Abstract
Purpose
The organic cation transporters (OCTs) and multi-drug and toxin extrusions (MATEs) together are regarded as an organic cation transport system critical to the disposition and response of many organic cationic drugs. Patient response to the analgesic morphine, a characterized substrate for human OCT1, is highly variable. This study was aimed to examine whether there is any organic cation transporter-mediated drug and drug interaction (DDI) between morphine and commonly co-administrated drugs.
Methods
The uptake of morphine and its inhibition by six drugs which are commonly co-administered with morphine in the clinic were assessed in human embryonic kidney 293 (HEK293) cells stably expressing OCT1, OCT2 and MATE1. The in vivo interaction between morphine and the select irinotecan was determined by comparing the disposition of morphine in the absence versus presence of irinotecan treatment in mice.
Results
The uptake of morphine in the stable HEK293 cells expressing human OCT1 and OCT2 was significantly increased by 3.56 and 3.04 fold, respectively, than that in the control cells, with no significant uptake increase in the cells expressing human MATE1. All of the six drugs examined, including amitriptyline, fluoxetine, imipramine, irinotecan, ondansetron, and verapamil, were inhibitors of OCT1/2-mediated morphine uptake. The select irinotecan significantly increased the plasma concentrations and decreased hepatic and renal accumulation of morphine in mice.
Conclusions
Morphine is a substrate of OCT1 and OCT2. Clinician should be aware that the disposition of and thus the response to morphine may be altered by co-administration of an OCT1/2 inhibitor, such as irinotecan.
Keywords: drug and drug interactions, irinotecan, morphine, organic cation transporters
INTRODUCTION
Morphine, a natural alkaloid derived from the immature seedpods of the opium poppy Papaver somniferum, was separated by Friedrich W. Serturner in 1803 (1). Morphine has been the most marked analgesic and sedative opioid for a long time (2) and is still frequently used for treatment of chronic and acute pain throughout the world (3,4). The consequences of morphine use include the desirable effect of analgesia and the undesirable side effects of reduced gastrointestinal peristalsis, nausea, vomiting, constipation, urinary retention, xerostomia, euphoria, respiratory depression, miosis, itch and sweating (5,6). The successful pain management with opioids would be adequate analgesic effect without excessive adverse effects (7). However, a significant proportion of patients (up to 30%) treated with morphine do not have such a successful outcome (2,8). To optimize the therapeutic strategy of morphine remains a major clinical challenge.
Before eliminated by the kidney, morphine undergoes extensive metabolism in the liver. The main metabolic pathway is the glucuronidation of morphine into the inactive morphine-3-glucuronide (M3G) and the analgesically active morphine-6-glucuronide (M6G) (9) that is catalyzed predominantly by uridine 5′-diphospho-glucuronosyltransferase 2B7 (UGT2B7) (10). However, the majority of morphine is positively charged at physiological pH 7.4 and it has been unclear for a long time how morphine crosses the cellular membrane to be metabolized in hepatocytes. The human organic cation transporter 1 (OCT1, or SLC22A1) is expressed mainly in the sinusoidal membrane of hepatocyte, mediating the hepatic uptake of many cationic drugs (11). Tzvetkov et al. have recently demonstrated that morphine is a substrate of OCT1, which is necessary for morphine uptake in the liver, and that loss-of-function OCT1 polymorphisms result in a significant decrease of morphine uptake (12). These findings were confirmed by Fukuda et al. who reported that the plasma concentration of morphine was significantly higher in pediatric patients with the genotypes conferring decreased function of OCT1 (13). Interestingly, the Met420del and Arg61Cys, two of OCT1 polymorphisms leading to decreased transport function, have been associated with increased incidence of morphine-related respiratory depression, nausea and vomiting, strongly suggesting that the function of OCT1 is a factor contributing to the clinical outcome of morphine treatment (14).
Morphine and its metabolites can be eliminated into the urine. The function of renal cationic drug transporters may be important in the disposition of morphine and its metabolites as well. Various drug transporters are located in human renal tubular cells, among which the basolateral OCT2 and the apical multidrug and toxin extrusion 1 and 2-K (MATE1, MATE2-K) are critically involved in renal accumulation and elimination of cationic drugs (15,16). However, whether these renal organic cation transporters involve in morphine disposition have yet to be determined.
Due to their role in drug disposition, hepatic and renal transporters have been widely viewed as a key player in drug-drug interactions (DDIs) (17–19). The patients, who require morphine for pain management in various diseases, such as cancer and other chronic disorders, commonly receive multiple medications that may significantly increase the incidence of adverse drug effects (2). The transporter-mediated DDIs might act as a potential mechanism underlying the side effects related to the usage of morphine. Certain drugs, such as ondansetron and irinotecan, which are frequently co-administered with morphine, have been previously shown to potently inhibit the cellular uptake of morphine via OCT1 (12). As OCTs and MATEs have significantly overlapping substrates and inhibitors (20), we speculate that other OCT and MATE transporters, in addition to OCT1, could be involved in the cellular uptake of morphine and its DDIs.
In the present study, we first sought to determine whether morphine was a substrate for organic cation transporters including OCT1, OCT2 and MATE1 in stable HEK293 cells. We then studied the inhibition of these morphine transporters by several drugs which are commonly co-administered with morphine in the clinic. Lastly, we investigated whether irinotecan, the most potent inhibitor of morphine uptake in our cellular studies, affected the plasma level and tissue distribution of morphine in mice.
