Abstract
The over-expressions of brain-derived neurotrophic factor (BDNF) and its tyrosine kinase receptor TrkB have been reported to induce chemo-resistance in neuroblastoma (NB) cells. In this study, we investigated the roles of P53 and BCL2 family members in the protection of BDNF/TrkB from etoposide-induced NB cell death. TB3 and TB8, two tetracycline (TET)-regulated TrkB-expressing NB cell lines, were utilized. The expressions of P53 and BCL2 family members were detected by Western blot or RT-PCR. Transfection of siRNAs was used to knockdown P53 or PUMA. Activated lentiviral was used to over-express PUMA. Cell survival was performed by MTS assay, and the percentage of cell confluence was measured by IncuCyte ZOOM. Our results showed that etoposide treatment induced significant and time-dependent increase of P53, which could be blocked by pre-treatment with BDNF, and knockdown P53 by transfecting siRNA attenuated etoposide-induced TrkB-expressing NB cell death. PUMA was the most significantly changed BCL2 family member after treatment with etoposide, and pre-treatment with BDNF blocked the increased expression of PUMA. Transfection with siRNA inhibited etoposide-induced increased expression of PUMA, and attenuated etoposide-induced NB cell death. We also found that over-expression of PUMA by infection of activated lentiviral induced TrkB-expressing NB cell death in the absence of etoposide, and treatment of BDNF protected NB cells from PUMA-induced cell death. Our results suggested that P53 and PUMA may be potential targets that mediated the protection of BDNF/TrkB from etoposide-induced NB cell death.
Keywords: Neuroblastoma, BDNF, TrkB, PUMA, P53, Etoposide
Introduction
Neuroblastoma (NB) derives from neural crest precursor cells and most frequently occurs in adrenal gland. It is one of the most common extracranial solid tumors in children [1, 2]. Patients with low-risk NB have a very good prognosis, but high-risk patients, aging older than 18 months, having MYCN (NB MYC oncogene) amplification, or accompanying with metastases, always have poor prognosis. Despite of multiple types of chemotherapy and stem cell transplantation, satisfactory response still couldn’t be achieved, and the long-term survival rate of high-risk patients is < 40% [2–5]. Chemo-resistance is one of the main challenges in the treatment of patients with high-risk NB, so studies on the mechanisms of chemo-resistance are urgently needed to help find more efficient therapy for high-risk NB patients.
Brain-derived neurotrophic factor (BDNF) is a member of neurotrophin family, and it is known as one of the key factors for the sympathetic nervous system development [6, 7]. Researchers have found that about 50–60% of high-risk NB patients have over-expressions of BDNF and/or its tyrosine kinase receptor TrkB in tumor tissues [3]. Previous studies from our group and other researchers have found that BDNF/TrkB induce chemo-resistance partially through activating phosphoinositied-3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK) signaling pathways in NB [6–9]. However, the mechanisms of BDNF/TrkB-induced protection from chemotherapy still need to be further investigated.
Escaping from chemotherapeutic drug-induced apoptosis is one of the mechanisms of chemo-resistance in cancer cells [10]. P53, a tumor suppressor gene, plays an important role in the chemotherapy-induced apoptosis. Studies found that over-expression of wide-type P53 increased the sensitivity of H1299 cells (non-small cell lung cancer cells) to etoposide treatment [11], the accumulation of P53 enhanced the cisplatin-induced apoptosis in HCT116 cells (colon cancer cells) [12], and P53 mutations mediated the resistance of head and neck squamous cell carcinoma (HNSCC) to cisplatin–fluorouracil neoadjuvant chemotherapy [13]. The BCL-2 family is a group of proteins that are also important in apoptosis [14–16]. The BCL2 family members are divided into two groups: the anti-apoptotic members such as BCL2, BCL-XL, and MCL; and the pro-apoptotic members such as BAX, BAK, BIM, BID, PUMA, and NOXA [10, 14–19]. Some of the BCL2 family members, such as PUMA, NOXA, BIM, and BID, were reported to be the downstream targets of P53. Studies found that upregulation of BCL2 contributed to the drug resistance to cisplatin, doxorubicin, and etoposide in small cell lung cancer [20]; and high expression of BAX increased chemo-sensitivity to 5-FU in colorectal cancer, and to 5-FU and capecitabine in rectal carcinoma [21, 22]. It was also reported that a combination of PI103 (a PI3K inhibitor) and doxorubicin induced apoptosis in several NB cell lines (SH-EP, SH-SY5Y, SK-N-AS, and Kelly) by up-regulating the ratio of pro-apoptotic proteins (NOXA and BIMEL) and anti-apoptotic protein (MCL1) [23].
Although we previously reported that BDNF/TrkB protected NB cells from paclitaxel-induced cell death by down-regulation of BIM [24], so far the roles of P53 and other BCL2 family members in the BDNF/TrkB protection have not been deeply investigated. In the present study, we systematically examined the expression changes of P53 and BCL2 family members after etoposide or BDNF treatment in TrkB-expressing NB cells, and further investigated the roles of P53 and PUMA in the BDNF/TrkB-mediated protection from etoposide-induced NB cell death.
Materials and methods
TrkB‒expressing cell lines and cell culture
TB3 and TB8, two tetracycline (TET)-regulated TrkB-expressing NB cell lines [8], were utilized. In the absence of TET, TrkB expression is induced in TB3 and TB8 cells [8], and all the experiments in this study are based on the induced expression of TrkB. TB3 and TB8 cells were cultured in RPMI 1640 media (Bioind, Israel) containing 10% fetal bovine serum (FBS; Bioind, Israel), 2 mM glutamine, antibiotics, and puromycin (0.5 μg/ml, Sigma Chemical Company) at 37 °С in 5% CO2 incubator.
Treatment
To study the protection of BDNF/TrkB, we cultured TB3 and TB8 cells in absence of TET for 3 days, and then seeded into 96-wells plates in triplicate. TB3 and TB8 cells were pre-treated with BDNF (100 ng/ml, PeproTech, Inc) for 1 h, followed by etoposide (1.0 μg/ml) treatment for 24 h, and the cell survival were measured by MTS assay. To test the effect of BDNF, TB3 and TB8 cells were treated with BDNF (100 ng/ml) for 1 h, and then the expression of Phospho-Akt (P-Akt, Ser473) (downstream target of BDNF/TrkB) was detected by Western blot. To study the regulations of P53 and BCL2 family members by BDNF/TrkB or/and etoposide, we treated TB3 and TB8 cells with BDNF (100 ng/ml) or/and etoposide (0.5 μg/ml) for different times, and the expressions of P53 and BCL2 family members were detected by Western blot or RT-PCR. To study the role of P53 or PUMA in etoposide-induced cell death, we first transfected P53 or PUMA siRNAs into TB3 and TB8 cells, then treated the cells with etoposide (0.5, 1.0 μg/ml) for 24 h, cell survival was detected by MTS assay. To study the role of PUMA in cell survival, we infected the activated PUMA lentiviral into TB3 and TB8 cells and cultured the cells in the presence or absence of BDNF (100 ng/ml) for 48 h, cell survival was detected by MTS assay.
