Abstract
Cell-permeable compounds provide a convenient and efficient approach to manipulate biological processes. A number of compounds controlling stem cell self-renewal, survival, differentiation, and reprogramming have been identified through high-throughput/content screens. Using these powerful chemical tools, strategies have been developed to direct human pluripotent stem cell (hPSC) differentiation to functional cells. Recently, hPSC-derived cells and organoids are used to model human diseases, which can be adapted to a high-throughput/content platform for chemical screens. The identified compounds provide novel tools for decoding the signaling pathways regulating disease progression and candidates for facilitating future drug discovery. Moreover, humanized mouse models carrying hPSC-derived cells enable an innovative system to evaluate the long-term in vivo efficacy of drug candidates on human cells. In summary, screening-based chemical approaches not only expedite strategy development of controlling stem cell fates, but also provide powerful tools for dissecting the molecular mechanisms regulating disease progression.
Keywords: high-throughput screen, high-content screen, human pluripotent stem cells, directed differentiation, self-renewal, survival, reprogramming, organoids, disease modeling, drug discovery
In this article, Chen et al. summarized the recent efforts to apply screen approach to identify small molecules controlling stem cell fates, and to establish hPSC-derived cells and organoids-based platforms for disease modeling and drug screening.

Dr. Shuibing Chen is an Associate Professor in the Department of Surgery and Biochemistry at Weill Cornell Medical College, New York. She received her BS and MS degrees in Chemistry from Tsinghua University in China. Then, she pursued her PhD under the advisement of Dr. Peter G. Schultz at the Scripps Research Institute. After graduation, she joined Dr. Doug Melton's laboratory at Harvard University to study the directed differentiation of human ESCs (hESCs) toward pancreatic lineage.
The major research interest in the Chen Laboratory at Weill Cornell focuses on studying the role of genetic factors and environmental factors on pancreatic β cells in type 1 and 2 diabetes. Dr. Chen has published more than 30 papers in peer-reviewed high impact journals, such as Nature Medicine, Cell Stem Cell, Nature Chemical Biology, etc. She has received many awards including New York Stem Cell Foundation Robertson Investigator, American Diabetes Association (ADA) Junior Faculty Award, ADA Innovative Award, NIH Director's New Innovator Award, American Association for Cancer Research Career Development Award, and ISSCR Dr. Susan Lim Award for Outstanding Young Investigator, etc.
Main Text
Chemical Approaches to Maintain Stem Cell Self-Renewal
Embryonic stem cells (ESCs), the ex vivo equivalent of the epiblast lineage of the blastocyst, were originally derived using medium containing serum with a feeder layer of mitotically inactivated fibroblasts. Later, leukemia inhibitory factor (LIF), and bone morphogenetic protein 4 (BMP4)-SMAD signals, which are associated with feeder layer cells, were found to be essential for maintaining mouse ESC (mESC) self-renewal (Niwa et al., 1998, Ying et al., 2003). From a high-content screen, we identified a new 3,4-dihydropyrimido(4,5-d)pyrimidine analog, pluripotin/SC1, which could maintain mESCs in the undifferentiated, pluripotent state under chemically defined conditions in the absence of feeder cells, serum, LIF, and BMP4 (Chen et al., 2006). Mechanistic studies revealed that SC1 directly binds and inhibits two target proteins involved in the differentiation-inducing signaling, extracellular signal-regulated kinases (ERK) and Ras GTPase-activating protein. This study provided a conceptual advance that mESC self-renewal can be achieved through inhibiting the differentiation-inducing signals, which highlights the power of chemical approaches in dissecting the complex biology of stem cells. Later, Ying et al. (2008) reported 2i medium, using CHIR99021, a glycogen synthase kinase-3 (GSK-3) inhibitor, and PD0325901, a MEK1/2 inhibitor, to maintain mESC self-renewal.
Chemical approaches were also used to facilitate hESC self-renewal. Watanabe et al. (2007) found Y-27632, a selective Rho-associated kinase (ROCK) inhibitor, which promotes the survival of dissociated single-cell hESCs without the loss of pluripotency. Consistently, Xu et al. (2010) identified thiazovivin from a high-content chemical screen, which increases hESC single-cell survival with enhanced self-renewal. Interestingly, affinity chromatography experiments revealed that thiazovivin enhances E-cadherin stability and cell-cell interactions through the inhibition of ROCK.
Synthetic Small Molecules Facilitate Achieving “Stemness”
In last two decades, a major breakthrough in the stem cell field is cellular reprogramming, in which lineage-committed cells overcome their intrinsic lineage-restriction upon exposure to a specific set of signals, and reverse back to the multipotent or even pluripotent stage. In 2004, we performed a high-throughput screen using C2C12 myoblasts and identified reversine, a 2,6-disubstituted purine, which reverses lineage-committed myoblasts back to the multipotent stage. The reversine-treated myoblasts can efficiently differentiate into osteoblasts and adipocytes only upon exposure to the appropriate differentiation conditions (Chen et al., 2004) (Table 1). Takahashi and Yamanaka (2006) reported the groundbreaking work to reprogram fibroblasts to the pluripotent stage by using overexpression of Oct4, Sox2, Klf4, and c-Myc. Subsequently, many chemical screens were carried out to identify the small molecules to replace the transcriptional factors in a stepwise manner. Using a phenotypic screen with Oct4 promoter-driven GFP expression, Shi et al. (2008) identified BIX-01294 and BayK8644, which enable reprogramming of Oct4/Klf4-transduced mouse embryonic fibroblasts. In a focused screen, Huangfu et al. (2008a) found that valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, improves reprogramming efficiency and induces reprogramming in the presence of c-Myc. Moreover, VPA enables the reprogramming of primary human fibroblasts with only two factors, Oct4 and Sox2 (Huangfu et al., 2008b). Through another chemical screen, kenpaullone was identified to enable the reprogramming of mouse embryonic fibroblasts in the absence of Klf4 (Lyssiotis et al., 2009). Ichida et al. (2009) identified RepSox, which replaces Sox2 during reprogramming by inhibiting transforming growth factor β (TGF-β) signaling. Li et al. (2011b) reported that the combination of VPA, tranylcypromine, CHIR99021, and 616452, was sufficient to induce reprogramming with a single transcription factor, Oct4. The treatment of EPZ004777, an inhibitor of the histone H3K79 methyltransferase DOT1-like, significantly increases the yield of induced pluripotent stem cell (iPSC) colonies, and could be substituted for Klf4 and c-Myc (Onder et al., 2012). Finally, Hou et al. (2013) reported a combination of seven compounds identified from a chemical screen, including VPA, tranylcypromine, CHIR99021, 616452, forskolin, 3-deazaneplanocin, and PD0325901, which induces mouse somatic cell reprogramming to PSCs in the absence of transgene overexpression.