MATERIALS AND METHODS
Chemical and Reagents
All chemicals and general reagents used in this study were of analytical grade or better. The Dulbecco’s modified Eagle’s medium (DMEM), Opti-MEM reduced serum medium, heat-inactived fetal bovine serum(FBS), phosphate-buffered saline (PBS), Lipofectamine 2000, penicillin, streptomycin, hygromycin, TRIzol, Flp-In transfection system, and trypsin/EDTA were obtained from Invitrogen. Unlabeled morphine, fluoxetine, imipramine, amitriptyline, irinotecan, ondansetron and verapamil were purchased from Sigma Chemical Co. (St. Louis, MO). 3H-morphine was purchased from American Radiolabeled Chemicals, Inc.
Cell Culture
Human embryonic kidney 293 cells(HEK293)were initially obtained from the American Type Culture Collection (ATCC) and maintained in our laboratory. HEK293 Flp-In cells stably expressing human (h) OCT1, hOCT2 and hMATE1 were established as we described previously (21). HEK293-mock, HEK293-hOCT1, HEK293-hOCT2 and HEK293-hMATE1 cells were maintained under humidity with 5% CO2 at 37°C in DMEM supplemented 10% (v/v) heat-inactivated FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 75 μg/ml hygromycin.
Uptake Experiments in HEK293 Cells
When the cells reached more than 90% confluence at forty-eight hours after seeding, HEK293-mock, HEK293-hOCT1, HEK293-hOCT2 and HEK293-hMATE1 cells were used for uptake experiments. For the uptake mediated by hOCT1 and hOCT2, the cells were carefully washed with pre-warmed KRH buffer containing 125 mM NaCl, 25 mM HEPES (pH 7.4), 5.6 mM glucose, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM CaCl2 and 1.2 mM KH2PO4 after removal of the culture medium, and then pre-incubated in KRH buffer for 10 min at 37°C. For the uptake mediated by hMATE1, the cells were carefully washed with pre-warmed KBB buffer containing 140 mM KCl, 0.8 mM MgSO4, 1.0 mM CaCl2,0.4 mM KH2PO4, 10 mM HEPES (pH 7.4), and 25 mM glucose. The HEK293-hMATE1 cells were then pre-incubated in KBB buffer supplemented with 30 mM NH4Cl (pH = 8.0) for 10 min at 37°C. This pre-incubation was to create an H+ gradient across the membrane that served as the driving force of hMATE1-mediated uptake as described previously (21). Pilot uptake experiments were performed to optimize the uptake time. The cells were eventually incubated with uptake buffer (KRH for hOCT1 and hOCT2; NH4Cl-free KBB for hMATE1) containing 0.2 μM morphine (3H-morphine: unlabeled morphine = 1:10000) for 2 min (hOCT1), 6 min (hOCT2) or 3 min (hMATE1). At the end of the incubation period, the uptake buffer was removed and the cells were carefully washed with ice-cold buffer (pH 7.4) three times. Then cells were lysed and the concentration of radioactivity in the cell lysate were measured as previously described (21). The uptake rate was corrected with cellular protein concentration in each assay. To normalize the results across different repeats of experiments, the uptake rates of morphine in HEK293-hOCT1, HEK293-hOCT2 and HEK293-hMATE1 cells were presented as a fold change relative to the respective control cells.
In Vitro Transporter Inhibition Assay
As describe above, the HEK293-hOCT1 and HEK293-hOCT2 were incubated in the reaction mixture with0.2 μM morphine (3H-morphine: unlabeled morphine = 1:10000) and various concentrations of different potential inhibitors for 2 min (hOCT1) or 6 min (hOCT1). The cells were lysed and the radioactivity of intracellular morphine was counted as described above.
Animal Studies
All animal studies were conducted in accordance with National Institutes of Health (NIH) guidelines for animal experimentation and according to a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the School of Pharmacy, University of Maryland at Baltimore. All mice used in the experiments were 10 to 12 weeks old C57BL/6 J mice. The mice were housed in a temperature-controlled environment of 23 ± 1°C with a 12-h light/dark cycle (lights on 08:00 AM). The mice were given a standard mouse diet, with water provided ad libitum.
Twelve C57BL/6 J mice were randomly assigned to control group (n = 6) and irinotecan treatment group (n = 6). Irinotecan dissolved in sterile saline. The mice in irinotecan-treated group were intraperitoneally injected with irinotecan at dose of 45 mg/kg and subsequently intraperitoneally injected with morphine at a dose of 1.45 mg/kg morphine (3H-morphine: unlabeled morphine = 1:10000) after 30 min. The mice in control group were treated with the vehicles (sterile saline) and subsequently morphine as described above. We chose two time points, 12 min and 30 min after intraperitoneal morphine injection, to compare the plasma concentrations of morphine between the irinotecan-treated mice and the saline-treated mice. Blood samples were centrifuged for 10 min at 4°C at 8000 g to separate plasma. Next, 6 μl of plasma was carefully added into 2 ml of scintillation buffer. The radioactivity of morphine was determined at a Beckman LS6500 scintillation counter (Brea, CA). After the second blood collection, the mice were euthanized. The liver and kidney tissues were isolated as described previously for the measurement of accumulated morphine (21).
Statistical Analysis
All results were presented as the mean ± standard deviation (SD). The IC50 (the concentration of inhibitor leads to 50% of inhibition) was calculated using GraphPad Prism 5(San Diego, CA) by fitting the experimental data with a built-in log(inhibitor) vs. response equation: where Y is the percentage of morphine uptake normalized to the control condition (no inhibitor, set to 100%), X is the log concentration of inhibitor, and the Hill Slope describes the steepness of the curve and is determined by the software. For parametric data, comparisons between two groups were performed using the unpaired 2-tailed Student’s t test, and multiple comparisons were analyzed using the one-way analysis of variance (ANOVA) followed by Dunnett’s test. P < 0.05 was considered as the level of significance.
