Abstract
Traumatic brain injury (TBI), causing neurological deficit in 70% of survivors, still lacks a clinically proven effective therapy. Transcranial direct current stimulation (tDCS) has emerged as a promising electroceutical therapeutic intervention possibly suitable for TBI; however, due to limited animal studies the mechanisms and optimal parameters are unknown. Using a mouse model of TBI we evaluated the acute effects of the anodal tDCS on cerebral blood flow (CBF) and tissue oxygenation, and assessed its efficacy in long-term neurologic recovery. TBI was induced by controlled cortical impact leading to cortical and hippocampal lesions with reduced CBF and developed hypoxia in peri-contusion area. Sham animals were subjected to craniotomy only. Repetitive anodal tDCS (0.1 mA/15min) or sham stimulation was done over 4 weeks for 4 consecutive days with 3-day intervals, beginning 1 or 3 weeks after TBI. Laser speckle contrast imaging (LSCI) revealed that anodal tDCS causes an increase in regional cortical CBF in both traumatized and Sham animals. On microvascular level, using in-vivo two-photon microscopy (2PLSM), we have shown that anodal tDCS induces arteriolar dilatation leading to an increase in capillary flow velocity and tissue oxygenation in both traumatized and Sham animals. Repetitive anodal tDCS significantly improved motor and cognitive neurologic outcome. The group with stimulation starting 3 weeks after TBI showed better recovery compared with stimulation starting 1 week after TBI, suggesting that the late post-traumatic period is more optimal for anodal tDCS.
1. Introduction
Traumatic brain injury (TBI) is a major health problem resulting in long-term neurological disabilities in 70% of survivors [1]. The primary injury is followed by secondary pathophysiological cascades persisting for months after injury which provides a wide treatment window. Unfortunately, no effective therapies have yet been proven for TBI [2]. Transcranial direct current stimulation (tDCS) has emerged as a promising electroceutical therapeutic intervention suggested for TBI [3]; however, the mechanisms and optimal stimulation parameters have not yet been determined due to the lack of pre-clinical studies. Altered neuronal excitability is believed to underlie the immediate effects of tDCS; however, recent studies have shown that astrocytes are another possible target. Several studies have also shown that tDCS modulates cerebral blood flow (CBF); however, the effects are under-investigated, especially at microvascular level [4]. On the other hand, reduced CBF and tissue hypoxia are common complications after TBI and their improvement could contribute to the effects of tDCS.
This study examined the acute effects of anodal tDCS on CBF and tissue oxygenation of mouse brain in the post-traumatic period and defined the optimal time window for long-term neurologic outcome improvement using a mouse controlled cortical impact (CCI) model of TBI.
2. Methods
Protocol #200247 was approved by the Institutional Animal Care and Use Committee of the University of New Mexico and the studies were conducted according the NIH Guide for the Care and Use of Laboratory Animals. Four groups of 10 mice each were used in the study: TBI, and Sham with and without stimulation. TBI was induced by a Benchmark Controlled Cortical Stereotaxic Impactor using a 3-mm flat-tip impounder deployed at a velocity of 5m/sec and depth of 2.0 mm from the cortical surface, as in our previous study [5]. Sham-controls were subjected to craniotomy only. Repetitive tDCS (0.1 mA/15min) or sham-stimulation (0 mA/15min) was done over 4 weeks for 4 consecutive days at 3-day intervals, beginning 1 or 3 weeks after TBI. The anode was placed around the craniotomy and the counter electrode on the thorax. Regional, and microvascular CBF concurrently with NADH were measured in-vivo by a custom-made laser speckle contrast imaging (LSCI) and two-photon laser scanning microscopy (2PLSM), respectively, before and after stimulation. Rotarod (motor function), passive avoidance (learning) and Y-maze (spatial memory) were used to evaluate neurological recovery at 1 week after the end of stimulation.
Two-Photon Laser Scanning Microscopy.
