Summary.
Background:
Neutrophil extracellular traps (NETs) are decondensed chromatin fibers that might play a role in the prothrombotic state of cancer patients.
Objectives:
To investigate whether the levels of citrullinated histone H3 (H3Cit), a biomarker for NET formation, cell-free DNA (cfDNA) and nucleosomes predict venous thromboembolism (VTE) in cancer patients.
Patients/Methods:
Nine-hundred and forty-six patients with newly diagnosed cancer or progression after remission were enrolled in this prospective observational cohort study. H3Cit, cfDNA and nucleosome levels were determined at study inclusion, and patients were followed for 2 years. VTE occurred in 89 patients; the cumulative 3-month, 6-month, 12-month and 24-month incidence rates of VTE were 3.7%, 6.0%, 8.1%, and 10.0%, respectively.
Results:
Patients with elevated H3Cit levels (> 75th percentile of its distribution, n 236) experienced a higher cumulative incidence of VTE (2-year risk of 14.5%) than patients with levels below this cut-off (2-year risk of 8.5%, n = 710). In a competing-risk regression analysis, a 100 ng ML−1 increase in H3Cit level was associated with a 13% relative increase in VTE risk (subdistribution hazard ratio [SHRI 1.13, 95% confidence interval [Cll 1.04—L22). This association remained after adjustment for high VTE risk and very high VTE risk tumor sites, D-dimer level, and soluble P-selectin level (SHR 1.13, Cl 1.04–1.22). The association of elevated nucleosome and cfDNA levels with VTE risk was time-dependent, with associations with a higher risk of VTE only during the first 3—6 months.
Conclusion:
These data suggest that biomarkers of NET formation are associated with the occurrence of VTE in cancer patients, indicating a role of NETs in the pathogenesis of cancerassociated thrombosis.
Keywords: cancer, H3Cit, neutrophil extracellular traps, thrombosis, venous thromboembolism
Introduction
Neutrophil extracellular traps (NETs) are decondensed chromatin fibers decorated with granular enzymes that are released from activated neutrophils. The antimicrobial properties of NETs were discovered first, showing that NETs immobilize and kill bacteria and fungi [1]. More recently, it has been shown that NETs also interact with the blood coagulation system [2]. Mechanisms include activation of factor XII [31, binding of von Willebrand factor [4], providing a scaffold for platelet adhesion and fibrin deposition [5], and inactivation of tissue factor pathway inhibitor [6]. In animal models, NETs accumulate early in growing thrombi [2], and treatment with DNAse 1 dismantles NETs, diminishing thrombus formation [3,7].
Patients with cancer have a high risk of developing venous thromboembolism (VTE) [8–10]. However, the mechanisms by which cancer induces a prothrombotic state leading to overt cancer-associated thrombosis are not yet fully understood. Experimental data indicate that NETs could play an important role in cancer-associated coagulation activation. In murine cancer models, leukocytes were primed by cancer cells to release NETs, and this was associated with spontaneous venous thrombus formation [11].
Recently, methods to indirectly quantify NETs in human plasma samples have been developed [12,13]. Citrullinated histone H3 (H3Cit) has been proposed as a target biomarker reflecting NET formation [11,12]. Other putative biomarkers for NETs include cell-free DNA (cfDNA) and nucleosomes, which may also have other sources than NET formation, such as cellular decay or apoptosis.
In recent years, clinical and laboratory risk factors for prediction of VTE in cancer patients have been identified, in order to stratify patients according to their risk of VTE during the course of disease [14]. In this prospective cohort study, we hypothesized that biomarkers reflecting NET formation in patients with cancer could predict the individual propensity to develop future VTE. The clinical investigation of NET-specific and NET-related biomarkers could also lead to novel insights regarding the pathogenesis of cancer-related VTE. To examine this hypothesis, we quantified H3Cit, cfDNA and nucleosome levels in the plasma of cancer patients, and followed them for the occurrence of VTE over a period of 2 years.
Methods
Study design and population
The Vienna Cancer and Thrombosis Study (CATS) is a single-center, ongoing, prospective, observational cohort study conducted at the Medical University of Vienna. Detailed inclusion and exclusion criteria have been reported previously [15, 16]. In brief, CATS enrols adult patients with: (i) newly diagnosed malignancy or progression of disease after remission; and (ii) the capacity to give written informed consent and comply with scheduled study procedures. Exclusion criteria are overt infection, thromboembolic events within the last 3 months, or continuous anticoagulation with low molecular weight heparin or vitamin K antagonists, radiotherapy or surgery within the last 2 weeks, and chemotherapy within the past 3 months. The primary endpoint of CATS is symptomatic or fatal, objectively confirmed VTE. Objective methods for diagnosis include computed tomography (CT) pulmonary angiography of the chest or ventilation/perfusion scan for symptomatic pulmonary embolism (PE), autopsy, and compression ultrasound or venography for deep vein thrombosis (DVT). At baseline, study patients underwent a detailed interview and chart review to ascertain clinical and pathological variables, and venous blood was drawn for biobank storage. Patients were followed up for 2 years. All study events were adjudicated by an independent panel of experts. So-called unsuspected PE (i.e. PE incidentally detected on restaging CT scans) and abdominal vein thromboses were included as events if the adjudication committee deemed the event to be of clinical significance. No routine screening for VTE was performed. Mortality was prespecified as a secondary endpoint. The study protocol was approved by the local institutional review board (EC number 126/2003; ethikkom@meduniwien.ac.at).
Recruitment in CATS started in 2003; however, unthawed plasma samples from patients recruited in 2003—2007 were already considerably limited. Furthermore, only samples from patients enrolled 2 years before the initiation of this study were analyzed, to ensure that VTE and mortality outcome data from the 2-year observation period were available. This gave a total of 1075 patients enrolled between 4 June 2007 and 28 October 2013, resulting in a median storage time between enrollment and measurement of 6.0 years (25th—75th percentile, 4.5—7.9; range, 2.6—9.3). Forty-four of these were immediately excluded because they did not meet the inclusion or exclusion criteria. A further 47 patients were excluded because no follow-up was available, and for 37 patients baseline blood samples were no longer available. After exclusion of one further patient with an implausible H3Cit reading (1022 times higher than the median H3Cit measurement), this left a final analysis population of 946 cancer patients. Potential influences of storage time on the association between NET biomarkers and VTE risk were specifically analyzed (see Results).
Laboratory analysis
Venous blood samples were collected at the time of study entry. Aliquots of platelet-poor plasma (centrifugation for 10 min at 3000 × g) were stored at — 80 oC. All biomarker analyses were performed in duplicate, and the duplicate mean was used for statistical analysis. H3Cit and cfDNA were expressed in ng mL-l, and nucleosomes were expressed as ratios to those in healthy controls.
Nucleosomes and cfDNA.