Western blot
Total protein was extracted with Whole Cell Lysis Assay kits (KeyGEN BioTECH) following the manufacturer’s protocol. Protein extracts (30 μg) in each condition were loaded onto SDS-PAGE gels, transferred to PVDF membrane, and probed with the anti-P-Akt (Ser473) (Cell Signaling Tech, 1:2000), anti-PUMA (Santa Cruz, USA, 1:500), anti-NOXA (Abcam, UK, 1:1000), anti-BAX (Santa Cruz, USA, 1:500), anti-BAK (Abcam, UK, 1:1000), anti-BID (Proteintech, China, 1:1000), anti-BCL2 (Santa Cruz, USA, 1:500), anti-BCL-XL (Abcam, UK, 1:1000), anti-MCL1 (Abcam, UK, 1:2000), anti-P53 (Abcam, UK, 1:2000) antibodies, or anti-α-TUBULIN antibody (Beyotime Biotechnology, China, 1:5000). Signals were detected using enhanced chemiluminescence reagents (Thermo Scientific, USA).
Real‒time quantitative PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen) according to the manufacturer’s instructions and 5 μg RNA was used to prepare cDNA using GoScript™ Reverse Transcription System kits (Promega, UK). qPCR was performed with the cDNA using SYBR Premix Ex Taq™ II (TaKaRa Clontech) according to the manufacturer’s instructions. GAPDH expression served as an internal control. Relative quantification of gene expression was performed with the 2(−ΔΔCt) method. Details of the PCR primers sequences were as follows: P53 sense 5′-TGC GTG TTT GTG CCT GTC CT-3′, P53 anti-sense 5′-GTG CTC GCT TAG TGC TCC CT-3′; PUMA sense 5′-GAC CTC AAC GCA CAGTACGAG-3′, PUMA anti-sense 5′-AGGAGTCCCATG ATG AGA TTGT-3′; NOXA sense 5′-CCT ACT GTG AAG GGA GAT GAC-3′, NOXA anti-sense 5′-CTG AAA AGC AAA ACA CCA AAA-3′; BAX sense 5′-TTG CTT CAG GGT TTC ATC CA-3′, BAX anti-sense 5′-AGA CAC TCG CTC AGC TTC TTG-3′; BAK sense 5′-GGA CGA CAT CAA CCG ACGCTAT-3′, BAK anti-sense 5′-TCAAACAGGCTGGTG GCA AT-3′; MCL1 sense 5′-AAA GCT GCA TCG AAC CAT TA-3′, MCL1 anti-sense 5′-AAG AAC TCC ACA AAC CCA TC-3′; BCL2 sense 5′-TGA TGG GAT CGT TGC CTT AT-3′, BCL2 anti-sense 5′-CCA ATT CCT TTC GGA TCT TTAT-3′; BCL-XL sense 5′-GGC AGG CGA CGA GTT TGA −3′, BCL-XL anti-sense 5′-CAT TGT TCC CAT AGA GTT CCA-3′; GAPDH sense 5′-GCA CCG TCA AGG CTG AGA AC-3′, GAPDH anti-sense 5′-TGG TGA AGA CGC CAG TGG A-3′.
Transfection and infection
To inhibit endogenous or etoposide-induced P53 or PUMA expression, we transfected small interfering RNAs (P53 siRNA, PUMA siRNA, siRNA control) (Ruibo, Guangzhou, China) into TB3 and TB8 cells. The target sequences of siRNAs were as follows: P53 siRNA#1 5′-CTG CCC TCA ACA AGA TGT T-3′; P53 siRNA#2 5′-GAA ATT TGC GTG TGG AGT A-3′; P53 siRNA#3 5′-GCA CAG AGG AAG AGA ATC T-3′; PUMA siRNA#1 5′-CGT GTG ACC ACT GCA TTC −3′; PUMA siRNA#2 5′-TCT CAT CAT GGG ACT CCT G-3′. TB3 and TB8 cells were cultured in 6-wells plates at a density of 50 × 104/well for 24 h before transfection. siRNAs were transfected into cells using jetPRIME (Polyplus Transfection, Illkirsch, France) according to manufacturer’s instructions. 20 h after transfection, the cells were seeded into 96-wells plates for cell survival analysis and 6-wells plates for Western blot and RT-PCR. 96-wells plates were placed into an IncuCyte ZOOM imaging system for real-time evaluation of cell confluence after treated with etoposide.
To over-express PUMA, TB3 and TB8 cells were cultured overnight in 96-wells plates or 6-wells plates, and then were infected with activated PUMA lentiviral, or empty vector (GENECHEM, Shanghai, China). 96-wells plates were placed into an IncuCyte ZOOM imaging system for real-time evaluation of cell confluence immediately after infection. 24 h after infection, BDNF (100 ng/ml) was added into 96-wells plates for cell survival analysis and into 6-wells plates for Western blot and RT-PCR.
Cell survival analysis
The MTS assay (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt assay) (Promega Corporation) was performed according to the manufacturer’s instructions. The percentage of cell survival was calculated by dividing the absorbance value of the treated samples by the absorbance value of the untreated control within each group.
IncuCyte ZOOM live cell imaging system
Real-time evaluation of cell confluence was performed using IncuCyte ZOOM (Essen BioSciences) equipment, and images were acquired every 4 or 8 h. The percentage of cell confluence was measured and analyzed by IncuCyte ZOOM software, and was used to indicate cell survival. The points showed three replicates, and presented as means ± standard deviation (SD).
Statistical analyses
Means ± SD of independent experiments were analyzed by Student’s t test. P values < 0.05 was considered as statistically significant. Data was analyzed by using GraphPad Prism software.