Fluorescent serum (i.v. tetramethylrhodamine isothiocyanate (TAMRA) dextran, 150 kDa in physiological saline, 5% wt/vol) was visualized using an Olympus BX 51WI upright microscope and water-immersion LUMPlan FL/IR 20X/0.50 W objective. Excitation was provided by a PrairieView Ultima multiphoton microscopy laser scan unit powered by a Millennia Prime 10 W diode laser source pumping a Tsunami Ti: Sapphire laser (Spectra-Physics, Mountain View, CA, USA) tuned to 750 nm center wavelength. Band-pass-filtered epifluorescence (570-600 nm for TAMRA and 425-475 nm for NADH) was collected by photomultiplier tubes of the Prairie View Ultima system. Images (512 X 512 pixels, 0.15 um/pixel in the x- and y-axes) or line scans were acquired using Prairie View software. Red blood cell flow velocity was measured in microvessels ranging from 3-50 μm diameter up to 500 μm below the surface of the parietal cortex, as we described previously [6]. Tissue hypoxia was assessed by measurement of NADH autofluorescence. In offline analyses using NIH ImageJ software, the three-dimensional anatomy of the vasculature in areas of interest was reconstructed from two-dimensional (planar) scans of the fluorescence intensity obtained at successive focal depths in the cortex (XYZ stack).
Behavioral testing.
Rotarod Performance Test for coordination and motor deficits was performed using a computer controlled Rotarod (San Diego Instruments, USA). The measured variable was the time to dismount from the rotating rod with increasing speed of rotation. Passive Avoidance Test for learning and memory, based on classical Pavlovian conditioning, was performed using a computer controlled Gemini Avoidance System (San Diego Instruments, USA). The avoidance chamber was partitioned into two sections, one light and one dark. As the mouse moved into the dark section a mild foot electric shock was delivered through the floor of the chamber. One day after training, the mouse was once again placed into the illuminated part of the chamber, and the time required for the mouse to move into the dark section was recorded. Spatial Alternation in the Y-Maze for measuring working memory was done using a computer controlled system (Noldus, EthoVision). Each animal was placed in the center of the Y-maze and allowed free exploration for 5 min. The total number of arm choices and number of spontaneous alternations (i.e. where the previous two arm choices differed from the third) was calculated, computed and analyzed from the recorded session.
Statistical analyses were done using GraphPad Prism software 6.0 (La Jolla, CA, USA) by Student’s t-test or Kholmogorov-Smirnov test where appropriate. Differences between groups were determined using two-way analysis of variance (ANOVA) for multiple comparisons and post-hoc testing using the Mann-Whitney U-test.
3. Results
CCI-induced moderate TBI caused tissue damage in the cortex and subcortical zones, including hippocampus in the ipsilateral hemisphere. Nissl staining revealed a shrunken hippocampus and obvious shrinkage of parietal somatosensory cortex with 18% counted neuronal loss compared to the contralateral hemisphere.
