Abstract
Introduction:
Current treatments for neuropathic pain are limited at least in part due to the incomplete understanding of its underlying mechanisms. Recent evidence reveals the dysregulated expression of long non-coding RNAs (lncRNAs) in the damaged nerve, dorsal root ganglion (DRG), and spinal cord dorsal horn following peripheral nerve injury. However, the role of the majority of lncRNAs in neuropathic pain genesis is still elusive. Unveiling the mechanisms of how lncRNAs participate in neuropathic pain may develop new strategies to prevent and/or treat this disorder.
Areas covered:
This review focuses on the dysregulation of lncRNAs in the DRG, spinal cord dorsal horn, and the injured peripheral nerves from preclinical rodent models of neuropathic pain. We provide evidence of how peripheral nerve injury causes the dysregulation of lncRNAs in these pain-related regions. The potential mechanisms of how dysregulated lncRNAs contribute to the pathogenesis of neuropathic pain are discussed.
Expert opinion:
The investigation on the role of the dysregulated lncRNAs in neuropathic pain might open up a novel avenue for therapeutic treatment of this disorder. However, current investigation is at the infancy stage, which challenges the translation of preclinical findings. More intensive study on lncRNAs is required before the preclinical findings are translated into therapeutic management for neuropathic pain.
Keywords: long non-coding RNA, neuropathic pain, dorsal root ganglion, spinal cord, epigenetics, RNA sequencing
1. Introduction
Neuropathic pain, a major public health problem worldwide, is a distressing and debilitating disease. It is characterized by spontaneous ongoing or intermittent burning pain, allodynia, and hyperalgesia. Neuropathic pain is often caused by trauma (e.g., peripheral nerve, spinal cord, or brain injury), and various diseases (e.g., stroke, multiple sclerosis, diabetics, cancer chemotherapy, human immunodeficiency virus, and cancer) [1, 2]. Epidemiological observation reveals that about 6.9–10% of the population suffers from neuropathic pain [3, 4]. In the United States, the cost of annual healthcare and productivity losses caused by neuropathic pain are estimated to be as much as $600 billion [5, 6]. Current treatments for this disorder including pharmacological interventions, such as anticonvulsants and opioids, physical therapies, and filed stimulations have limited effects and/or produce several adverse effects [7–9]. Thus, identifying the mechanisms of neuropathic pain is essential for the discovery of novel treatments and preventive tactics for this disorder.
The transcriptional and translational changes in gene expression in the dorsal root ganglion (DRG), spinal cord dorsal horn, and pain-related brain regions following peripheral nerve injury are considered to participate in neuropathic pain genesis [10, 11]. In the past decade, the advances of high throughput approaches, including gene microarray and next-generation sequencing, have provided revolutionary strategies for transcriptome research. RNA sequencing (RNA-Seq) utilizing diverse next-generation deep sequencing protocols paired with bioinformatics pipelines has made it a possibility to detect expressional changes not only of the transcripts of known genes, but also of the transcripts not previously annotated [12]. Non-coding RNA investigation has benefited from these approaches due to the fact that non-coding RNAs cannot be translated to detectable protein. Long non-coding RNAs (lncRNAs, > 200 nucleotides) interact with proteins, DNA, and other RNAs and participate in transcriptional silencing, translational inhibition, or modulating partners of multiprotein complexes. They are emerging as potent and multifunctional regulators in physiological and pathological processes (e.g. embryonic development, cancer, inflammation, and cardiovascular and neurobiological diseases), despite being previously considered as the spurious byproducts of gene transcription [13, 14]. An intriguing association between lncRNAs and neuropathic pain has also been demonstrated recently [10, 11]. A large number of lncRNAs have been comprehensively identified in the pain-related regions (e.g. DRG and spinal cord) of mouse, rat, and human nervous systems [15–18]. The lncRNAs are dysregulated in these regions following peripheral inflammation or nerve injury [15, 19–21]. Moreover, these functional studies reveal that lncRNAs contribute to the development and maintenance of neuropathic pain by regulating pain-associated genes and increasing neuronal excitability in DRG primary sensory neurons [19, 21]. Undoubtedly, lncRNAs are new players in the mechanisms of neuropathic pain.
