Abstract
Background:
Accumulating evidence indicates a link between posttraumatic stress disorder (PTSD) and cannabis use and suggests that this link may vary as a function of PTSD symptom cluster type. Consistent with negative reinforcement models of substance use, individuals with elevated Cluster D (Hyperarousal) symptoms may be more likely to use cannabis in response to elevated state anxiety and experience decreases in state anxiety after using cannabis.
Objectives:
We aimed to test hypotheses that the interaction of Cluster D and state anxiety would be related to subsequent cannabis use and that those with elevated Cluster D symptoms who used cannabis would report the greatest decreases in state anxiety. To test specificity, we tested whether Clusters B (re-experiencing) and C (avoidance) showed similar relationships.
Methods:
The present study used ecological momentary assessment to examine cannabis use among 87 cannabis-using individuals with PTSD symptoms (64.4% male, 56.3% non-Hispanic Caucasian). State anxiety and cannabis use were assessed over the two-week period via signal contingent (six random prompts per day), interval contingent (each bedtime), and event contingent (cannabis use episodes) assessments.
Results:
Consistent with negative reinforcement models, participants with clinically significant Cluster D symptoms with elevated state anxiety had a greater likelihood of subsequent cannabis use and cannabis use resulted in less subsequent anxiety. The negative reinforcement hypothesis was only partially supported for those with Cluster B and C symptoms.
Conclusions:
Results suggest that negative reinforcement models may be especially relevant to understanding cannabis use among those with clinically elevated Cluster D symptoms.
Keywords: cannabis, marijuana, posttraumatic stress, ecological momentary assessment, negative reinforcement
Introduction
Posttraumatic stress disorder (PTSD) is associated with cannabis use and cannabis-related problems. Data from three large national epidemiological studies demonstrate a link between PTSD and cannabis use and cannabis use disorder (CUD).1–3 To illustrate, the National Comorbidity Study Replication found that 65% of those with a lifetime diagnosis of PTSD reported lifetime cannabis use and rates of current cannabis use were higher among those with PTSD (14%) compared to individuals without a lifetime diagnosis of PTSD (9.2%).3 Further, the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) found that a lifetime PTSD diagnosis was significantly associated with both CUD (OR = 1.217) and lifetime cannabis use (OR = 0.992).1 Additionally, the NESARC-III found that individuals with a past 12-month diagnosis of CUD had significantly greater odds of having a comorbid PTSD diagnosis (OR = 4.3) than those without CUD.2
Among veterans, those with a CUD had significantly higher rates of PTSD than those with other substance use disorders4 and PTSD symptom severity was incrementally positively associated with cannabis-related problems and withdrawal severity during a previous quit attempt, after controlling for other substance use and co-occurring anxiety and mood disorders.5 Additionally, 14.6% of veterans seeking treatment at a Veterans Affairs PTSD specialty clinic reported past six-month cannabis use and after controlling for a wide range of relevant variables (e.g., combat exposure, age, race), past six-month cannabis use was significantly associated with PTSD symptom severity.6 Similarly, veterans who screened positive for PTSD were significantly more likely to have used cannabis in their lifetime and within the past month than veterans who did not screen positive for PTSD (68.3% vs. 54.5% and 27.9% vs. 12%, respectively).7 Taken together, there appears to be a consistent link between PTSD and cannabis/CUD among representative United States populations, as well as veterans.
Negative reinforcement models of substance use propose that substances may be used in an attempt to relieve unpleasant physical and/or emotional states such as withdrawal, craving, and negative affect.8, 9 Consistent with these models, substance use should be maintained if the desired effect is achieved (i.e., substance produces alleviation of negative state). More recent models highlight the importance of desire to escape from and avoid aversive internal states, such as negative affect, in motivation to use substances.8 This model also highlights the importance of considering level of negative affect, as the model proposes that individuals are more likely to use substances at higher (versus more moderate) levels of negative affect.8 In line with these models, persons with post-traumatic symptomatology (which includes elevated negative affect) may be particularly likely to use cannabis during periods of elevated negative affect and may also experience greater effects of these substances. In support of this hypothesis, PTSD symptom severity is related to more coping-motivated cannabis use (i.e., using cannabis to cope with negative affective states) among general cannabis-using adult populations10, 11 and veterans.5 Further, cannabis-using veterans expected cannabis to relieve symptoms related to PTSD, particularly symptoms associated with arousal and intrusion, and these expectancies mediated the relationship between these PTSD symptoms and quantity of cannabis used per month.12 Similarly, among young adult veterans, expectancies related to tension-reduction and relaxation moderated the relationship between PTSD and past-month cannabis use, such that the participants who screened positive for PTSD and endorsed higher levels of these expectancies were most likely to report past-month cannabis use.7
Emerging data suggest that the PTSD clusters are differentially related to cannabis use, indicating a need to examine the relation of PTSD symptomatology separately by cluster. To illustrate, re-experiencing symptoms (Cluster B) and hyperarousal symptoms (Cluster D) were positively associated with severity of cannabis use among methadone maintenance patients.13 Greater frequency of cannabis use following residential PTSD treatment was found among military veterans with less change in PTSD avoidance/numbing symptoms (Cluster C) and Cluster D symptoms between treatment intake and discharge.14 Further, a diagnosis of CUD was predictive of lower levels of change in Cluster C and Cluster D symptoms between treatment intake and discharge among veterans.15 Moreover, veterans with PTSD reported that using cannabis helped to alleviate sleep disturbance (i.e., a Cluster D symptom)16 and medical-cannabis users with probable PTSD were more likely to use cannabis to improve sleep than those without PTSD.17 Additionally, among veterans, coping motives related to sleep mediated the relationship between PTSD and cannabis use frequency, cannabis-related problems, and CUD.18 Taken together, and consistent with negative reinforcement models of substance use8, cannabis appears to be most consistently related to Cluster D, which largely consists of aversive internal states (in contrast to Cluster C, which largely consists of attempts to avoid experiencing aversive internal states).19
Consistent with negative reinforcement models of substance use,8 cannabidiol (CBD), one of the two main constituents of cannabis, exhibits anxiolytic effects (i.e., reduces a negative affective state).20 The other main constituent of cannabis, Δ9-tetrahydrocannabinol (Δ9-THC) can produce anxiogenic effects, especially when taken in high doses or among cannabis-naïve individuals.21, 22 Yet, Δ9-THC taken in low doses has been found to produce anxiolytic effects among laboratory rodents23 and sleep-inducing effects among humans.24 In two pilot studies of PTSD patients, both Δ9-THC and nabilone (a synthetic cannabinoid) reduced nightmares and Δ9-THC improved sleep quality and Cluster D symptoms.25, 26 A recent positron emission tomography study found that individuals with PTSD evidenced greater CB1 receptor availability than both healthy controls and trauma-exposed controls,27 thereby pointing to greater CB1 receptor availability as a potential mechanism by which cannabis alleviates PTSD symptoms. Taken together, the pharmacological effects of cannabis are relevant to the negative reinforcement model of substance use broadly and PTSD symptomatology specifically. Consistent with negative reinforcement hypotheses regarding likelihood of substance use in a state of heightened negative affect,8 Cluster D may be especially related to cannabis use if individuals with elevated Cluster D symptoms are vulnerable to using cannabis during periods of elevated state anxiety in an attempt to manage this arousal. Yet, no known studies have tested this hypothesis.