Nucleosomes and cfDNA were analyzed from plasma samples with Cell Death Detection ELISAPLUS (Roche Diagnostics, Mannheim, Germany) and a Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA), respectively, and according to the manufacturers’ instructions. Nucleosome results were compared with those from pooled plasma from five young male healthy controls to obtain a multiple of the median.
H3Cit ELISA.
H3Cit levels were determined according to Thalin et al. [12]. In detail, 96-well plates were coated with an anti-histone antibody (Cell Death Detection ELISA; Sigma Aldrich, st Louis, MO, USA) overnight at 4 oC, and blocked with the incubation buffer. H3Cit standards were obtained by incubation of recombinant human peptidylarginine deiminase 4 (PAD4) (Cayman Chemicals, Ann Arbor, MI, USA) and recombinant human histone 113.1 (New England Biolabs, Evry, France). H3Cit standards and plasma samples were incubated at room temperature and washed with 0.05% phosphate-buffered saline (PBS) containing Tween-20, and anti-H3Cit antibody (l : 1000 ab5103; Abeam, Cambridge, MA, USA; diluted in 1% PBS—bovine serum albumin [BSA]), was added for 1.5 h. After another washing step, anti-rabbit horseradish peroxidase (HRP)-conjugated antibody (1 : 5000 goat anti-rabbit lgG HRP [Bio-Rad Labora-tones, Hercules, CA, USA] in PBS-BSA) was incubated for 1 h, and washing was performed again. After incubation with 3,3’,5,5’-tetramethylbenzidine (Sigma Aldrich) for 25 min, the reaction was stopped with 2% sulfuric acid and read at 450 nm. When the absorbance of a patient reading was lower than that of the buffer blank, the 1–13Cit measurement was set to zero ng mL−1.
Statistical analysis
All statistical analyses were performed with STATA (Version 14.0; stata corp., Houston, TX, USA). Continuous variables were reported as medians [25th—75th percentile], and count data were summarized as absolute frequencies (percentages). Correlations were assessed with Spearman’s rank-based correlation coefficient. The distribution of continuous variables between two or more groups, such as 1–13Cit levels between patients with and without a history of thrombosis, or between different tumor sites, was compared by the use of Wilcoxon’s rank-sum tests and Kruskal—Wallis tests, respectively. The median follow-up was estimated with the method of Schemper and Smith [17]. Competing-risk estimators with 95% confidence intervals (CIS) were used for estimation of the cumulative incidence of VTE (STATA routine stcompet) [18]. VTE functions between two or more groups were compared by the use of Gray’s test (self-written command stgrays) [19]. Univariable and multivariable modeling of time to VTE was performed with Fine and Gray proportional subdistribution hazards models [20]. For multivariable analysis, variables with a known and/or strong univariable association with VTE were selected. We also included neutrophil count and metastatic disease, because they are of interest mechanistically. In all of these analyses, death from causes other than fatal VTE was considered to be the competing event of interest [21]. The proportional hazards assumption was assessed by fitting interactions between the variables of interest and follow-up time. To further explore non-proportionality of hazards, time-dependent VTE rates were predicted from flexible parametric timeto-event models (user-contributed routine stpm2) [22]. Here, modeling was performed on the log cumulative VTE subdistribution function after addition of timedependent weights (user-contributed routine stcrprep) [23]. H3Cit, cfDNA and nucleosome levels were dichotomized into binary variables at the 75th percentile of their distribution [24]. To compare the magnitude of associations between H3Cit levels and previously published biomarkers for cancer-associated VTE risk, we Z-standardized these variables by subtracting the mean and dividing by the standard deviation, and compared them univariably by use of a forest plot [15].
For multivariable modeling, tumor sites with a high or very high VTE risk were defined according to Khorana et al. [25], with additions by Ay et al. [24] (high risk – lung, colon, kidney, myeloma, lymphoma, and gynecologiCal; very high risk brain, stomach, and pancreas).
Results
Analysis at baseline
Nine-hundred and forty-six patients were included in the analysis. At baseline, most patients had newly diagnosed disease (n = 701, 74.1%), and the median age was 62 years (25th—75th percentile: 52—69). The most frequent tumor sites were lung (n — 182, 19.2%), lymphoma (n - 160, 16.9%), and breast (n - 132, 14.0%) (Table 1).
Table 1.
Distribution of baseline variables overall and by citrullinated histone H3 (H3Cit) levels (n = 946)
| variable | n(% missing) | Overall (n=946) | H3Cit ≤ Q3 (n=710) | H3Cit > Q3 (n=236) | P | Rho(P) |
|---|---|---|---|---|---|---|
| Clinical variables | ||||||
| Age at entry (years) | 946 (0.0) | 61.7 (51.6–68.5) | 61.9 (52.0–68.4) | 60.7 (49.6–69.2) | 0.45 | 0.00 (0.90) |
| BMI (kg/m2) | 944 (0.2) | 25.1 (22.4–28.4) | 25.1 (22.4–28.3) | 24.9 (22.2–28.4) | 0.96 | 0.01 (0.86) |
| Female gender | 946 (0.0) | 443 (46.8) | 322 (45.4) | 121 (51.3) | 0.11 | – |
| Tumor site | 946 (0.0) | – | – | – | 0.11 | – |
| Brain | – | 129 (13.6) | 89 (12.5) | 40 (17.0) | – | – |
| Breast | – | 132 (14.0) | 99 (13,9) | 33 (14.0) | – | – |
| Bronchus | – | 182 (19.2) | 142 (20.0) | 40 (17.0) | – | – |
| Stomach | – | 26 (2.8) | 18 (2.5) | 8 (3.4) | – | – |
| Colorectal | – | 68 (7.2) | 51 (7.2) | 17 (7.2) | – | – |
| Pancreas | – | 75 (7.9) | 54 (7.6) | 21 (8.9) | – | – |
| Kidney | – | 22 (2.3) | 19 (2.7) | 3 (1.3) | – | – |
| Prostate | – | 40 | 30 (4.2) | 10 (4.2) | – | – |
| Myeloma | – | 29 (3.1) | 26 (3.7) | 3 (1.3) | – | – |