RESULTS
Uptake of Morphine by HEK-293 Cells Expressing hOCT1,hOCT2 and hMATE1
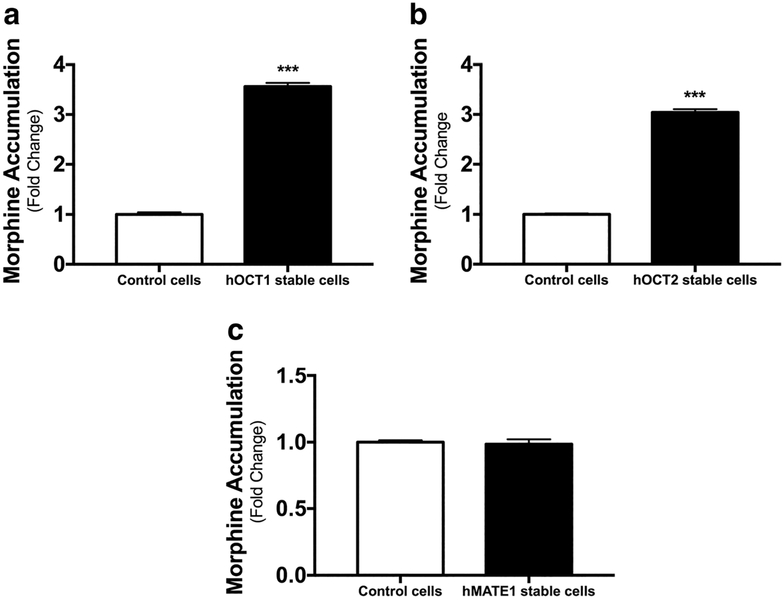
To determine if morphine is a substrate for hOCT1, hOCT2 and hMATE1, the uptake assays were conducted in HEK-293 cells stably expressing these transporters (HEK293-hOCT1, HEK293-hOCT2, and HEK293-hMATE1) and in the mock cells expressing the empty vector (HEK293-mock). The transporter function of these cells has been well characterized in our previous reports by using classical substrates such as metformin (21,22). In this study, the cells were incubated with 0.2 μM morphine (3H-morphine: unlabeled morphine = 1:10000) for 2 min (hOCT1), 6 min (hOCT2) and 3 min (hMATE1), respectively. The uptake of morphine in HEK293-hOCT1 and HEK293-hOCT2 was significantly increased by 3.56 and 3.04 fold, respectively, than that in the mock cells (Fig. 1a, b). However, there was no difference in the uptake of morphine between HEK-hMATE1 and the mock cells (Fig. 1c). As hMATE1 and hMATE-2 K have significantly overlapping substrates and inhibitors, we did not further investigate the role of hMATE-2 K in morphine transport. The results of our uptake assays indicated that morphine is a substrate of hOCT1 and hOCT2 but not of hMATE1.
Fig. 1. Uptake of morphine in stable HEK293 cells expressing human (h) OCT1, OCT2, and MATE1.
The HEK293 cells stably overexpressing hOCT1 (a), hOCT2 (b), hMATE1 (c), and the control empty vectors were incubated with 0.2 μM morphine (3H-morphine: unlabeled morphine = 1:10000) for 2 min (hOCT1), 6 min (hOCT2) and3 min (hMATE1), respectively. Dates are shown as mean ± SD (n = 6). ***P < 0.001.
Inhibition of hOCT1 and hOCT2-Mediated Cellular Morphine Uptake by Different Drugs
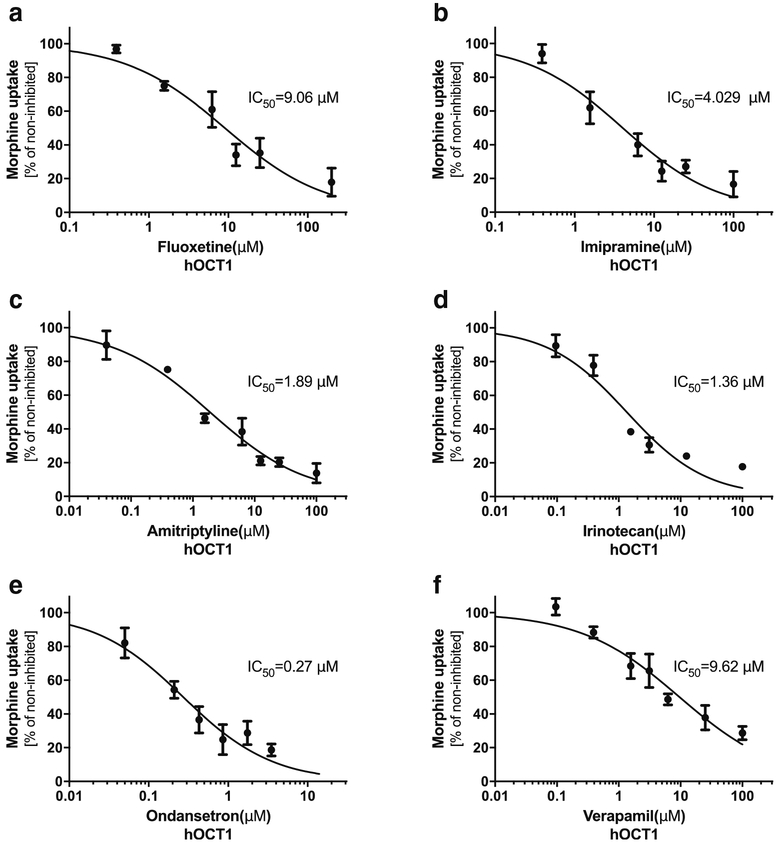
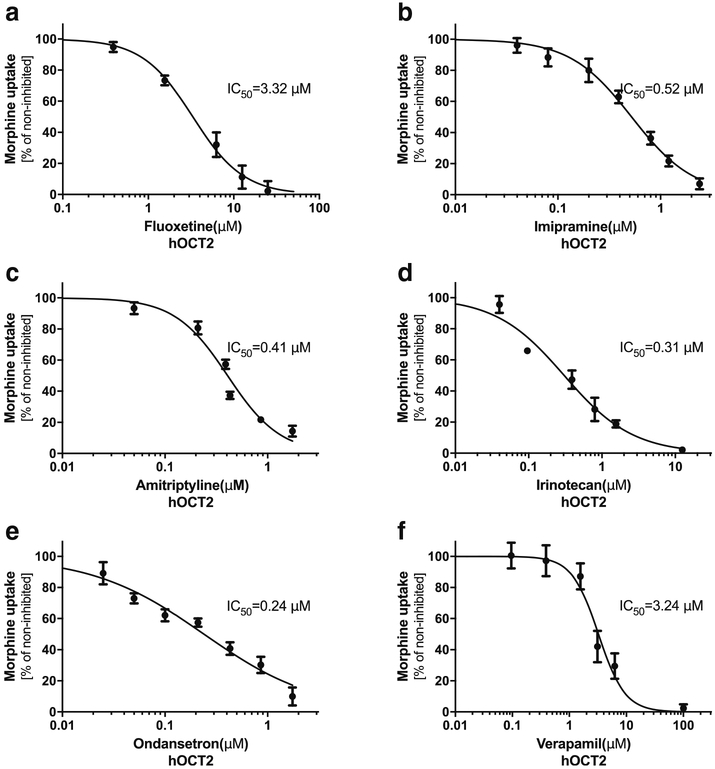
The identification of hOCT1 and hOCT2 as the uptake transporters of morphine has implications in DDIs. We tested the inhibition potency against the hOCT1- and hOCT2-mediated uptake of morphine for six drugs that are commonly co-prescribed with morphine in the clinic. The inhibition on the uptake of morphine by all tested drugs was concentration-dependent in HEK293-hOCT1 and HEK293-hOCT2 cells (Figs. 2 and 3). The IC50 (half maximal inhibitory concentration) by these drugs for hOCT1 varied between 0.27 μM (ondansetron) to 9.62 μM (verapamil) and for hOCT2 between 