The present study used ecological momentary assessment (EMA) to collect real-world data about cannabis use episodes over a two-week period to test the utility of the negative reinforcement8 hypothesis in regards to cannabis use among those with and without elevated PTSD symptoms in a racially diverse sample of community-recruited adult cannabis users. Benefits of EMA include: collection of data in real-world environments; minimization of retrospective recall bias; and aggregation of observations over multiple assessments facilitating within-subject assessments across time and context, permitting the examination of both antecedents and consequences of use.28 It was predicted that the interaction of Cluster D and state anxiety would be prospectively related to cannabis use such that those with elevated Cluster D symptoms and elevated state anxiety would be the most likely to use cannabis. It was also predicted that those with elevated Cluster D symptoms who used cannabis would report the greatest decreases in state anxiety. Specifically, for the within-person effect, we hypothesized that on those occasions in which an individual experienced greater state anxiety, that individual would be more likely to use cannabis at the next study assessment. To test the specificity of these relationships, we also tested whether Clusters B and C evinced similar relationships to state anxiety and cannabis use during the monitoring period.
Material and methods
Participants and Procedures
Participants were recruited via community advertisements (e.g., flyers, newspaper ads) for a study of the relation between anxiety and cannabis use.29 The current study is based on secondary analyses of those data. Interested participants completed a screening (on-line or telephone) and baseline appointment to determine eligibility. Participants were asked to refrain from cannabis use the day of their appointment. Eligibility criteria included being between 18–45 years old, current cannabis use (confirmed via urine sample using a 50 ng/ml positive cutoff), cannabis as drug of choice, and no interest in, or current receipt of, substance use disorder treatment. Of the 125 people who attended a baseline appointment, 1 refused to participate and 14 were excluded due to: negative biological verification of cannabis use (n=6), being under the influence of cannabis during assessment (n=1), meeting DSM criteria for primary substance dependence other than cannabis dependence (n=3), and meeting criteria for other diagnoses (e.g., psychosis) that would preclude participation (n=4). Of the 110 participants enrolled, 8 dropped out during the monitoring period and 9 were excluded due to: equipment malfunction (n=7), non-compliance with protocol during check-in appointments (n=1), and non-compliance with EMA data collection (n=1; described below). An additional 6 were excluded from the current study due to denial of PTSD symptoms.
The final sample was composed of 87 cannabis users (35.6% female) aged 18–36 years (M=21.0, SD=2.7). The racial composition was: 56.3% non-Hispanic Caucasian, 24.1% African American or Black, 3.4% Hispanic Caucasian, 1.1% American Indian, 1.1% Asian, 10.3% mixed, and 3.4% other. The majority (79.3%) were college students from a variety of local colleges (i.e., the state’s Flagship University, a Historically Black College, a community college, a regional university) with 14.9% employed full-time and 39.1% employed part-time. Mean age of first cannabis use was 15.9 (SD=2.1; range=11–19). At baseline, participants reported using cannabis 7–90 (M=70.1, SD=20.5) days in the past 90 days. All participants endorsed at least weekly use in the past month (with 81.6% endorsing daily use).
Baseline Measures
The Posttraumatic Stress Disorder Checklist-Civilian (PCL-C) version 4 was used to assess PTSD symptoms at baseline.30 Instructions asked participants to rate the degree to which they have experienced 17 symptoms of posttraumatic stress during the past month in response to an extremely stressful event such as being in combat, being attacked, being sexually assaulted, being physically or sexually abused, seeing someone killed or injured, or being in a fire, flood, or natural disaster. Items are assessed on a five point Likert scale (ranging from 1 [not at all] to 5 [extremely]) and correspond to the symptoms for PTSD in the Diagnostic and Statistical Manual for Mental Disorders-IV.19 Symptoms rated as 3 (moderately) or higher are considered clinically significant. Cluster B (re-experiencing) is clinically significant if at least one re-experiencing symptom is rated as 3 or higher. Cluster C (avoidance) is clinically significant if three or more avoidance symptoms are rated as 3 or higher. Cluster D (hyperarousal) is clinically significant if two or more hyperarousal symptoms are rated as 3 or higher. We categorized participants into PTSD symptom+ (any clinically significant PTSD symptom cluster) and PTSD symptom− (no clinically significant PTSD symptom clusters). The PCL-C items may be summed to yield a total symptoms severity score and/or the items assessing specific PTSD clusters (i.e., re-experiencing, avoidance, and hyperarousal) may be summed to derive a cluster specific symptom severity score. The PCL has been used to assess PTSD symptoms in numerous samples.31–33 Internal consistency in the current sample was excellent (α=.90).