Table 1.
Summary of Compounds Identified from Stem Cell-Based Screens
| Compound | Starting Population | Screening Readout | Biological Process Affected | Mechanism of Action | References |
|---|---|---|---|---|---|
| Cellular Reprogramming | |||||
| Reversine | C2C12 cells | alkaline phosphatase as an osteoblast marker | reprogramming from lineage-committed myoblasts to multipotent stage | a dual inhibitor of MEK1 and nonmuscle myosin II heavy chain | Chen et al. (2004) |
| BIX-01294 | Oct4, Klf4-transduced mouse embryonic fibroblasts (MEFs) | ESC colony and staining with alkaline phosphatase as a pluripotency marker | reprogramming of Oct4/Klf4-transduced mouse embryonic fibroblasts | a potential G9a histone methyltransferase inhibitor | Shi et al. (2008) |
| BayK8644 | a potential L-channel calcium agonist | ||||
| Valproic acid | Oct4-, Sox2-, Klf4-, and c-Myc-transduced MEFs | the percentage of Oct4-GFP+ cells | improve reprogramming efficiency of mouse and human fibroblasts | a potential histone deacetylase inhibitor | Huangfu et al., 2008a, Huangfu et al., 2008b |
| Kenpaullone | Oct4-, Sox2-, and c-Myc-transduced MEFs | Nanog-Luc activity | induce reprogramming in the absence of Klf4 | a potential GSK-3β inhibitor | Lyssiotis et al. (2009) |
| RepSox | Oct4-, Klf4-, and c-Myc-transduced MEFs | the number of Oct4-GFP + colonies | induce reprogramming in the absence of Sox2 | inhibit TGF-β signaling | Ichida et al. (2009) |
| Forskolin | Sox2-, Klf4-, and c-Myc-transduced MEFs | the number of Oct4-GFP + colonies | induce reprogramming in the absence of Oct4 | a potential cAMP activator | Hou et al. (2013) |
| (S)-(+)-Dimethindene maleate | primed H9 hESCs |
activate the OCT4 distal enhancer |
support the conversion and maintenance of dome-shaped hPSCs from primed hPSCs |
a potential M2 muscarinic receptor antagonist |
Yang et al. (2017) |
| minocycline hydrochloride | a potential metalloproteinase inhibitor | ||||
| Directed Differentiation | |||||
| PD407824 | C2C12 cells | Id2-Luc reporter | synergize with BMP4 to direct hPSC differentiation toward mesoderm or cytotrophoblast stem cells | inhibit checkpoint kinase 1 | Feng et al. (2016) |
| Atauprimide | mESCs | immunostaining using SOX17 antibody | prime mouse and human ESC differentiation | interact with NME2 and inhibit nuclear localization | Zhu et al. (2009) |
| IDE1 | mESC | Sox17 promoter-driven tdTomato | induce nearly 80% of ESCs to form definitive endoderm | activate TGF-β signaling | Borowiak et al. (2009) |
| IDE2 | |||||
| Indolactam V | hESC-derived endodermal cells | immunostaining using PDX1 antibody | induce differentiation toward pancreatic progenitors | activate PKC | Chen et al. (2009) |
| H1152 | hESC-derived pancreatic progenitors | immunostaining using insulin antibody | increase the generation of insulin-secreting cells | inhibit ROCKII | Ghazizadeh et al. (2017) |
| CI-994 | hPSC-derived immature hepatocytes | albumin-Venus reporter | promote hPSC differentiation toward hepatocyte-like cells | inhibit histone deacetylase | Li et al. (2018) |
| SU5402 CHIR99021 DAPT |
hPSC-derived neuroectoderm | immunostaining using PAX6 and β3-tubulin antibodies | promote hPSC differentiation toward neurons | a potential VEGFR inhibitor | Chambers et al. (2012) |
| a potential GSK-3β inhibitor | |||||
| a potential Notch inhibitor | |||||
| Selamectin | mESC | immunostaining using tyrosine hydroxylase antibody | transform mPSCs into neurons | a potential GABAA agonist | Sun et al. (2013) |
| Phenanthroline | hESC-derived lens placode | SIX1 H2B::GFP reporter | promote differentiation from nonneural ectoderm to cranial placode | a potential metalloprotease inhibitor | Tchieu et al. (2017) |
| KY02111 | monkey ESCs | αMHC promoter-driven EGFP | PSC differentiation toward cardiomyocytes | NA | Minami et al. (2012) |
| Sodium nitroprusside | mESC-derived mesodermal cells | CCS:lacz reporter | promote the generation of cardiac Purkinje fiber-like cells | increase intracellular cAMP | Tsai et al. (2015) |
| Stem Cell-Based Disease Models | |||||
| SKF-86466 | wild-type and familial dysautonomia iPSC-derived neural crest cells | alamarBlue to detect cell survival qRT-PCR to detect IKBKAP expression |
rescue IKAP protein expression and the disease-specific loss of autonomic neuronal marker expression | a potential α2-adrenoceptor antagonist | Lee et al. (2012) |
| Kenpaullone | wild-type and SOD1G93A HB9::GFP mESC-derived motor neuron | imaging-based assay to count GFP+ cell number | prolong the healthy survival both mouse and human motor neurons | a potential dual inhibitor of GSK-3 and HGK kinases | Yang et al. (2013) |
| Alsterpaullone | fibroblasts from parental SMA carriers | immunostaining using SMN antibody | increase cellular SMN | a potential GSK-3 inhibitor | Makhortova et al. (2011) |
| E34 | a co-culture of mESC-derived motor neurons, astrocytes, and interferon-γ- and lipopolysaccharide-activated microglial cells | the total neurite length of all neurites of the motor neurons | prevent the neurons from cytokine and lipopolysaccharide-induced degeneration | inhibit nitric oxide synthase | Hoing et al. (2012) |
| Pepstatin A | EDNRB-null mutant hESC-derived enteric nervous system precursors | cell migration assay | rescue loss of EDNRB-induced cell migration defects | inhibit BACE2 | Fattahi et al. (2016) |
| Avermectins | TS21 iPSC-derived cortical neurons | the activity of lactate dehydrogenase in medium | increase the relative production of short Aβ peptides at the expense of longer, potentially more toxic peptides | NA | Brownjohn et al. (2017) |
| Gardiac glycosides | familial hypercholesterolemia iPSC-derived hepatocyte-like cells | ELISA-based assay to detect apolipoprotein B (apoB) production | reduce the production of apoB | potential inhibitors of the membrane sodium-potassium (NA+-K+) pump | Cayo et al. (2017) |
| T5524 | CDKAL1−/− hESC-derived pancreatic β-like cells | staining with PI and insulin antibody | rescues loss of CDKAL1 induced increased sensitivity to glucolipotoxcity | inhibit the FOS/JUN pathway | Zeng et al. (2016) |
| Galunisertib | GLIS3−/− hESC-derived pancreatic β-like cells | staining with PI and insulin antibody | rescues loss of CDKAL1 induced pancreatic β cell death | inhibit TGF-β pathway | Amin et al. (2018) |
| Emricasan | hNPCs | caspase-3/7 activity assay | block ZIKV-induced hNPC death | a pan-caspase inhibitor | Xu et al. (2016) |
| Niclosamide | inhibits ZIKV replication | NA | |||
| Hippeastrine hydrobromide | hNPCs | staining with ZIKV antibody | eliminate ZIKV infection | NA | Zhou et al. (2017) |
| Geneticin | familial adenomatous polyposis-iPSC-derived colonic organoids | staining with CDX2 and Ki67 antibodies | rescue early stop codon caused APC protein decrease and increased cell proliferation | NA | Crespo et al. (2017) |
NA, not applicable.
