Abstract
We report a simple and effective method to remove IrIMes homogeneous polarization transfer catalysts from solutions where NMR Signal Amplification By Reversible Exchange (SABRE) has been performed, while leaving intact the substrate’s hyperpolarized state. Following microTesla SABRE hyperpolarization of 15N spins in metronidazole, addition of SiO2 microparticles functionalized with 3-mercaptopropyl or 2-mercaptoethyl ethyl sulfide moieties provides removal of the catalyst from solution well within the hyperpolarization decay time at 0.3 T (T1>3 mins)—and enabling transfer to 9.4 T for detection of enhanced 15N signals in the absence of catalyst within the NMR-detection region. Successful catalyst removal from solution is supported by the inability to “re-hyperpolarize” 15N spins in subsequent attempts, as well as by 1H NMR and ICP-MS. Record-high 15N nuclear polarization of up to ~34% was achieved, corresponding to >100,000-fold enhancement at 9.4 T, and approximately 5/6th of the 15N hyperpolarization is retained after ~20-second-long purification procedure. Taken together, these results help pave the way for future studies involving in vivo molecular imaging using agents hyperpolarized via rapid and inexpensive parahydrogen-based methods.
TOC Graphic
Introduction
MRI is a powerful imaging method not only because its ability to distinguish anatomical boundaries of soft tissues without ionizing radiation, but also because of its potential to spectrally discern among different biochemical species and physiological states.1, 2 However, the need for much greater detection sensitivity for dilute species has led to growing interest in hyperpolarization3–5—the generation of highly non-equilibrium population distributions of nuclear spins to dramatically increase the detectable magnetization. Various methods for hyperpolarization have been developed with an eye toward biomedical applications, including Spin-Exchange Optical Pumping (SEOP),6, 7 dissolution Dynamic Nuclear Polarization (d-DNP),8–11 ParaHydrogen Induced Polarization (PHIP),12–14 and Signal Amplification By Reversible Exchange (SABRE).15–17 In particular, SABRE—along with its microTesla variant dubbed “SABRE-SHEATH” (for SABRE in SHield Enables Alignment Transfer to Heteronuclei)18, 19–22—have garnered increasing attention because they are rapid and inexpensive to perform, scalable, and do not require major instrumentation. A wide range of biomolecules is amendable to SABRE-SHEATH.4, 23 However, reliance on a heavy-metal (Ir-based) catalyst to mediate polarization transfer to a biocompatible substrate presents an obstacle to envisioned clinical applications because of its presence in the same solution as the hyperpolarized (HP) agent. Efficient removal of the catalyst while preserving the HP state of the substrate is thus likely necessary before studies with human subjects can be considered.
Generating a pure HP substrate via SABRE necessitates that the catalyst is either heterogeneous (enabling ready separation of the catalyst from the dissolved substrate), or that the homogeneous catalyst is removed somehow from the solution post-hyperpolarization transfer to target nuclei. While progress has been made,24–29 each approach demonstrated so far has drawbacks and none have enabled the production of catalyst-free solutions with agents possessing nuclear spin polarizations of several percent. Indeed, such approaches are further complicated by the necessity of the HP nuclei to retain their HP state during (and following) catalyst removal, enabling it to survive all the way through separation, agent administration, and ultimate MRI detection. The present work is thus motivated by the desire for simple methods to efficiently remove standard SABRE catalysts from solution while retaining the HP state of the substrate.
Experimental/Background
Metronidazole, a molecule that has a 15N T1>3 min at 0.3 T,30 is relatively easy to hyperpolarize,31, 32 and possesses biological significance (it is an FDA-approved antibiotic of interest for probing tissue hypoxia),31,33 was utilized in the present study. Indeed, we note that metronidazole can be administered in relatively large dose (~2 g per patient34). Moreover, metronidazole contains a nitroimidazole moiety, a structure which is frequently employed in positron emission tomography (PET) molecular probes for hypoxia sensing.33, 35–38 Correspondingly, we anticipate that when hyperpolarized, this agent will potentially be able to distinguish between hypoxic and normoxic tissues via the 15N chemical shift differences that are expected for structures in healthy versus pathological tissues.