Cannabis-related problems were assessed using the Marijuana Problems Scale (MPS; Stephens et al., 2000), a list of 19 cannabis-related problems experienced in the past 90 days. Endorsed items were summed. The MPS has demonstrated good internal consistency in prior research (Buckner et al., 2010b).
Frequency of cannabis use during the 90 days prior to baseline was assessed with the Timeline Follow Back.34 Participants were asked to report for each day how many cigarette-sized joints of cannabis they used. Days on which cannabis was used were summed to determine frequency of cannabis use. This measure has demonstrated good test-retest reliability.35
EMA Measures
EMA assessments were completed on a personal digital assistant (PDA) using Satellite Forms 5.2 by Pumatech. Three types of assessments were collected several times per day over the two-week period:36 signal contingent (completed in response to a signal from the PDA at six semi-random times within 20 minutes of the following anchor times: 9:20am, 11:40am, 1:00pm, 3:20pm, 5:40pm, and 7:20pm), interval contingent (completed at bedtime), and event contingent (completed immediately prior to using cannabis). Participants were presented with the below questions regardless of assessment type.
State anxiety.
Subjective Units of Distress (SUDS)37 were used to assess state anxiety. Participants were asked to “Please indicate your current level of anxiety by choosing the number that best corresponds with the way you are feeling RIGHT NOW” from 0 (Totally relaxed, on the verge of sleep) to 10 (The highest anxiety you have ever experienced). SUDS ratings during EMA monitoring have been found to correlate with longer measures of anxiety.38
Cannabis use.
Because participants were instructed to complete an EMA assessment immediately prior to cannabis use, participants indicated whether they were about to use cannabis (yes or no). “Yes” responses were considered cannabis use episodes. In prior work, this EMA measure was related to baseline self-report accounts of cannabis use.38
Procedures
Study procedures were approved by the University’s Institutional Review Board and informed consent was obtained prior to data collection. Participants were trained on PDA use and given printed PDA instructions. Participants were instructed to not complete assessments when it was inconvenient (e.g., in class) or unsafe (e.g., driving) and asked to respond to any PDA signals within one hour if possible.
Consistent with other EMA protocols,39 participants completed two days of practice data (not used for analyses) then returned to the lab to receive feedback on compliance. Participants then completed EMA assessments for two weeks, given that this timeframe appears sufficient to monitor substance use.38, 40, 41 Participants were paid $25 for completing the baseline assessment and $100 for each week of EMA data completed. A $25 bonus was given for completing at least 85% of the random prompts during the monitoring period.
Data Analyses
Analyses were conducted using mixed effects functions in SPSS version 22.0. Models were random intercept, random slope designs. Pseudo R-squared values were calculated using error terms from the unrestricted and restricted models as described by Kreft and de Leeuw.42 The relationships of antecedents (baseline PTSD symptom cluster status [clinically elevated: positive vs negative] X EMA-assessed state anxiety measured continuously with the SUDS) to cannabis use were evaluated in two ways. First, generalized linear models (GLM) with a logistic response function (0=no cannabis use, 1=cannabis use) were used to conduct cross-lagged analyses to test whether PTSD cluster status interacted with state anxiety at one time point to predict cannabis use at the next time point that day. Second, GLM was used to conduct cross-lagged analyses to test whether PTSD cluster status interacted with cannabis use at one time point to predict anxiety at the next time point. The SUDS and lagged SUDS variables were person-mean centered.
Compliance with the EMA protocol was assessed by determining mean percentage of random prompts, of end of day assessments, and of both random and end of day assessments completed per participant. Consistent with prior work,38, 41, 43 one participant was excluded for completing less than 20% of assessments. Remaining participants completed a mean of 68.5% (SD=18.5%; range=23%−98%) of random signals, 60.7% (SD=23.6%; range=7%−100%) of end of day assessments, and 67.4% (SD=18.0%; range=26%−95%) of both random and end of day assessments, with compliance rates slightly higher on cannabis use days (68.8%) than non-use days (63.8%). Compliance was evenly distributed among hours of the day and days of the week. These rates are comparable to other EMA studies of cannabis users.38, 41 Participants completed 4,828 signal contingent (M=55.9, SD=15.2 per participant), 726 interval contingent (M=8.5, SD=3.3 per participant), and 1,011 event contingent (M=13.1, SD=11.5 per participant) assessments. Signal contingent assessments were completed on average 29.1 (SD=55.4) minutes after the signal occurred and there was an average of 122.1 (SD = 110.9) minutes between same-day assessments. Participants recorded 1,812 cannabis use entries (M=22.2, SD=14.2 per participant), suggesting some cannabis use was recorded during signal and interval contingent assessments. Participants reported an average of 1.8 (SD=2.3) cannabis use episodes per day. Possible reactivity to EMA recording was assessed by ratings during the first and last four days of the monitoring period. Mean ratings of affect, F(1, 84) = 0.54, p = .465, d = .11, and number of cannabis use episodes, F(1, 86) = 0.25, p = .618, d = .08, did not significantly differ. In other words, there was no evidence of reactivity in the current study.
Results
Baseline Sample Descriptives
Based on PCL scores, 41.4% reported clinically significant Cluster B (Re-experiencing) symptoms (n = 36), 31.0% reported clinically significant Cluster C (Avoidance) symptoms (n = 27), and 43.7% reported clinically significant Cluster D (Hyperarousal) symptoms (n = 38). The PTSD groups did not differ on any demographic variable other than anxiety treatment history (Table 1), such that more participants with clinically significant PTSD cluster symptoms (PTSD symptom+) reported more anxiety treatment than those without clinically significant PTSD symptoms (PTSD symptom−). PTSD symptom+ participants reported more cannabis-related problems, although they did not differ from PTSD symptom− participants on frequency of cannabis use in the two weeks prior to the baseline appointment. Baseline cannabis use frequency was positively correlated with use during the monitoring period, r = .38, p = .001.
