinal stem cells and mice from radiation. Sci. Rep. 2015;5:8566. doi: 10.1038/srep08566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K.S., Chia L.A., Li X., Ootani A., Su J., Lee J.Y., Su N., Luo Y., Heilshorn S.C., Amieva M.R. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. U S A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Huang Y.-F., Kek C., Bulavin D.V. Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell. 2013;12:298–303. doi: 10.1016/j.stem.2013.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.