Each sample utilized here contains a 20 mM methanol-d4 solution of metronidazole and 1 mM Ir-catalyst precursor [IrCl(COD)(IMes)] (where IMes = 1,3-bis(2,4,6-trimethylphenyl)-imidazol-2-ylidene and COD = cyclooctadiene)39,40; ~75–85% parahydrogen (at 75 psi) is administered via bubbling through a 5 mm NMR tube with a flow rate of 150 sccm using an experimental setup described elsewhere.41 The catalyst was activated by bubbling for at least ~5–10 mins prior to initial NMR acquisition. Additional experimental details can be found in the Supporting Information (SI) document.
Results and Discussion
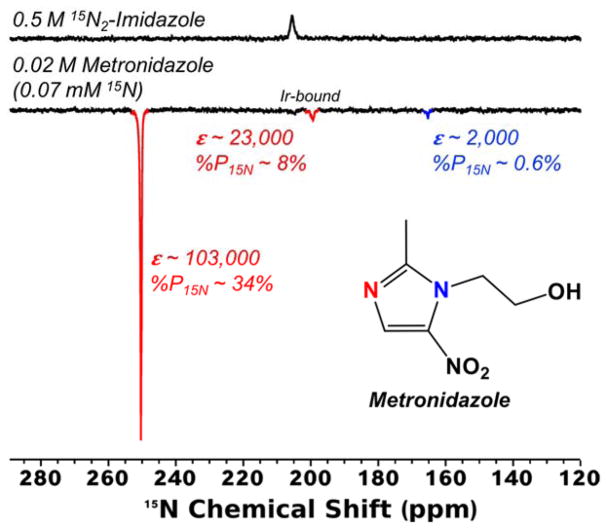
A SABRE-SHEATH mixing field of ~1 μT for 15N was found to result in 15N nuclear spin polarization of up to ~34% for the Ir-binding nitrogen in the free substrate (Figure 1), which corresponds to an enhancement factor (ε) of ~103,000-fold at 9.4 T and 300 K.
Figure 1.
Bottom: 15N spectrum from metronidazole exhibiting 34% 15N polarization achieved via SABRE-SHEATH. The Ir-binding nitrogen site of the free substrate exhibited the greatest polarization; the resonance for that nitrogen site for the Ir-bound substrate and the adjacent imidazole nitrogen were polarized 8% and 0.6%, respectively. Top: 15N spectrum from thermally polarized 15N2-imidazole, used as a reference for calculating polarization enhancement.
To our knowledge, such a value represents the greatest 15N polarization yet reported amongst all hyperpolarization methods (moreover, if nearly 100% pH242 were employed, and assuming an original fraction of ~85%, P of ~42% and ε of ~129,000 would be achieved4). At 15N natural abundance (0.364%), we also observed HP 15N signals from that nitrogen in catalyst-bound species and the adjacent imidazole nitrogen of free species (8% and 0.6% polarization, respectively; see Table S1 for a summary of calculations of polarization enhancement). With a long hyperpolarization lifetime30 and high 15N polarization values, metronidazole represents an ideal candidate to attempt catalyst removal post-hyperpolarization. We also note that SABRE hyperpolarization of this compound in organic solvent (vs. that in aqueous medium) may be desirable, because of metronidazole and parahydrogen have significantly greater solubility in organic solvents enabling preparation of highly concentrated and highly polarized liquids; such a HP liquid prepared in this fashion potentially can be diluted with isotonic buffer in a manner suitable for in vivo injection (e.g. 5 mg/ml solution).31, 41
The functionalized SiO2 microparticles (3-mercaptopropyl and 2-mercaptoethyl ethyl sulfide) investigated here are commercially available (Sigma 538086 and Sigma 745111), and do not require post-synthetic modifications. The surface functional moieties are terminated with strongly binding sulfur atoms that in principle can rapidly remove the catalyst from solution (ideally, on a timescale ≪ T1). To provide a rough estimate of the amount of functionalized SiO2 microparticles necessary to complete catalyst removal, 1H SABRE enhancement (several hundred-fold) as a function of time (post-SiO2 particle addition) was studied first, using 40 mM solutions of a test substrate (pyridine, also containing 4 mM catalyst in methanol-d4; Figure S1). These preliminary experiments were initiated by activating the catalyst with ~5–10 min. of parahydrogen bubbling, performing SABRE at ~10 mT, and then measuring the SABRE enhancement at 9.4 T prior to particle addition; once a 1H enhancement baseline was established, functionalized (3-mercaptopropyl) or non-functionalized (control) SiO2 microparticles were added to the solution. 1H SABRE experiments were then repeated and the decreasing 1H SABRE enhancement was recorded over time. Indeed, subsequent 1H SABRE spectra showed drastic reductions in enhancement as a function of time for solutions containing functionalized SiO2 microparticles, where the fastest decay (within ~250 s) occurred for microparticle:catalyst molar ratios of 30:1 (Figure S2). However, corresponding solutions of non-functionalized SiO2 microparticles did not show a change in enhancement over ~2,000 s following an initial loss likely attributable to physisorption of the catalyst.