Table 1.
Demographic and baseline cannabis variables by those with and without clinically elevated PTSD cluster symptoms.
| PTSD symptom− (n = 33) |
PTSD symptom+ (n = 54) |
F or χ2 | p |
d or Cramer’s V |
|
|---|---|---|---|---|---|
| Age | 20.6 (1.9) | 21.2 (3.0) | 0.99 | .322 | 0.23 |
| Gender (% male) | 72.7 | 59.3 | 1.62 | .203 | 0.14 |
| Ethnicity (% non-Hispanic/Latino) | 84.8 | 92.6 | 1.32 | .250 | 0.12 |
| Race (% Caucasian) | 60.6 | 59.3 | 1.42 | .922 | 0.13 |
| Estimated family income | 152,911 (369,329) | 113,647 (107,747) | 0.47 | .496 | 0.14 |
| Employment status (% unemployed) | 48.5 | 42.6 | 0.87 | .832 | 0.10 |
| Class (% second year) | 30.3 | 25.9 | 2.54 | .771 | 0.17 |
| Marital status (% single) | 93.9 | 90.7 | 3.17 | .531 | 0.19 |
| Anxiety treatment history (%) | 6.1 | 25.9 | 5.39 | .020 | 0.25 |
| Substance treatment history (%) | 6.1 | 11.1 | 0.63 | .429 | 0.09 |
| Cannabis problems | 3.2 (2.2) | 6.9 (3.7) | 27.15 | <.001 | 1.22 |
| Past 2-week cannabis use (days) | 11.1 (3.1) | 11.7 (3.1) | 0.71 | .402 | 0.19 |
Note. PTSD symptom− = no clinically significant PTSD symptom cluster. PTSD symptom+ = at least one clinically significant PTSD cluster. PTSD symptom cluster status based on PCL-C 30.
Baseline PTSD Clusters’ Relationships with State Anxiety and Cannabis Use during the Monitoring Period
For descriptive purposes, EMA data were aggregated by participant and analyses of variance conducted. During the monitoring period, participants with clinically elevated Cluster B symptoms (Cluster B+; M = 2.6, SD = 1.3) reported significantly greater mean state anxiety than participants without clinically elevated Cluster B symptoms (Cluster B-; M = 1.7, SD = 1.3), F(1, 85) = 10.04, p = .002, d = .69. Cluster C+ participants (M = 2.6, SD = 1.4) reported significantly greater mean state anxiety than Cluster C− participants (M = 1.9, SD = 1.3), F(1, 85) = 5.30, p = .024, d = .52 and Cluster D+ participants (M = 2.7, SD = 1.4) reported significantly greater mean state anxiety than Cluster D− participants (M = 1.6, SD = 1.2), F(1, 85) = 14.08, p < .001, d = .86.
During the monitoring period, Cluster B+ participants (M = 22. 8, SD = 14.8) did not differ from Cluster B− participants (M = 19.5, SD = 12.4) on frequency of cannabis use (i.e., number of times cannabis was used during the monitoring period), F(1, 85) = 1.29, p = .259, d = .25. Cluster C+ participants (M = 21.6, SD = 12.5) did not differ from Cluster C− participants (M = 20.5, SD = 14.0) on cannabis use frequency, F(1, 85) = 0.11, p = .738, d = .08. Cluster D+ participants (M = 20.7, SD = 11.9) did not differ from Cluster D− participants (M = 20.9, SD = 14.7) on cannabis use frequency, F(1, 85) = 0.00, p = .956, d = .01.
PTSD Clusters X State Anxiety in the Prediction of Subsequent Cannabis Use
Cross-lagged analyses tested whether state anxiety at one assessment point interacted with PTSD Cluster to predict cannabis use at the subsequent assessment (Table 2). The within-subjects interaction was significant for Cluster D such that subsequent cannabis use was greatest among Cluster D+ participants with higher SUDS ratings (Figure 1A). The within-subjects interaction was also significant for Cluster C but was not significant for Cluster B.
Table 2.
Separate Generalized Estimating Equations Analyses of the Lagged Effects of State Anxiety on Cannabis Use.
| Parameter Estimates | B | SE | Wald χ2 | df | p |
|---|---|---|---|---|---|
| For the model involving the predictor variable of Cluster D status | |||||
| Cluster D status | 0.77 | 0.31 | 6.03 | 1 | .014 |
| Between-person mean lagged state anxiety | 0.02 | 0.03 | 0.41 | 1 | .523 |
| Within-person mean lagged state anxiety | 0.22 | 0.07 | 10.36 | 1 | .001 |
| Cluster D status * Between-person mean lagged state anxiety | −0.05 | 0.05 | 1.01 | 1 | .315 |
| Cluster D status * Within-person mean lagged state anxiety |
−0.41 | 0.13 | 9.82 | 1 | .002 |
| For the model involving the predictor variable of Cluster B status | |||||
| Cluster B status | 0.54 | 0.41 | 1.69 | 1 | .194 |
| Between-person mean lagged state anxiety | −0.00 | 0.03 | 0.01 | 1 | .929 |
| Within-person mean lagged state anxiety | 0.19 | 0.11 | 2.93 | 1 | .087 |
| Cluster B status * Between-person mean lagged state anxiety | 0.01 | 0.04 | 0.03 | 1 | .858 |
| Cluster B status * Within-person mean lagged state anxiety | −0.26 | 0.14 | 3.57 | 1 | .059 |
| For the model involving the predictor variable of Cluster C status | |||||
| Cluster C status | 1.01 | 0.28 | 12.64 | 1 | <.001 |
| Between-person mean lagged state anxiety | −0.01 | 0.04 | 0.05 | 1 | .832 |
| Within-person mean lagged state anxiety | 0.28 | 0.06 | 19.76 | 1 | <.001 |
| Cluster C status * Between-person mean lagged state anxiety | 0.02 | 0.05 | 0.11 | 1 | .743 |
| Cluster C status * Within-person mean lagged state anxiety | −0.36 | 0.10 | 13.85 | 1 | <.001 |
Figure 1.
