Somatic, de novo mutations affecting pluripotent cells and occurring early in embryogenesis can generate lesions in distinct germ layers (Hall, 1988; Youssoufian & Pyeritz, 2002). Such events give rise to syndromic mosaic disorders including Schimmelpenning-Feuerstein-Mims syndrome (SFM), Cutaneous Skeletal Hypophosphatemia syndrome, and McCune-Albright syndrome (MAS), wherein a multipotent cell acquires a postzygotic mutation in the Ras subfamily or GNAS, respectively, prior to its replication and differentiation into segments of mutant neural, endocrine, skeletal, and skin tissues (Groesser et al., 2012; Lim et al., 2014; Weinstein et al., 1991). Consequently, multiple end organs present a constellation of symptoms: SFM features ipsilateral keratinocytic or sebaceous nevi associated with central nervous system disorders including epilepsy, seizures, and mental retardation, as well as ocular, skeletal, cardiovascular, and genitourinary anomalies (Groesser et al., 2012), while MAS patients exhibit melanotic skin patches, polyostotic fibrous dysplasia of the bones, and endocrinopathies (Robinson, Collins, & Boyce, 2016; Weinstein et al., 1991). Unless the postzygotic mutation affects gonadal tissues as in germline (mutation present in gametes) or gonosomal (mutation in both soma and gametes) mosaicism, such disorders are not transmitted to subsequent generations (Happle, 2016). Certain mutations, like the AKT1 variants that cause the Proteus syndrome, only manifest as mosaic disorders, as they are likely constitutionally lethal (Lindhurst et al., 2011). Patterned distributions of lesional tissue such as unilateral or linear presentations suggest genetic mosaicism (Happle, 2016). However, exceptions such as the coat-like pattern of giant congenital melanocytic nevi arising most commonly from somatic activating mutation in neuroblastoma RAS viral oncogene homolog (NRAS), exist (Charbel et al., 2014).
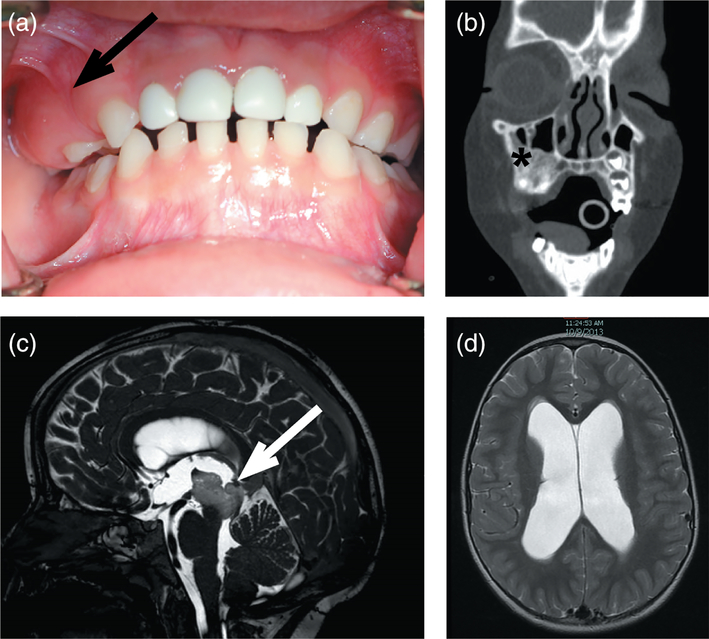
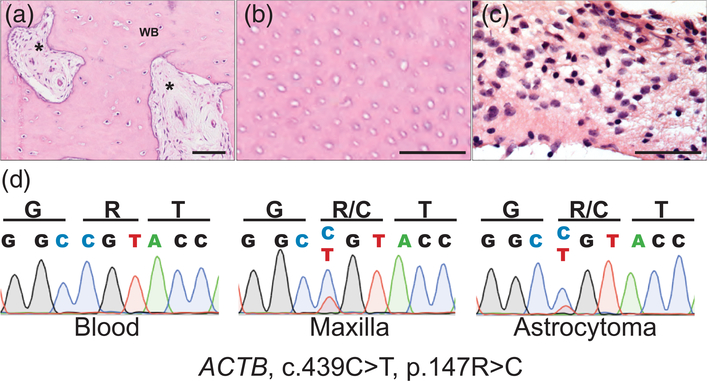
Here, we report a 5-year-old boy (MOS100) who presented at 3 years of age with asymptomatic expansion of the right maxillary alveolar ridge (Figure 1a). His parents gave informed consent for him to be evaluated under a NIDCR IRB-approved protocol and a Yale Human Investigation committee-approved study protocol. Dental radiographic imaging and computed tomography (CT) revealed a fibro-osseous lesion suggestive of fibrous dysplasia (Figure 1b), but without “ground-glass” appearance. The primary teeth exhibited abnormal growth and one permanent tooth bud was missing. Endocrine workup was unremarkable, and a bone series did not indicate evidence of extragnathic fibro-osseous bone lesions. A 6-month follow-up CT and magnetic resonance imaging (MRI) revealed a slow expansion of the maxillary lesion, and incidental findings of a new brain stem mass with mild obstructive hydrocephalus (Figure 1c,d). Histopathologic assessment of the maxillary lesion demonstrated hypermineralized woven bone, with regions of dentinal tubules (Figure 2a,b) suggestive of a tooth cell origin. The patient underwent biopsy of brain stem lesion and placement of ventriculoperitoneal shunt, and histopathology was consistent with pilocytic astrocytoma (Figure 2c). The tumor was not mitotically active, with a mildly elevated Ki67 proliferation index (5%). Florescence in situ hybridization using the D7Z1 DNA Probe (chromosome 7α satellite DNA) at 7p11.1-q11.1 and homebrew probes RP11-767F15 and RP11-60F17 did not identify BRAF duplication at 7q34 in the brain lesion (Tian et al., 2011) and Sanger sequencing did not identify hotspot mutations in BRAF (V600) and GNAS (R201) mutation in the brain and maxillary lesions (data not shown).