In this review, we focus on the frontier findings of lncRNAs and the changes of their expression in the DRG, spinal cord dorsal horn, and the injured peripheral nerves from preclinical rodent models of neuropathic pain and in the serum from neuropathic pain patients. We also provide in vitro and in vivo evidence of how peripheral nerve injury causes the dysregulation of lncRNAs in the pain-related regions. The potential mechanisms of how the dysregulated lncRNAs contributes to the pathogenesis of neuropathic pain are discussed. This review provides up-to-date knowledge regarding the role of lncRNAs in neuropathic pain.
2. LncRNAs expressed in DRG primary sensory neurons
Pain signals start as noxious stimuli detected by nociceptors located in peripheral nerve fiber terminals of DRG primary sensory neurons and are transmitted via the DRG primary afferents into the central nervous system, including the spinal cord dorsal horn and pain-associated brain regions. The DRG appears to play a key role in neuropathic pain [22, 23]. Transcriptome analysis of the injured DRG following peripheral nerve injury by microarray and RNA-seq identified a large number of deferentially expressed lncRNAs [20, 24–26]. Evidence indicates the potential involvement of the dysregulated DRG lncRNAs in neuropathic pain.
The first intensively investigated lncRNA in neuropathic pain is an endogenous voltage-gated potassium channel (Kv) Kcna2 antisense (AS) RNA [19]. This new regulatory gene is highly conserved among humans, monkeys, mice, and rats. The Kcna2 AS RNA (2.52 kb in size) is classified as a natural antisense transcript (NAT), which is defined as a processed transcript that is complementary to the corresponding sense transcript in exon regions. It is located in the cytoplasm and specifically targets Kcna2 mRNA, which encodes membrane Kv1.2 subunit involved in the induction of neuropathic pain (Figure 1) [19] [27]. The Kcna2 AS RNA is up-regulated time-dependently in the injured rat DRG after spinal nerve ligation (SNL). Mimicking this upregulation through the delivery of adeno-associated virus (AAV) harboring the full-length Kcna2 AS RNA into the DRG neurons of naive rats significantly reduced basal Kcna2 mRNA level both in vitro and in vivo [19]. Electrophysiological experiments further identified that Kcna2 AS RNA reduced total Kv current and increased excitability of DRG neurons [19]. Animals that received DRG injection of AAV-Kcna2 AS RNA exhibited neuropathic pain symptoms as evidenced by the mechanical and cold hypersensitivities in naive rats [19]. Blocking the nerve injury-induced up-regulation of DRG Kcna2 AS RNA through microinjection of the AAV expressing a complement segment from Kcna2 sense RNA into the injured DRG rescued DRG Kv1.2 expression and alleviated neuropathic pain [19]. Kcna2 AS RNA may be an endogenous trigger in neuropathic pain development and maintenance.
Figure 1.
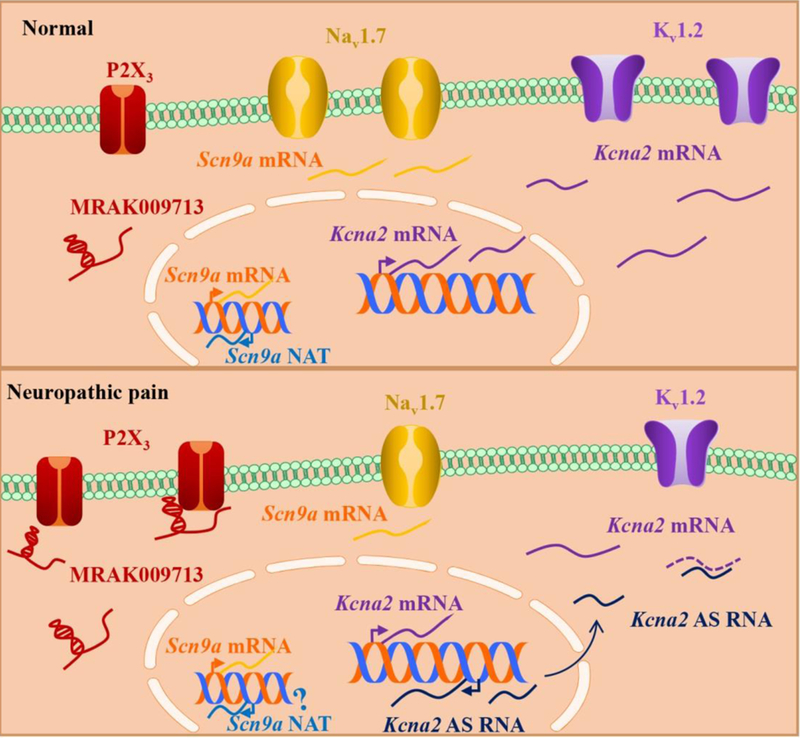
Long noncoding RNAs in dorsal root ganglion neurons contribute to neuropathic pain. Under normal conditions, the expressional level of Kcna2 AS RNA is relatively low. In contrast, Kcna2 AS RNA is up-regulated after nerve injury and specifically inhibits the expression of Kcna2 mRNA in the cytoplasm of the dorsal root ganglion neurons, resulting in the decrease in membrane Kv1.2 and the increased excitability of primary sensory neurons [18]. The Scn9a NAT is found to act as a negative regulator of Scn9a. The role of Scn9a NAT in neuropathic pain condition is still elusive [24]. MRAK009713 contributes to neuropathic pain by directly binding to the nociceptive P2X3 receptors and then regulating its expression and function in dorsal root ganglion neurons [20]. Kcna2: potassium voltage-gated channel subfamily A member 2 gene; Kv1.2: voltage-gated potassium channel 1.2; mRNA: messenger RNA; AS: antisense; P2X3: purinergic receptor P2X 3; Scn9a: sodium voltage-gated channel alpha subunit 9; Nav1.7: voltage-gated sodium channel 1.7; NAT: natural antisense transcript.