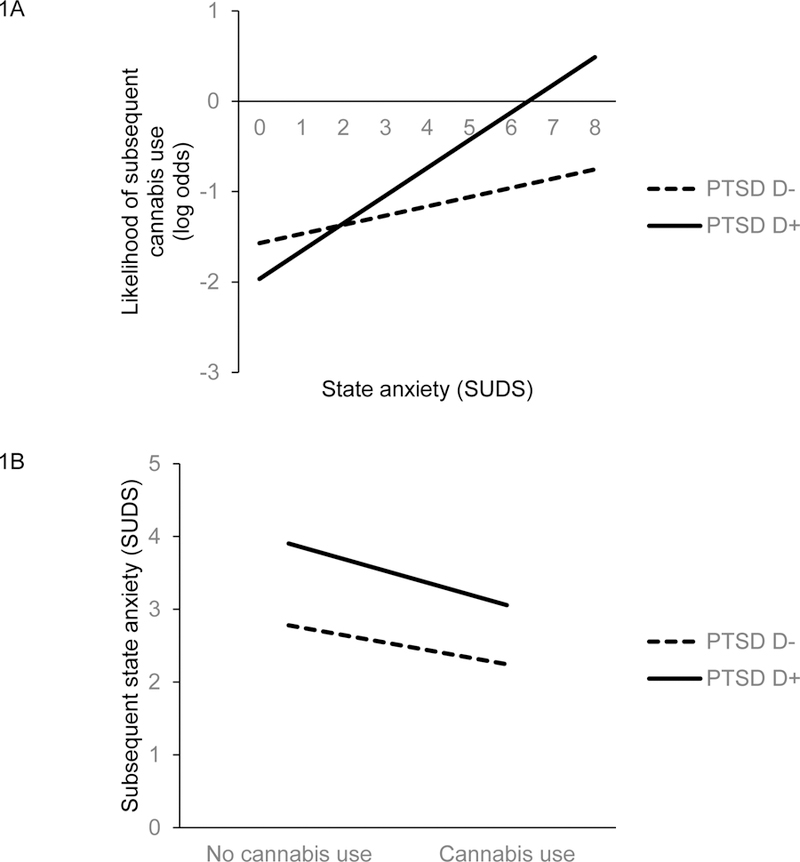
The interaction of PTSD Cluster D status with (A) state anxiety to predict subsequent cannabis use and (B) cannabis use to predict subsequent state anxiety.
PTSD Clusters X Cannabis Use in the Prediction of Subsequent State Anxiety
Next, cross-lagged analyses tested whether Cluster D status interacted with cannabis use at one assessment point to predict state anxiety at the subsequent assessment (Table 3). Cluster D+ participants reported significantly greater state anxiety, although this significant main effect is qualified by the significant interaction indicating that subsequent state anxiety decreased among those who used cannabis and the rate of this decline was greatest among Cluster D+ participants (Figure 1B). Cluster B+ and Cluster C+ participants reported significantly greater state anxiety than B− and C− participants. The interaction was also significant for Cluster B, but was not for Cluster C.
Table 3.
Separate Generalized Linear Modeling Analyses of the Lagged Effects of Cannabis use on State Anxiety.
| Parameter Estimates | B | SE | df | t | p |
|---|---|---|---|---|---|
| For the model involving the predictor variable of Cluster D status | |||||
| Intercept | 2.71 | ||||
| Cluster D status | 1.11 | 0.27 | 82.62 | −4.14 | <.001 |
| Between-Person mean lagged cannabis use | −6.38 | 2.09 | 82.57 | 3.05 | .003 |
| Within-Person mean lagged cannabis use | 0.56 | 0.07 | 6700.74 | −8.46 | <.001 |
| Cluster D status * Between-Person mean lagged cannabis use | 8.43 | 2.67 | 82.51 | −3.16 | .002 |
| Cluster D status * Within-Person mean lagged cannabis use | −0.35 | 0.09 | 6700.97 | 3.94 | <.001 |
| For the model involving the predictor variable of Cluster B status | |||||
| Intercept | 2.60 | ||||
| Cluster B status | 0.93 | 0.29 | 82.83 | −3.25 | .002 |
| Between-Person mean lagged cannabis use | −3.72 | 1.98 | 82.76 | 1.88 | .064 |
| Within-Person mean lagged cannabis use | 0.57 | 0.07 | 6701.78 | −8.51 | <.001 |
| Cluster B status * Between-Person mean lagged cannabis use | 5.65 | 2.77 | 82.69 | −2.04 | .044 |
| Cluster B status * Within-Person mean lagged cannabis use | −0.36 | 0.09 | 6701.22 | 4.02 | <.001 |
| For the model involving the predictor variable of Cluster C status | |||||
| Intercept | 2.59 | ||||
| Cluster C status | 0.74 | 0.30 | 82.69 | −2.44 | .017 |
| Between-Person mean lagged cannabis use | −7.68 | 2.63 | 82.23 | 2.93 | .004 |
| Within-Person mean lagged cannabis use | 0.32 | 0.08 | 6700.72 | −4.15 | <.001 |
| Cluster C status * Between-Person mean lagged cannabis use | 8.90 | 3.08 | 82.35 | −2.89 | .005 |
| Cluster C status * Within-Person mean lagged cannabis use | 0.06 | 0.09 | 6700.81 | −0.68 | .496 |
Discussion
The current study tested negative reinforcement-based models of cannabis use8 in a racially diverse sample of cannabis-using individuals with elevated symptoms of specific PTSD clusters. We simultaneously examined antecedents and consequences of cannabis use among cannabis-using individuals with and without elevated PTSD symptom clusters. Findings contribute to our understanding of cannabis use in several substantial ways. First, in line with negative reinforcement hypotheses, participants with clinically elevated PTSD symptoms should be more likely to use cannabis after experiencing elevated state anxiety and they should experience decreases in anxiety following cannabis use. This pattern was observed for participants with clinically significant Cluster D (Hyperarousal) symptoms, as cannabis use was especially likely among participants with elevated Cluster D symptoms and state anxiety and cannabis use resulted in less subsequent anxiety. This is consistent with previous findings that cannabis-using veterans expect that cannabis will alleviate arousal symptoms associated with PTSD12 and that veterans reported that using cannabis alleviates sleep disturbance.16
Second, there was some evidence of the specificity of the utility of negative reinforcement models in understanding cannabis use amongst cannabis users with elevated Cluster D symptoms, in that the negative reinforcement hypothesis was only partially supported for those with Cluster B (Re-experiencing) and C (Avoidance) symptoms. Specifically, among Cluster C+ participants, elevated anxiety predicted greater likelihood of subsequent cannabis use but cannabis use did not result in less subsequent anxiety. On the other hand, among Cluster B+ participants, cannabis use was not associated with state anxiety. Although not fully in line with negative reinforcement models of substance use, these results are partially consistent with prior findings that cannabis-using veterans expect cannabis to relieve PTSD symptoms related to arousal and intrusion.12 Further, these findings suggest cannabis may reduce anxiety among individuals with elevated Cluster B and D symptoms, which is consistent with prior work finding that cannabis exhibits anxiolytic effects.20