FIGURE 1.
Maxillary lesion and brain stem mass in MOS100. (a) Expansion of the maxillary bone was noted on routine dental examination (arrow). (b) Coronal CT demonstrated an area of dysplastic bone with a mostly sclerotic appearance (asterisk). (c) T2 sagittal MRI of the brain identified a central, soft tissue mass (arrow), which on biopsy was found to be a low-grade astrocytoma, and (d) associated hydrocephalus in the lateral ventricles [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
ACTB mutation in maxillary lesion and pilocytic astrocytoma. (a) Representative 20× histology from an area of the maxillary lesion, which included areas of fibrosis (asterisk) within areas of woven bone (WB). (b) 40× view of tubular-like structures consistent with dentinal tubules. (c) 40× histology of smear of brain stem lesion demonstrates monomorphic population of glial tumor cells with elongated pilocytic processes in myxoid matrix, without Rosenthal fibers or eosinophilic granular bodies. (d) Sanger sequencing of ACTB demonstrates multilineage somatic c.439C>T, p.147R>C mutation, present in both the maxillary bone lesion (middle) and astrocytoma (right), which is absent in blood (left) [Color figure can be viewed at wileyonlinelibrary.com]
Paired whole exome sequencing was performed using genomic DNA isolated from blood and biopsy of the maxillary lesion (Supporting Information Supplementary Methods), identifying a single somatic c.439C>T, p.147R>C mutation in beta-actin (ACTB). The mutation was confirmed via Sanger sequencing of DNA isolated from cells cultured from affected bone tissue (Supplementary Methods and Supporting Information Table 1). Suspecting that the subject’s astrocytoma resulted from widespread ACTB mosaicism, we performed targeted sequencing of ACTB in brain biopsy DNA. In so doing, we found the identical c.439C>T, p.147R>C ACTB mutation (Figure 2d), confirming multilineage ACTB mosaicism affecting the mesoderm (maxilla) and ectoderm (astrocytes) (Figure 2d).
Beta-actin is one of six isoforms of the highly conserved, ubiquitous “housekeeping” actin, a cytoskeletal protein involved in cell motility, adhesion, and embryonic development (Bunnell, Burbach, Shimizu, & Ervasti, 2011). Distinct germline mutations in ACTB including p.74G>S, p.117E>K, p.120T>I, and p.196R>H have been reported to cause Baraitser-Winter Cerebrofrontofacial syndrome (BWCFF) (Di Donato et al., 2014; Riviere et al., 2012), an autosomal dominant developmental disorder that features characteristic facies with hypertelorism, ptosis, broad nasal bridge, and pointed chin, along with mental disability and structural brain abnormalities due presumably to impaired neuronal migration (Verloes, Drunat, Pilz, & Di Donato, 2015). Functional analyses of BWCFF ACTB mutations found altered cell adhesion and polymer instability (Johnston et al., 2013). Other germline ACTB mutations including p.183R>W cause juvenile-onset dystonia with hearing loss and developmental delay (Conboy et al., 2017), and this mutation led to alter depolymerization dynamics leading to a morphologically abnormal actin cytoskeleton (Procaccio et al., 2006).