In addition to cellular reprogramming, chemical screens were used to identify the compounds that facilitate the conversion between prime and naive pluripotent status. By screening compounds that activate the OCT4 distal enhancer, Yang et al. (2017) established a minimal condition consisting of human LIF, CHIR99021, (S)-(+)-dimethindene maleate, and minocycline hydrochloride. This minimal condition supports the conversion and long-term maintenance of extended PSCs, which are capable of chimerizing both embryonic and extraembryonic tissues.
Directed hPSC Differentiation Using Chemical Approaches
Human pluripotent stem cells (hPSCs) provide unlimited starting material to generate differentiated cells that can be used to build a functional organ. Essential to this pursuit is an efficient way to differentiate hPSCs into specific types of mature cells. The rational-based chemical approach has been widely used to manipulate the key pathways involved in PSC differentiation. For example, CHIR99021, which activates the Wnt pathway, is commonly used to promote hPSC differentiation (Davidson et al., 2012). Chambers and coworkers found that the combination of SB431542, a TGF-β inhibitor, and, dorsomorphin, a BMP inhibitor, efficiently induces hESC differentiation toward the neural lineage (Chambers et al., 2009, Kim et al., 2010). Li et al. (2011a) reported that the combination of CHIR99021, SB431542, and compound E can efficiently convert hESCs into homogeneous primitive neuroepithelium. Fattahi et al. (2016) found that CHIR99021 promotes the differentiation to neural crest, and that retinoic acid facilitates differentiation to enteric neural crest lineages. In addition, retinoic acid, LDN193189 (a BMP pathway inhibitor), KAAD-cyclopamine (a sonic hedgehog pathway inhibitor) are used to promote pancreatic induction from hESCs (D'Amour et al., 2006). Although chemical agonists and antagonists provide powerful tools for hPSC differentiation, chemical agonists of several key development pathways, such as BMP, TGF-β, epidermal growth factor (EGF), and fibroblast growth factor (FGF), are still missing. Using a high-throughput screening platform based on C2C12 cells containing Id2-Luc reporter, we discovered PD407824, a checkpoint kinase 1 inhibitor that increases the sensitivity of cells to sub-threshold BMP4. We showed the utility of PD407824 in the directed differentiation of hESCs toward mesoderm or cytotrophoblast stem cells (Feng et al., 2016).
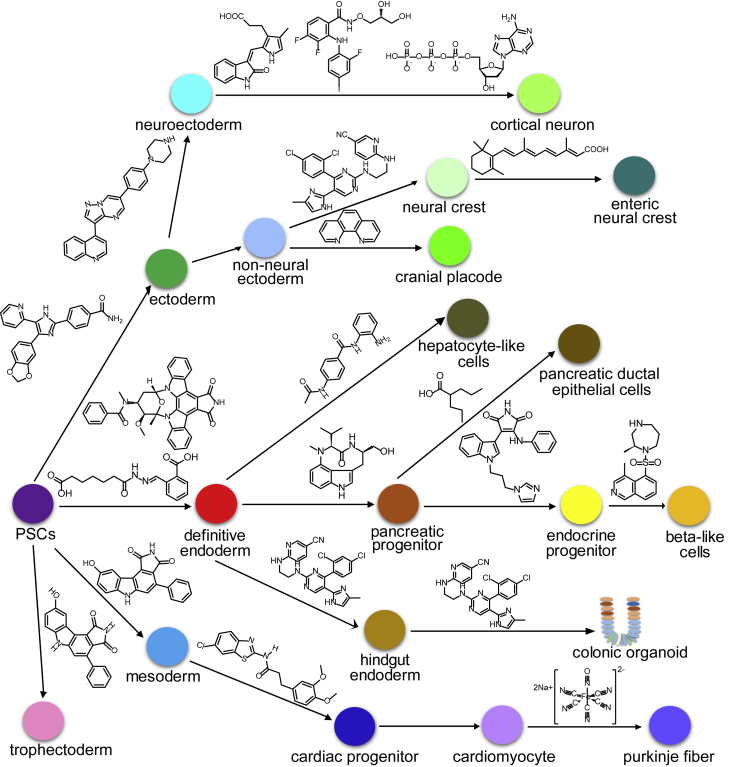
Although rational-based chemical approaches have provided useful tools to induce hPSC differentiation, the molecular mechanism controlling organogenesis is not fully understood. Screening-based chemical approaches can also be applied to identify the small molecules directing stem cell differentiation (Figure 1). More importantly, the identified small molecules function as tools to discover the pathways controlling cell fate decision. For example, Desbordes et al. (2008) reported a high-throughput screen for the identification of compounds regulating self-renewal and differentiation in hESCs. By monitoring SOX17 expression, Zhu et al. (2009) screened 20,000 compounds and identified stauprimide, which promotes efficient induction of SOX17 in both mouse and human ESCs synergistically with low concentrations of activin A. Using Sox17 promoter-driven tdTomato as a reporter, Borowiak et al. (2009) screened 4,000 compounds and found two small molecules, inducer of definitive endoderm 1 (IDE1) and IDE2, that induce endoderm differentiation in both mESCs and hESCs. In addition, we performed a high-content chemical screen using hESC-derived definitive endoderm and identified (−)-indolactam V, a protein kinase C (PKC) activator, which promotes the generation of pancreatic progenitors (Chen et al., 2009). Now, a PKC activator has become a standard component of most pancreatic β cell differentiation protocols (Maehr et al., 2009, Pagliuca et al., 2014, Rezania et al., 2014, Vegas et al., 2016). Recently, we screened 2,000 compounds and found that H1152, a ROCKII inhibitor, increases the generation of insulin-secreting cells from hPSCs and improves β cell maturation (Ghazizadeh et al., 2017). Using an albumin-Venus reporter line, Li et al. (2018) identified, CI-994, an HDAC inhibitor that promotes hPSC differentiation toward hepatocyte-like cells.