The Scn9a NAT is another identified antisense lncRNA coded by the complementary strand of DNA that is opposite to its target Scn9a gene, with the two genes partially overlapping (Figure 1) [28]. The expression levels of these two genes show a discordant pattern in the DRG, with higher levels of Scn9a mRNA and lower level of Scn9a NAT [28]. The Scn9a NAT is proved to act as a negative regulator of Scn9a mRNA. Overexpressing Scn9a NAT reduced Scn9a mRNA, its coding Nav1.7 protein, and Nav1.7 currents in DRG neurons [28]. However, the levels of neither Scn9a mRNA nor Scn9a NAT change significantly in the ipsilateral L4-L6 DRG 3 days following chronic constriction injury (CCI) of the sciatic nerve [28]. Interestingly, Nav1.7 protein and current are both increased in the DRG from a rat model of painful diabetic neuropathy [29, 30], whereas the amount of Nav1.7 protein is decreased in the injured DRG after SNL, spinal nerve injury (SNI), and sciatic nerve axotomy in the animals [31, 32]. The regulation of Nav1.7 by Scn9a NAT under these neuropathic pain conditions is still unknown and remains to be further elucidated.
Using Gene2DGE [33] transcriptome screening in the DRG of rats, several research groups have identified dysregulated lncRNA transcripts associated with diabetes [34, 35]. Among these transcripts, the lncRNA uc.48+ [34, 36], BC168687 [37–39], and NONRATT021972 [35, 40, 41] were found to be involved in diabetic neuropathic pain through purinergic receptors or TRPV1. The levels of these three lncRNAs are markedly increased in the DRG of diabetic neuropathic pain rats [34, 35, 38, 41]. Blocking these increases via intrathecal or intravenous injection of each corresponding siRNA, respectively, alleviated mechanical allodynia and thermal hyperalgesia in diabetic rats [34, 35, 38, 41]. Knockdown of DRG uc.48+ attenuated P2X3 receptor- or P2X7 receptor-mediated immune and inflammatory responses, including cytokine secretion, reactive oxygen species formation, and activation of the extracellular signal-regulated kinase (ERK) 1/2 signaling pathway in the diabetic DRG [34, 36]. Intrathecal BC168687 siRNA blocked the increases in the levels of P2X7 mRNA and protein, phosphorylated-ERK1/2 (p-ERK1/2), and phosphorylated p38 in the DRG and the amounts of nitric oxide, tumor necrosis factor-α (TNF-α), and interleukin-β in serum of diabetic rats [38, 39]. BC168687 siRNA also reduced TRPV1 expression in diabetic DRG [39]. NONRATT021972 was also found to be increased in the serum of patients with type 2 diabetes mellitus [40, 41]. Intravenous injection of NONRATT021972 siRNA attenuated the increases in the levels of P2X3, P2X7, GFAP, p-ERK1/2, and TNF-α in the DRG of diabetic rats [35, 40, 41]. In addition, the lncRNA MRAK009713 was also significantly up-regulated in the injured DRG from CCI rats and contributed to CCI-induced neuropathic pain through regulating the expression and function of DRG P2X3 receptors (Figure 1) [21]. Taken together, these studies indicate the association of these lncRNAs with P2X3 receptor, P2X7 receptor, TRPV1 and downstream signals under neuropathic pain conditions, but the detailed mechanisms of how these lncRNAs regulate purinergic receptors and TRPV1 are still unclear. Moreover, this siRNA strategy has potential off-target effects. Single siRNA effects from these reports should be verified by other approaches. Therefore, the role of these lncRNAs in neuropathic pain remains to be confirmed.