0.24 μM (ondansetron) to 3.32 μM (fluoxetine). We compared the IC50 values of these tested drugs with their Cmax,unbound from the literature (Table I). A ratio of Cmax,unbound/IC50 more than 0.1 may suggest a clinically relevant inhibition of transporter activity by the inhibitor (24). Since human OCT1 is highly expressed in the sinusoidal membrane of hepatocytes, the maximal unbound plasma concentration in the portal vein (Cmax port, unbound) may be more appropriate to assess the potential DDI risk involving OCT1. Therefore, we also compared the IC50 values of these tested drugs with their Cmax port, unbound (Table I). Of note, the Cmax, unbound of irinotecan is much higher than its IC50 against both hOCT1 and hOCT2 with the Cmax,unbound/IC50 of 2.86 and12.55, respectively. The Cmax port,unbound of irinotecan is nearly equal to that of Cmax,unbound (Table I). We further examined the inhibitory potential of irinotecan towards the OCTs. Irinotecan was pre-incubated with HEK293-hOCT1 and HEK293-hOCT2 cells for 15 min, and then the cells were thoroughly washed before performing morphine uptake. Interestingly, the inhibition of irinotecan on morphine uptake after irinotecan washes was almost the same as the above condition of co-incubation of irinotecan and morphine, suggesting a tight, irreversible binding of irinotecan to OCT transporters (data not shown). These data suggested that irinotecan could be a potent inhibitor of OCT1 and OCT2 in vivo, affecting the disposition of morphine.
Fig. 2. Inhibition of human (h) OCT1-mediated morphine uptake by different drugs.
The stable HEK293 cells expressing hOCT1 were incubated for 2 min with 0.2 μM morphine (3H-morphine: unlabeled morphine = 1:10000) in the absence or in the presence of increasing concentrations of select drugs that are commonly co-administrated with morphine in the clinic. The data represents mean ± SD from at least three independent experiments.
Fig. 3. Inhibition of human (h) OCT2-mediated morphine uptake by different drugs.
The stable HEK293 cells expressing hOCT2 were incubated for 6 min with 0.2 μM morphine (3H-morphine: unlabeled morphine = 1:10000) in the absence or in the presence of increasing concentrations of select drugs that are commonly co-administrated with morphine in the clinic. The data represents mean ± SD from at least three independent experiments.
Table I.