Results of the current study suggest that negative reinforcement models of substance use may apply differentially to PTSD symptom clusters. This model posits that substance use tends to be reinitiated during times of negative affect and stress and that withdrawal symptoms can contribute.8 Consistent with this, our results suggest that experiencing Cluster D symptomatology combined with elevated state anxiety, potentially from withdrawal, may place individuals at a high risk for using and that using cannabis decreases these symptoms, thereby providing negative reinforcement. Given that individuals with elevated Cluster C symptoms used cannabis after experiencing elevated anxiety but their anxiety did not subsequently decrease, there may be other factors driving their cannabis use besides negative reinforcement.
Of note, we found that PTSD symptom+ participants and PTSD symptom− participants did not differ significantly in terms of their use of cannabis during the two weeks prior to the monitoring period, although the magnitude of the effect was nearly in the small range, suggesting this finding may be partially explained by our small sample size. Yet, PTSD symptom+ participants endorsed significantly more cannabis-related problems than PTSD symptom− participants, suggesting something about their use makes them more vulnerable to cannabis-related impairment. These results are consistent with prior work finding a stronger relation between PTSD and cannabis-related problems than PTSD and cannabis use.1
Results have important clinical implications. For example, data suggest that patients with PTSD symptoms may benefit from tailored interventions based on which PTSD symptom clusters they are currently experiencing. To illustrate, given that participants with clinically elevated Cluster D symptoms were more likely to use cannabis after experiencing elevated state anxiety and experience decreases in anxiety following cannabis use, these patients may especially benefit from learning more adaptive means to manage elevated anxiety. Given that discontinuation of cannabis use is associated with a number of symptoms synonymous with Cluster D symptoms (i.e., sleep disturbance, irritability, anxiety),44, 45 it may be especially difficult for individuals who experience elevated hyperarousal symptoms to quit using cannabis without more adaptive skills to manage increases in negative affectivity such as anxiety. On the other hand, individuals with elevated Cluster C+ symptoms may benefit from psychoeducation that although they may use cannabis in response to elevations in anxiety, cannabis use may not result in less subsequent anxiety.
Results of the present study should be considered in light of limitations that point to additional avenues of work in this area. First, we did not collect data on type of trauma and future work could benefit from testing whether negative reinforcement hypotheses are more relevant to specific types of trauma. Second, data were collected via self-report and future work could benefit from biological verification of cannabis use during the monitoring period. Relatedly, although we cannot know compliance rates for event-contingent assessments, rates for signal- and interval-contingent assessments were approximately 67%. Thus, biological verification of use could help inform compliance of event-contingent assessment and it is recommended that future work strive to improve overall compliance. Third, although recruited from the community, the present sample comprised undergraduate and graduate students from several local colleges (e.g., community college, Historically Black University). Thus, although our data are thereby generalizable to groups particularly vulnerable to cannabis use and cannabis-related impairment (i.e., young adults, college students),46, 47 future study is needed to examine whether the observed relations generalize to other cannabis-using populations. Similarly, our sample consisted of frequent cannabis users (e.g., over 80% of the sample reported daily use) and it is unknown whether these results would generalize to infrequent cannabis users or cannabis users using other substances concurrently. Additionally, much of the extant research that informed our hypotheses was conducted among veterans; thus, an important next step would be to examine whether our findings generalize to cannabis-using veteran populations. Fourth, participants were not treatment-seeking, permitting the examination of factors that maintain cannabis use among non-treatment seekers with and without clinically elevated PTSD symptom clusters. Given that anxiety is related to poorer cannabis cessation outcomes,47, 48 an important next step will be to identify factors related to lapse and/or relapse vulnerability among cannabis users in cannabis cessation treatment. Fifth, although compliance rates were comparable to other EMA studies with cannabis users,38, 41, 49 these rates are lower than those obtained with other populations and future work could benefit from testing ways to increase EMA protocol compliance among cannabis users. Sixth, we did not collect data on the use of other substances during the monitoring period. Given that PTSD is related to problems related to use of other substances,50, 51 future work could benefit from investigation of the role of other substances in cannabis use among those with PTSD. Seventh, future work could benefit from larger samples with greater representation of female participants to test whether gender moderates observed effects. Eighth, we categorized PTSD clusters dichotomously based on PCL cut scores and future work would benefit from examining whether utilizing severity of PTSD clusters or number of PTSD symptoms per cluster evince similar relations.