| Lymphoma | – | 160 (16.9) | 128 (18.0) | 32 (13.6) | – | – |
| Other site | – | 83 (8.8) | 54 (7.6) | 29 (12.3) | – | – |
| VTE risk tumor sites* | 946 (0.0) | 0.01 | – | |||
| Low/moderate VTE risk | – | 172 (18.2) | 129 (18.2) | 43 (18.2) | – | – |
| High VTE risk | – | 544 (57.5) | 420 (59.2) | 124 (52.5) | – | – |
| very high VTE risk | – | 230 (24.3) | 161 (22.7) | 69 (29.2) | – | – |
| Tumor stage† | 593 (20.2) | – | – | – | 0.01 | – |
| I | – | 64 (10.8) | 53 (12.1) | 11 (7.1) | – | – |
| II | – | 129 (21.8) | 92 (21.0) | 37 (23.9) | – | – |
| III | – | 132 (22.3) | 108 (24.7) | 24 (15.5) | – | – |
| IV | – | 268 (45.2) | 185 (42.2) | 83 (53.6) | – | – |
| Laboratory parameters and biomarkers | ||||||
| Hemoglobin (g dL−1 ) | 944 (0.2) | 13.0 (11.7–14.1) | 13.1 (11.8–14.1) | 12.9 (11.4–14.0) | 0.22 | 0.06 (0.08) |
| Leukocyte count (g L−1 ) | 943 (0.3) | 7.3 (5.7–9.7) | 7.0 (5.6–9.3) | 8.2 (6.0–10.6) | 0.001 | 0.14 (< 0.001) |
| Absolute neutrophil count (g L−1) | 808 (14.6) | 5.0 (3.6–6.9) | 4.8 (3.6–6.5) | 5.5 (3.8–7.9) | 0.001 | 0.14 (<0.001) |
| Platelet count (g L−1 ) | 944 (0.2) | 252 (199–317) | 251 (200–315) | 254 (196–327) | 0.91 | 0.04 (0.22) |
| D-dimer (pg mL−1) | 939 (0.7) | 0.6 (0.3–1.4) | 0.6 (0.3–1.2) | 0.8 (0.3–2.1) | 0.001 | 0.12 (< 0.001) |
| sP-selectin (ng mL−1 ) | 942 (0.4) | 34.7 | 33.9 (25.244.1) | 35.3 (25.848.8) | 0.04 | 0.09 (0.005) |
| Factor VII(%) | 881 (6.9) | 193 (153–246) | 191 (149–243) | 208 (166–258) | 0.005 | 0.10 (0.002) |
| Prothrombin fragment 1 + 2 (pmol L−1) | 928 (1.9) | 199 (144−−277) | 194 (141–268) | 218 (152–323) | 0.003 | 0.11 (<0.001) |
| Fibrinogen (mg dL−1 ) | 936 (1.1) | 369 (297450) | 367 (297–446) | 374 (297466) | 0.71 | 0.06 (0.09) |
BMI, body mass index; (23, 75th percentile of the H3Cit distribution; sP-selectin, soluble P-selectin; VTE, venous thromboembolism. Categorical variables are reported as absolute frequencies and percentages; continuous data are reported as medians with QI—Q3 (with Q1—Q3 representing the first and third quartiles of the H3Cit distribution); the cut-off for Q3 is set at 88.3 ng mL—1. P-values are from chi-squared tests for categorical variables, and rank-sum tests for continuous variables. Rho (P) indicates Spearman’s rank correlation coefficient between H3Cit and the respective variable with P-value (no adjustment for multiplicity performed).
High VTE risk or very high VTE risk tumor sites are defined according to Khorana et al. [25], with additions by Ay et al. [24] (high risk— lung, colon, kidney, myeloma, lymphoma, and gynecological; very high risk — brain, stomach, and pancreas).
Staging cannot be provided for some patients, because no UICC-conforming staging system (i.e. stages I, II, III, and IV) is defined for some tumor entities (such as glioblastoma and multiple myeloma); thus, the missingness proportion was based on 788 patients with potentially observable staging instead of the 946 overall patients.
The H3Cit level was below the detection limit in 219 patients (23.2%). H3Cit, cfDNA and nucleosome levels were directly correlated with each other, with weak correlations being seen between H3Cit and cfDNA levels (Spearman’s rho − 0.16, P < 0.0001) and H3Cit and nucleosome levels (rho 0.18, P < 0.0001), and a moderate correlation being seen between cfDNA and nucleosome levels (rho 0.50, P < 0.0001). The absolute neutrophil count was weakly correlated with H3Cit levels (rho = 0.14, P = 0.0001), cfDNA (rho = 0.17, P < 0.0001), and nucleosome levelss (rho = 0.15, P < 0.0001). Patients with an elevated H3Cit level (defined as H3Cit level > 75th percentile of its distribution, i.e. Q3, n 236) were more likely to have metastatic disease than patients with levels below this cut-off (Table 1). Furthermore, patients with elevated H3Cit levels had higher average levels of some previously reported biomarkers of cancer-associated VTE risk, such as FVIll and prothrombin fragment 1 + 2. Average T13Cit, cfDNA and nucleosome levels differed among tumor types (Kruska1-Wa11is P = 0.02, P = 0.0001, and P = 0.0001, respectively; Table 2). The highest H3Cit levels were observed in prostate cancer, and the lowest levels in multiple myeloma.
Table 2.
Levels of citrullinated histone 113 (H3Cit), cell-free DNA (cfDNA) and nucleosomes by venous thromboembolism (VTE) event status and tumor type
| H3Cit (ng mL−l) |
cfDNA (ng mL −l) |
Nucleosomes (MoM) |
||||
|---|---|---|---|---|---|---|
| Patient group | Median | Q1-Q3 | Median | Q1-Q3 | Median | Q1-Q3 |
| Overall (n = 946) | 26.0 | 2.0–88.3 | 359.2 | 303.6–442.6 | 1.2 | 0.5–3.0 |
| No VTE during follow-up (n = 857) | 24.1 | 1.5–84.o | 355.8 | 302.04–40.7 | 1.2 | 0.5–3.0 |
| VTE during follow-up (n= 89) | 52.4 | 11.8–153.0 | 384.5 | 322.0–461.4 | 1.3 | 0.6–3.2 |
| Brain (n= 129) | 38.0 | 8.3–108.6 | 370.6 | 318–0436.9 | 1.4 | 0.6–2.9 |
| Breast (n= 132) | 23.1 | 5.6–88.4 | 315.9 | 264.1–373.6 | 0.8 | 0.4–1.5 |
| Bronchus (n= 182) | 22.0 | 0–80.2 | 378.0 | 320.7–457.2 | 1.3 | 0.6–3.2 |
| Stomach (n= 26) | 33.5 | 0–96.3 | 440.2 | 304.8–560.7 | 2.0 | 0.8–3.8 |
| Colorectal (n= 68) | 23.0 | 1.3–86.3 | 359.8 | 310.6–463.1 | 0.8 | 0.5–2.3 |
| Pancreas (n= 75) | 33.2 | 10.3–129.3 | 402.2 | 316.6–477.1 | 1.1 | 0.6–3.1 |
| Kidney (n= 22) | 19.5 | 0–81.0 | 385.4 | 313.3–442.3 | 1.8 | 0.9–3.1 |
| Prostate (n=40) | 44.9 | 4.0–100.1 | 328.2 | 297.5–393.6 | 1.3 | 0.7–3.6 |
| Myeloma (n= 29) | 21.0 | 0–61.8 | 344.5 | 277.1–414.7 | 1.1 | 0.5–2.3 |
| Lymphoma (n= 160) | 21.0 | 0–61.8 | 378.4 | 309.1–459.7 | 1.9 | 0.8–3.8 |
MoM, multiple of the median; QI and Q3, first and third quartiles of the biomarker’s distribution. Parameters of ‘other site’ tumor are not given, as this group contains various sites of cancer.