Figure 1.
Summary of Small Molecules Regulating Pluripotent Stem Cell Differentiation
Both high-throughput and focused chemical screens were used to develop compounds or compound combinations directing hPSC differentiation to ectodermal lineages. Using a focused screen, Chambers et al. (2012) developed a combination of five chemical pathway inhibitors, LDN193189 and SB431542, SU5402, CHIR99021, and DAPT, which yields hPSC-derived neurons at >75% efficiency. Sun et al. (2013) reported a high-content screen, which revealed that selamectin, an anti-helminthic therapeutic compound, promotes neural differentiation of PSCs. A focused screen by combinatorial application of six pathway inhibitors revealed that XAV939 promotes anterior central nervous system identity; SU5402 and PD0325901 accelerate hPSC differentiation toward neuroectodermal lineages; and the highly transient anterior neuroectodermal precursor state is driven toward post-mitotic cortical fates in the presence of DAPT and SU5402/PD0325901 (Qi et al., 2017). Tchieu et al. (2017) screened the LOPAC library (Sigma) and identified phenanthroline, a metal chelator that can act as a metalloprotease inhibitor, enhances cranial placode differentiation.
Finally, high-throughput or high-content screens also facilitate discovery of compounds inducing mesodermal differentiation. A cell-based screen led to the discovery of KY02111, which promotes hPSC differentiation to cardiomyocytes (Minami et al., 2012). We carried out a high-throughput screen using CCS:lacz and Contactin2:egfp reporter ESC lines, and discovered sodium nitroprusside efficiently promotes the generation of cardiac Purkinje fiber-like cells (Tsai et al., 2015).
hPSC-Derived Cells for Disease Modeling and Drug Screening
Patient-specific hPSCs represent a novel system for modeling human genetic diseases, which can be adapted to a high-throughput/content manner to screen for gene/mutation-specific drug candidates. The identified drug candidates will also facilitate dissecting entangled mechanisms under specific pathological settings. For example, Lee et al. (2012) reported a high-content screen using neural crest precursors derived from iPSCs that were generated from individuals with familial dysautonomia, a rare, fatal genetic disorder affecting neural crest lineages. One hit compound, SKF-86466, rescues IKAP protein expression and the disease-specific loss of autonomic neuronal marker expression. By applying a small-molecule survival screen using motor neurons from both wild-type and mutant SOD1 (a gene associated with amyotrophic lateral sclerosis) mESCs, Yang et al. (2013) discovered that kenpaullone, a dual inhibitor of GSK-3 and HGK (hematopoietic progenitor kinase) kinases, prolongs the healthy survival both mouse and human motor neurons. Spinal muscular atrophy results from mutations that lead to low levels of the ubiquitously expressed protein, survival of motor neuron (SMN). Makhortova et al. (2011) executed an image-based screen and discovered compounds targeting the receptor tyrosine kinase-phosphatidylinositol 3-kinase-AKT-GSK-3 signaling cascade, which increase cellular SMN. A neuroprotective screen was carried out by adding interferon-γ- and lipopolysaccharide-activated microglial cells into the co-culture of mESC-derived motor neurons and astrocytes. This screen identified a number of neuroprotective small molecules that prevented the neurons from degeneration through diverse mechanisms, including inhibition of nitric oxide production by microglia, stimulation of the nuclear factor erythroid 2-related factor 2 pathway in microglia and astrocytes, and direct protection of neurons from cell death (Hoing et al., 2012). By modeling Hirschsprung disease-related migration defects using EDNRB-null hESC-derived mutant enteric nervous system precursors, we identified pepstatin A as a candidate therapeutic target for the treatment of Hirschsprung disease (Fattahi et al., 2016). Amyloid precursor protein (APP) processing and the accumulation of APP-derived amyloid β peptides are key processes in Alzheimer's disease. Brownjohn et al. (2017) designed a phenotypic chemical screen to identify modulators of APP processing in trisomy 21/Down syndrome neurons, a complex genetic model of Alzheimer's disease. Avermectins, which are commonly used anthelmintics, were found to increase the relative production of short Aβ peptides at the expense of longer, potentially more toxic peptides. Finally, using hepatocyte-like cells generated from homozygous familial hypercholesterolemia iPSCs, Cayo et al. (2017) identified cardiac glycosides that can potentially be repurposed to lower serum low-density lipoprotein cholesterol.