Despite these limitations, these findings contribute to our understanding of co-occurring PTSD symptomatology and cannabis use. Individuals with elevated Cluster D symptoms may be especially vulnerable to the reinforcing effects of cannabis in response to elevated anxiety given that their anxiety subsequently reduces after using cannabis. Thus, these individuals in particular may benefit from interventions that teach them more adaptive strategies to manage elevated negative affective states.
Acknowledgments
Funding: This research was supported in part by grants from the National Institute of Drug Abuse (5R21DA029811-02, 1R34DA031937-01A1). NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Financial Disclosures: The authors report no relevant financial conflicts or other conflicts of interest.
References
- 1.Kevorkian S, Bonn-Miller MO, Belendiuk K, Carney DM, Roberson-Nay R, Berenz EC. Associations among trauma, posttraumatic stress disorder, cannabis use, and cannabis use disorder in a nationally representative epidemiologic sample. Psychology of Addictive Behaviors 2015;29(3):633–8. doi:10.1037/adb0000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, et al. Prevalence and Correlates of DSM-5 Cannabis Use Disorder, 2012–2013: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions-III. American Journal of Psychiatry 2016;173(6):588. doi:10.1176/appi.ajp.2015.15070907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cougle JR, Bonn-Miller MO, Vujanovic AA, Zvolensky MJ, Hawkins KA. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychology of Addictive Behaviors 2011;25(3):554–8. doi:10.1037/a0023076. [DOI] [PubMed] [Google Scholar]
- 4.Bonn-Miller MO, Harris AHS, Trafton JA. Prevalence of cannabis use disorder diagnoses among veterans in 2002, 2008, and 2009. Psychological Services 2012;9(4):404–16. doi:10.1037/a0027622. [DOI] [PubMed] [Google Scholar]
- 5.Boden MT, Babson KA, Vujanovic AA, Short NA, Bonn‐Miller MO. Posttraumatic stress disorder and cannabis use characteristics among military veterans with cannabis dependence. American Journal on Addictions 2013;22(3):277–84. [DOI] [PubMed] [Google Scholar]
- 6.Gentes EL, Schry AR, Hicks TA, Clancy CP, Collie CF, Kirby AC, et al. Prevalence and correlates of cannabis use in an outpatient VA posttraumatic stress disorder clinic. Psychology Of Addictive Behaviors: Journal Of The Society Of Psychologists In Addictive Behaviors 2016;30(3):415–21. doi:10.1037/adb0000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant S, Pedersen ER, Neighbors C. Associations of posttraumatic stress disorder symptoms with marijuana and synthetic cannabis use among young adult U.S. veterans: a pilot investigation. Journal of Studies on Alcohol and Drugs 2016;77(3):509–14. doi:10.15288/jsad.2016.77.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review 2004;111(1):33–51. doi:10.1037/0033-295x.111.1.33. [DOI] [PubMed] [Google Scholar]
- 9.Conger JJ. Alcoholism: theory, problem and challenge II. Reinforcement theory and the dynamics of alcoholism. Quarterly Journal of Studies on Alcohol 1956;17(2):296–305. [PubMed] [Google Scholar]
- 10.Bonn-Miller MO, Vujanovic AA, Feldner MT, Bernstein A, Zvolensky MJ. Posttraumatic stress symptom severity predicts marijuana use coping motives among traumatic event-exposed marijuana users. Journal of Traumatic Stress 2007;20(4):577–86. doi:10.1002/jts.20243. [DOI] [PubMed] [Google Scholar]
- 11.Potter CM, Vujanovic AA, Marshall-Berenz EC, Bernstein A, Bonn-Miller MO. Posttraumatic stress and marijuana use coping motives: the mediating role of distress tolerance. Journal of Anxiety Disorders 2011;25(3):437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earleywine M, Bolles JR. Marijuana, expectancies, and post-traumatic stress symptoms: a preliminary investigation. Journal of Psychoactive Drugs 2014;46(3):171–7. doi:10.1080/02791072.2014.920118. [DOI] [PubMed] [Google Scholar]
- 13.Villagonzalo K-A, Dodd S, Ng F, Mihaly S, Langbein A, Berk M. The relationship between substance use and posttraumatic stress disorder in a methadone maintenance treatment program. Comprehensive Psychiatry 2011;52:562–6. doi:10.1016/j.comppsych.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Bonn-Miller MO, Vujanovic AA, Drescher KD. Cannabis use among military veterans after residential treatment for posttraumatic stress disorder. Psychology of Addictive Behaviors 2011;25(3):485–91. [DOI] [PubMed] [Google Scholar]
- 15.Bonn-Miller MO, Boden MT, Vujanovic AA, Drescher KD. Prospective investigation of the impact of cannabis use disorders on posttraumatic stress disorder symptoms among veterans in residential treatment. Psychological Trauma: Theory, Research, Practice, and Policy 2013;5(2):193–200. doi:10.1037/a0026621. [Google Scholar]
- 16.Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. American Journal of Psychiatry 1996;153(3):369–75. [DOI] [PubMed] [Google Scholar]
- 17.Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug and Alcohol Dependence 2014;136:162–5. doi:10.1016/j.drugalcdep.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metrik J, Jackson K, Bassett SS, Zvolensky MJ, Seal K, Borsari B. The mediating roles of coping, sleep, and anxiety motives in cannabis use and problems among returning veterans with PTSD and MDD. Psychology of Addictive Behaviors 2016;30(7):743–54. doi:10.1037/adb0000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 20.Crippa JAdS Zuardi AW, Garrido GEJ, Wichert-Ana L, Guarnieri R, Ferrari L, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 2004;29(2):417–26. doi:10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- 21.Manzanares J, Urigüen L, Rubio G, Palomo T. Role of endocannabinoid system in mental diseases. Neurotoxicity Research 2004;6(3):213–24. doi:10.1007/BF03033223. [DOI] [PubMed] [Google Scholar]