Storage time did not influence H3Cit levels. In detail, H3Cit levels increased by 1.3 ng mL−1 per year of storage (95% CI – 3.9 co 6.4, P- 0.636), and storage time explained only 0.002% of the variation in H3Cit levels (R2 — 0.0002). Storage time minimally influenced cfDNA levels, which increased by 11.5 ng mL−1 per year of storage (95% CI 5.6 –17.4, P < 0.01), but explained only 1.6% of the variation in cfDNA levels (R2 - 0.0155, P 0.51). In contrast, nucleosome levels significantly increased, by 0.5-fold per year of storage time (95% CI 0.45—0.63, P < 0.0001), and storage time explained 13% of the total variation in nucleosome levels (R2 = 0.134). Multivariable adjustment of the association between the levels of the three biomarkers and VTE risk for storage time did not alter the previously observed results (Table S1).
Occurrence of VTE
During the follow-up period, we observed 89 VTE events. The most frequent types of event were PE (n — 36, 40.4%) and lower-extremity DVT (n – 30, 33.7%). Upper-arm/jugular vein DVT occurred in eight patients (9.0%), concomitant PE and DVT in six patients (6.7%), and fatal PE in four patients (4.5%). The remaining five events (5.6%) were splanchnic vein thromboses. In competing risk analysis accounting for death from any cause except fatal VTE as the competing event, the cumulative 3-month, 6-month, 12-month, and 24-month incidence rates of VTE were 3.7% (95% Cl 2.6–5.1), 6.0% (95% Cl 4.6–7.7), 8.1% (95 0/0 Cl 6.5–10.0), and 10.0% (95% CI 8.1—12. l), respectively. With 352 deaths and a 24-month mortality of 39.8% (95% Cl 36.6–43.1), death was clearly present as a competing risk.
H3Cit, cfDNA and nucleosome levels and the risk of VTE
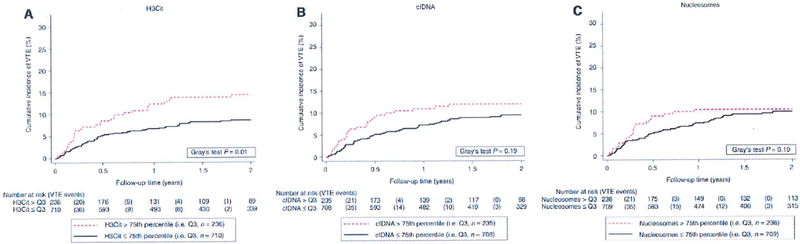
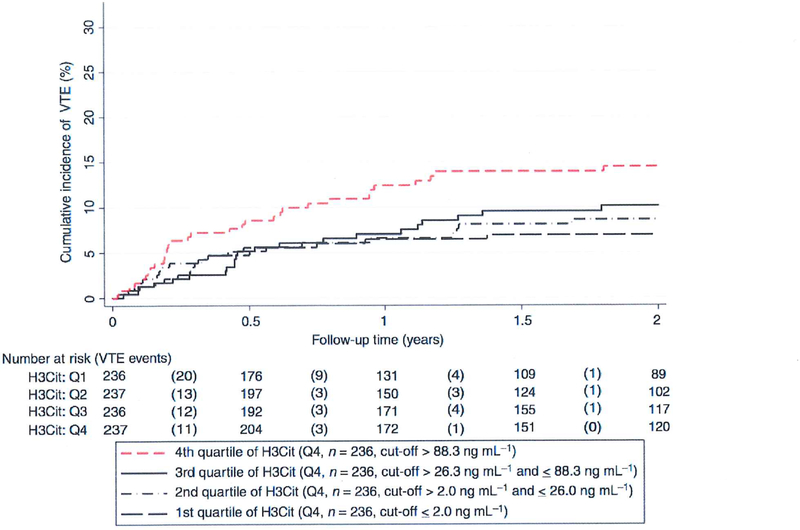
Average levels of H3Cit (P - 0.005), but not of cfDNA (P — 0.08) or of nucleosomes (P — 0.95), were statistically significantly higher in patients who developed VTE during the 2-year follow-up period (Table 2). In competing-risk analysis, patients with elevated H3Cit levels had a higher VTE risk. In detail, in the 236 patients with an H3Cit baseline measurement > 75th percentile (88.3 ng mL-l ) of its distribution, the cumulative VTE risks after 6 months, 1 year and 2 years were 8.6% (95% Cl 5.4–12.6), 12.4 % (95% CI 8.5–17.1), and 14.5% (95% CI 10.2–19.5), as compared with 5.2% (95% CI 3.7–7.0), 6.70/0 (95% CI 5.0–8.7) and 8.5 % (95% CI 6.6–10.8) in the 710 patients with H3Cit levels at or below this cut-off (Gray’s test P = 0.01; Fig. 1A). The corresponding 2-year risks for cfDNA levels > 75th percentile versus 75th percentile were 12.0% (95% CI 8.1–16.6) and 9.4% (95% CI 7.3–11.8) (Gray’s test P = 0.19; Fig. 1B), and those for nucleosome levels > 75th percentile versus 75th percentile were 0.4% (95% CI 6.9–14.8) and 9.9% (95% CI 7.8–12.4) (Gray’s test P- 0.60; Fig. 1C). In competing-risk time-to-VTE regression, a 100 ng mL increase in H3Cit level was associated with a 13% relative increase in VTE risk (subdistribution hazard ratio [SHR] 1.13, Cl 1.04–1.22, P = 0.003; Table 3). This association remained after multivariable adjustment for high and very high VTE risk tumor entities (adjusted SHR for H3Cit 1.13, Cl 1.04–1.22, P = 0.004), and after further adjustment for D-dimer and soluble P-selectin (sP-selectin) levels. Moreover, we included body mass index, neutrophil count and tumor stage in the univariable model (Table 3) and multivariable model (Table S2). In contrast to previous studies [25,26], we found no association between VTE risk and leukocyte count, which was therefore not included in our multivariable model. In multivariable models I and 2, the adjusted SHR for H3Cit levels was 1.11 (95% Cl 1.03–1.20, P = 0.008; Table 3). A multivariable adjustment for ‘newly diagnosed’ patients showed only negligible changes (data not shown). Whereas patients with elevated H3Cit levels (i.e. > Q3) had an elevated risk of VTE, non-linear analysis showed comparable VTE risks between the three lower quartiles (Fig. 2).
Fig. 1.