3. LncRNAs expressed in the dorsal horn of the spinal cord
The spinal cord dorsal horn, where the second order of sensory neurons are located, is responsible for relaying and modulating pain-related signals from nociceptors to the supraspinal cord regions [2, 21]. Using the microarray-based profiling approach, a mouse lncRNA gene chip including 25,376 lncRNA probes identified 511 differentially expressed (> 2-fold) lncRNAs in the ipsilateral spinal cord dorsal horn at 10 days after SNL [15]. Pathway annotation and gene ontology analysis showed that the 35 differentially expressed lncRNA-mRNA pairs coordinated in the genome [15] were involved in Toll-like receptor signaling, cytokine-cytokine receptor interaction, and peroxisome proliferator-activated receptor signaling pathway [15]. Recently, sequencing analysis showed that 35, 44, 25, and 15 lncRNAs were up-regulated and 59, 135, 101, and 129 lncRNAs were down-regulated at 1, 3, 7, and 14 days, respectively, in the spinal cord after SNI [17]. These dysregulated lncRNAs are associated with genes that have significant enriched molecular functions, including transporter activity, calcium ion binding, protein binding, anion binding, structural molecular activity, lipid binding, and receptor binding [17]. Dou L et al reported that the expression of lncRNA colon cancer associated transcript-1 (CCAT1) was reduced in the spinal cord dorsal horn, DRG, hippocampus, and anterior cingular cortex from day 1 to day 5 after CCI [42]. Over-expression of lncRNA CCAT1 alleviated CCI-induced mechanical allodynia [42]. However, how lncRNA CCAT1 participates in CCI-induced pain hypersensitivity is unknown. Thus, functional study of lncRNA in the spinal cord dorsal horn is not well documented.
Circular RNAs (circRNAs) are highly stable and have circularized transcripts by back splicing of exons from mRNAs and antisense RNAs [43]. The majority of circRNAs are conserved across species and lack the potency of translation, despite the existence of cap-independent translation [44, 45]. RNA-seq analysis of transcripts from the spinal cord of SNI rats identified that 188 circRNAs as well as 134 lncRNAs and 12 microRNAs (miRNAs) were significantly altered on day 14 after SNI [46]. Another study revealed that up to 363 circRNAs were significantly upregulated and 106 were downregulated in the ipsilateral dorsal horn after CCI [47]. The circRNA-miRNA-mRNA network was constructed in silico by bioinformatics analyses, suggesting that these circRNAs function as miRNA sponges and target mRNAs under neuropathic pain conditions [46, 47]. Although the functioning of circRNAs as efficient miRNA sponges is still under debate, they have been called the competing endogenous RNAs (ceRNAs) to competitively bind the miRNAs and inhibit their biological functions, therefore positively regulating the target genes of miRNA [48]. For example, the deficiency of the circRNA, Cdr1as, down-regulates mature miR-7 expression in excitatory neurons of the brain, resulting in disinhibition of immediate early genes’ expression and in dysfunction of excitatory synaptic transmission [49]. The lncRNA, Cyrano, which is also expressed in neurons of the brain, prevents repression of miR-7-targeted mRNAs and enables accumulation of Cdr1as through directing potent multiple-turnover destruction of miR-7 [50]. After knock-out of Cyrano, excess miR-7 causes cytoplasmic degradation of Cdr1as in mouse neurons, in part through enhanced silence of Cdr1as by a second miRNA, miR-671 [50]. Thus, the interactions among different types of non-coding RNAs may form a sophisticated regulatory network [49, 50]. However, whether and how the dysregulated circRNAs participate in neuropathic pain is still unknown and merits to be further investigated.