Results
BDNF/TrkB protected NB cells from etoposide–induced cell death through down‒regulation of P53
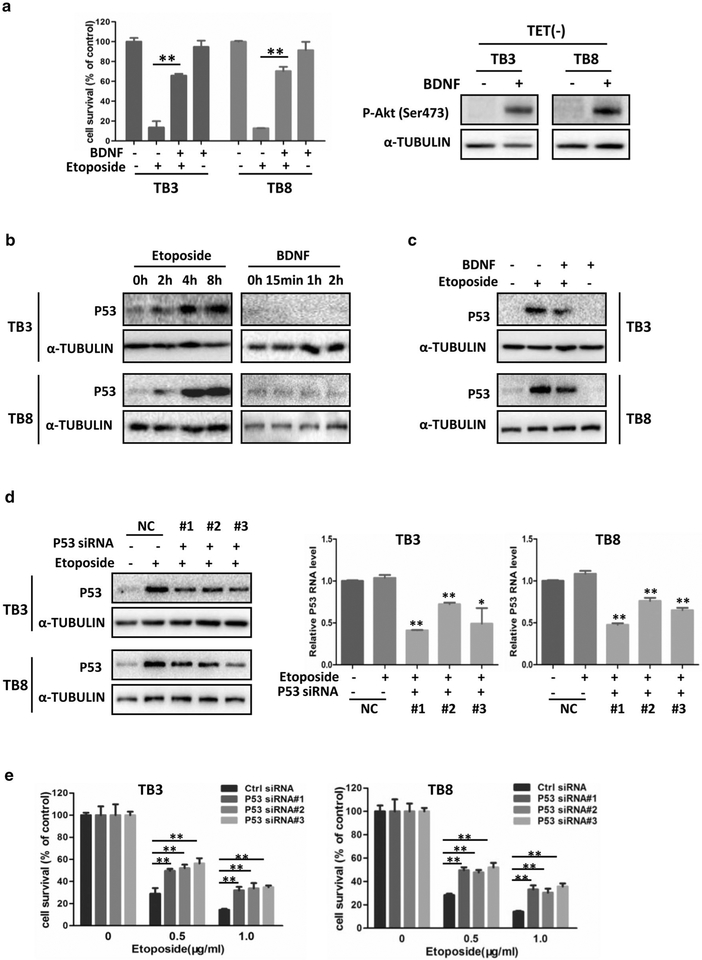
Previously we reported that BDNF activation of TrkB protected NB cells from etoposide-induced cell death, and the PI3K/AKT pathway partially mediated the protection of BDNF/TrkB. In the present study, similar results were achieved in both TrkB-expressing TB3 and TB8 cells. Pre-treatment with BDNF (100 ng/ml) protected TB3 and TB8 cells from etoposide-induced (1.0 μg/ml) cell death (P < 0.01, P < 0.01), and BDNF treatment activated its downstream target Akt (P-Akt, Ser473) (Fig. 1a). To study whether or not P53 is involved in the protection of BDNF/TrkB, we first treated the TrkB-expressing TB3 and TB8 cells with etoposide (0.5 μg/ml) or BDNF (100 ng/ml) individually, harvested the cells at different time points, and detected the P53 expression by Western blot. Figure 1b showed that etoposide treatment induced a time-dependent increase of P53 expression in TB3 and TB8 cells. As the basal level of P53 in TB3 and TB8 cells was low, we observed a trend to decrease of the P53 expression after BDNF treatment (Fig. 1b). Then we detected the P53 expression in TB3 and TB8 cells which were pretreated with BDNF (100 ng/ml) for 1 h, followed by etoposide treatment (0.5 μg/ml) for 8 h. Figure 1c showed that the etoposide-induced increase of P53 expression was partially blocked by pretreatment of BDNF in TB3 and TB8 cells.
Fig. 1.
BDNF/TrkB protected NB cells from etoposide-induced cell death through down-regulation of P53. a TB3 and TB8 cells were cultured and seeded into 96-well plate as described in “Materials and methods”, and cell survival analysis was tested by MTS assay. Bars, SD P-value were tested by Student’s t test. **P < 0.01. After treated with BDNF (100 ng/ml) for 1 h, the expression of P-Akt (ser473) was detected by Western blot in TB3 and TB8 cells. b The cell lysates from etoposide (0.5 μg/ml) or BDNF (100 ng/ml) treated TB3 and TB8 cells were used to test the expression of P53 by Western blot. c TB3 and TB8 cells were pre-treated with BDNF (100 ng/ml) for 1 h, followed by treatment with etoposide (0.5 μg/ml) for 8 h, and cells were harvested. The cell lysates were used to test the expression of P53 by Western blot. d P53 siRNA and siRNA control were transfected into TB3 and TB8 cells as described in “Materials and methods”, and the expression of P53 was detected by Western blot (protein level) and RT-PCR (mRNA level). Bars, SD P-value were tested by Student’s t test. *P < 0.05, **P < 0.01. e The cell survival of etoposide-treated P53 siRNA-transfected TB3 or TB8 cells was tested by MTS assay. Bars, SD P-value were tested by Student’s t test. **P < 0.01
To clarify the role of P53 in etoposide-induced cell death, we down-regulated the P53 expression by transfecting P53 siRNAs into etoposide-treated cells, and then detected the expression of P53 by Western blot and RTPCR, and measured cell survival by MTS assay. Three P53 siRNAs were used and all of them could reduce the etoposide-induced P53 expression at both protein and mRNA level (Fig. 1d). We examined the cell survival at 24 h treatment of etoposide (0.5, 1 μg/ml) in the NB cells. There were a 17–27% increase of cell survival in TB3 cells (P < 0.01) and 16–24% increase of cell survival in TB8 cells (P < 0.01) in the P53 siRNA-transfected cells, compared to the control siRNA-transfected cells (Fig. 1e). These data indicated that BDNF/TrkB protected NB cells from etoposide-induced cell death via down-regulation of P53.