Notably, the same postzygotic p.147R>C mutation in ACTB as in our subject was recently identified in a majority (14 of 23 examined, 61%) of Becker’s nevi (BN) and Becker’s nevus syndrome (BNS) subjects, along with a p.147R>S mutation. BN are common benign hamartomas affecting approximately 1 in 200 individuals, with syndromic cases having variable symptoms of cardiomyopathy, developmental delay, and unilateral breast hypoplasia (Cai et al., 2017). The mutation is localized to the pilar muscle of hair follicles, and was not shown to affect cytoskeletal actin organization or MAPK signaling. In mutation-expressing myoblasts treated with smoothened agonist, however, increased Gli1 expression was found, suggesting that the ACTB p.147R>C mutation in tissues leads to increased Hedgehog (Hh) signaling (Cai et al., 2017). In our case of fibro-osseous dysplasia with pilocytic astrocytoma, the mechanism by which the p.147R>C mutation gives rise to the neoplasms is unclear, though Gli-dependent aberrant activation of the Hh pathway is a feature of multiple solid tumors and affects tooth development (Hanna & Shevde, 2016; Hardcastle, Mo, Hui, & Sharpe, 1998). Moreover, the Hh pathway has been shown to regulate the growth of gliomas, and higher levels of GLI1 as well as PTCH, a transcriptional target of the Hh pathway, was demonstrated in pilocytic astrocytomas (Rush, Abel, Valadez, Pearson, & Cooper, 2010). Finally, ACTB has been demonstrated to play a role in the development of gliomas by facilitating interactions between heat shock proteins and 14-3-3 proteins, which are known to play a role in the patho-genesis of gliomas (Com et al., 2012). BNS can often feature skeletal defects, though they tend to be structural abnormalities such as hypoplasia of the shoulder girdle and extremities, scoliosis, and pectus excavatum (Happle & Koopman, 1997).
Our findings identify a novel clinical phenotype arising from multilineage ACTB mosaicism and extend the phenotypic spectrum of somatic ACTB mutation diseases to include a unique dental stem cell related facial skeletal disease and pilocytic astrocytoma. The presence of identical somatic mutation in two end organs arising from distinct germ layers confirms early postzygotic mutagenesis affecting a multipotent progenitor.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from NIAMS/NIH (R01AR071491) to KAC. Y.H.L. is supported by the Medical Scientist Training Program at Yale University (NIH/NIGMS T32 GM007205). A.B.B., M.S.R., and M.T.C. were supported by the Division of Intramural Research, National Institute of Dental and Craniofacial Research, NIH.
Funding information
National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Number: R01AR071491; National Institute of General Medical Sciences, Grant/ Award Number: T32GM007205
Footnotes
CONFLICT OF INTEREST
There is no conflict of interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Bunnell TM, Burbach BJ, Shimizu Y, & Ervasti JM (2011). Beta-actin specifically controls cell growth, migration, and the G-actin pool. Molecular Biology of the Cell, 22(21), 4047–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ED, Sun BK, Chiang A, Rogers A, Bernet L, Cheng B, … Sarin KY (2017). Postzygotic mutations in beta-actin are associated with Becker’s nevus and Becker’s nevus syndrome. The Journal of Investigative Dermatology, 137(8), 1795–1798. [DOI] [PubMed] [Google Scholar]
- Charbel C, Fontaine RH, Malouf GG, Picard A, Kadlub N, El-Murr N, … Guégan S (2014). NRAS mutation is the sole recurrent somatic mutation in large congenital melanocytic nevi. The Journal of Investigative Dermatology, 134(4), 1067–1074. [DOI] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, … Lifton RP (2009). Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proceedings of the National Academy of Sciences of the United States of America, 106(45), 19096–19101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, … Getz G (2013). Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature Biotechnology, 31(3), 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Com E, Clavreul A, Lagarrigue M, Michalak S, Menei P, & Pineau C (2012). Quantitative proteomic isotope-coded protein label (ICPL) analysis reveals alteration of several functional processes in the glioblastoma. Journal of Proteomics, 75(13), 3898–3913. [DOI] [PubMed] [Google Scholar]