Cumulative incidence of VTE according to baseline H3Cit, cfDNA and nucleosome levels. The cumulative incidence of VTE was estimated accounting for death-from-any-cause (except fatal VTE which is a VTE event) as a competing risk; Note the scaling of the y-axis from 0% to of VTE risk. cfDNA, cell-free DNA; H3Cit, citrullinated histone H3; VTE, venous thromboembolism. [Color figure can be viewed at wileyonlinelibrary.com]
Table 3.
Association between citrullinated histone H3 (H3Cit), cell-free DNA (cfDNA), nucleosomes, and time to venous thromboembolism (VTE) (univariable and multivariable competing-risk regression models)
| Analysis | Variable | SHR | 95% Cl | P-value |
|---|---|---|---|---|
| Univariable analysis | H3Cit (per 100 ng mL−1 increase) | 1.13 | 1.04–1.22 | 0.003 |
| H3Cit > Q3 (i.e. 75th percentile) | 1.76 | 1.14–2.72 | 0.01 | |
| H3Cit (per one standard deviation increase) | 1.21 | 1.07–1.37 | 0.003 | |
| cfDNA (per 100 ng mL −1 increase) | 1.03 | 0.96–1.10 | 0.42 | |
| cfDNA > Q3 (i.e. 75th percentile) | 1.36 | 0.86–2.14 | 0.19 | |
| Nucleosomes (per one unit increase) | 0.95 | 0.89–1.02 | 0.16 | |
| Nucleosomes > Q3 (i.e, 75th percentile) | 1.12 | 0.70–1.80 | 0.64 | |
| Low/moderate VTE risk tumor sites (n= 172) | Ref. | Ref. | Ref. | |
| High VTE risk tumor sites (n= 544) | 1.89 | 0.93–3.85 | 0.08 | |
| Very high VTE risk tumor sites (n= 230) | 2.59 | 1.22–5.51 | 0.01 | |
| D-dimer (per 10 μg mL−1 increase) | 1.49 | 1.15–1.94 | 0.002 | |
| sP-selectin (per 10 ng mL−1 increase) | 1.12 | 1.01–1.25 | 0.03 | |
| BMI (per 5 kg m−2 increase) | 1.33 | 1.09–1.63 | 0.005 | |
| Absolute neutrophil count (per doubling) | 1.40 | 1.01–1.85 | 0.04 | |
| Stage IV (i.e. metastatic disease) | 1.25 | 0.80–1.94 | 0.32 | |
| Multivariable model 1 | H3Cit (per 100 ng mL −1 increase) | 1.13 | 1.04–1.22 | 0.004 |
| Low/moderate VTE risk tumor sites (n= 172) | Ref. | Ref. | Ref. | |
| High VTE risk tumor sites (n= 544) | 1.94 | 0.96–3.96 | 0.07 | |
| Very high VTE risk tumor sites (n= 230) | 2.58 | 1.21–5.49 | 0.01 | |
| Multivariable model 2 | H3Cit (per 100 ng mL −1 increase) | 1.11 | 1.03–1.20 | 0.008 |
| Low/moderate VTE risk tumor sites (n= 172) | Ref. | Ref. | Ref. | |
| High VTE risk tumor sites (n= 544) | 1.80 | 0.87–3.69 | 0.11 | |
| Very high VTE risk tumor sites (n= 230) | 2.21 | 1.02–4.81 | 0.05 | |
| D-dimer (per 10 μg mL−1 increase) | 1.31 | 1.00–1.73 | 0.05 | |
| sP-selectin (per 10 ng mL−1 increase) | 1.05 | 0.93–1.18 | 0.44 |
BMI, body mass index; Cl, confidence interval; Q3, 75th percentile of the variable’s distribution; Ref., reference category; SHR, subdistribution hazard ratio; sP-selectin, soluble P-selectin. All data are derived from Fine and Gray proportional subdistribution hazard models. The tumor site variable is defined as in Table 1.
Fig. 2.
Cumulative incidence of VTE according to quartiles of H3Cit levels. The cumulative incidence of VTE was estimated accounting for death-from-any-cause (except fatal VTE which is a VTE event) as a competing risk; Note the scaling of the y-axis from 0% to 30% of VTE risk. H3Cit cut-offs at the 25th, 50th, and 75th percentile were 2.0ng/mL, 26.0ng/mL, and 88.3ng/mL, respectively. 1–13Cit, citrullinated histone H3; VTE, venous thromboembolism. [Color figure can be viewed at wileyonlinelibrary.com]
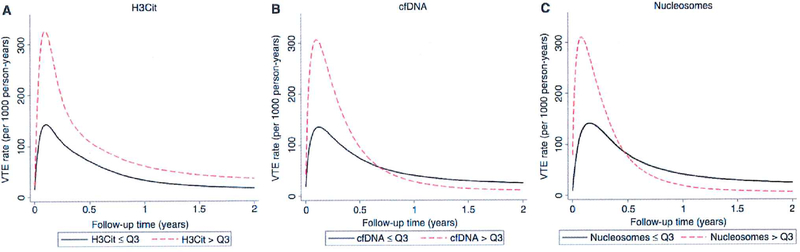
The association between high H3Cit levels and VTE was constant during the whole 2-year follow-up (Fig. 3A), whereas the association of cfDNA and nucleosome levels with VTE was strongly time-dependent (Fig. 3B, C). By fitting an interaction between linear follow-up time and elevated cfDNA levels (interaction SHR 0.26, 95% CI 0.08—0.85, P 0.03), we estimated that the magnitude of the association between cfDNA levels and VTE risk declines by 74% for every year after baseline. This ‘weakening effect’ of cfDNA was confirmed in flexible parametric regression, where we observed a strong association between cfDNA levels and VTE risk shortly after follow-up, which then rapidly vanished (Fig. 3B). In Fine and Gray competing risk regression, the SHRs for elevaled cfDNA levels (i.e. > Q3) were 2.30 (P = 0.02), 1.86 (P = 0.03), 1.59 (P = 0.06) and 1.36 (P = 0.19) for the prediction of 3-month, 6-month, 12-month and 24-month VTE risks, respectively. A highly similar pattern of nonproportionality was observed for nucleosome levels (SHR for interaction with follow-up time 0.13, 95% CI 0.040.45, P = 0.001) (Fig. 3C). Here, the SHRs for elevated nucleosome levels (i.e. > Q3) were 1.39 (P = 0.36), 1.83 (P = 0.03), 1.47 (P = 0.12) and 1.12 (P = 0.64) for the prediction of 3-month, 6-month, 12-month and 24-month VTE risks, respectively.
Fig. 3.