In addition to modeling monogenic diseases, hPSC-derived cells can also be used to study the complicated diseases caused by a combination of genetic susceptibility and environmental factors. Genome-wide association studies (GWAS) have generated a large database of disease-associated SNPs and loci. One major challenge is to construct a robust system to systematically evaluate the role of these SNPs/loci using disease-relevant cells. Using isogenic hESC-derived pancreatic β-like cells, we found that mutations in CDKAL1, KCNQ1, and KCNJ11, diabetes-associated genes identified from GWAS, led to impaired glucose secretion. CDKAL1 mutant insulin+ cells were also hypersensitive to glucolipotoxicity. A high-content chemical screen identified T5524 that rescues CDKAL1-specific defects in vitro and in vivo by inhibiting the FOS/JUN pathway (Zeng et al., 2016). Using these isogenic mutant hESCs, we found an unexpected interaction of two diabetes-associated genes, CDKAL1 and metallothionein 1E (MT1E). Forced MT1E expression rescues both hypersensitivity of CDKAL1 mutant cells to glycolipotoxicity and pancreatic β cell dysfunction (Guo et al., 2017). We also discovered the haploinsufficiency and dosage-sensitive requirements for GATA6 and GATA4, two diabetes-associated genes, in the formation of both definitive endoderm and pancreatic progenitor cells (Shi et al., 2017). Finally, we studied GLIS3, one of the only two genes associated with type 1, type 2, and neonatal diabetes. Using a “minimal component” protocol to generate mono-hormonal pancreatic β-like cells, we discovered that GLIS3−/− hESCs show impaired differentiation with significant death of β-like cells. Furthermore, we performed a high-content chemical screen and identified a drug candidate targeting the TGF-β pathway, which rescues mutant GLIS3-associated β cell death both in vitro and in vivo (Amin et al., 2018). These examples highlight the power of isogenic hPSCs to evaluate GWAS-identified genetic factors and to identify novel gene/mutation-specific drug candidates for precision medicine.
Finally, hPSC-derived cells also facilitate the drug discovery and evaluation for infectious diseases. A focused screen using hiPSC-CMs infected with a luciferase-expressing coxsackievirus B3 strain was used to evaluate anti-viral compounds including interferon β1, ribavirin, pyrrolidine dithiocarbamate, and fluoxetine (Sharma et al., 2014). A recent large outbreak first observed in Brazil brought Zika virus (ZIKV) to the forefront of public health attention. ZIKV infection is associated with an increased incidence of congenital defects, most prominently microcephaly (Mlakar et al., 2016, Rasmussen et al., 2016). In infected adults, ZIKV is associated with serious neurological complications such as meningoencephalitis and myelitis (Cao-Lormeau et al., 2016, Carteaux et al., 2016, Mecharles et al., 2016, Parra et al., 2016). The World Health Organization and the NIH have compelled the scientific community to find solutions to the ZIKV threat. Xu et al. (2016) used hPSC-derived human cortical neural progenitors (hNPCs) to screen for anti-ZIKV drugs, and identified emricasan, a pan-caspase inhibitor, which protects hNPCs; cyclin-dependent kinases inhibit ZIKV replication; and niclosamide inhibits ZIKV replication. Concurrently, we performed a high-content screen and identified a novel anti-ZIKV compound, hippeastrine hydrobromide. Strikingly, hippeastrine hydrobromide suppresses viral propagation when administered to adult mice with active ZIKV infection, highlighting its therapeutic potential (Zhou et al., 2017).
hPSC-Derived 3D Organoids for Disease Modeling and Drug Screening
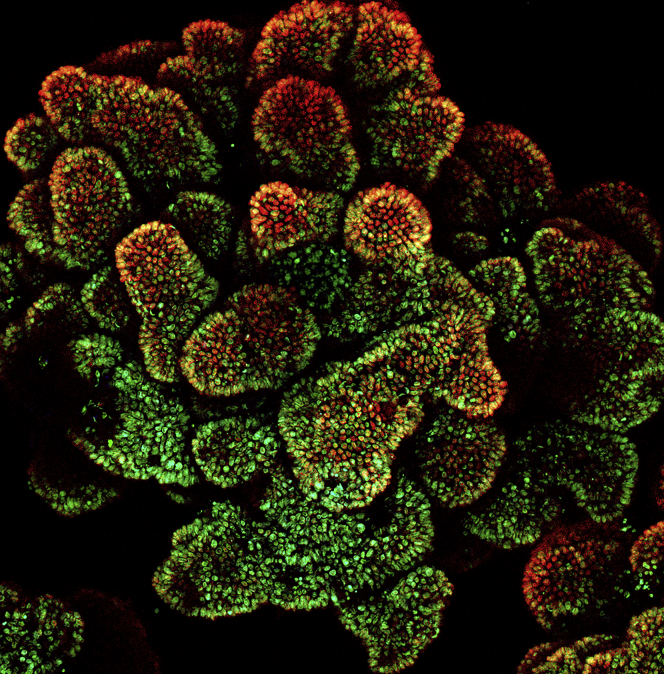
In last decade, significant progresses has been made in 3D organoids. Strategies have been reported to differentiate hPSCs to 3D self-organizing organoids representing intestinal (Spence et al., 2011), gastric (McCracken et al., 2014), brain (Lancaster et al., 2013), liver (Takebe et al., 2013), lung (Dye et al., 2015), pancreas (Huang et al., 2015), and esophageal (Trisno et al., 2018) “mini-organs.” Concurrently, we (Crespo et al., 2017, Munera et al., 2017) reported strategies to efficiently generate colonic organoids (COs) from hESCs. We made COs from the iPSCs derived from familial adenomatous polyposis (FAP) patients, which show enhanced WNT activity and increased epithelial cell proliferation (Figure 2). A focused screen identified geneticin targets abnormal WNT activity and thereby corrects the deregulated proliferation specific to FAP COs (Crespo et al., 2017). In addition to hPSC-based organoids, organoids derived from primary tumor tissues were also used for drug screens. Broutier et al. (2017) developed a platform to propagate primary liver cancer (PLC) organoids. Screening using PLC-derived organoids identified an ERK inhibitor, SCH772984, as a potential therapeutic agent for PLC. Finally, we evaluated the anti-ZIKV compounds identified from a chemical screen and showed that hippeastrine hydrobromide rescues ZIKV-induced growth and differentiation defects in human fetal-like forebrain organoids, which highlights the value of human forebrain organoids in long-term evaluation of drug candidates in infectious diseases (Zhou et al., 2017).
Figure 2.
Projection Image of Colonic Organoids Derived from FAP-iPSCs
Red, CDX2; green, CCND1.