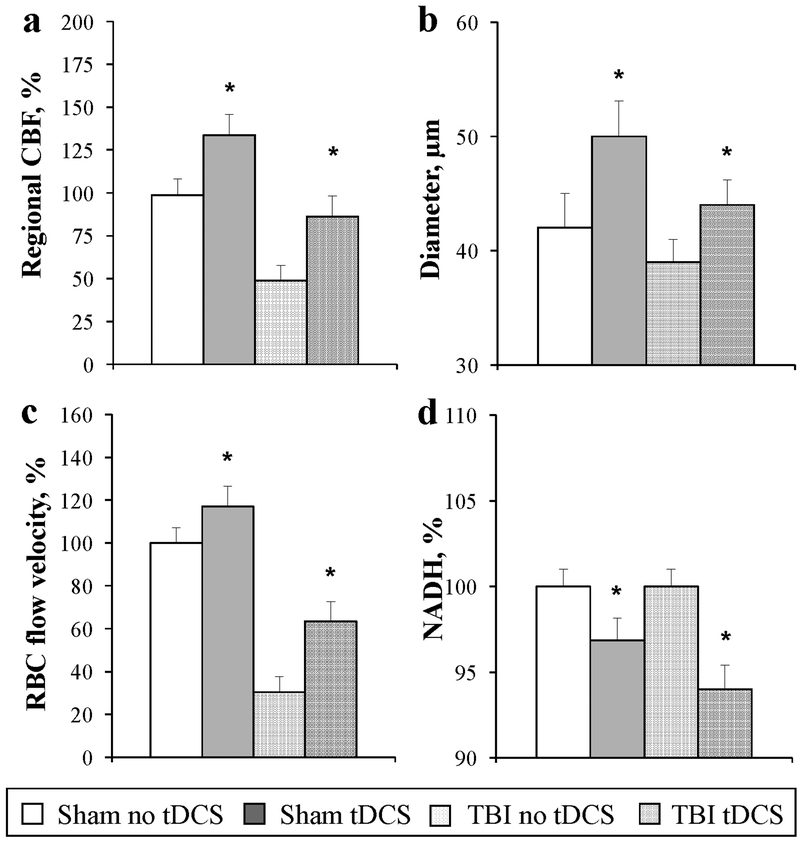
Regional CBF in the peri-contusion area was 48.8 ± 8.8% from that in the ipsilateral hemisphere (Fig. 1a). Anodal tDCS increased rCBF bilaterally in the cortex in both Sham and TBI mice (Fig. 1a, P<0.05). In vivo 2PLSM of the peri-contusion cortex in traumatized brain showed a decrease in arteriolar diameters, and a decrease in microvascular CBF and tissue oxygenation (Fig. 1b-d). Anodal tDCS dilated arterioles led to an increase in blood flow volume (Fig. 1b, P<0.01), which increased blood flow velocity in capillaries (Fig. 1c, P<0.001). The improved perfusion in capillaries after tDCS caused a prolonged increase in tissue oxygenation, as reflected by NADH autofluorescence decrease in TBI and Sham mouse brain (Fig. 1d, P<0.01).
Fig. 1.
Anodal tDCS improves cerebral blood flow and tissue oxygenation impaired by TBI: a. Increase in regional cortical CBF; b. Dilatation of arterioles; c. Increase in capillary red blood cells (RBC) flow velocity; and d. Increase in tissue oxygenation (NADH autofluorescence decrease). N = 10 for each group, mean ± SEM, *P<0.05.
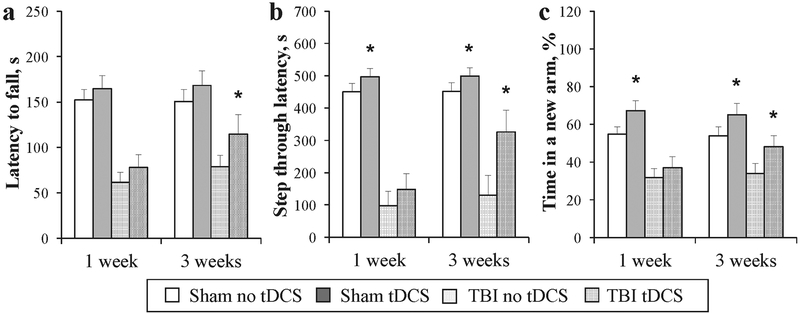
TBI impaired motor and coordination functions, as shown using the Rotarod test by a decrease in the latency period to fall from a rotating rod, compared to Sham animals (Fig. 2a). Repetitive tDCS significantly attenuated motor deficit in the TBI group with tDCS started at 3 weeks after TBI; the latency to fall in the stimulated group was longer than in the sham-stimulated group (Fig. 2a, P<0.05). The passive avoidance test revealed impaired learning and memory in traumatized mice (Fig. 2b). In the tDCS TBI group, where stimulation was started 3 weeks after trauma, learning and memory were better preserved; step-through retention latency was significantly longer than that of sham-stimulated mice (Fig. 2b, P<0.01). Interestingly in sham-operated mice, the tDCS group performed better than sham-stimulated, P<0.05. The Y-maze showed that TBI impaired spatial working memory, as mice spent an equal amount of time in all three arms (Figure 2c). Anodal tDCS-stimulated mice, where stimulation was started at 3 weeks after TBI, entered into the newly-opened arm more frequently compared to sham- stimulated, reflecting better preserved spatial working memory (Fig. 2c, P–0.05). In the sham-operated group, stimulation also significantly enhanced spatial working memory (Fig. 2c, P<0.05). Despite that mice in the TBI group, where stimulation was started at 1 week after trauma, performed better than sham-stimulated, the difference was not statistically significant.
