Abstract
Purpose
Current in vitro disintegration methods for polymeric films are qualitative and introduce significant user bias. The goal of these studies is to develop a novel, quantitative disintegration technique which can be used to characterize polymeric films in vitro.
Methods
A method was developed using a Texture Analyzer instrument to evaluate film disintegration. Solvent casted, clinically advanced, anti-HIV, vaginal films as well as marketed vaginal films were used throughout these studies. Method development followed a Quality by Design (QbD) process and was used to evaluate film products.
Results
The current method developed provided reproducible, quantitative disintegration times for the commercially available Vaginal Contraceptive Film (57.88 ± 5.98 sec.). It distinguished between two clinically advanced antiretroviral containing films based on disintegration time (p value < 0.001); the tenofovir film (41.28 ± 3.35 sec.) and the dapivirine film (88.36 ± 10.61 sec.). This method could also distinguish between tenofovir and dapivirine films which had been altered in terms of volume (p<0.0001) and formulation (p<0.0001) based on disintegration time.
Conclusions
This method can be applied for pharmaceutical films for ranging indications as part of vigorous in vitro characterization. Parameters of the test can be altered based on site of application or indication.
Keywords: Polymeric films, disintegration, texture analyzer, quality by design (QbD)
Introduction
The thin polymeric film is a versatile dosage form that can be used to deliver a wide range of pharmaceuticals. There has been wide application of this technology for oral and vaginal drug delivery and potential use in topical wound care, diagnostic devices, and pH dependent dissolution in the gastrointestinal tract [1]. Currently, there are many marketed oral thin film products for indications including nausea from cancer and chemotherapeutic treatments, opioid dependence, and pain management. These products include Zuplenz® (Galena Biopharma) for the delivery of ondansetron; Bunavil® (BioDelivery Sciences International, Inc.) and Suboxone® (Indivior, Inc.) for the delivery of buprenorphine and naloxone; Onsolis® (BioDelivery Sciences International, Inc.) for the delivery of fentanyl; and Belbuca® (Endo Pharmaceuticals, Inc.) for the delivery of buprenorphine. There are over-the-counter vaginal films such as the Vaginal Contraceptive Film® (VCF), VCF Scented Film® and VCF Lubricating Film® all manufactured and distributed by Apothecus Pharmaceutical Corporation. Vaginal films represent an emerging technology in the field of topical pre-exposure prophylactics (PrEP) [2] and various film platforms are currently being developed in an attempt to reduce the HIV infection rate in women. The most clinically advanced films contain either the antiretroviral tenofovir or dapivirine [3–6], and a film containing the monoclonal antibody Mapp66 has recently entered into the clinic and this trial is expected to be completed in October 2018 [7, 8]. As the application of this dosage form has been more widely applied in the area of drug delivery, it is important to establish certain characterization parameters in order to standardize evaluations of the physical attributes of this dosage form.
Preclinical film product development includes rigorous physical and chemical testing to evaluate product functionality and uniformity. Common testing protocols exist regardless of the route of administration and chemical entity in the product. Standard testing for films include drug content, and drug content uniformity, contact angle, water content, dissolution, disintegration, and mechanical properties such as tensile strength, puncture strength, elongation, Young's modulus, and folding endurance [1, 9–13]. All of these evaluations aim to establish the variability within and functionality of the film product and are commonly used in stability assessments. Dosage form disintegration and dissolution are two of the most critical parameters that dictate achieving efficacious levels of drug at the desired site. Disintegration is a process in which, the dosage form breaks down into smaller particles after coming in contact with the physiological fluid. The nature of disintegration (e.g. time taken to disintegrate) impacts the downstream dissolution process and ultimately drug dissolution. In vitro disintegration is a valuable tool used to evaluate a crucial dosage form parameter that can predict the behavior of film in vivo and provide a means of comparison between other products. While this test can be extremely valuable, there is not a well-defined, bio-relevant, quantitative method that introduces no user bias. In 1997, The Food and Drug Administration (FDA) issued a guidance for industry for orally disintegrating tablets [14], which refers to the United States Pharmacopeia (USP 701) disintegration testing methods [15]. This method uses a 1 L beaker in which a basket and rack consisting of six plastic tubes with a wire mesh basket at the bottom of the tube. A mechanical arm consistently raises and lowers the tubes in and out of the fluid. Fluid volume in the beaker must be enough so that at the highest point, the wire mesh is still 15 mm below the surface of the fluid and at the lowest point the mesh is at least 25 mm from the bottom of the beaker [15]. However, this test is specifically designed for the disintegration of tablets and capsules and lacks firmly established guidelines for films.