Humanized Animal Models for Long-Term In Vivo Drug Evaluation
Animal models have proven to be a powerful tool in drug discovery. However, due to the species differences, some animal models fail to precisely predict the clinical outcome, which is one of the major reasons of the high failure rate of clinical trials. There is a strong need to develop more predictive in vivo models to evaluate the efficacy, pharmacokinetic profile, and toxicological properties of leading compounds. Humanized mice carrying hPSC-derived cells will provide a powerful tool to evaluate the long-term in vivo efficacy of drug candidates on human cells. In our recent studies, we created humanized mice carrying hESC-derived pancreatic β-like cells. The mouse pancreatic β cells were destroyed by the administration of streptozotocin. Thus, the humanized mice depend on the transplanted human pancreatic β-like cells to maintain glucose homeostasis. We found that the mice transplanted with CDKAL1−/− hESC-derived cells show impaired glucose secretion, coinciding with defective glucose homeostasis. The humanized mice were applied to evaluate the drug candidate identified from a high-content screen. We found that T5224 treatment significantly increases the level of insulin secretion and restores the capacity of the humanized mice to maintain glucose homeostasis (Zeng et al., 2016). In another study using humanized mice transplanted with GLIS3−/− hESC-derived pancreatic β-like cells, we found that galunisertib rescues cell death induced by loss of GLIS3 in vivo (Amin et al., 2018). Using avatar mice harboring hepatocyte-like cells generated from homozygous familial hypercholesterolemia iPSCs, Cayo et al. (2017) showed that cardiac glycosides reduce the production of apolipoprotein B in the serum of humanized mice. Finally, Verissimo et al. (2016) used xenotransplanted mice carrying RAS mutant organoids to confirm the growth arrest effect of pan-HER/MEK combination therapy.
Conclusion and Prospective
High-throughput/content chemical screening is an emerging, critical approach to identify the small molecules controlling stem cell self-renewal, differentiation, and reprogramming. Using the small molecules identified from focused or unbiased screens, significant progress has been made to derive functional cells or organoids from hPSCs. However, several issues remain to be addressed to apply chemical approaches for broader applications. First, a better chemical toolkit to manipulate developmental pathways, such as chemical agonists of BMP, TGF-β, EGF, and FGF signals, are required. Second, additional chemical screens are needed to identify small molecules inducing differentiation to some poorly characterized cell types, such as cells in the cardiac conduction system, neuroendocrine cells, etc. Third, maturation is still a major concern for most directed differentiation protocols. With the appropriate experimental design, chemical screens will expedite the development of strategies promoting cell maturation.
hPSC-derived cells or organoids are widely used to study the role of genetic and environmental factors in human disease progression, which could provide a source of cells for large-scale drug discovery. Indeed, the chemical screens using hESC/iPSC-derived cells or organoids have identified candidates that help future drug development. In the meanwhile, these identified small molecules facilitate dissecting the molecular mechanism under pathological conditions. Most of current screens using hPSC-derived cells are phenotypic-based screens. Additional work needs to be done to further improve the throughput of these types of screens. Moreover, hPSC-derived 3D organoids show large variation due to their complicated structure. Appropriate assays need to be designed to adapt the organoid-based screen to a high-throughput/content manner. Finally, initial attempts of humanized mice have proven the power of this innovative model to evaluate long-term drug efficacy on human cells in vivo. Improved humanized mouse models, which contain multiple human organs, and/or the human immune system, will facilitate establishing more predictive in vivo models for drug discovery.
In summary, screening-based chemical approaches have been proven to be a powerful tool for controlling stem cell fate decisions, as well as decoding complicated disease progression. With the discovery of novel hPSC differentiation strategies, disease models, and humanized mouse models, the high-throughput/content chemical screens will significantly contribute to the discovery of a novel “toolkit” for regenerative medicine and precision therapy.
Acknowledgment
The author would like to give thanks for the long-term support from peer-reviewed competitive grant funds from the New York Stem Cell Foundation, the NIH, the American Diabetes Association, the American Heart Association, and the American Association for Cancer Research.
References
- Amin S., Cook B., Zhou T., Ghazizadeh Z., Lis R., Zhang T., Khalaj M., Crespo M., Perera M., Xiang J.Z. Discovery of a drug candidate for GLIS3-associated diabetes. Nat. Commun. 2018;9:2681. doi: 10.1038/s41467-018-04918-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak M., Maehr R., Chen S., Chen A.E., Tang W., Fox J.L., Schreiber S.L., Melton D.A. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broutier L., Mastrogiovanni G., Verstegen M.M., Francies H.E., Gavarro L.M., Bradshaw C.R., Allen G.E., Arnes-Benito R., Sidorova O., Gaspersz M.P. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownjohn P.W., Smith J., Portelius E., Serneels L., Kvartsberg H., De Strooper B., Blennow K., Zetterberg H., Livesey F.J. Phenotypic screening identifies modulators of amyloid precursor protein processing in human stem cell models of Alzheimer's disease. Stem Cell Reports. 2017;8:870–882. doi: 10.1016/j.stemcr.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau V.M., Blake A., Mons S., Lastere S., Roche C., Vanhomwegen J., Dub T., Baudouin L., Teissier A., Larre P. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteaux G., Maquart M., Bedet A., Contou D., Brugieres P., Fourati S., Cleret de Langavant L., de Broucker T., Brun-Buisson C., Leparc-Goffart I. Zika virus associated with meningoencephalitis. N. Engl. J. Med. 2016;374:1595–1596. doi: 10.1056/NEJMc1602964. [DOI] [PubMed] [Google Scholar]