The expression changes of the BCL–2 family members after treatment with etoposide or BDNF in NB cells
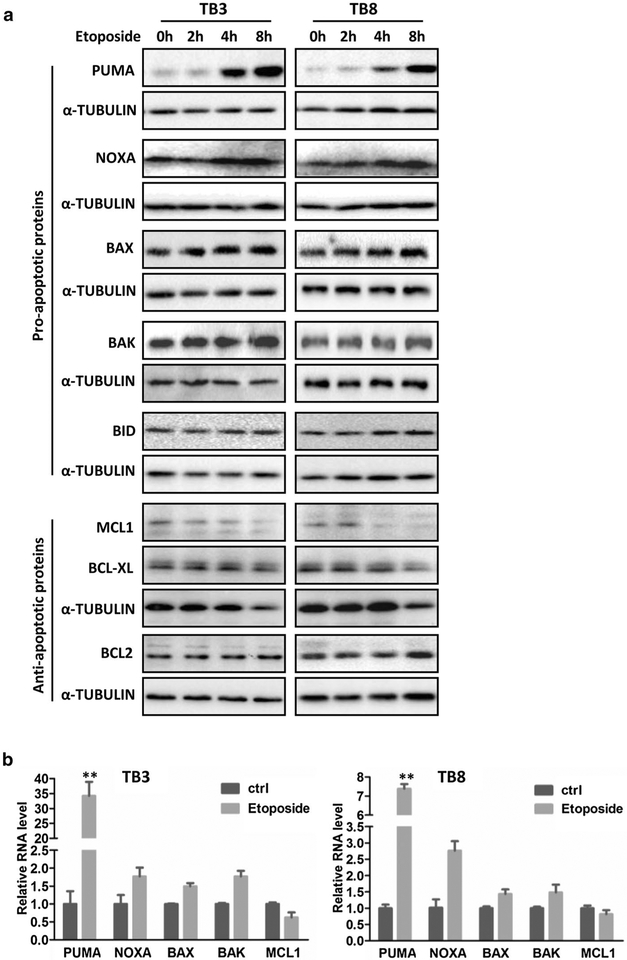
BCL-2 family is a group of proteins that play important role in the intrinsic apoptosis, and some of them are the downstream targets of P53 [25]. To investigate whether or not BCL-2 family members are involved in the BDNF/TrkB protection from etoposide-induced cell death, we treated the TB3 and TB8 cells with etoposide (0.5 μg/ml) (Fig. 2) or BDNF (100 ng/ml) (Fig. 3), harvested the cells at different time points, and examined the expressions of the pro-apoptotic members (PUMA, NOXA, BAX, BAK, BID) and anti-apoptotic members (BCL-2, MCL-1, BCL-XL) by Western blot or RT-PCR. At protein level, the expression of PUMA began to increase after 4 h treatment of etoposide and continued to increase at 8 h in TB3 and TB8 cells. There were also increased expressions of NOXA, BAX, and BAK after etoposide treatment, which were not as significant as that of PUMA. A decreased expression of MCL-1 was detected after etoposide treatment. While there were no significant changes for the expressions of BID, BCL-2 and BCL-XL after etoposide treatment (Fig. 2a). The expressions of PUMA, NOXA, BAX, BAK, and MCL-1 at mRNA level after etoposide treatment (4 h) were detected by RT-PCR. The increased expressions of PUMA, NOXA, BAX and BAK in TB3 and TB8 cells were detected, and PUMA was the one that had the most significant increase after etoposide treatment (P < 0.01). There was a decreased trend for the MCL-1 expression after etoposide treatment in TB3 and TB8 cells which didn’t reach statistically significant (Fig. 2b).
Fig. 2.
The expression changes of the BCL-2 family members after treatment with etoposide in NB cells. TB3 and TB8 cells were treated with etoposide (0.5 μg/ml), and then cellswere harvested at different time points, and Western blot was done to detect the expressions of BCL2 family members in protein level (a). b The expressions of PUMA, NOXA, BAX, BAK, and MCL1 in mRNA level (treated with etoposide for 4 h) were tested by real-time PCR representatively. Bars, SD P-value were tested by Student’s t test. **P < 0.01
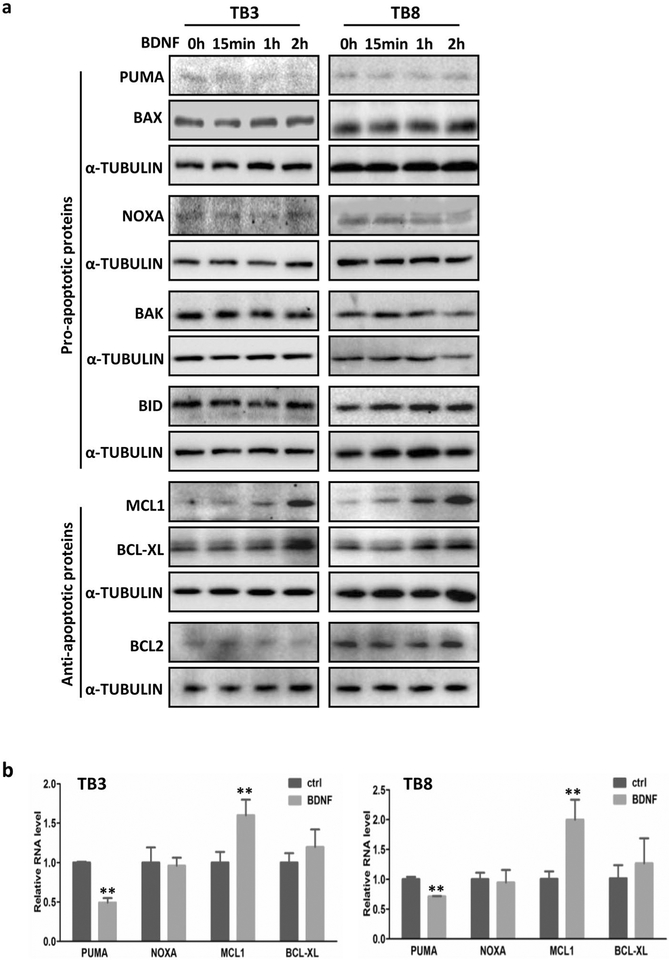
Fig. 3.
The expression changes of the BCL-2 family members after treatment with BDNFin NB cells. TB3 and TB8cells were treated with BDNF (100 ng/ml), and then cells were harvested at different time points, and Western blot was done to identify the expressions of BCL2 family members in protein level (a). b The expressions of PUMA, NOXA, MCL, and BCL-XL in mRNA level (treated with BDNF for 2 h) were tested by real-time PCR representatively. Bars, SD P-value were tested by Student’s t test. **P < 0.01
The effect of BDNF on the expression of BCL-2 family members was showed in Fig. 3. There were no significant changes for the pro-apoptotic members (NOXA, BAX, BAK, and BID) and anti-apoptotic member BCL2 at protein level after BDNF treatment in TB3 and TB8 cells, while the expressions of anti-apoptotic members (MCL-1, and BCL-XL) increased after BDNF treatment, and the expression of pro-apoptotic member PUMA showed a trend to decrease (Fig. 3a). The mRNA expressions of PUMA, NOXA, BCL-XL and MCL-1 were detected by RT-PCR, and the results showed an unchanged expression of NOXA, a decreased expression of PUMA, and increased expressions of BCL-XL and MCL-1 after BDNF treatment (2 h) (Fig. 3b), but the change of BCL-XL was not as significant as PUMA and MCL1.