4. LncRNAs expressed in peripheral nerves
The demyelination of peripheral sensory fibers after injury participates in neuropathic pain [51]. An antisense lncRNA, which is transcribed for the opposite strand of the proximal promoter of Egr2, was discovered by Martinez-Moreno et al in both mouse and rat sciatic nerves [52]. After peripheral nerve injury, the expression of Egr2 AS RNA is increased and affects the expression of Egr2 mRNA via an epigenetic mechanism [52]. Ectopic expression of Egr2 AS RNA in mouse DRG cultures inhibits the expression of Egr2 mRNA and attenuates myelination of Schwann cells [52]. This work sheds light on the investigation of lncRNAs in peripheral nerves. A microarray-based analysis revealed that a large number of lncRNAs are differentially expressed in the distal segment of the sciatic nerve at different time points following injury [53]. The ectopic expression of the lncRNA, NONMMUG014387, promotes the proliferation of mouse Schwann cells [53]. Yao et al analyzed the microarray data of lncRNAs in rat DRG after sciatic nerve injury and reported the expressional change of a down-regulated lncRNA, uc.217, in regenerative DRG neuronal outgrowth. Knock-down of uc.217 expression could significantly promote neurite outgrowth in cultured DRG neurons [54]. These studies show the regulatory function of plasticity in peripheral nerves by lncRNAs. Nevertheless, it is still obscure if lncRNAs play a role in neuropathic pain genesis.
5. Conclusion
Compared to miRNAs, lncRNAs may specifically target the gene expression as they are much longer in size and selectively bind to specific genes. For example, blocking nerve injury-induced increases in DRG Kcna2 antisense RNA specifically and selectively rescued downregulation of Kcna2 mRNA and alleviated neuropathic pain, without affecting acute pain and locomotor function [19]. Given that virus-mediated gene therapy has been used in clinical trial [55, 56], virus-mediated DRG delivery of Kcna2 sense RNA fragment may open a new avenue for neuropathic pain management. Thus, investigations of the dysregulated lncRNAs in neuropathic pain not only provide insights into the mechanisms underlying this disorder but also open a door to develop new analgesics with greater efficacy and fewer side effects. However, current investigation on the role of lncRNAs in neuropathic pain is in the infancy stage, which challenges the translation of preclinical findings. The clinical efficacy and risks associated with lncRNAs’ therapy also needs to be systematically evaluated through rigid trials.
6. Expert opinion
It’s becoming feasible to profile lncRNAs by high-throughput analysis, such as next generation RNA-seq nowadays. The pioneering studies described above have suggested that a subset of lncRNAs, including circRNAs, are involved in neuropathic pain in different pain-related regions. Except for a few lncRNAs (e.g. Kcna2 AS) [19, 21], the specific functions of most lncRNAs in neuropathic pain are still elusive. Given that the lncRNAs orchestrate the upstream changes in gene transcription, such as transcriptional factors [13, 57], manipulating the key lncRNAs’ expression in pain pathways may effectively prevent and/or treat the development and maintenance of neuropathic pain. Therefore, it is optimistic that lncRNAs will become promising targets for the management of this disorder with the combined efforts of pain researchers and clinical physicians.
7. Future and focus of research
Research regarding the role of lncRNAs in neuropathic pain is still at an early stage compared to other fields (such as cancer research). As discussed above, some lncRNAs from the damaged peripheral nerve, DRG, and spinal cord dorsal horn may play a critical role in neuropathic pain, but the detailed mechanisms of how the majority of these lncRNAs (except for Kcna2 antisense RNA) contribute to this disorder is still elusive. Moreover, the role of most dysregulated lncRNAs identified from RNA sequencing in neuropathic pain is still unclear. It is worthy to explore the functions of lncRNAs in the process of central sensitization in the spinal cord dorsal horn and brain pain-related regions (such as the amygdala and anterior cingulate cortex) [58]. Due to the heterogeneity and complexity of neuropathic pain, investigations of the types of neuropathic pain (e.g., orofacial neuropathic pain vs somatic neuropathic pain) might reveal distinct functional lncRNAs. In addition, single-cell RNA sequencing [59] may disclose specific expression patterns of lncRNAs in sub-populations of DRG sensory neurons and dorsal horn projection neurons or excitatory/inhibitory interneurons. The integration of multiple approaches will enable efficient characterization of lncRNAs in neuropathic pain.