Predicted time-dependent VTE rates over 2 years of follow-up according to baseline levels of H3Cit, cfDNA and nucleosomes. Rates were predicted using a flexible parametric model with restricted cubic splines allowing for a time-dependent change in both the log(cumulative baseline hazard) function and the log(relative hazard). Death was incorporated as a competing risk, which was achieved by time-dependent weighting of the data (Stata routine stcrprep). cfDNA, cell-free DNA; H3Cit, citrullinated histone H3; VTE, venous thromboembolism. [Color figure can be viewed at wileyonlinelibrary.com]
Comparison of H3Cit with previously identified biomarkers for VTE in patients with cancer
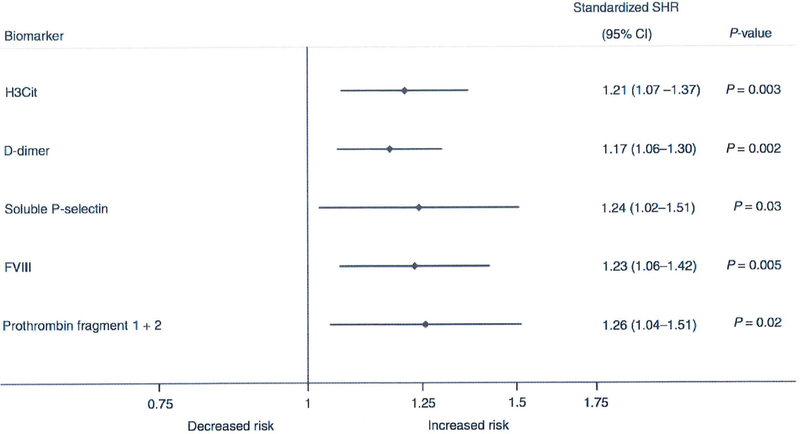
The levels of both H3Cit and other biomarkers such as D-dimer and sP-selectin were Z-standardized in order to enable comparison on a common scale. Here, H3Cit levels had a magnitude of association with VTE risk comparable to those of these previously identified biomarkers (Fig. 4).
Fig. 4.
Forest plot comparing Z-scores of H3Cit and other VTE biomarkers as predictors ofVTE risk. Comparability between biomarkers is achieved by transforming all variables on a common scale with mean zero and standard deviation of I (Z-standardization); the standardized subdistribution hazard ratio (SHR) of each variable can then be interpreted as the multiplicative increase in VTE risk for I standard deviation increase in the variable. Cl, Confidence Interval; FVIII, coagulation factor Vlll; H3Cit, citrullinated histone H3; Stand. SHR, standardized subdistribution hazard ratio.
Analysis of H3Cit levels in selected tumor types
To enable exploration of the role of H3Cit levels in the occurrence of VTE in various tumor entities, the VTE risk of single tumor types according to their H3Cit levels was calculated. In the analysis, only tumor entities with at least five thrombotic events were included. The result showed a significantly increased risk of VTE in patients with pancreas and lung cancer. Patients with breast cancer also had an increased risk of VTE. For patients with brain and colorectal cancer, as well as for ‘other sites’, no association between H3Cit levels and VTE was found. Patients with lymphoma showed an inverse correlation of VTE and 1–13Cit levels (Fig. S1. These results must be looked at very cautiously, as the study was not designed and was underpowered for tumor-type specific analyses.
Discussion
In this prospective observational cohort study, we found an association between high H3Cit plasma levels and an increased risk of cancer-associated VTE. This association remained over a follow-up period of 2 years, and was also independent of other risk factors for VTE, such as high VTE risk tumor sites, and elevated D-dimer and sP-selectin. Elevated cfDNA and nucleosome levels were also associated with the risk of cancer-associated VTE, although the prognostic role of these biomarkers was only relevant for short-term thrombotic risk prediction.
H3CiL has been established as a marker for NET formation in different experimental thrombosis and sepsis models, [3,11,27–30] as citrullination of histone H3 by PAD4 leads to chromatin decondensation and subsequent NET formation [31]. In an inferior vena cava stenosis model, for example, it was shown that a minority of mice incapable of histone H3 citrullination produce thrombi, whereas this was the case in 90% of wild-type mice [27]. Moreover, H3Cit levels were found in plasma of tumorbearing mice that were prone to NET formation and developed microthrombi in the lungs [11]. Data from clinical studies that have investigated H3Cit and coagulation are very limited. In one small study, high H3Cit levels correlated with the presence of cancer and acute ischemic stroke [12]. In patients with thrombotic microangiopathies, elevated levels of NET-related markers, including myeloperoxidase and cfDNA, were found, but H3Cit levels were not determined [32]. Furthermore, a recent study including patients with and without malignancy and objectively diagnosed DVT showed increased nucleosome levels in plasma of patients with malignancy and DVT as compared with patients without DVT. [33].
Our present study adds clinical data from a large cohort of prospectively followed patients with cancer showing that an increased H3Cit level is a strong and enduring risk factor for VTE in these patients.
The results of H3Cit subgroup analysis need to be discussed. We found the highest H3Cit levels in patients with prostate cancer, a tumor entity that is associated with a low risk of developing VTE [34]. One explanation could be that prostate cancer cells strongly express PAD4 [35], which hypothetically could citrullinate circulating histones from different sources than neutrophils, and thereby could generate H3Cit that is not NET-derived. We detected low H3Cit levels in patients with myeloma, a tumor entity that is regarded to be strongly associated with an increased VTE risk. However, this is primarily true for myeloma patients receivrng an angiogenesis inhibitor (lenalidomide or thalidomide) in combination with dexamethasone [36]. Therefore, the pathogenesis of VTE in myeloma seems to be primarily treatment-related, and NETs could play a minor role. In patients with brain tumors, which are among the most prothrombotic malignancies, we found no association between H3Cit levels and VTE risk. Previously, we demonstrated that glioma cells activate platelets via podoplanin [37]. Podoplanin in tumor specimens was strongly associated with the occurrence of thrombosis in these patients, and could be the main mechanism of coagulation activation in primary brain tumors. Surprisingly, we found an inverse correlation between H3Cit levels and VTE risk in lymphoma patients, which we cannot explain, but plan to investigate in future studies.
In addition to H3Cit levels, we also determined the levels of cfDNA and nucleosomes, which are essential components of NETs [2]. In recent studies, it was shown that such NET breakdown products rather than intact NETs induce coagulation [38,39]. However, elevated levels as such do not necessarily indicate increased NET formation, as apoptotic and necrotic cell death are other potential sources of cfDNA and nucleosome release [40]. This is true particularly for cancer patients, in whom the amount of cell death is usually increased [41,42]. Data indicate that endonucleosomal cleavage during apoptosis rather than NET formation could be the main source of cfDNA and nucleosomes in plasma of cancer patients [43,44]. Consistently, we found only weak correlations between H3Cit and cfT)NA levels and between H3Cit and nucleosome levels in our study. Nevertheless, these correlations were statistically significant, indicating that NET formation could contribute to overall cfDNA and nucleosome levels. The presence of a more substantial correlation between cfDNA and nucleosome levels in our study supports the close structural and functional relationships between these parameters.