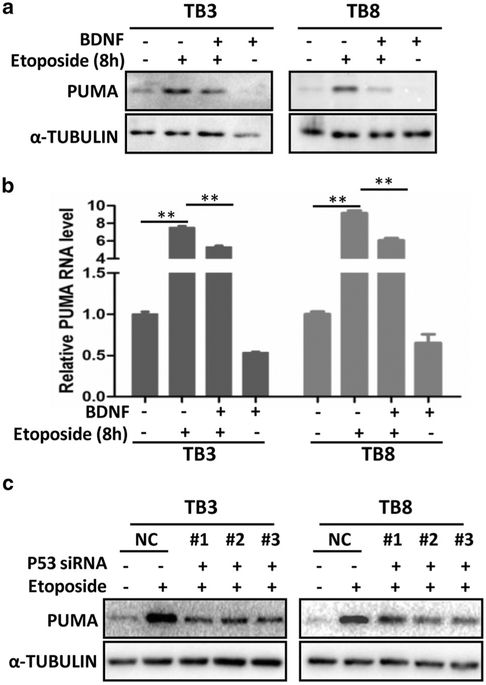
BDNF blocked the etoposide–induced increase of PUMA in NB cells
As the expression of PUMA showed a decrease trend after BDNF treatment (Fig. 3a), we wonder whether BDNF could block the etoposide-induced increase of PUMA (like P53, Fig. 1c) to exert its protective function in TrkB-expressing cells. We pretreated the TB3 and TB8 cells with BDNF (100 ng/ml) for 1 h followed by etoposide (0.5 μg/ml) treatment for 8 h, harvested the cells and detected the PUMA expression by Western blot and RT-PCR. Results showed that BDNF partially blocked the etoposide-induced increase of PUMA at protein level (Fig. 4a) and mRNA level (P < 0.01) (Fig. 4b) in both TB3 and TB8 cells. As PUMA is reported to be a down-stream target of P53, to identify the regulation of PUMA by P53 in our TrkB-expressing NB cells, we examined the PUMA expression in the P53 siRNA-transfected cells. Figure 4c showed that the etoposide-induced increase of PUMA expression could be attenuated by knockdown of P53 via siRNA transfection. These data indicated that PUMA was regulated by P53 in the TrkB-expressing TB3 and TB8 cells.
Fig. 4.
BDNF inhibited the increased expression of PUMA induced by etoposide. a, b TB3 and TB8 cells were pre-treated with BDNF (100 ng/ml) for 1 h, followed by treatment with etoposide (0.5 μg/ml) for 8 h, and cells were harvested. The expression of PUMA was detected by Western blot (a) and RT-PCR (b). Bars, SD P-value were tested by Student’s t test. **P < 0.01. c The expression of PUMA was detected by Western blot after transfection of P53 siRNA into TB3 and TB8 cells
PUMA mediated the BDNF/TrkB protection of NB cells from etoposide–induced cell death
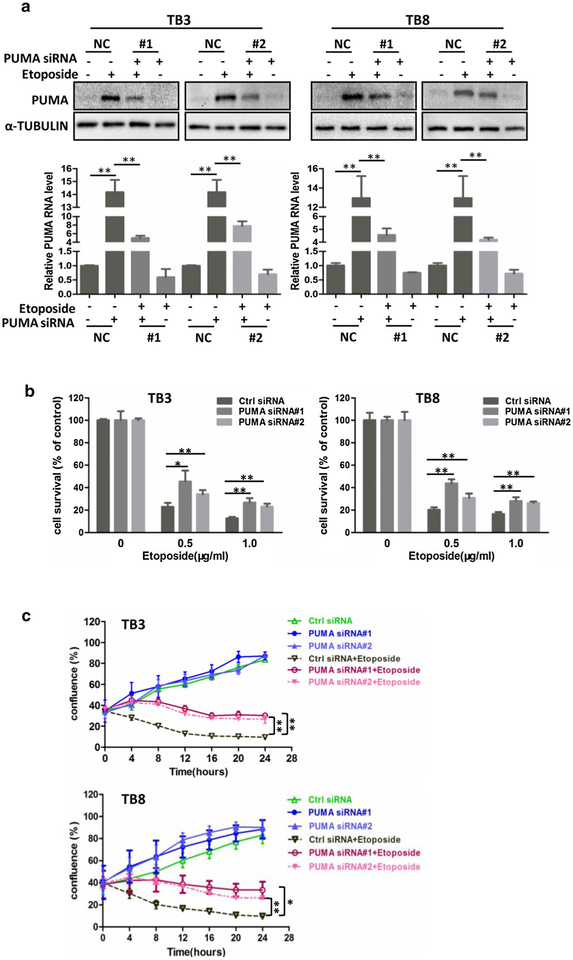
To study the role of PUMA in the etoposide-induced cell death, we down-regulated the PUMA expression by transfecting PUMA siRNA into the TB3 and TB8 cells which were treated with etoposide, to either examine the PUMA expression by Western blot and RT-PCR (8 h of etopo-side treatment, Fig. 5a), or detect the cell survival by MTS assay (24 h of etoposide treatment, Fig. 5b). Two PUMA siRNAs were used. Figure 5a showed that the etoposide-induced increase of PUMA expression could be attenuated by transfecting PUMA siRNA into TB3 and TB8 cells at both protein and mRNA level (P < 0.01, P < 0.01). Using these two PUMA siRNAs, we examined the cell survival and found that down-regulation of PUMA protected TB3 and TB8 cells from etoposide-induced cell death (Fig. 5b). Knockdown of PUMA expression by transfection with siRNA induced 11–22% increase in TB3 cell survival (P < 0.05, P < 0.01) and 10–23% increase in TB8 cell survival (P < 0.01, P < 0.01) after etoposidie treatment (Fig. 5b). We also studied the effect of PUMA in the etoposide-induced cell death by detecting the percentage of cell confluence using IncuCyte ZOOM equipment after transfection of PUMA siRNA into etoposide (0.5 μg/ml)-treated NB cells. The results showed that knockdown of PUMA expression significantly increased the percentage of cell confluence in TB3 (P < 0.01, P < 0.01) and TB8 cells (P < 0.05, P < 0.01) (Fig. 5c), that was consistent with cell survival results detected by MTS assay.
Fig. 5.