Inhibition of the Human (h) OCT1 and OCT2-Mediated Morphine Uptake by the Drugs Commonly Co-Administered with Morphine
| Drug | Cmax,unbounda [μM] |
Cmax port,unboundb [μM] |
hOCT I | hOCT2 | |||
|---|---|---|---|---|---|---|---|
| IC50 [μM] | Cmax,unbound/IC50 | Cmax,unbound/IC50 | IC50 [μM] | Cmax,unbound/IC50 | |||
| Fluoxetine | 0.01 | 0.7 | 9.06 ± 3.24 | 0.001 | 0.08 | 3.32 ± 0.76 | 0.003 |
| Imipramine | 0.07 | 4.5 | 4.03 ± 1.62 | 0.02 | 1.12 | 0.52 ± 0.1 1 | 0.13 |
| Amitriptyline | 0.01 | 4.3 | 1.89 ± 0.77 | 0.005 | 2.28 | 0.41 ± 0.05 | 0.02 |
| Irinotecan | 3.89 | 3.9 | 1.36 ± 0.43 | 2.86 | 2.87 | 0.3 1 ± 0.07 | 12.55 |
| Ondansetron | 0.08 | 0.08 | 0.27 ± 0.10 | 0.30 | 0.30 | 0.24 ± 0.05 | 0.33 |
| Verapamil | 0.06 | 1.7 | 9.62 ± 3.63 | 0.04 | 0.18 | 3.24 ± 0.70 | 0.02 |
Cmax,unbound: maximal plasma concentration of the unbound drug; Cmax port,unbound, maximal unbound plasma concentration in the portal vein
The maximal plasma concentration (Cmax) was obtained from Goodman and Gilman’s The Pharmacological Basis of Therapeutics except for the Cmax of ondansetron (Zofran, information for prescribers) and fluoxetine (23)
The maximal unbound plasma concentration in the portal vein (Cmax prot,unbound,) was calculated by Tzvetkov et al. (12)
Effects of Irinotecan on the Plasma and Tissue Concentrations of Morphine in Mice
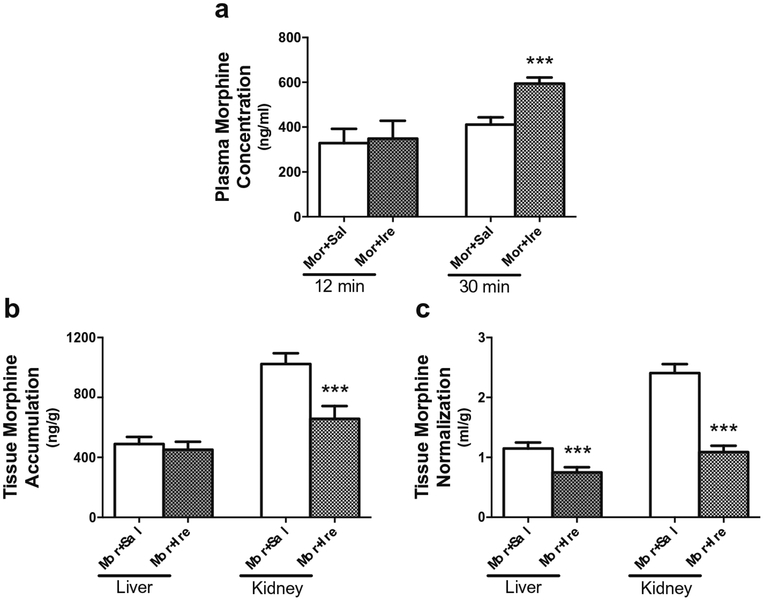
To determine the effect of irinotecan on morphine disposition in vivo, we further determined the plasma and tissue concentrations of morphine in two groups (n = 6/group) of mice. These two groups of mice were treated with irinotecan or sterile saline control at 30 min before intraperitoneally injection of morphine. The plasma concentrations of morphine at an early time point of 12 min after morphine injection showed no significant differences between the two groups. However, there was a significant difference in the plasma concentration of morphine at 30 min after morphine injection between the irinotecan-treated and control mice. The plasma concentration of morphine was 1.44-fold greater in the mice received morphine and irinotecan than that in control mice (594 ± 27.7 vs. 411 ± 32.8 ng/ml) (Fig. 4a). The tissue accumulation of morphine were measured at 30 min after intraperitoneal morphine injection. As expected from the fact that Oct1 and Oct2 are highly expressed in mouse kidney (25), we found a significant decrease in renal morphine accumulation in the irinotecan-treated mice (Fig. 4b). After normalized by the plasma morphine concentration at 30 min after its intraperitoneal injection, this decrease was more apparent (Fig. 4c). We did not observe any significant difference in accumulation in the liver (Fig. 4b) and other tissues (data not shown) between these two groups of mice before normalization by the terminal plasma concentration of morphine. After the normalization, however, there was a significant decrease in hepatic morphine accumulation in the irinotecan-treated mice (Fig. 4c).
Fig. 4. Effect of irinotecan on the plasma concentrations and tissue accumulation of morphine in mice.
(a) The plasma concentrations of morphine in the mice administrated with irinotecan or saline. Mice at age of 10–12 weeks were randomly divided into 2 groups and injected intraperitoneally with 45 mg/kg irinotecan (n = 6) and 0.9% saline (n = 6), respectively. Thirty minutes later, all mice were given 1.45 mg/kg of morphine (3H-morphine: unlabeled morphine = 1:10000). The blood samples were collected at 12 min and 30 min after morphine injection. (b) Comparison of liver and kidney accumulation of morphine between the mice administrated with saline plus morphine and those with irinotecan plus morphine. The tissues were isolated at 30 min after morphine injection. (c) Comparison of liver and kidney accumulation of morphine between the two groups, as that in (b), after normalization by the terminal plasma concentration. ***P < 0.001, significantly different in the mice received with irinotecan as compared with those received saline control.
DISCUSSION
In this study, we have not only confirmed morphine as a substrate for hOCT1 but also demonstrated, for the first time, the drug as a substrate for hOCT2. Moreover, we found that the cellular uptake of morphine by hOCT1 and hOCT2 may be easily subject to inhibition by other drugs that are co-prescribed with morphine in the clinic. All of the six drugs examined in this study, including fluoxetine, imipramine, amitriptylin, irinotecan, ondansetron, and verapamil, were the potent inhibitors of morphine uptake mediated by both hOCT1 and hOCT2. Among the six drugs, we speculated that irinotecan was most likely to affect the disposition of morphine in the body in consideration of its inhibitory potency against the two transporters and the reported free drug concentrations in the plasma. Consistently, we have demonstrated that irinotecan affected the plasma concentrations and tissue accumulations of morphine, likely via inhibition of Oct activities, in mice.