Table 1.
LncRNAs Associated with Neuropathic Pain.
| Neuropathic Pain Models |
Tissue | lncRNAs | Change in Expression |
Targets | References |
|---|---|---|---|---|---|
| SNL | Injured DRG | Kcna2 AS RNA | ↑ | Kcna2 | Zhao et al. [19] |
| SNL | Injured DRG | H19, Gm21781 et al. | ↑/↓ | Unknown | Wu et al. [20] |
| CCI | Injured DRG | MRAK009713 | ↑ | P2X3 receptor | Li et al. [21] |
| Diabetic neuropathy |
DRG | uc.48+ | ↑ | P2X3 receptor | Wang et al. [34] |
| Diabetic neuropathy |
DRG | NONRATT021972 | ↑ | P2X7 receptor, P2X3 receptor, TNF-α et al. |
Liu et al. [35], Yu et al. [40] and Yu et al. [41] |
| Diabetic neuropathy |
DRG | BC168687 | ↑ | P2X7 receptor. TRPV1 et al. |
Liu et al. [38] and Liu et al. [39] |
| SNL | Spinal cord | Speer7-ps, Uc007pbc.1, et al. |
↑/↓ | Unknown | Jiang et al. [15] |
| SNI | Spinal cord | XLOC_041439, Mlxipl, Rn50_X_0739.1 |
↑/↓ | Unknown | Zhou et al. [17] |
| CCI | Spinal cord, DRG, hippocampus, and ACC |
CCAT1 | ↓ | microRNA-155 and SGK3 |
Dou et al. [42] |
| SNI | Spinal cord | rno circ 0004058, Rn50_8_0646.1 et al. |
↑/↓ | Unknown | Zhou et al. [46] |
| CCI | Spinal cord | rno_circRNA_008973, rno_circRNA_007512 et al. |
↑/↓ | Unknown | Cao et al. [47] |
| Sciatic nerve transection |
Peripheral nerves | Egr2 AS RNA | ↓ | Egr2 | Martinez-Moreno et al. [52] |
ACC: anterior cingulate cortex; AS: antisense; CCI: chronic constriction injury; DRG: dorsal root ganglion; LncRNAs: long noncoding RNAs; SNI: spared nerve injury; SNL: spinal Nerve ligation.
Article highlights box.
A large number of lncRNAs, including antisense RNAs and circular RNAs, are dysregulated in pain-related regions in the peripheral and central nerve systems following peripheral nerve injury.
The Kcna2 antisense RNA participates in neuropathic pain through peripheral nerve injury-induced upregulation, and through specific and selective silence of Kcan2 mRNA expression in the injured dorsal root ganglion.
The role of the majority of dysregulated lncRNAs in neuropathic pain is still elusive and remains to be determined.
Acknowledgments
Funding
This work was supported by NIH grants: R01NS094664, R01NS094224, and R01DA033390.
Footnotes
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
Reference List
- 1.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006;52:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieleman JP, Kerklaan J, Huygen FJ, et al. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008;137:681–8. [DOI] [PubMed] [Google Scholar]
- 4.van HO, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155:654–62. [DOI] [PubMed] [Google Scholar]
- 5.Gilron I, Dickenson AH. Emerging drugs for neuropathic pain. Expert Opin Emerg Drugs 2014;19:329–41. [DOI] [PubMed] [Google Scholar]
- 6.Holmes D The pain drain. Nature 2016;535:S2–S3. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85:S3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L, Zhang Y, Huang Y. Advances in the Treatment of Neuropathic Pain. Adv Exp Med Biol 2016;904:117–29. [DOI] [PubMed] [Google Scholar]
- 9.Duehmke RM, Derry S, Wiffen PJ, et al. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev 2017;6:CD003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bali KK, Kuner R. Noncoding RNAs: key molecules in understanding and treating pain. Trends Mol Med 2014;20:437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz BM, Bekker A, Tao YX. Noncoding RNAs: new players in chronic pain. Anesthesiology 2014;121:409–17.**The first review paper to discuss the role of non-coding RNAs including long non-coding RNAs in chronic neuropathic and inflammatory pain.