Fig. 2.
Repetitive anodal tDCS attenuates TBI-induced neurologic impairment: a. Rotarod test showed improved motor function; b. Passive avoidance showed improved learning and memory; and c. The Y-maze test showed improved spatial working memory. N = 10 for each group, mean ± SEM, *P<0.05.
4. Discussion
Anodal tDCS acutely increases cerebral microvascular flow and tissue oxygenation by dilatation of arterioles in both traumatized and Sham mice. The vasodilatory effects of anodal tDCS on cerebral arterioles could be induced by nitric oxide through activation of nitric oxide synthases (NOS), as was shown in our previous studies on healthy rat brain with another type of electroceutical – pulsed electromagnetic field stimulation [6]. What cellular target and what isoform of NOS (endothelial or neuronal) is primarily involved in tDCS-induced vasodilatation need to be determined in further studies. Diminished CBF and focal tissue hypoxia are identified components of TBI pathophysiology [7], therefore, long-term repeated increase of CBF may contribute to the demonstrated post-TBI improvement in recovery. The group in which stimulation started 3 weeks after TBI made a better recovery than when stimulation started 1 week after TBI, suggesting that the late post-traumatic period is more optimal for anodal tDCS efficacy. Acute pathophysiological cascades, including blood brain barrier and CBF autoregulation dysfunction, and repair processes, including angiogenesis, that are still active at 1 week after TBI [8], might be the reason for inefficiency of anodal tDCS. This emphasize the significance of therapeutic time window of tDCS application and show that care is needed when considering effective interventions to increase CBF in the early recovery period.
5. Conclusion
Our studies show that anodal tDCS acutely increases brain microvascular flow and tissue oxygenation by dilatation of arterioles in both traumatized and Sham mouse brain that could contribute to neurologic improvement.
Acknowledgments
Supported by NIH-NIGMS P20GM109089 and RSF NO 17-75-20069.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG (2010) Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control [Google Scholar]
- 2.Jain KK (2008) Neuroprotection in traumatic brain injury. Drug Discovery Today 13:1082–9 [DOI] [PubMed] [Google Scholar]
- 3.Clayton E, Kinley-Cooper SK, Weber RA et al. (2016) Brain stimulation: Neuromodulation as a potential treatment for motor recovery following traumatic brain injury. Brain Res 1;1640(Pt A): 130–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wachter D, Wrede A, Schulz-Schaeffer W et al. (2011) Transcranial direct current stimulation induces polarity-specific changes of cortical blood perfusion in the rat. Exp Neurol 227(2):322–7 [DOI] [PubMed] [Google Scholar]
- 5.Chohan MO, Bragina O, Kazim SF et al. (2015) Enhancement of neurogenesis and memory by a neurotrophic peptide in mild to moderate traumatic brain injury. Neurosurgery 76(2):201–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bragin DE, Statom GL, Hagberg S et al. (2015) Increases in microvascular perfusion and tissue oxygenation via pulsed electromagnetic fields in the healthy rat brain. J Neurosurg 122(5):1239–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroder ML, Muizelaar JP, Bullock MR et al. (1995) Focal ischemia due to traumatic contusions documented by stable xenon-CT and ultrastructural studies. J Neurosurg 82 (6):966–971 [DOI] [PubMed] [Google Scholar]
- 8.Pop V, Badaut J (2011) A neurovascular perspective for long-term changes after brain trauma. Transl Stroke Res 2(4):533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]