In contrast to H3Cit levels, elevated plasma cfDNA and nucleosome levels predicted VTE only in the short term, at 3-month and 6-month cut-offs for follow-up, but not at later time pomts. Possible explanations for this are that both parameters were also strong predictors of surVival (data not shown), or that patients with elevated H3Cit levels had different prognostic variables than patients with elevated cfDNA or nucleosome levels.
To minimize confounding, we adjusted the association between H3Cit levels and VTE for important other prognostic variables, such as elevated D-dimer and sP-selectin levels, which have previously been identified. We also adjusted for high VTE risk and very high VTE risk cancer sites, which are important components of a wellknown VTE risk assessment model for cancer patients [45]. In multivariable analysis adjusting for these variables, the H3Cit level remained an independent biomarker for prediction of VTE. In further analyses, we compared H3Cit levels with those of the previously published cancer-associated VTE biomarkers D-dimer, sPselectin, prothrombin fragment 1 + 2, and FVIII [14], and found that H3Cit level was an equally strong predictor of VTE.
The procoagulant and prothrombotic properties of NETs or NET breakdown products were discovered only recently [38]. Therefore, H3Cit as a biomarker of NET formation could reflect a novel pathomechanism of cancer-associated VTE. These findings support the hypothesis that NETs could serve as novel target structures for the prevention of cancer-associated VTE. One candidate agent that targets NETs is DNase L In murine models, DNase I was protective against DVT, myocardial infarction, and stroke [2]. In a clinical study, endogenous DNase activity correlated negatively with coronary NET burden [46]. Whether intravenous DNase I is safe and effective in humans remarns to be investigated. Other candidate agents that could prevent VTE in patients with cancer without interfering with physiological blood coagulation are PAD4 inhibitors, which prevent histone citrullination and subsequent NET formation [47].
Some limitations of the present study need to be addressed. We did not quantify NETs directly, but indirectly via biomarkers reflecting NET formation. We chose this approach because direct NET quantification has not yet been standardized, hampering its applicability in a large clinical study Our H3Cit ELISA, on the other hand, can be applied to plasma samples, and has previously been used in a clinical study [12]. The cut-off at the 75th percentile of H3Cit, cfDNA and nucleosome levels was chosen empirically, and we did not search for other cut-offs to preserve the type I error rate. Importantly, storage time did not alter H3Cit and cfDNA levels, or their association with VTE risk. However, nucleosome levels were affected by storage time, but adjustment for storage time also did not reveal a significant association between nucleosome levels and 2-year VTE risk. In summary, these analyses show that storage time is a major preanalytical determinant of nucleosome levels but not of H3Cit or cfDNA levels. To circumvent the impact of storage time on nucleosome levels, this biomarker should be measured as soon as possible after blood sample collection.
Another limitation is that we did not take into account data on antitumor treatments after baseline, such as surgical or radiotherapeutic interventions. Although these interventions can have a prognostic impact on VTE risk, we refrained from including them in our analysis, because the aim of this study was to assess the potential of NET parameters as VTE risk biomarkers that may support risk assessment for cancer-associated VTE, rather than to analyze postbaseline treatments that may modify VTE risk.
It also needs to be clearly stated that this study was not designed and powered for statistical analysis in specific cancer subtypes. Subgroup analysis therefore needs to be interpreted with caution. However, we believe that findings from specific cancer types are of great interest for researchers investigating NETs, and need to be reported. Finally, it is a limitation that multiple biomarkers, including D-dimer and sP-selectin, were previously tested in the CATS study, which introduces multiplicity and hence potential for type 1 errors. Therefore, the post hoc nature of this analysis should be taken into account.
We conclude that H3Cit levels are independently associated with the occurrence of VTE in cancer patients. Nucleosome and cfDNA levels seem to have a role in short-term VTE risk prediction. The data from our large clinical study support experimental evidence indicating an important role of NETs in the pathogenesis of cancer-related VTE.
Supplementary Material
Fig. S1 Forest plot comparing Z-scores of H3Cit within selected tumor types.
Table S1 Association between H3Cit, cfDNA, nucleosomes and time to VTE (univariable and multivariable competing-risk regression models).
Table S2. Supplementary multivariable competing-risk regression models for VTE.
Essentials.
Neutrophil extracellular traps (NETs) might play a role in cancer-related coagulopathy.
We determmed NET biomarkers and followed cancer patients for venous thromboembolism (VTE).
We found a constant association with VTE for citrullinated histone H3.
Biomarkers of NET formation could reflect a novel pathomechanism of cancer-related VTE.
Acknowledgements
We would like to thank the Austrian Science Fund (FWF), Special Research Program (SFB) 54 and the Austrian National Bank (Anniversary Fund, project 14744) for funding this project. We thank the members of the adjudication committee: A. Willfort-Ehringer (Department of Angiology, Medical University of Vienna) and S. Metz-Schimmerl (Department of Diagnostic Radiology, Medical University of Vienna).
Footnotes
Disclosure of Conflict of Interests
D. D. Wagner has patent US 9,642,822 B2 issued. The other authors state that they have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Addendum
I. Pabinger, C. Ay, and J. Thaler designed the study. L.-M. Mauracher, K. Martinod, T. Däullary, L. Hell, C. Brostjan, and D. D. Wagner designed and performed the experiments. J. Thaler, F. Posch, E. Grilz, and C. Ay recruited patients. L.-M. Mauracher, J. Thaler, C. Zielinsk, C. Ay, and I. Pabinger analyzed and interpreted the data. F. Posch performed statistical analyses. L.-M. Mauracher and J. Thaler: wrote the article. All authors reviewed, edited and approved the final article.