- Cayo M.A., Mallanna S.K., Di Furio F., Jing R., Tolliver L.B., Bures M., Urick A., Noto F.K., Pashos E.E., Greseth M.D. A drug screen using human iPSC-derived hepatocyte-like cells reveals cardiac glycosides as a potential treatment for hypercholesterolemia. Cell Stem Cell. 2017;20:478–489.e5. doi: 10.1016/j.stem.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Qi Y., Mica Y., Lee G., Zhang X.J., Niu L., Bilsland J., Cao L., Stevens E., Whiting P. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol. 2012;30:715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Borowiak M., Fox J.L., Maehr R., Osafune K., Davidow L., Lam K., Peng L.F., Schreiber S.L., Rubin L.L. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat. Chem. Biol. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- Chen S., Do J.T., Zhang Q., Yao S., Yan F., Peters E.C., Scholer H.R., Schultz P.G., Ding S. Self-renewal of embryonic stem cells by a small molecule. Proc. Natl. Acad. Sci. U S A. 2006;103:17266–17271. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhang Q., Wu X., Schultz P.G., Ding S. Dedifferentiation of lineage-committed cells by a small molecule. J. Am. Chem. Soc. 2004;126:410–411. doi: 10.1021/ja037390k. [DOI] [PubMed] [Google Scholar]
- Crespo M., Vilar E., Tsai S.Y., Chang K., Amin S., Srinivasan T., Zhang T., Pipalia N.H., Chen H.J., Witherspoon M. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017;23:878–884. doi: 10.1038/nm.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour K.A., Bang A.G., Eliazer S., Kelly O.G., Agulnick A.D., Smart N.G., Moorman M.A., Kroon E., Carpenter M.K., Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Davidson K.C., Adams A.M., Goodson J.M., McDonald C.E., Potter J.C., Berndt J.D., Biechele T.L., Taylor R.J., Moon R.T. Wnt/beta-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. U S A. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbordes S.C., Placantonakis D.G., Ciro A., Socci N.D., Lee G., Djaballah H., Studer L. High-throughput screening assay for the identification of compounds regulating self-renewal and differentiation in human embryonic stem cells. Cell Stem Cell. 2008;2:602–612. doi: 10.1016/j.stem.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye B.R., Hill D.R., Ferguson M.A., Tsai Y.H., Nagy M.S., Dyal R., Wells J.M., Mayhew C.N., Nattiv R., Klein O.D. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4 doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattahi F., Steinbeck J.A., Kriks S., Tchieu J., Zimmer B., Kishinevsky S., Zeltner N., Mica Y., El-Nachef W., Zhao H. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531:105–109. doi: 10.1038/nature16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Cook B., Tsai S.Y., Zhou T., LaFlamme B., Evans T., Chen S. Discovery of a small-molecule BMP sensitizer for human embryonic stem cell differentiation. Cell Rep. 2016;15:2063–2075. doi: 10.1016/j.celrep.2016.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh Z., Kao D.I., Amin S., Cook B., Rao S., Zhou T., Zhang T., Xiang Z., Kenyon R., Kaymakcalan O. ROCKII inhibition promotes the maturation of human pancreatic beta-like cells. Nat. Commun. 2017;8:298. doi: 10.1038/s41467-017-00129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Zhang T., Dong X., Xiang J.Z., Lei M., Evans T., Graumann J., Chen S. Using hESCs to probe the interaction of the diabetes-associated genes CDKAL1 and MT1E. Cell Rep. 2017;19:1512–1521. doi: 10.1016/j.celrep.2017.04.070. [DOI] [PubMed] [Google Scholar]
- Hoing S., Rudhard Y., Reinhardt P., Glatza M., Stehling M., Wu G., Peiker C., Bocker A., Parga J.A., Bunk E. Discovery of inhibitors of microglial neurotoxicity acting through multiple mechanisms using a stem-cell-based phenotypic assay. Cell Stem Cell. 2012;11:620–632. doi: 10.1016/j.stem.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Hou P., Li Y., Zhang X., Liu C., Guan J., Li H., Zhao T., Ye J., Yang W., Liu K. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- Huang L., Holtzinger A., Jagan I., BeGora M., Lohse I., Ngai N., Nostro C., Wang R., Muthuswamy L.B., Crawford H.C. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med. 2015;21:1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D., Maehr R., Guo W., Eijkelenboom A., Snitow M., Chen A.E., Melton D.A. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D., Osafune K., Maehr R., Guo W., Eijkelenboom A., Chen S., Muhlestein W., Melton D.A. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat. Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- Ichida J.K., Blanchard J., Lam K., Son E.Y., Chung J.E., Egli D., Loh K.M., Carter A.C., Di Giorgio F.P., Koszka K. A small-molecule inhibitor of TGF-beta signaling replaces Sox2 in reprogramming by inducing Nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.S., Lee J.S., Leem J.W., Huh Y.J., Kim J.Y., Kim H.S., Park I.H., Daley G.Q., Hwang D.Y., Kim D.W. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. 2010;6:270–281. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Ramirez C.N., Kim H., Zeltner N., Liu B., Radu C., Bhinder B., Kim Y.J., Choi I.Y., Mukherjee-Clavin B. Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat. Biotechnol. 2012;30:1244–1248. doi: 10.1038/nbt.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Li M., Liu X., Yang Y., Wei Y., Chen Y., Qiu Y., Zhou T., Feng Z., Ma D. Genetic and chemical screenings identify HDAC3 as a key regulator in hepatic differentiation of human pluripotent stem cells. Stem Cell Reports. 2018;11:22–31. doi: 10.1016/j.stemcr.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Sun W., Zhang Y., Wei W., Ambasudhan R., Xia P., Talantova M., Lin T., Kim J., Wang X. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. U S A. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang Q., Yin X., Yang W., Du Y., Hou P., Ge J., Liu C., Zhang W., Zhang X. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2011;21:196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssiotis C.A., Foreman R.K., Staerk J., Garcia M., Mathur D., Markoulaki S., Hanna J., Lairson L.L., Charette B.D., Bouchez L.C. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc. Natl. Acad. Sci. U S A. 2009;106:8912–8917. doi: 10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr R., Chen S., Snitow M., Ludwig T., Yagasaki L., Goland R., Leibel R.L., Melton D.A. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. U S A. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhortova N.R., Hayhurst M., Cerqueira A., Sinor-Anderson A.D., Zhao W.N., Heiser P.W., Arvanites A.C., Davidow L.S., Waldon Z.O., Steen J.A. A screen for regulators of survival of motor neuron protein levels. Nat. Chem. Biol. 2011;7:544–552. doi: 10.1038/nchembio.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken K.W., Cata E.M., Crawford C.M., Sinagoga K.L., Schumacher M., Rockich B.E., Tsai Y.H., Mayhew C.N., Spence J.R., Zavros Y. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecharles S., Herrmann C., Poullain P., Tran T.H., Deschamps N., Mathon G., Landais A., Breurec S., Lannuzel A. Acute myelitis due to Zika virus infection. Lancet. 2016;387:1481. doi: 10.1016/S0140-6736(16)00644-9. [DOI] [PubMed] [Google Scholar]