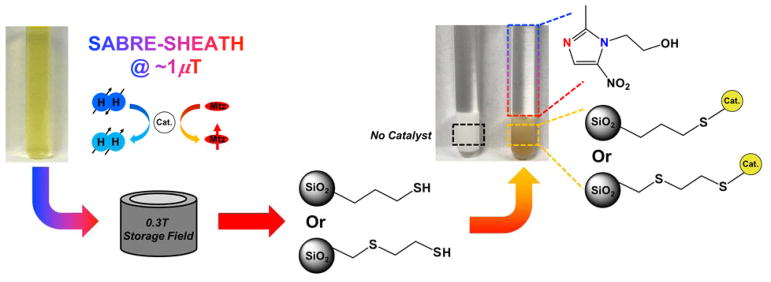
Informed by the above results (which indicated the need for large molar ratios of surface functionalization to catalyst), the experimental procedure used for obtaining enhanced 15N spectra from metronidazole with catalyst removal is summarized in Figure 2.
Figure 2.
Schematic of the experiment. After polarization transfer to 15N of metronidazole (Mtz) at ~1 μT (within a magnetic shield, not shown), the solution is transferred to a 0.3 T storage field, where the 15N T1 is long. The sample is depressurized and functionalized (3-mercaptopropyl or 2-mercaptoethyl ethyl sulfide) or non-functionalized (control) SiO2 microparticles are added, which in the former cases, cause the catalyst to be removed from solution while retaining high levels of the substrate’s 15N polarization.
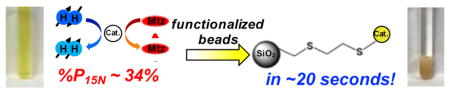
To rapidly remove homogeneous catalyst species from solution, microparticle:catalyst molar ratios of ~170:1 (3-mercaptopropyl) and ~142:1 (2-mercaptoethyl ethyl sulfide) were respectively used. Immediately following hyperpolarization transfer to 15N at ~1 μT, the solution is transferred to a 0.3 T storage field (where the 15N T1 is > 3 min30), depressurized to 1 atm, and then ~85 mg of the functionalized or non-functionalized SiO2 microparticles were added to the solution. The solution was bubbled with parahydrogen for ~5 s to ensure good mixing, and then immediately transferred to 9.4 T for detection. This entire process takes ~20 s and can likely be significantly accelerated (and even automated) with a more streamlined apparatus.
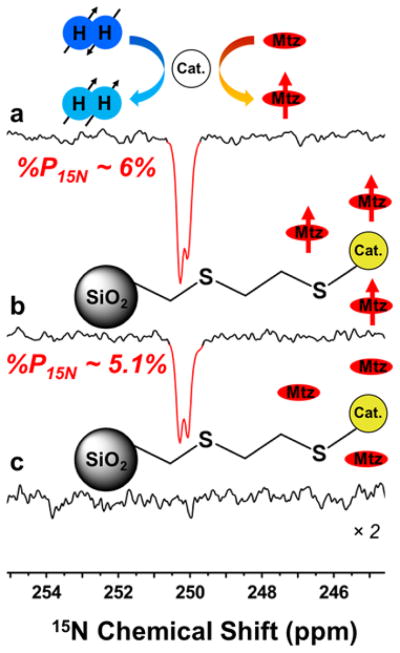
Results obtained with metronidazole (naturally abundant in 15N spins, hyperpolarized via SABRE-SHEATH) are summarized in Figure 3 and Figures S3, S4; all enhancement calculations are summarized in Table S2 of the SI.
Figure 3.
15N SABRE-SHEATH spectra from metronidazole (naturally abundant in 15N spins) at different stages: First, high levels of 15N polarization prior to microbead addition was seen (a). 15N hyperpolarization is retained after addition of 2-mercaptoethyl ethyl sulfide functionalized silica (b). Any attempt to re-hyperpolarize the solutions yielded no enhancements for the functionalized SiO2 solutions (c).