- 12.Weirick T, Militello G, Muller R, et al. The identification and characterization of novel transcripts from RNA-seq data. Brief Bioinform 2016;17:678–85. [DOI] [PubMed] [Google Scholar]
- 13.Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell 2013;154:26–46.*Imoportant review on lincRNAs.
- 14.Lekka E, Hall J. Noncoding RNAs in disease. FEBS Lett 2018;592:2884–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang BC, Sun WX, He LN, et al. Identification of lncRNA expression profile in the spinal cord of mice following spinal nerve ligation-induced neuropathic pain. Mol Pain 2015;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duran RC, Yan H, Zheng Y, et al. The systematic analysis of coding and long non-coding RNAs in the sub-chronic and chronic stages of spinal cord injury. Sci Rep 2017;7:41008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Fan Y, Chen H. Analyses of long non-coding RNA and mRNA profiles in the spinal cord of rats using RNA sequencing during the progression of neuropathic pain in an SNI model. RNA Biol 2017;14:1810–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray P, Torck A, Quigley L, et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018;159:1325–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Tang Z, Zhang H, et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 2013;16:1024–31.**The first report to demonstrate the role of a long non-coding RNA in neuropathic pain.
- 20.Wu S, Marie LB, Miao X, et al. Dorsal root ganglion transcriptome analysis following peripheral nerve injury in mice. Mol Pain 2016;12.*Using high throughput RNA sequencing approach to report the dysregulaed expression of long interspersed non-coding RNAs in the injured DRG from neuropathic pain mice.
- 21.Li G, Jiang H, Zheng C, et al. Long noncoding RNA MRAK009713 is a novel regulator of neuropathic pain in rats. Pain 2017;158:2042–52. [DOI] [PubMed] [Google Scholar]
- 22.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006;52:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krames ES. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med 2014;15:1669–85. [DOI] [PubMed] [Google Scholar]
- 24.Raju HB, Tsinoremas NF, Capobianco E. Emerging Putative Associations between Non-Coding RNAs and Protein-Coding Genes in Neuropathic Pain: Added Value from Reusing Microarray Data. Front Neurol 2016;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raju HB, Englander Z, Capobianco E, et al. Identification of potential therapeutic targets in a model of neuropathic pain. Front Genet 2014;5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao P, Li CR, Zhang SZ, et al. Transcriptomic differential lncRNA expression is involved in neuropathic pain in rat dorsal root ganglion after spared sciatic nerve injury. Braz J Med Biol Res 2018;51:e7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan L, Guan X, Wang W, et al. Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons. Mol Pain 2014;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koenig J, Werdehausen R, Linley JE, et al. Regulation of Nav1.7: A Conserved SCN9A Natural Antisense Transcript Expressed in Dorsal Root Ganglia. PLoS One 2015;10:e0128830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chattopadhyay M, Mata M, Fink DJ. Continuous delta-opioid receptor activation reduces neuronal voltage-gated sodium channel (NaV1.7) levels through activation of protein kinase C in painful diabetic neuropathy. J Neurosci 2008;28:6652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S, Morrow TJ, Paulson PE, et al. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J Biol Chem 2004;279:29341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berta T, Poirot O, Pertin M, et al. Transcriptional and functional profiles of voltage-gated Na(+) channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol Cell Neurosci 2008;37:196–208. [DOI] [PubMed] [Google Scholar]
- 32.Kim CH, Oh Y, Chung JM, Chung K. Changes in three subtypes of tetrodotoxin sensitive sodium channel expression in the axotomized dorsal root ganglion in the rat. Neurosci Lett 2002;323:125–8. [DOI] [PubMed] [Google Scholar]