- Minami I., Yamada K., Otsuji T.G., Yamamoto T., Shen Y., Otsuka S., Kadota S., Morone N., Barve M., Asai Y. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep. 2012;2:1448–1460. doi: 10.1016/j.celrep.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Mlakar J., Korva M., Tul N., Popovic M., Poljsak-Prijatelj M., Mraz J., Kolenc M., Resman Rus K., Vesnaver Vipotnik T., Fabjan Vodusek V. Zika virus associated with microcephaly. N. Engl. J. Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Munera J.O., Sundaram N., Rankin S.A., Hill D., Watson C., Mahe M., Vallance J.E., Shroyer N.F., Sinagoga K.L., Zarzoso-Lacoste A. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell. 2017;21:51–64.e6. doi: 10.1016/j.stem.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Burdon T., Chambers I., Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder T.T., Kara N., Cherry A., Sinha A.U., Zhu N., Bernt K.M., Cahan P., Marcarci B.O., Unternaehrer J., Gupta P.B. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012;483:598–602. doi: 10.1038/nature10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra B., Lizarazo J., Jimenez-Arango J.A., Zea-Vera A.F., Gonzalez-Manrique G., Vargas J., Angarita J.A., Zuniga G., Lopez-Gonzalez R., Beltran C.L. Guillain-Barre syndrome associated with Zika virus infection in Colombia. N. Engl. J. Med. 2016;375:1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- Qi Y., Zhang X.J., Renier N., Wu Z., Atkin T., Sun Z., Ozair M.Z., Tchieu J., Zimmer B., Fattahi F. Combined small-molecule inhibition accelerates the derivation of functional cortical neurons from human pluripotent stem cells. Nat. Biotechnol. 2017;35:154–163. doi: 10.1038/nbt.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S.A., Jamieson D.J., Honein M.A., Petersen L.R. Zika virus and birth defects – reviewing the evidence for causality. N. Engl. J. Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., O'Dwyer S., Quiskamp N., Mojibian M., Albrecht T. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- Sharma A., Marceau C., Hamaguchi R., Burridge P.W., Rajarajan K., Churko J.M., Wu H., Sallam K.I., Matsa E., Sturzu A.C. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus B3-induced myocarditis and antiviral drug screening platform. Circ. Res. 2014;115:556–566. doi: 10.1161/CIRCRESAHA.115.303810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Desponts C., Do J.T., Hahm H.S., Scholer H.R., Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Shi Z.D., Lee K., Yang D., Amin S., Verma N., Li Q.V., Zhu Z., Soh C.L., Kumar R., Evans T. Genome editing in hPSCs reveals GATA6 haploinsufficiency and a genetic interaction with GATA4 in human pancreatic development. Cell Stem Cell. 2017;20:675–688.e6. doi: 10.1016/j.stem.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J.R., Mayhew C.N., Rankin S.A., Kuhar M.F., Vallance J.E., Tolle K., Hoskins E.E., Kalinichenko V.V., Wells S.I., Zorn A.M. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Dong Z., Jin T., Ang K.H., Huang M., Haston K.M., Peng J., Zhong T.P., Finkbeiner S., Weiss W.A. Imaging-based chemical screening reveals activity-dependent neural differentiation of pluripotent stem cells. Elife. 2013;2:e00508. doi: 10.7554/eLife.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.R., Ueno Y., Zheng Y.W., Koike N. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- Tchieu J., Zimmer B., Fattahi F., Amin S., Zeltner N., Chen S., Studer L. A modular platform for differentiation of human PSCs into all major ectodermal lineages. Cell Stem Cell. 2017;21:399–410.e7. doi: 10.1016/j.stem.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisno S.L., Philo K.E.D., McCracken K.W., Cata E.M., Ruiz-Torres S., Rankin S.A., Han L., Nasr T., Chaturvedi P., Rothenberg M.E. Esophageal organoids from human pluripotent stem cells delineate Sox2 functions during esophageal specification. Cell Stem Cell. 2018;23:501–515.e7. doi: 10.1016/j.stem.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S.Y., Maass K., Lu J., Fishman G.I., Chen S., Evans T. Efficient generation of cardiac Purkinje cells from ESCs by activating cAMP signaling. Stem Cell Reports. 2015;4:1089–1102. doi: 10.1016/j.stemcr.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegas A.J., Veiseh O., Gurtler M., Millman J.R., Pagliuca F.W., Bader A.R., Doloff J.C., Li J., Chen M., Olejnik K. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verissimo C.S., Overmeer R.M., Ponsioen B., Drost J., Mertens S., Verlaan-Klink I., Gerwen B.V., van der Ven M., Wetering M.V., Egan D.A. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. Elife. 2016;5 doi: 10.7554/eLife.18489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J.B., Nishikawa S., Nishikawa S., Muguruma K. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Xu M., Lee E.M., Wen Z., Cheng Y., Huang W.K., Qian X., Tcw J., Kouznetsova J., Ogden S.C., Hammack C. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016;22:1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhu X., Hahm H.S., Wei W., Hao E., Hayek A., Ding S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl. Acad. Sci. U S A. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liu B., Xu J., Wang J., Wu J., Shi C., Xu Y., Dong J., Wang C., Lai W. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell. 2017;169:243–257.e25. doi: 10.1016/j.cell.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.M., Gupta S.K., Kim K.J., Powers B.E., Cerqueira A., Wainger B.J., Ngo H.D., Rosowski K.A., Schein P.A., Ackeifi C.A. A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell. 2013;12:713–726. doi: 10.1016/j.stem.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.L., Nichols J., Chambers I., Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Guo M., Zhou T., Tan L., Chong C.N., Zhang T., Dong X., Xiang J.Z., Yu A.S., Yue L. An isogenic human ESC platform for functional evaluation of genome-wide-association-study-identified diabetes genes and drug discovery. Cell Stem Cell. 2016;19:326–340. doi: 10.1016/j.stem.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Tan L., Cederquist G.Y., Fan Y., Hartley B.J., Mukherjee S., Tomishima M., Brennand K.J., Zhang Q., Schwartz R.E. High-content screening in hPSC-neural progenitors identifies drug candidates that inhibit Zika virus infection in fetal-like organoids and adult brain. Cell Stem Cell. 2017;21:274–283.e5. doi: 10.1016/j.stem.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Wurdak H., Wang J., Lyssiotis C.A., Peters E.C., Cho C.Y., Wu X., Schultz P.G. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell. 2009;4:416–426. doi: 10.1016/j.stem.2009.04.001. [DOI] [PubMed] [Google Scholar]