First, enhanced 15N spectra obtained prior to particle addition for three different runs are shown in Figures 3a, Figure S3a, and Figure S4a. Figures 3b, Figure S3b, and Figure S4b show significant retention of 15N hyperpolarization after each type of SiO2 microparticles were added. Importantly, the functionalized microparticles (Figure 3b and Figure S3b) took on a pale-yellow color similar to the catalyst while the supernatant liquid became clear (see photos in Figure 2), consistent with rapid removal of catalyst molecules from the solution as a result of binding to the microparticles. The decreases in signal intensity (and hence hyperpolarization level) are likely due to T1 losses; for example, Figure 3 demonstrates that ~84% of the initial 15N polarization was retained after the ~20-second-long purification procedure. After detection at 9.4 T, we attempted to “re-hyperpolarize” the 15N spins of the substrate, but observed no detectable 15N enhancement for the two samples containing functionalized SiO2 microparticles, again supporting the effective absence of catalysts in the supernatant solution. However, we were still able to achieve similar hyperpolarization levels for the sample containing non-functionalized SiO2 microparticles (SI). These results are also corroborated by corresponding 1H SABRE studies with these types of samples (Figure S5). Moreover, high-resolution 1H NMR of the supernatant liquid following addition of 3-mercaptopropyl-functionalized microparticles does not contain signals from the SABRE catalyst (Figure S6). Finally, ICP-MS elemental analysis of solutions obtained using similar concentrations and approach (and with volumes scaled up by ~25-fold) found that >98% of the catalyst had been removed (see SI)—consistent with the NMR results.
Conclusion
In summary, a simple and effective method is reported for removal of the most potent homogeneous IrIMes SABRE catalyst from solutions containing HP agent. The method uses inexpensive and commercially available microparticles, 5 mm NMR tubes, and it is sufficiently rapid (≪T1) to enable detection of NMR signals from substrates with intact HP states, in the apparent absence of dissolved catalysts (maintaining 15N polarization levels of up to >84% of the initial value). We note that the entire procedure from beginning of hyperpolarization to the end of the purification process requires less than 1.5 minutes. We envision that the purified HP metronidazole organic solution can be transferred from 5-mm-NMR-tube-based setup into a syringe partially filled with isotonic saline buffer via a catheter and particle filter for subsequent in vivo injection. Moreover, larger volumes with higher concentrations catalyst, parahydrogen, and agent (as well as agent isotopic labeling) should be easily amenable to the present approach—along with the use of other agents (including our recent demonstration of SABRE-hyperpolarized cleavable metabolic agents43). Current work is also focusing on developing cartridges or other devices that can be integrated with our setups to increase the efficiency of the process while minimizing polarization losses during catalyst removal and/or agent separation. These results, combined with observation of record 15N polarization of up to 34% in metronidazole, bode well for a wide range of envisioned in vivo molecular imaging applications.
Supplementary Material
Table S1. Calculations of 15N signal enhancements and polarizations for Figure 1.
Table S2. Calculations of 15N signal enhancements and polarizations for Figure 3.
Table S3. ICP-MS elemental analysis of residual Ir in solution.
Figure S1. 1H SABRE spectra of pyridine with varying quantities of functionalized and non-functionalized SiO2 microparticles.
Figure S2. 1H SABRE enhancement of pyridine as a function of time after microparticle addition.
Figure S3. 15N SABRE-SHEATH spectra from metronidazole after microparticles were added.
Figure S4. 15N SABRE-SHEATH spectra from metronidazole after control microparticles.
Figure S5. 1H SABRE enhancement of metronidazole after microparticle and control were added.
Figure S6. High-resolution 1H spectra of metronidazole before and after microparticle filtration.
Acknowledgments
We thank the City of Carbondale Public Works Central Laboratory and staff K.A. Cole and E.L. Stuart for ICP-MS measurements, and M. Kinsel (SIUC Chemistry & Biochemistry) for advice and assistance with preliminary analytical experiments. The US team thanks the following funding sources for support. This work was supported by NSF CHE-1416268, CHE-1836308, and CHE-1416432, NIH 1U01CA202229, 1R21EB020323, and R21CA220137, DOD CDMRP BRP W81XWH-12-1-0159/BC112431, DOD PRMRP awards W81XWH-15-1-0271 and W81XWH-15-1-0272, and RFBR (17-54-33037-OHKO_a). K.V.K. thanks the Russian Science Foundation (grant 17-73-20030) for support. I.V.K. thanks the Federal Agency for Scientific Organizations (project #0333-2017-0002) for support.