Downregulation of PUMA decreased the cell death induced by etoposide both in TB3 and TB8 cells. PUMA siRNA and siRNA control were transfected into TB3 and TB8 cells as described in “Materials and methods”, and the expression of PUMA was detected by Western blot and RT-PCR (a), and after transfection of PUMA siRNA into etoposide-treated TB3 and TB8 cells, cell survival was tested by MTS assay (b). Bars, SD P-value were tested by Student’s t test. *P < 0.05, **P < 0.01. c After transfection of PUMA siRNA into etoposide-treated TB3 and TB8 cells, real-time evaluation of cell confluence was performed by IncuCyte ZOOM. The points showed three replicates. Bars, SD P-value were tested by Student’s t test. *P < 0.05, **P < 0.01
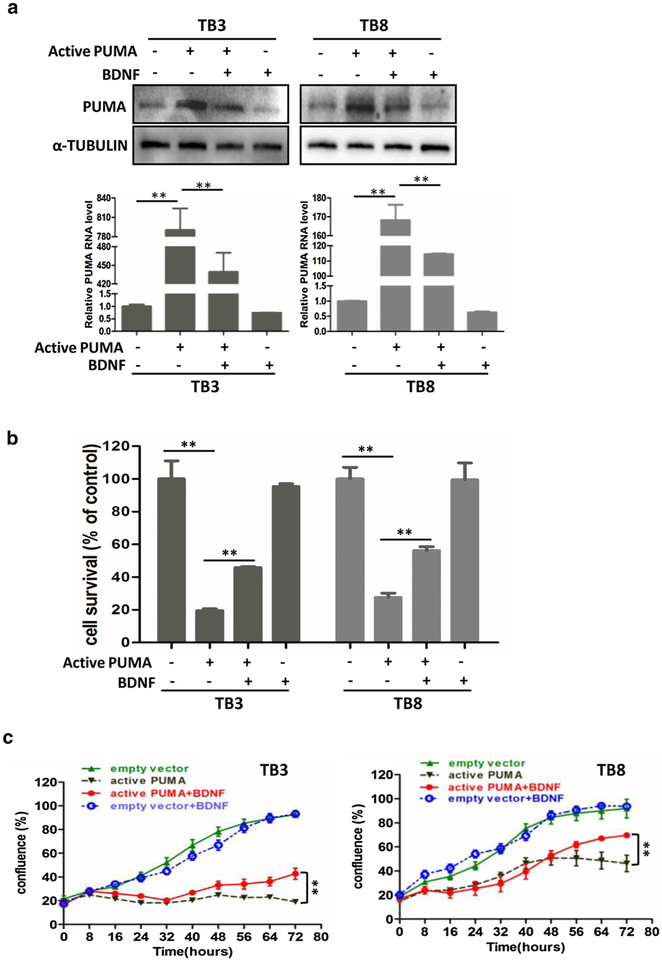
To further investigate the role of PUMA in cell survival, we over-expressed the PUMA expression by infecting activated PUMA lentiviral into TB3 and TB8 cells. After 24 h of infection, BDNF (100 ng/ml) was added into 6-well plates or 96-well plates. The expression of PUMA was detected by Western blot and RT-PCR (treatment with BDNF for 3 h, Fig. 6a), and the cell survival was detected by MTS assay (treatment with BDNF for 48 h, Fig. 6b). The expression of PUMA was significantly increased by infection of activated PUMA lentiviral in both TB3 and TB8 cells, and treatment with BDNF partially blocked the increased expression of PUMA at both protein and mRNA level (P < 0.01, P < 0.01) (Fig. 6a). Figure 6b showed that the over-expression of PUMA in NB cells induced cell death. 72 h after infection, the survival rate is 20% in TB3 cells (P < 0.01) and 27% in TB8 cells (P < 0.01), compared to cells infected with control lentiviral. Treatment with BDNF, the cell survival rate was increased to 46% in TB3 cells (P < 0.01) and 56% in TB8 cells (P < 0.01). We also analyzed the percentage of cell confluence using IncuCyte ZOOM, and results showed that BDNF significantly increased the percentage of cell confluence of TB3 and TB8 cells after infection of activated PUMA lentiviral (P < 0.01, P < 0.01) (Fig. 6c).
Fig. 6.
BDNF protected TrkB-expressing TB3 and TB8 cells from over-expression PUMA induced cell death. TrkB-expressing TB3 and TB8 cells were infected with activated PUMA lentiviral or empty vector, 24 h after infection, BDNF (100 ng/ml) was added. Treated with BDNF for 3 h, cells were harvested, and the expression of PUMA was tested by Western blot and RT-PCR (a). The cell survival was tested by MTS assay after treated with BDNF for 48 h (b). Bars, SD P-value were tested by Student’s t test. **P < 0.01. c After treated PUMA-lentiviral-infected TB3 and TB8 cells with BDNF (100 ng/ml), real-time evaluation of cell confluence was performed by IncuCyte ZOOM. The points showed three replicates. Bars, SD P-value were tested by Student’s t test. **P < 0.01
These data suggested that PUMA mediated the protection of BDNF/TrkB from etoposide-induced cell death in NB cells.
Discussion
In this study, we found that BDNF partially blocked the etoposide-induced increases of P53 and PUMA in TrkB-expressing NB cells; knockdown expression of P53 or PUMA increased the cell survival of etoposide-treated TrkB-expressing NB cells; and BDNF partially protected TrkB-expressing NB cells from over-expression PUMA-induced cell death. These suggested that P53 and PUMA were the new potential targets of BDNF/TrkB mediate chemo-resistance in NB cells.
Etoposide is a chemotherapeutic drug commonly used for treatment of various cancer types, including NB. It inhibits DNA synthesis by forming complex with topoisomerase II and DNA, and this complex induces DNA damage [26, 27]. P53 is a tumor suppressor, and induces apoptosis in response to many kinds of cellular stresses, including DNA damage [25, 28]. Studies have confirmed that P53 mediates etoposide-induced cell death in many cell types, including, human fibrosarcoma and colon cancer cells, and cortical neurons [29, 30]. The expression of P53 was significantly increased by treatment with etoposide in human fibrosarcoma HT1080 cells and colon cancer HCT116 cells, and knockdown P53 by transfection of siRNAs significantly decreased etoposide-induced apoptosis through down-regulation of cleaved caspase3 [29]. Etoposide-induced DNA strand breaks (DSBs) could lead to P53-dependent apoptosis in cortical neurons [30]. The role of P53 in etoposide-induced NB cells death have been studied. Results showed that the activity of P53 was blocked by Wip1 (wild-type p53-inducible phosphatase 1), which could be reactivated by Wip1 inhibitor GSK2830371, and reactivated P53 enhanced etoposide-induced cell death in NB cell lines (IMR-32, NGP, NB-19, CHLA-255, and SH-SY5Y) [31]. Similarly, our results showed that etoposide increased the expression of P53, and knockdown P53 by transfection with siRNAs decreased etoposide-induced NB cells (TB3 and TB8) death. Furthermore, we also found that BDNF blocked the increased expression of P53 induced by etoposide in TrkB-expressing TB3 and TB8 cells. Our results suggest that P53 is a new potential target of BDNF/TrkB to protect NB cells from etoposide induced cell death.