Morphine undergoes extensive hepatic metabolism before renal elimination. It has been reported that the drug has very limited cellular membrane permeability by diffusion and carrier-mediated transport plays a critical role (12). Several transporters including OCT1, ABCB1 and ABCB3 have been known to involve in the disposition of morphine and its metabolites (10,26–29). These transporters are mainly expressed in the liver. In particular, OCT1 is an uptake transporter highly expressed in the sinusoidal membrane of human hepatocyte. Our confirmation of the previous findings for OCT1 as a morphine transporter suggests that this transporter may be a major determinant of morphine distribution and metabolism in the liver. hOCT1 is highly polymorphic in human populations (11,30). Those hOCT1 variants with decreased function led to not only a reduced morphine uptake in cell models but also an increased plasma concentration of morphine in human subjects due to a reduced liver distribution and consequently a reduced metabolism (31). Interestingly, the genetic difference in OCT1 polymorphism has been recently implicated as an underlying reason for a higher plasma concentration of morphine in white American children as compared to those of African American children (32). On the other hand, the efflux transporters ABCB1, which is primarily expressed on the canalicular side of hepatocytes, and ABCC3, which is located at the basolateral membranes of hepatocytes, also transport morphine and its metabolites M3G and M6G (10,26,27,29). Genetic polymorphisms of these efflux transporters may affect morphine clearance as well (29).
The transporters in the kidney may also play a role in morphine disposition. The basolateral OCT2 and the apical MATEs of human proximal tubules constitute an important system which can mediate the elimination of cationic drugs from the circulation to the urine (21,25,33). In our study, we demonstrated that morphine is a substrate for OCT2, but not for MATE1. hOCT2 genetic polymorphisms have been clearly shown to affect the disposition of certain substrate drugs. For example, the human subjects with OCT2 Thr199Ile, Thr201Met and/or Ala270Ser were reported to have significantly different systemic exposure of metformin from those carrying only reference alleles (34–36). Filipski et al. demonstrated that the carriers of the Ala270Ser appear to be at a lower risk of nephrotoxicity associated with cisplatin and the patients only bearing the reference sequence showed a significantly higher serum creatinine level (31,37,38). Future studies can be carried to clarify whether OCT2 polymorphisms alter the disposition, efficacy and side effects of morphine.
Increasing evidence has indicated that a drug transporter can be a site of significant DDIs where a ‘perpetrator’ drug can affect the clearance and the systemic exposure of a ‘victim’ drug, resulting in exaggerated/diminished therapeutic and/or side effects (39). Morphine is commonly co-administrated with other drugs which may serve as “perpetrator” agents via transporter inhibition and cause undesirable outcomes of morphine. In the present study, we studied six prescription drugs commonly co-administered with morphine. These tested drugs inhibited the uptake of morphine mediated by both hOCT1 and hOCT2 in a dose-dependent manner. The observed IC50 values for hOCT1 inhibition were comparable to those reported in the previous study (12). We compared the IC50 values for both hOCT1 and hOCT2 with the unbound Cmax of these drugs (Table I). It has been proposed that an inhibitor causing clinically appreciable inhibition of transporter activity may have a ratio of Cmax,unbound/IC50 more than0.1 (24). Accordingly, among the six drugs studied, irinotecan and ondansetron are most likely to interact with morphine in the body via inhibition of OCT1 and OCT2. This is supported by our follow-up in vivo studies in mice. At the single dose of 45 mg/kg, which was scaled from a clinical dose of 125 mg/m2 [FDA CAMPTOSAR clinical pharmacology], irinotecan significantly decreased renal and hepatic accumulation and increased plasma concentrations of morphine in mice. Of note, there are certain differences in the tissue expression pattern of OCTs between humans and rodents. In rodents, Oct1 has a high level of expression in both kidney and liver that is comparable to that of Oct2, whereas hOCT2 is only predominantly expressed in human kidney and hOCT1 in human liver (40). The inhibition of hOCT2 (Cmax,unbound/IC50:12.55) by irinotecan was more potent than that of OCT1 (Cmax port, unbound/IC50: 2.87), suggesting that the effect of irinotecan on morphine renal clearance could be even greater in humans. Moreover, given the relatively long half-life of irinotecan (more than 10 h) (41) and the high ratio of Cmax,unbound/IC50 for both OCT1 and OCT2, it is likely that irinotecan has a sustained inhibition on these transporters in humans, i.e., not only at its Cmax but also at a trough concentration in the plasma. We have to point out that the levels of morphine and its metabolites were measured in total as we dosed the radiolabeled morphine in mice in the present study. In humans, UGT2B7 converts morphine into M3G and M6G, whereas mice only form M3G. In our in vivo study, we measured the levels of morphine and M3G in total. The majority of morphine is excreted as its metabolites. Only approximately 10% of a morphine dose is excreted as unchanged morphine in urine from the body (42). The significantly increase in renal accumulation of total morphine by irinotecan treatment in mice is intriguing. Although our data in mice, in consistent with the cellular results, supports a role of OCT function in the interaction between morphine and irinotecan in vivo, the exact contribution by OCTs remains unclear. Further studies are needed to clarify whether there is any significant species difference in morphine metabolism and whether the cellular uptake of morphine metabolites such as M3G and M6G into the kidney is dependent on OCT function. Alternatively, it is also likely that irinotecan is an inhibitor of the transporters involved in the excretion of morphine and its metabolites, such as ABCC3.