References
- 1.Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, et al. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissleder R. Molecular Imaging in Cancer. Science. 2006;312(5777):1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 3.Nikolaou P, Goodson BM, Chekmenev EY. NMR Hyperpolarization Techniques for Biomedicine. Chem Eur J. 2015;21:3156–3166. doi: 10.1002/chem.201405253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovener J, Pravdivtsev AN, Kidd BE, Bowers R, Gloggler S, Kovtunov KV, Plaumann M, Katz-Brull R, Buckenmaier K, Jerschow A, et al. Parahydrogen-based Hyperpolarization for Biomedicine. Angew Chem Int Ed. 2018 doi: 10.1002/anie.201711842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovtunov K, Pokochueva E, Salnikov O, Cousin S, Kurzbach D, Vuichoud B, Jannin S, Chekmenev E, Goodson B, Barskiy D, et al. Hyperpolarized NMR: d-DNP, PHIP, and SABRE. Chem Asian J. 2018 doi: 10.1002/asia.201800551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker TG, Happer W. Spin-Exchange Optical Pumping of Noble-Gas Nuclei. Rev Mod Phys. 1997;69:629–642. [Google Scholar]
- 7.Goodson BM. Nuclear Magnetic Resonance of Laser-Polarized Noble Gases in Molecules, Materials, and Organisms. J Magn Reson. 2002;155:157–216. doi: 10.1006/jmre.2001.2341. [DOI] [PubMed] [Google Scholar]
- 8.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in Signal-to-Noise Ratio of > 10,000 Times in Liquid-State NMR. Proc Natl Acad Sci US A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comment A. Dissolution DNP for In Vivo Preclinical Studies. J Magn Reson. 2016;264:39–48. doi: 10.1016/j.jmr.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Okuno Y, Cavagnero S. Sensitivity Enhancement in Solution NMR: Emerging Ideas and New Frontiers. J Magn Reson. 2014;241:18–31. doi: 10.1016/j.jmr.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardenkjaer-Larsen JH. On the Present and Future of Dissolution-DNP. J Magn Reson. 2016;264:3–12. doi: 10.1016/j.jmr.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Bowers CR, Weitekamp DP. Para-Hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J Am Chem Soc. 1987;109:5541–5542. [Google Scholar]
- 13.Bowers CR. eMagRes. John Wiley & Sons, Ltd; 2007. Sensitivity Enhancement Utilizing Parahydrogen. [Google Scholar]
- 14.Duckett SB, Mewis RE. Application of Parahydrogen Induced Polarization Techniques in NMR Spectroscopy and Imaging. Acc Chem Res. 2012;45:1247–1257. doi: 10.1021/ar2003094. [DOI] [PubMed] [Google Scholar]
- 15.Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Khazal IG, Lopez-Serrano J, Williamson DC. Reversible Interactions With Para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science. 2009;323:1708–1711. doi: 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
- 16.Adams RW, Duckett SB, Green RA, Williamson DC, Green GGR. A Theoretical Basis for Spontaneous Polarization Transfer in Non-Hydrogenative Parahydrogen-Induced Polarization. J Chem Phys. 2009;131:194505. doi: 10.1063/1.3254386. [DOI] [PubMed] [Google Scholar]
- 17.Rayner PJ, Duckett S. Signal Amplification by Reversible Exchange (SABRE): From Discovery to Diagnosis. Angew Chem Int Ed. 2018 doi: 10.1002/anie.201710406. [DOI] [PubMed] [Google Scholar]