- Conboy E, Vairo F, Waggoner D, Ober C, Das S, Dhamija R, Klee EW, Pichurin P (2017). Pathogenic variant in ACTB, p.Arg183Trp, causes juvenile-onset dystonia, hearing loss, and developmental delay without midline malformation. Case Reports in Genetics(9184265, 2017, 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Donato N, Rump A, Koenig R, Der Kaloustian VM, Halal F, Sonntag K, … Verloes A (2014). Severe forms of Baraitser-Winter syndrome are caused by ACTB mutations rather than ACTG1 mutations. European Journal of Human Genetics, 22(2), 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groesser L, Herschberger E, Ruetten A, Ruivenkamp C, Lopriore E, Zutt M, … Hafner C (2012). Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nature Genetics, 44(7), 783–787. [DOI] [PubMed] [Google Scholar]
- Hall JG (1988). Review and hypotheses: Somatic mosaicism: Observations related to clinical genetics. American Journal of Human Genetics, 43(4), 355–363. [PMC free article] [PubMed] [Google Scholar]
- Hanna A, & Shevde LA (2016). Hedgehog signaling: Modulation of cancer properties and tumor microenvironment. Molecular Cancer, 15, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happle R (2016). The categories of cutaneous mosaicism: A proposed classification. American Journal of Medical Genetics, Part A, 170A(2), 452–459. [DOI] [PubMed] [Google Scholar]
- Happle R, & Koopman RJ (1997). Becker nevus syndrome. American Journal of Medical Genetics, 68(3), 357–361. [PubMed] [Google Scholar]
- Hardcastle Z, Mo R, Hui CC, & Sharpe PT (1998). The Shh signaling pathway in tooth development: Defects in Gli2 and Gli3 mutants. Development, 125(15), 2803–2811. [DOI] [PubMed] [Google Scholar]
- Johnston JJ, Wen KK, Keppler-Noreuil K, McKane M, Maiers JL, Greiner A, … Biesecker LG (2013). Functional analysis of a de novo ACTB mutation in a patient with atypical Baraitser–Winter syndrome. Human Mutation, 34(9), 1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, & Durbin R (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 25(14), 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YH, Ovejero D, Sugarman JS, Deklotz CM, Maruri A, Eichenfield LF, … Choate KA (2014). Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Human Molecular Genetics, 23(2), 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, … Biesecker LG (2011). A mosaic activating mutation in AKT1 associated with the Proteus syndrome. The New England Journal of Medicine, 365(7), 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccio V, Salazar G, Ono S, Styers ML, Gearing M, Davila A, … Wainer BH (2006). A mutation of beta-actin that alters depolymerization dynamics is associated with autosomal dominant developmental malformations, deafness, and dystonia. American Journal of Human Genetics, 78(6), 947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere JB, van Bon BW, Hoischen A, Kholmanskikh SS, O’Roak BJ, Gilissen C, … Dobyns WB (2012). De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nature Genetics, 44(4), 440–4 S1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Collins MT, & Boyce AM (2016). Fibrous dysplasia/McCune-Albright syndrome: Clinical and translational perspectives. Current Osteoporosis Reports, 14(5), 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush SZ, Abel TW, Valadez JG, Pearson M, & Cooper MK (2010). Activation of the hedgehog pathway in pilocytic astrocytomas. Neuro Oncology, 12(8), 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Rich BE, Vena N, Craig JM, Macconaill LE, Rajaram V, … Ligon KL (2011). Detection of KIAA1549-BRAF fusion transcripts in formalin-fixed paraffin-embedded pediatric low-grade gliomas. The Journal of Molecular Diagnostics, 13(6), 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, … MA DP (2013). From FastQ data to high confidence variant calls: The genome analysis toolkit best practices pipeline. Current Protocols in Bioinformatics, 43, 11.10.1–11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verloes A, Di Donato N, Masliah-Planchon J, Jongmans M, AbdulRaman OA, Albrecht B, … Pilz DT (2015). Baraitser-Winter cerebrofrontofacial syndrome: delineation of the spectrum in 42 cases. European Journal of Human Genetics, 23(3), 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, & Hakonarson H (2010). ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Research, 38(16), e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, & Spiegel AM (1991). Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. The New England Journal of Medicine, 325(24), 1688–1695. [DOI] [PubMed] [Google Scholar]
- Youssoufian H, & Pyeritz RE (2002). Mechanisms and consequences of somatic mosaicism in humans. Nature Reviews Genetics, 3(10), 748–758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.