References
- 1.Brinkmann V, Reichard U, Goosmann C, Fauler B, Ulllemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–5. [DOI] [PubMed] [Google Scholar]
- 2.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood 2014; 123: 2768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Timiceriu A, Coletti R, Kolinberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012; 209: 819–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carestia A, Rivadeneyra L, Romaniuk MA, Fondevila C, Negrotto S, Schattner M. Functional responses and molecular mechanisms involved in histone-mediated platelet activation. Thromb Haemost 2013; 110: 1035–45. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 2010; 107: 15880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010; 16: 887–96. [DOI] [PubMed] [Google Scholar]
- 7.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 2012; 10: 136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost 2017; 117: 219–30. [DOI] [PubMed] [Google Scholar]
- 9.Falanga A, Marchetti M, Russo L. The mechanisms of cancer-associated thrombosis. Thromb Res 2015; 135(Suppl. l): S8–ll. [DOI] [PubMed] [Google Scholar]
- 10.Khorana AA. Cancer-associated thrombosis: updates and controversies. Hematology Am Soc Hematol Educ Program 2012; 2012: 626–30. [DOI] [PubMed] [Google Scholar]
- 11.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA 2012; 109: 13076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thalin C, Demers M, Blomgren B, Wong SL, von Arbin M, von Heijne A, Laska AC, Wallen H, Wagner DD, Aspberg S. NET0sis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res 2016; 139: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessenbrock K, Krumbholz Ms Schonermarck U, Back W, Gross WL, Werb Z, Grone HI, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009; 15: 623–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood 2013; 122: 2011–18. [DOI] [PubMed] [Google Scholar]
- 15.Posch F, Thaler J, Zlabinger GJ, Konigsbrugge O, Koder S, Zielinski C, Pabinger I, Ay C. Soluble vascular endothelial growth factor (sVEGF) and the risk of venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis study (CATS). Clin Cancer Res 2016; 22: 200–6. [DOI] [PubMed] [Google Scholar]
- 16.Konigsbrugge O, Posch F, Riedl J, Reitter EM, Zielinski C, Pabinger I, Ay C. Association between decreased serum albumin with risk of venous thromboembolism and mortality in cancer patients. Oncologist 2016; 21: 252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–6. [DOI] [PubMed] [Google Scholar]
- 18.Coviello V, Bogges M. Cumulative incidence estimation in the presence of competing risks. Stata J 2004; 4: 103–12. [Google Scholar]
- 19.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statistics 1988; 16: 1141–54. [Google Scholar]
- 20.Fine JP, Gray RJA. Proportional hazards model for the subdistribution of a competing risk, J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 21.Ay C, Pasch F, Kaider A, Zielinski C, Pabinger 1. Estimating risk of venous thromboembolism in patients with cancer in the presence of competing mortality. J Thromb Haemost 2015; 13: 390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert PC, Royston P. Flexible Parametric Alternatives to the cox Model. UK Stata User Group 2009, 2009.
- 23.Lamber P STCRPREP: Stata module to prepare data for competing risks analysis using time-dependent weights. https://ideas.repec.org/c/boc/bocode/s458015.html.
- 24.Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, Quehenberger P, Zielinski C, Pabinger I. Prediction of venous thromboembolism in cancer patients. Blood 2010; 116: 5377–82. [DOI] [PubMed] [Google Scholar]
- 25.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008; 111: 4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly GC, Khorana AA, Kuderer NM, Culakova E, Francis CW, Lyman GH. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res 2010; 126: 113–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peplidylarginine denninase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA 2013; 110: 8674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, Wang Y, Wagner DD. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 2015; 125: 1948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 2010; 207: 1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng H, Yalavarthi S, Kanthi Y, Mazza LF, Elfline MA, Luke CE, Pinsky DJ, Henke PK, Knight JS. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol 2017; 69: 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.wang Y, Li M, Stadier S, Correll S, Li P, wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009; 184: 205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lammle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood 2012; 120: 1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Montfoort ML, Stephan F, Lauw MN, Hutten BA, Van Mierlo GJ, Solati S, Middeldorp S, Meijers JC, Zeerleder S. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler Thromb Vasc Biol 2013; 33: 147–51. [DOI] [PubMed] [Google Scholar]
- 34.Ording AG, Horvath-Puho E, Lash TL, Ehrenstein V, Borre M, Vyberg M, Sorensen HT. Prostate cancer, comorbidity, and the risk of venous thromboembolism: a cohort study of 44,035 Danish prostate cancer patients, 1995—2011. Cancer 2015; 121: 3692–9. [DOI] [PubMed] [Google Scholar]
- 35.Kholia S, Jorfi S, Thompson PR, Causey CP, Nicholas AP, Inal JM, Lange S. A novel role for peptidylarginine deiminases in microvesicle release reveals therapeutic potential of PAD inhibition in sensitizing prostate cancer cells to chemotherapy. J Extra-cell Vesicles 2015; 4: 26192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thaler J, Ay C, Pabinger I. Venous thromboembolism in cancer patients — risk scores and recent randomised controlled trials. Thromb Haemost 2012; 108: 1042–8. [DOI] [PubMed] [Google Scholar]
- 37.Riedl J, Preusser M, Nazari PM, Posch F, Panzer S, Marosi C, Birner P, Thaler J, Brostjan C, Lotsch D, Berger W, Hainfellner JA, Pabinger I, Ay C. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 2017; 129: 1831–9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noubouossie DF, Whelihan MF, Yu YB, Sparkenbaugh E, Pawlinski R, Monroe DM, Key NS. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood 2017; 129: 1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz C, Massberg S. Demystifying the prothrombotic role of NETs. Blood 2017; 129: 925–6. [DOI] [PubMed] [Google Scholar]
- 40.Holdenrieder S, Nagel D, Schalhorn A, Heinemann V, Wilkowski R, von Pawel J, Railh H, Feldmann K, Kremer AE, Muller S, Geiger S, Hamann GF, Seidel D, Stieber P. Clinical relevance of circulating nucleosomes in cancer. Ann N Y Acad Sci 2008; 1137: 180–9. [DOI] [PubMed] [Google Scholar]
- 41.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011; 11: 426–37. [DOI] [PubMed] [Google Scholar]
- 42.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994; 73: 2013–26. [DOI] [PubMed] [Google Scholar]
- 43.Giacona MB, Ruben GC, lczkowski KA, Roos TB, Porter DM, Sorenson GD- Cell-free DNA in human blood plasma: length measurements in patients with pancreatic cancer and healthy controls. Pancreas 1998; 17: 89–97. [DOI] [PubMed] [Google Scholar]
- 44.Rumore PM, Steinman CR. Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J Clin Invest 1990; 86: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis 2016; 41: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenbock A, Simon D, Laimer D, Bangert C, Kammerlander A, Mascherbauer J, Winter MP, Distelmaier K, Adlbrecht C, Preissner KT, Lang IM. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors or ST-segment resolution and infarct size. Circ Res 2015; 116: 1182–92. [DOI] [PubMed] [Google Scholar]
- 47.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, Bicker KL, Bingham RP, Campbell M, Chen YH, Chung CW, Craggs PD, Davis RP, Eberhard D, Joberty G, Lind KE, Locke K, Mailer C, Martinod K, Patten C, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol 2015; 11: 189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brinkmann V, Goosmann C, Kuhn LI, Zychlinsky A. Automatic quantification of in vitro NET formation. Front Immunol 2012; 3: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vorobjeva NV, Pinegin BV. Neutrophil extracellular traps: mechanisms of formation and role in health and disease. Bio-chemistry (Mosc) 2014; 79: 1286–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Forest plot comparing Z-scores of H3Cit within selected tumor types.
Table S1 Association between H3Cit, cfDNA, nucleosomes and time to VTE (univariable and multivariable competing-risk regression models).
Table S2. Supplementary multivariable competing-risk regression models for VTE.