- 18.Theis T, Truong ML, Coffey AM, Shchepin RV, Waddell KW, Shi F, Goodson BM, Warren WS, Chekmenev EY. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J Am Chem Soc. 2015;137:1404–1407. doi: 10.1021/ja512242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong ML, Theis T, Coffey AM, Shchepin RV, Waddell KW, Shi F, Goodson BM, Warren WS, Chekmenev EY. 15N Hyperpolarization By Reversible Exchange Using SABRE-SHEATH. J Phys Chem C. 2015;119:8786–8797. doi: 10.1021/acs.jpcc.5b01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shchepin RV, Truong ML, Theis T, Coffey AM, Shi F, Waddell KW, Warren WS, Goodson BM, Chekmenev EY. Hyperpolarization of “Neat” Liquids by NMR Signal Amplification by Reversible Exchange. J Phys Chem Lett. 2015;6:1961–1967. doi: 10.1021/acs.jpclett.5b00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barskiy DA, Shchepin RV, Tanner CPN, Colell JFP, Goodson BM, Theis T, Warren WS, Chekmenev EY. The Absence of Quadrupolar Nuclei Facilitates Efficient 13C Hyperpolarization via Reversible Exchange with Parahydrogen. ChemPhysChem. 2017;18:1493–1498. doi: 10.1002/cphc.201700416. [DOI] [PubMed] [Google Scholar]
- 22.Shchepin RV, Goodson BM, Theis T, Warren WS, Chekmenev EY. Toward Hyperpolarized 19F Molecular Imaging via Reversible Exchange with Parahydrogen. ChemPhysChem. 2017;15:1961–1965. doi: 10.1002/cphc.201700594. [DOI] [PubMed] [Google Scholar]
- 23.Colell JFP, Logan AWJ, Zhou Z, Shchepin RV, Barskiy DA, Ortiz GX, Wang Q, Malcolmson SJ, Chekmenev EY, Warren WS, et al. Generalizing, Extending, and Maximizing Nitrogen-15 Hyperpolarization Induced by Parahydrogen in Reversible Exchange. J Phys Chem C. 2017;121:6626–6634. doi: 10.1021/acs.jpcc.6b12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi F, Coffey AM, Waddell KW, Chekmenev EY, Goodson BM. Heterogeneous Solution NMR Signal Amplification by Reversible Exchange. Angew Chem Int Ed. 2014;53:7495–7498. doi: 10.1002/anie.201403135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi F, Coffey AM, Waddell KW, Chekmenev EY, Goodson BM. Nanoscale Catalysts for NMR Signal Enhancement by Reversible Exchange. J Phys Chem C. 2015;119:7525–7533. doi: 10.1021/acs.jpcc.5b02036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovtunov KV, Kovtunova LM, Gemeinhardt ME, Bukhtiyarov AV, Gesiorski J, Bukhtiyarov VI, Chekmenev EY, Koptyug IV, Goodson BM. Heterogeneous Microtesla SABRE Enhancement of 15N NMR Signals. Angew Chem Int Ed. 2017;56:10433–10437. doi: 10.1002/anie.201705014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iali W, Olaru A, Green G, Duckett S. Achieving High Levels of NMR-Hyperpolarization in Aqueous Media With Minimal Catalyst Contamination Using SABRE. Chem Eur J. 2017;23:10491–10495. doi: 10.1002/chem.201702716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manoharan A, Rayner P, Iali W, Burns M, Perry V, Duckett S. Achieving Biocompatible SABRE: An invitro Cytotoxicity Study. ChemMedChem. 2018;13:352–359. doi: 10.1002/cmdc.201700725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mewis RE, Fekete M, Green GGR, Whitwood AC, Duckett SB. Deactivation of Signal Amplification by Reversible Exchange Catalysis, Progress Towards In Vivo Application. Chem Commun. 2015;51:9857–9859. doi: 10.1039/c5cc01896j. [DOI] [PubMed] [Google Scholar]
- 30.Shchepin RV, Jaigirdar L, Chekmenev EY. Spin-Lattice Relaxation of Hyperpolarized Metronidazole in Signal Amplification by Reversible Exchange in Micro-Tesla Fields. J Phys Chem C. 2018;122:4984–4996. doi: 10.1021/acs.jpcc.8b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barskiy DA, Shchepin RV, Coffey AM, Theis T, Warren WS, Goodson BM, Chekmenev EY. Over 20% 15N Hyperpolarization in Under One Minute for Metronidazole, an Antibiotic and Hypoxia Probe. J Am Chem Soc. 2016;138:8080–8083. doi: 10.1021/jacs.6b04784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shchepin R, Jaigirdar L, Theis T, Warren W, Goodson B, Chekmenev E. Spin Relays Enable Efficient Long-Range Heteronuclear Signal Amplification by Reversible Exchange. J Phys Chem C. 2017;121:28425–28434. doi: 10.1021/acs.jpcc.7b11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kizaka-Kondoh S, Konse-Nagasawa H. Significance of Nitroimidazole Compounds and Hypoxia-Inducible Factor-1 for Imaging Tumor Hypoxia. Cancer Sci. 2009;100:1366–1373. doi: 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson SH, Oppenheim GL, Smith GH. Metronidazole in Breast Milk. Obstet Gynecol. 1981;57:48–50. [PubMed] [Google Scholar]