Morphine use is associated with a large unpredictable variability in analgesia and adverse effects (31). DDIs are contributing factors. For example, the concomitant use of cimetidine, a histamine H2 receptor antagonist, could potentiate the efficacy of morphine and increase the risk of respiratory depression, profound sedation, hypotension, coma, and death [MORPHABOND ER product insert] (43). Interestingly, cimetidine is a well-characterized inhibitor of OCT2 (36). Irinotecan usually co-administration with morphine in advanced colorectal cancer (12,44). Our present study demonstrated that irinotecan had the most potent inhibitory effect among these tested drugs on the uptake of morphine mediated by the hepatic OCT1 and by the renal OCT2. In the present study, even though we did not directly establish that irinotecan could exaggerate therapeutic effects and/or exacerbate the adverse reaction of morphine, we have characterized that irinotecan significantly increased the systemic exposure of morphine, possibly via inhibition of OCT transporters. Other drugs validated as OCT inhibitors in the present study include adjuvant therapies in pain treatment (fluoxetine, imipramine and amitriptyline), an anti-emetic given in cancer chemo-therapy or peri- and post-operation (ondansetron), and the calcium channel blocker verapamil. In addition, morphine is used with antibiotics in antidiarrheal therapy. Important antibiotics such as fluoroquinolones (gatifloxacin, prulifloxacin and moxifloxacin) have been identified as potent inhibitors of hOCTs (45,46).
In summary, we have demonstrated that human OCT1 and OCT2 mediate the cellular uptake of morphine and the inhibition of OCT activities by irinotecan may alter the disposition of morphine in the body. Given that the patients requiring morphine for pain management commonly receive concomitant medications, clinicians should be aware that the therapeutic and/or toxic effects of morphine may be significantly affected by those co-administrated inhibitors and/or substrates of morphine uptake transporters.
ACKNOWLEDGMENTS AND DISCLOSURES
The present study was partially supported by the National Natural Science Foundation (NNSF) of China (81,570,533, Q.L.) and the Natural Science Foundation for Young Scientist of Hunan Province, China (2018JJ3829, Q.L.). Dr. Yan Shu received funding supports from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award R01GM099742 (Y.S.) and the US Food and Drug Administration (FDA) under Award U01FD004320 (J.E.P, Y.S.). Dr. Dong Guo is an M-CERSI Scholar (FDA 1U01FD005946). Dr. Yan Shu is a co-founder for and owns equity in Optivia Biotechnology. Designed Research: Yan Shu, Qing Li, Wei Zhang, James E. polli, Honghao Zhou. Performed Research: Peng Zhu, Zhi Ye, Zongping Xiong, Shiqiong Huang, Dong Guo. Analyzed Data: Peng Zhu, Zhi Ye, Qing Li, Dong Guo, Jun Guo. Wrote Manuscript: Peng Zhu, Qing Li, Yan Shu. All authors approved the version to be submitted.
REFERENCES
- 1.Andersen G, Christrup L, Sjogren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manag. 2003;25:74–91. [DOI] [PubMed] [Google Scholar]
- 2.Cherny N, Ripamonti C, Pereira J, Davis C, Fallon M, McQuay H, et al. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19:2542–54. [DOI] [PubMed] [Google Scholar]
- 3.Sverrisdottir E, Lund TM, Olesen AE, Drewes AM, Christrup LL, Kreilgaard M. A review of morphine and morphine-6-glucuronide’s pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur J Pharm Sci. 2015;74:45–62. [DOI] [PubMed] [Google Scholar]
- 4.Wiffen PJ, Wee B, Moore RA. Oral morphine for cancer pain. Cochrane Database Syst Rev:CD003868. 2013. [DOI] [PubMed] [Google Scholar]
- 5.Babul N, Provencher L, Laberge F, Harsanyi Z, Moulin D. Comparative efficacy and safety of controlled-release morphine suppositories and tablets in cancer pain. J Clin Pharmacol. 1998;38:74–81. [DOI] [PubMed] [Google Scholar]
- 6.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686–91. [DOI] [PubMed] [Google Scholar]
- 7.Morphine in cancer pain: modes of administration. Expert Working Group of the European Association for Palliative Care. BMJ. 1996. 312:823–826. [PMC free article] [PubMed] [Google Scholar]
- 8.Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987;59:850–6. [DOI] [PubMed] [Google Scholar]
- 9.Brokjaer A, Kreilgaard M, Olesen AE, Simonsson US, Christrup LL, Dahan A, et al. Population pharmacokinetics of morphine and morphine-6-glucuronide following rectal administration–a dose escalation study. Eur J Pharm Sci. 2015;68:78–86. [DOI] [PubMed] [Google Scholar]
- 10.Coffman BL, Rios GR, King CD, Tephly TR. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab Dispos. 1997;25: 1–4. [PubMed] [Google Scholar]
- 11.Shu Y, Leabman MK, Feng B, Mangravite LM, Huang CC, Stryke D, et al. Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc Natl Acad Sci U S A. 2003;100:5902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzvetkov MV, dos Santos Pereira JN, Meineke I, Saadatmand AR, Stingl JC, Brockmoller J. Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochem Pharmacol. 2013;86:666–78. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda T, Chidambaran V, Mizuno T, Venkatasubramanian R, Ngamprasertwong P, Olbrecht V, et al. OCT1 genetic variants influence the pharmacokinetics of morphine in children. Pharmacogenomics. 2013;14:1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balyan R, Zhang X, Chidambaran V, Martin LJ, Mizuno T, Fukuda T, et al. OCT1 genetic variants are associated with postoperative morphine-related adverse effects in children. Pharmacogenomics. 2017;18:621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koepsell H Organic cation transporters in intestine, kidney, liver, and brain. Annu Rev Physiol. 1998;60:243–66. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Ye Z, Zhu P, Guo D, Yang H, Huang J, et al. Indinavir alters the pharmacokinetics of lamivudine partially via inhibition of multidrug and toxin extrusion protein 1 (MATE1). Pharm Res. 2018;35:14. [DOI] [PubMed] [Google Scholar]