- 22.Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of Δ9-tetrahydrocannabinol in man. European Journal of Pharmacology 1974;28:172–7. doi:10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- 23.Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology 2002;163(1):111–7. doi:10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- 24.Cousens K, DiMascio A. (−)D9THC as an hypnotic: an experimental study of three dose levels. Psychopharmacologia 1973;33(4):355–64. doi:10.1007/BF00437513. [DOI] [PubMed] [Google Scholar]
- 25.Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Δ-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clinical Drug Investigation 2014;34(8):587–91. [DOI] [PubMed] [Google Scholar]
- 26.Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 2015;51:585–8. doi:10.1016/j.psyneuen.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Molecular Psychiatry 2013;18(9):1034–40. doi:10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology 2008;4:1–32. doi:10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 29.Buckner JD, Zvolensky MJ, Crosby RD, Wonderlich SA, Ecker AH, Richter AA. Antecedents and consequences of cannabis use among racially diverse cannabis users: an analysis from ecological momentary assessment. Drug and Alcohol Dependence 2015;147:20–5. doi:10.1016/j.drugalcdep.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist: reliability, validity, and diagnostic utility. Paper presented at: 9th Annual Meeting of the International Society for Traumatic Stress Studies; 1993. October; San Antonio, TX. [Google Scholar]
- 31.Brewin CR. Systematic review of screening instruments for adults at risk of PTSD. Journal of Traumatic Stress 2005;18(1):53–62. doi:10.1002/jts.20007. [DOI] [PubMed] [Google Scholar]
- 32.Cheng HL, Mallinckrodt B. Racial/ethnic discrimination, posttraumatic stress symptoms, and alcohol problems in a longitudinal study of Hispanic/Latino college students. Journal of Counseling Psychology 2015;62(1):38–49. doi:10.1037/cou0000052. [DOI] [PubMed] [Google Scholar]
- 33.Holland JM, Malott J, Currier JM. Meaning made of stress among veterans transitioning to college: examining unique associations with suicide risk and life-threatening behavior. Suicide and Life-Threatening Behavior 2014;44(2):218–31. doi:10.1111/sltb.12061. [DOI] [PubMed] [Google Scholar]
- 34.Sobell LC, Sobell MB. Timeline FollowBack user’s guide: a calendar method for assessing alcohol and drug use Toronto: Addiction Research Foundation; 1996. [Google Scholar]
- 35.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The Timeline Followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. Journal of Consulting and Clinical Psychology 2000;68(1):134–44. doi:10.1037/0022-006X.68.1.134. [DOI] [PubMed] [Google Scholar]
- 36.Wheeler L, Reis HT. Self-recording of everyday life events: origins, types, and uses. Journal of Personality 1991;59(3):339–54. doi:10.1111/1467-6494.ep9110141804. [Google Scholar]
- 37.Wolpe J Psychotherapy by reciprocal inhibition. Conditional Reflex 1968;3(4):234–40. [DOI] [PubMed] [Google Scholar]
- 38.Buckner JD, Crosby RD, Wonderlich SA, Schmidt NB. Social anxiety and cannabis use: an analysis from ecological momentary assessment. Journal of Anxiety Disorders 2012;25(1):297–304. doi:10.1016/j.janxdis.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crosby RD, Wonderlich SA, Engel SG, Simonich H, Smyth J, Mitchell JE. Daily mood patterns and bulimic behaviors in the natural environment. Behaviour Research and Therapy 2009;47(3):181–8. doi:10.1016/j.brat.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freedman MJ, Lester KM, McNamara C, Milby JB, Schumacher JE. Cell phones for ecological momentary assessment with cocaine-addicted homeless patients in treatment. Journal of Substance Abuse Treatment 2006;30(2):105–11. doi:10.1016/j.jsat.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Buckner JD, Zvolensky MJ, Ecker AH. Cannabis use during a voluntary quit attempt: an analysis from ecological momentary assessment. Drug and Alcohol Dependence 2013;132(3):610–6. doi:10.1016/j.drugalcdep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreft I, de Leeuw J. Introducing multivlevel modeling Thousand Oaks, CA: Sage Publications, Inc; 1998. [Google Scholar]
- 43.Hopper JW, Su Z, Looby AR, Ryan ET, Penetar DM, Palmer CM, et al. Incidence and patterns of polydrug use and craving for ecstasy in regular ecstasy users: an ecological momentary assessment study. Drug and Alcohol Dependence 2006;85(3):221–35. doi:10.1016/j.drugalcdep.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Kouri EM, Pope HG Jr. Abstinence symptoms during withdrawal from chronic marijuana use. Experimental and Clinical Psychopharmacology 2000;8(4):483–92. doi:10.1037/1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- 45.Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, et al. Sleep disturbance in heavy marijuana users. Sleep 2008;31(6):901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldeira KM, Arria AM, O’Grady KE, Vincent KB, Wish ED. The occurrence of cannabis use disorders and other cannabis-related problems among first-year college students. Addictive Behaviors 2008;33(3):397–411. doi:10.1016/j.addbeh.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckner JD, Carroll KM. Effect of anxiety on treatment presentation and outcome: results from the Marijuana Treatment Project. Psychiatry Research 2010;178(3):493–500. doi:10.1016/j.psychres.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonn-Miller MO, Moos RH. Marijuana discontinuation, anxiety symptoms, and relapse to marijuana. Addictive Behaviors 2009;34(9):782–5. doi:10.1016/j.addbeh.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Shrier LA, Walls C, Rhoads A, Blood EA. Individual and contextual predictors of severity of marijuana use events among young frequent users. Addictive Behaviors 2013;38(1):1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaher RM, Simons JS, Hahn AM, Hofman NL, Hansen J, Buchkoski J. An experience sampling study of PTSD and alcohol-related problems. Psychology of Addictive Behaviors 2014;28(4):1013–25. doi:10.1037/a0037257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry 2003;60(3):289–94. doi:10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]