- 33.Tang X, Deng L, Zhang D, et al. Gene2DGE: a Perl package for gene model renewal with digital gene expression data. Genomics Proteomics Bioinformatics 2012;10:51–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Xu H, Zou L, et al. LncRNA uc.48+ is involved in diabetic neuropathic pain mediated by the P2X3 receptor in the dorsal root ganglia. Purinergic Signal 2016;12:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Zou L, Xie J, et al. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol Brain 2016;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H, Wen F, Jiang M, et al. LncRNA uc.48+ is involved in the diabetic immune and inflammatory responses mediated by P2X7 receptor in RAW264.7 macrophages. Int J Mol Med 2018;42:1152–60. [DOI] [PubMed] [Google Scholar]
- 37.Liu CL, Deng ZY, Du ER, Xu CS. Long noncoding RNA BC168687 small interfering RNA reduces high glucose and high free fatty acidinduced expression of P2X7 receptors in satellite glial cells. Mol Med Rep 2018;17:5851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Tao J, Wu H, et al. Effects of LncRNA BC168687 siRNA on Diabetic Neuropathic Pain Mediated by P2X7 Receptor on SGCs in DRG of Rats. Biomed Res Int 2017;2017:7831251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Li C, Deng Z, et al. Long Non-coding RNA BC168687 is Involved in TRPV1-mediated Diabetic Neuropathic Pain in Rats. Neuroscience 2018;374:214–22. [DOI] [PubMed] [Google Scholar]
- 40.Yu W, Zhao GQ, Cao RJ, et al. LncRNA NONRATT021972 Was Associated with Neuropathic Pain Scoring in Patients with Type 2 Diabetes. Behav Neurol 2017;2017:2941297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng H, Zou L, Xie J, et al. lncRNA NONRATT021972 siRNA Decreases Diabetic Neuropathic Pain Mediated by the P2X3 Receptor in Dorsal Root Ganglia. Mol Neurobiol 2017;54:511–23. [DOI] [PubMed] [Google Scholar]
- 42.Dou L, Lin H, Wang K, et al. Long non-coding RNA CCAT1 modulates neuropathic pain progression through sponging miR-155. Oncotarget 2017;8:89949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van RD, Verheijen BM, Pasterkamp RJ. Circular RNAs: Novel Regulators of Neuronal Development. Front Mol Neurosci 2016;9:74.Imoportant review on circRNAs.
- 44.You X, Vlatkovic I, Babic A, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci 2015;18:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pamudurti NR, Bartok O, Jens M, et al. Translation of CircRNAs. Mol Cell 2017;66:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J, Xiong Q, Chen H, et al. Identification of the Spinal Expression Profile of Non-coding RNAs Involved in Neuropathic Pain Following Spared Nerve Injury by Sequence Analysis. Front Mol Neurosci 2017;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao S, Deng W, Li Y, et al. Chronic constriction injury of sciatic nerve changes circular RNA expression in rat spinal dorsal horn. J Pain Res 2017;10:1687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature 2013;495:384–8. [DOI] [PubMed] [Google Scholar]
- 49.Piwecka M, Glazar P, Hernandez-Miranda LR, et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 2017;357. [DOI] [PubMed]
- 50.Kleaveland B, Shi CY, Stefano J, Bartel DP. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell 2018; [DOI] [PMC free article] [PubMed]
- 51.Ueda H Peripheral mechanisms of neuropathic pain - involvement of lysophosphatidic acid receptor-mediated demyelination. Mol Pain 2008;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez-Moreno M, O’Shea TM, Zepecki JP, et al. Regulation of Peripheral Myelination through Transcriptional Buffering of Egr2 by an Antisense Long Non-coding RNA. Cell Rep 2017;20:1950–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan B, Zhou HX, Liu Y, et al. Time-dependent differential expression of long non-coding RNAs following peripheral nerve injury. Int J Mol Med 2017;39:1381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao C, Wang J, Zhang H, et al. Long non-coding RNA uc.217 regulates neurite outgrowth in dorsal root ganglion neurons following peripheral nerve injury. Eur J Neurosci 2015;42:1718–25. [DOI] [PubMed] [Google Scholar]
- 55.Fink DJ, Wechuck J, Mata M, et al. Gene therapy for pain: results of a phase I clinical trial. Ann Neurol 2011;70:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfe D, Mata M, Fink DJ. Targeted drug delivery to the peripheral nervous system using gene therapy. Neurosci Lett 2012;527:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Briggs JA, Wolvetang EJ, Mattick JS, et al. Mechanisms of Long Non-coding RNAs in Mammalian Nervous System Development, Plasticity, Disease, and Evolution. Neuron 2015;88:861–77. [DOI] [PubMed] [Google Scholar]
- 58.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015;18:145–53. [DOI] [PubMed] [Google Scholar]