- 17.Koepsell H Role of organic cation transporters in drug-drug interaction. Expert Opin Drug Metab Toxicol. 2015;11:1619–33. [DOI] [PubMed] [Google Scholar]
- 18.Konig J, Muller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–66. [DOI] [PubMed] [Google Scholar]
- 19.Maeda K Organic anion transporting polypeptide (OATP)1B1 and OATP1B3 as important regulators of the pharmacokinetics of substrate drugs. Biol Pharm Bull. 2015;38:155–68. [DOI] [PubMed] [Google Scholar]
- 20.Nies AT, Damme K, Kruck S, Schaeffeler E, Schwab M. Structure and function of multidrug and toxin extrusion proteins (MATEs) and their relevance to drug therapy and personalized medicine. Arch Toxicol. 2016;90:1555–84. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, et al. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol Appl Pharmacol. 2013;273:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo D, Yang H, Li Q, Bae HJ, Obianom O, Zeng S, et al. Selective inhibition on organic cation transporters by carvedilol protects mice from cisplatin-induced nephrotoxicity. Pharm Res. 2018;35:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moraes MO, Lerner FE, Corso G, Bezerra FA, Moraes ME, De Nucci G. Fluoxetine bioequivalence study: quantification of fluoxetine and norfluoxetine by liquid chromatography coupled to mass spectrometry. J Clin Pharmacol. 1999;39:1053–61. [DOI] [PubMed] [Google Scholar]
- 24.Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, et al. Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013;56:781–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011:105–67. [DOI] [PubMed] [Google Scholar]
- 26.Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83:559–66. [DOI] [PubMed] [Google Scholar]
- 27.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of P-glycoprotein in the intestinal absorption and clinical effects of morphine. Clin Pharmacol Ther. 2003;74:543–54. [DOI] [PubMed] [Google Scholar]
- 28.Tzvetkov MV, Vormfelde SV, Balen D, Meineke I, Schmidt T, Sehrt D, et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther. 2009;86:299–306. [DOI] [PubMed] [Google Scholar]
- 29.Venkatasubramanian R, Fukuda T, Niu J, Mizuno T, Chidambaran V, Vinks AA, et al. ABCC3 and OCT1 genotypes influence pharmacokinetics of morphine in children. Pharmacogenomics. 2014;15:1297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzvetkov MV. OCT1 pharmacogenetics in pain management: is a clinical application within reach? Pharmacogenomics. 2017;18: 1515–23. [DOI] [PubMed] [Google Scholar]
- 32.Sadhasivam S, Krekels EH, Chidambaran V, Esslinger HR, Ngamprasertwong P, Zhang K, et al. Morphine clearance in children: does race or genetics matter? J Opioid Manag. 2012;8:217–26. [DOI] [PubMed] [Google Scholar]
- 33.Fenner KS, Troutman MD, Kempshall S, Cook JA, Ware JA, Smith DA, et al. Drug-drug interactions mediated through P-glyco-protein: clinical relevance and in vitro-in vivo correlation using digoxin as a probe drug. Clin Pharmacol Ther. 2009;85:173–81. [DOI] [PubMed] [Google Scholar]
- 34.Song IS, Shin HJ, Shim EJ, Jung IS, Kim WY, Shon JH, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther. 2008;84:559–62. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Li S, Brown C, Cheatham S, Castro RA, Leabman MK, et al. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet Genomics. 2009;19:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang ZJ, Yin OQ, Tomlinson B, Chow MS. OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics. 2008;18:637–45. [DOI] [PubMed] [Google Scholar]
- 37.Lazar A, Zimmermann T, Koch W, Grundemann D, Schomig A, Kastrati A, et al. Lower prevalence of the OCT2 Ser270 allele in patients with essential hypertension. Clin Exp Hypertens. 2006;28: 645–53. [DOI] [PubMed] [Google Scholar]
- 38.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanyuk A, Livio F, Biollaz J, Buclin T. Renal drug transporters and drug interactions. Clin Pharmacokinet. 2017;56:825–92. [DOI] [PubMed] [Google Scholar]
- 40.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–51. [DOI] [PubMed] [Google Scholar]
- 41.Denlinger CS, Blanchard R, Xu L, Bernaards C, Litwin S, Spittle C, et al. Pharmacokinetic analysis of irinotecan plus bevacizumab in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;65:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christrup LL. Morphine metabolites. Acta Anaesthesiol Scand. 1997;41:116–22. [DOI] [PubMed] [Google Scholar]
- 43.Lam AM. Potentially lethal interaction of cimetidine and morphine. Can Med Assoc J. 1981;125:820. [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita K, Kubota Y, Ishida H, Sasaki Y. Irinotecan, a key chemo-therapeutic drug for metastatic colorectal cancer. World J Gastroenterol. 2015;21:12234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulgaonkar A, Venitz J, Grundemann D, Sweet DH. Human organic cation transporters 1 (SLC22A1), 2 (SLC22A2), and 3 (SLC22A3) as disposition pathways for fluoroquinolone antimicrobials. Antimicrob Agents Chemother. 2013;57:2705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]