- 35.Procissi D, Claus F, Burgman P, Koziorowski J, Chapman JD, Thakur SB, Matei C, Ling CC, Koutcher JA. In Vivo 19F Magnetic Resonance Spectroscopy and Chemical Shift Imaging of Tri-Fluoro-Nitroimidazole as a Potential Hypoxia Reporter in Solid Tumors. Clin Cancer Res. 2007;13:3738–3747. doi: 10.1158/1078-0432.CCR-06-1563. [DOI] [PubMed] [Google Scholar]
- 36.Komar G, Seppänen M, Eskola O, Lindholm P, Grönroos TJ, Forsback S, Sipilä H, Evans SM, Solin O, Minn H. 18F-EF5: A New PET Tracer for Imaging Hypoxia in Head and Neck Cancer. J Nucl Med. 2008;49:1944–1951. doi: 10.2967/jnumed.108.053785. [DOI] [PubMed] [Google Scholar]
- 37.Fleming IN, Manavaki R, Blower PJ, West C, Williams KJ, Harris AL, Domarkas J, Lord S, Baldry C, Gilbert FJ. Imaging Tumour Hypoxia With Positron Emission Tomography. Br J Cancer. 2015;112:238–250. doi: 10.1038/bjc.2014.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masaki Y, Shimizu Y, Yoshioka T, Tanaka Y, Nishijima K, Zhao S, Higashino K, Sakamoto S, Numata Y, Yamaguchi Y, et al. The Accumulation Mechanism of the Hypoxia Imaging Probe “FMISO” by Imaging Mass Spectrometry: Possible Involvement of Low-Molecular Metabolites. Sci Rep. 2015;5:1–8. doi: 10.1038/srep16802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazquez-Serrano LD, Owens BT, Buriak JM. The Search for New Hydrogenation Catalyst Motifs Based on N-Heterocyclic Carbene Ligands. Inorganica Chim Acta. 2006;359:2786–2797. [Google Scholar]
- 40.Cowley MJ, Adams RW, Atkinson KD, Cockett MCR, Duckett SB, Green GGR, Lohman JAB, Kerssebaum R, Kilgour D, Mewis RE. Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer from Para-Hydrogen. J Am Chem Soc. 2011;133:6134–6137. doi: 10.1021/ja200299u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truong ML, Shi F, He P, Yuan B, Plunkett KN, Coffey AM, Shchepin RV, Barskiy DA, Kovtunov KV, Koptyug IV, et al. Irreversible Catalyst Activation Enables Hyperpolarization and Water Solubility for NMR Signal Amplification by Reversible Exchange. J Phys Chem B. 2014;18:13882–13889. doi: 10.1021/jp510825b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng B, Coffey AM, Colon RD, Chekmenev EY, Waddell KW. A Pulsed Injection Parahydrogen Generator and Techniques for Quantifying Enrichment. J Magn Reson. 2012;214:258–262. doi: 10.1016/j.jmr.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kidd BE, Mashni JA, Limbach MN, Shi F, Chekmenev EY, Hou Y, Goodson BM. Toward Cleavable Metabolic/pH Sensing “Double Agents” Hyperpolarized via NMR Signal Amplification by Reversible Exchange. Chem Eur J. 2018 doi: 10.1002/chem.201802622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Calculations of 15N signal enhancements and polarizations for Figure 1.
Table S2. Calculations of 15N signal enhancements and polarizations for Figure 3.
Table S3. ICP-MS elemental analysis of residual Ir in solution.
Figure S1. 1H SABRE spectra of pyridine with varying quantities of functionalized and non-functionalized SiO2 microparticles.
Figure S2. 1H SABRE enhancement of pyridine as a function of time after microparticle addition.
Figure S3. 15N SABRE-SHEATH spectra from metronidazole after microparticles were added.
Figure S4. 15N SABRE-SHEATH spectra from metronidazole after control microparticles.
Figure S5. 1H SABRE enhancement of metronidazole after microparticle and control were added.
Figure S6. High-resolution 1H spectra of metronidazole before and after microparticle filtration.