BCL-2 family is a group of proteins that regulate programmed cell death and play important role in the intrinsic apoptosis, some of them are the downstream targets of P53 [25]. The involvement of BCL2 family members in chemotherapy-induced cells death have been widely studied in many diseases, including diffuse large B-cell lymphoma (DLBCL) cells, triple-negative breast cancer (TNBC) cells, acute myeloid leukemia (AML) cells, and non-small cell lung cancer (NSCLC) cells [32–35]. Down-regulation of BCL2 expression (anti-apoptotic protein) by ABT-199, a third-generation BH3 mimetic, increased the sensitivity of DLBCL cells to both doxorubicin and etoposide [32]. In TNBC cell lines, the expressions of BCL2 and MCL1 could be down-regulated by siRNA transfection-induced blockage of PIM1 (a PIM kinase that control cancer cells proliferation and apoptosis), which increased the sensitivity to paclitaxel or eribulin [33]. It has been reported that the decreased expression of PUMA induced by the loss of fragile histidine triad (FHIT), a tumor suppressor that could induce apoptosis and inhibit proliferation of tumor cells, mediated the resistance to cisplatin in NSCLC cell lines [35]. In this study, we also examined the role of BCL2 family members in BDNF/TrkB-induced resistance to etoposide in NB cells. Similarly, our results showed that knockdown expression of PUMA (pro-apoptotic member) by transfection of siRNA increased cell survival in etoposide-treated NB cells. Furthermore, we found that BDNF could partially block etoposide-induced increased expression of PUMA, and down-regulate activated PUMA lentiviral induced increased expression of PUMA in TrkB-expressing NB cells, and BDNF could protect NB cells from PUMA-induced cell death. Our results also showed that the increased expression of PUMA induced by etoposide could be blocked by transfection of P53 siRNAs. All these results suggest that PUMA was a downstream target of P53 in NB cells, and mediated the protection of BDNF/TrkB from etoposide-induced cell death.
There is a complex network of interactions between pro- and anti-apoptotic BCL2 family proteins in response to apoptosis. The anti-apoptotic BCL2 family members (e.g. BCL2, BCL-XL) promote cell survival by inhibiting effectors (BAX and BAK) through binding with them. The BH3-only proteins, sensitizers (e.g. BAD, NOXA, BIK) and activators (e.g. BIM, PUMA, BID), could bind with anti-apoptotic BCL2 proteins through the BH3 domain and lead to the release of BAX and/or BAK from anti-apoptotic proteins, inducing apoptosis. The activators (e.g. BIM, PUMA, BID) could also directly interact with BAX and/or BAK, and induce the activation of BAX and/or BAK [36, 37]. There are also studies investigating the role of BCL2 family members in chemotherapy-induced apoptosis by detecting the ratio of pro-apoptotic proteins with the anti-apoptotic proteins [23]. In our study, we detected the expression changes of most of the BCL2 family members after treatment with etoposide or BDNF, PUMA was the most significantly but not the only changed molecular. The expressions of proapoptotic proteins (NOXA, BAX, and BAK) were increased by treatment with etoposide, and the expression of anti-apoptotic protein MCL1 was decreased. Treatment with BDNF increased the expressions of anti-apoptotic proteins (MCL1, and BCL-XL). In the future, we will investigate the interaction among these BCL-2 family members and their roles in the protection of BDNF/TrkB from chemotherapy-induced NB cell death. In the present study, transfection with P53 or PUMA siRNAs neither completely blocked the increased expression of P53 or PUMA, nor the etoposide-induced cell death. These indicated that there might be other targets involved in this, and another reason is the limitation of transfection efficiency. Another limitation of this study is that only one chemotherapeutic agent (etoposide) was used. Our future studies will be aimed to investigate the roles of P53 and PUMA in BDNF/TrkB-induced resistance to cisplatin or paclitaxel or other chemotherapeutic agents in NB cells.
In conclusion, our present study provides evidence that P53 and PUMA may be potential targets that mediate the protection of BDNF/TrkB from etoposide-induced NB cell death.
Acknowledgements
We thank the staff from Medical Research Center of Shengjing Hospital who gave us support throughout the experiments.
Funding This work was supported by National Natural Science Foundation of China (Nos. 81472359, 81272538), Natural Science Foundation of Liaoning Province (No. 201602855), and 2013 Liaoning Climbing Scholar Foundation.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- TrkB
Tropomyosin-related kinase B
- NB
Neuroblastoma
- MYCN
Neuroblastoma MYC oncogene
- PI3K
Phosphoinositied-3-kinase
- MAPK
Mitogen-activated protein kinase
- HNSCC
Head and neck squamous cell carcinoma
- TET
Tetracycline
- FBS
Fetal bovine serum
- DSBs
DNA strand breaks
- Wip1
Wild-type p53-inducible phosphatase 1
- DLBCL
Diffuse large B-cell lymphoma
- TNBC
Triple-negative breast cancer
- AML
Acute myeloid leukemia
- NSCLC
Non-small cell lung cancer
- FHIT
Fragile histidine triad
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare no conflict of interest.
References
- 1.Brodeur GM (2003) Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 3(3):203–216 [DOI] [PubMed] [Google Scholar]
- 2.Shohet J, Foster J (2017) Neuroblastoma. BMJ 357:j1863. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM, Iyer R, Croucher JL, Zhuang T, Higashi M, Kolla V (2014) Therapeutic targets for neuroblastomas. Expert Opin Ther Targets 18(3):277–292. https://doi.org/10.1517/14728222.2014.867946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vo KT, Matthay KK, Neuhaus J, London WB, Hero B, Ambros PF, Nakagawara A, Miniati D, Wheeler K, Pearson AD, Cohn SL, DuBois SG (2014) Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol 32(28):3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodeur GM, Bagatell R (2014) Mechanisms of neuroblastoma regression. Nat Rev Clin Oncol 11(12):704–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiele CJ, Li Z, McKee AE (2009) On Trk—the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res 15(19):5962–5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Thiele CJ (2007) Targeting Akt to increase the sensitivity of neuroblastoma to chemotherapy: lessons learned from the brain-derived neurotrophic factor/TrkB signal transduction pathway. Expert Opin Ther Targets 11(12):1611–1621. https://doi.org/10.1517/14728222.11.12.1611 [DOI] [PubMed] [Google Scholar]
- 8.Jaboin J, Kim CJ, Kaplan DR, Thiele CJ (2002) Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3′-kinase pathway. Cancer Res 62(22):6756–6763 [PubMed] [Google Scholar]
- 9.Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, Camoratto AM, Evans AE, Brodeur GM (2002) Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res 62(22):6462–6466 [PubMed] [Google Scholar]
- 10.Kachalaki S, Ebrahimi M, Mohamed Khosroshahi L, Mohammadinejad S, Baradaran B (2016) Cancer chemoresistance; biochemical and molecular aspects: a brief overview. Eur J Pharm Sci 89:20–30 [DOI] [PubMed] [Google Scholar]
- 11.Jia L, Lu XA, Liu G, Wang S, Xu M, Tian Y, Zhang S, Fu Y, Luo Y (2017) Endostatin sensitizes p53-deficient non-small-cell lung cancer to genotoxic chemotherapy by targeting DNA-dependent protein kinase catalytic subunit. J Pathol 243(2):255–266. https://doi.org/10.1002/path.4952 [DOI] [PubMed] [Google Scholar]
- 12.Paek AL, Liu JC, Loewer A, Forrester WC, Lahav G (2016) Cell-to-cell variation in p53 dynamics leads to fractional killing. Cell 165(3):631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabelguenne A, Blons H, de Waziers I, Carnot F, Houllier AM, Soussi T, Brasnu D, Beaune P, Laccourreye O, Laurent-Puig P (2000) p53 alterations predict tumor response to neoadjuvant chemotherapy in head and neck squamous cell carcinoma: a prospective series. J Clin Oncol 18(7):1465–1473. https://doi.org/10.1200/JCO.2000.18.7.1465 [DOI] [PubMed] [Google Scholar]
- 14.Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT (2011) Anti-apoptosis and cell survival: a review. Biochim Biophys Acta 1813(1):238–259 [DOI] [PubMed] [Google Scholar]
- 15.Hengartner MO (2000) The biochemistry of apoptosis. Nature 407(6805):770–776. https://doi.org/10.1038/35037710 [DOI] [PubMed] [Google Scholar]
- 16.Czabotar PE, Lessene G, Strasser A, Adams JM (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15(1):49–63 [DOI] [PubMed] [Google Scholar]
- 17.Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116(2):205–219 [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann SH, Vaux DL (2003) Alterations in the apoptotic machinery and their potential role in anticancer drug resistance. Oncogene 22(47):7414–7430 [DOI] [PubMed] [Google Scholar]
- 19.Radha G, Raghavan SC (2017) BCL2: a promising cancer therapeutic target. Biochim Biophys Acta 1868(1):309–314 [DOI] [PubMed] [Google Scholar]
- 20.Sartorius UA, Krammer PH (2002) Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer 97(5):584–592 [DOI] [PubMed] [Google Scholar]
- 21.Katkoori VR, Suarez-Cuervo C, Shanmugam C, Jhala NC, Cal-lens T, Messiaen L, Posey J 3rd, Bumpers HL, Meleth S, Grizzle WE, Manne U (2010) Bax expression is a candidate prognostic and predictive marker of colorectal cancer. J Gastrointest Oncol 1(2):76–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang HJ, Jung KH, Kim DY, Jeong SY, Choi HS, Kim YH, Sohn DK, Yoo BC, Lim SB, Kim DH, Ahn JB, Kim IJ, Kim JM, Yoon WH, Park JG (2005) Bax, a predictive marker for therapeutic response to preoperative chemoradiotherapy in patients with rectal carcinoma. Hum Pathol 36(4):364–371 [DOI] [PubMed] [Google Scholar]
- 23.Bender A, Opel D, Naumann I, Kappler R, Friedman L, von Schweinitz D, Debatin KM, Fulda S (2011) PI3K inhibitors prime neuroblastoma cells for chemotherapy by shifting the balance towards pro-apoptotic Bcl-2 proteins and enhanced mitochondrial apoptosis. Oncogene 30(4):494–503 [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Zhang J, Liu Z, Woo CW, Thiele CJ (2007) Downregulation of Bim by brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from paclitaxel but not etoposide or cisplatin-induced cell death. Cell Death Differ 14(2):318–326 [DOI] [PubMed] [Google Scholar]
- 25.Kastenhuber ER, Lowe SW (2017) Putting p53 in context. Cell 170(6):1062–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen PB, Sehested M (1997) DNA topoisomerase II rescue by catalytic inhibitors: a new strategy to improve the antitumor selectivity of etoposide. Biochem Pharmacol 54(7):755–759 [DOI] [PubMed] [Google Scholar]
- 27.Hande KR (1998) Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer 34(10):1514–1521 [DOI] [PubMed] [Google Scholar]
- 28.Zuckerman V, Wolyniec K, Sionov RV, Haupt S, Haupt Y (2009) Tumour suppression by p53: the importance of apoptosis and cellular senescence. J Pathol 219(1):3–15. https://doi.org/10.1002/path.2584 [DOI] [PubMed] [Google Scholar]
- 29.Sun B, Ross SM, Rowley S, Adeleye Y, Clewell RA (2017) Contribution of ATM and ATR kinase pathways to p53-mediated response in etoposide and methyl methanesulfonate induced DNA damage. Environ Mol Mutagen 58(2):72–83. https://doi.org/10.1002/em.22070 [DOI] [PubMed] [Google Scholar]
- 30.Slomnicki LP, Hallgren J, Vashishta A, Smith SC, Ellis SR, Hetman M (2016) Proapoptotic requirement of ribosomal protein L11 in ribosomal stress-challenged cortical neurons. Mol Neurobiol 55:538–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Wang L, Yao D, Yang T, Cao WM, Dou J, Pang JC, Guan S, Zhang H, Yu Y, Zhao Y, Wang Y, Xu X, Shi Y, Patel R, Vasudevan SA, Liu S, Yang J, Nuchtern JG (2016) Wip1 inhibitor GSK2830371 inhibits neuroblastoma growth by inducing Chk2/p53-mediated apoptosis. Sci Rep 6:38011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Pongtornpipat P, Tiutan T, Kendrick SL, Park S, Persky DO, Rimsza LM, Puvvada SD, Schatz JH (2015) Synergistic induction of apoptosis in high-risk DLBCL by BCL2 inhibition with ABT-199 combined with pharmacologic loss of MCL1. Leukemia 29(8):1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braso-Maristany F, Filosto S, Catchpole S, Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L, Gazinska P, Liccardi G, Meier P, Gris-Oliver A, Cheang MC, Perdrix-Rosell A, Shafat M, Noel E, Patel N, McEachern K, Scaltriti M, Castel P, Noor F, Buus R, Mathew S, Watkins J, Serra V, Marra P, Grigoriadis A, Tutt AN (2016) PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat Med 22(11):1303–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson LA, Burwell EA, Thompson KA, Shahnaz S, Chen AR, Loeb DM (2006) The antiapoptotic gene A1/BFL1 is a WT1 target gene that mediates granulocytic differentiation and resistance to chemotherapy. Blood 107(12):4695–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu DW, Lee MC, Hsu NY, Wu TC, Wu JY, Wang YC, Cheng YW, Chen CY, Lee H (2015) FHIT loss confers cisplatin resistance in lung cancer via the AKT/NF-kappaB/Slug-mediated PUMA reduction. Oncogene 34(19):2505–2515 [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui WA, Ahad A, Ahsan H (2015) The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol 89(3):289–317. https://doi.org/10.1007/s00204-014-1448-7 [DOI] [PubMed] [Google Scholar]
- 37.Hata AN, Engelman JA, Faber AC (2015) The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov 5(5):475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]