In lieu of an acceptable method specific for polymeric films, other methods have been adapted for use for this dosage form as there is no official guidelines for films [16]. These methods can be classified based on volume of media used throughout the experiment. Small volume methods include visual methods and the slide frame method [17]. Visual disintegration methods use a holder for the films (i.e. petri dish) and a known amount of media is applied to the films, ranging from 2mL– 25 mL [9, 17]. Disintegration time is user-defined by when the film disintegrates. There are no standardized guidelines for this endpoint. The slide frame method places the film in a slide frame which is then laid on a Petri dish. Media is added to the film and time until the film disintegrates is measured. This test has been performed with amount of media ranging from drops to 2 mL [18–20]. Again, this is a visual method and there are no specific indications which can be used across users to define film disintegration. Larger volume tests are mainly modifications to the USP disintegration method for tablets and capsules as described previously. The disintegration apparatus as described previously and 500mL– 1L of media are used for these tests [17]. In one method using this setup, one end of the film is clamped to a weight and the other end to a sample holder. Films are then submerged in media, or continuously dipped in and out of media, and the time until the weight drops to the bottom of the vessel is measured [21]. Other tests using the disintegration apparatus mimic what is done for tablets and capsules, but include a way to secure the film in the basket or to the arm of the apparatus [17]. There are pitfalls with both the large and small volume methods as they have user defined endpoints, use non-biologically relevant volumes of media or introduce a large amount of user bias into the final measurement of disintegration. In addition, these tests can be difficult to replicate and control (i.e. droplet size) and can have large deviations [18, 17]. With this wide range of in vitro methods, it is difficult to draw meaningful comparisons between film products.
Standardizing the disintegration test to characterize film dosage forms would allow for comparisons between various films that range in application, delivery site, and release profiles. Therefore, the goal of this communication is to establish a standard testing method for film disintegration that provides objective and quantitative data. It will be of high importance to design and qualify this testing method through Quality by Design (QbD) approach [22, 23]. This manuscript will follow QbD as described by Borman et al. and will focus on the establishment of: the design intent or the performance requirements of the method, the design selection or method development, control definition and control verification [24, 25]. The goal of this method is to establish a quantitative disintegration method that introduces less user bias than visual methods. Specific parameters utilized for this test were tailored towards vaginal films. It is anticipated that these parameters can be modified for other film applications.
Materials and Methods
Materials
Tenofovir was provided by CONRAD (Arlington, Virginia, USA) and dapivirine was provided by the International Partnership for Microbicides (IPM, Silver Spring, MD, USA). Film excipients were purchased from the following; polyvinyl alcohol (PVA) (Emprove®, EMD Millipore), glycerin (Spectrum Chemical), hydroxmethylcellulose (HPMC) (Dow Pharmaceutical Solutions), propylene glycol (Spectrum Chemical), polyethylene glycol (PEG) 8000 (Dow Pharmaceutical Solutions), hydroxyethyl cellulose (HEC) (Ashland), carboxymethylcellulose sodium (NaCMC) (Spectrum Chemical), polyvinylpyrrolidone (PVP) (Fluka) and sodium hydroxide (Spectrum Chemical).
Methods
Film Manufacture
The DPV film formulation was composed of a base polymer of PVA as well as glycerin, HPMC, propylene glycol and PEG 8000 [3, 9, 4]. The TFV film formulation was composed of three cellulose-based polymers; HEC, HPMC and NaCMC, glycerin and sodium hydroxide [26]. Laboratory films were made using the solvent cast manufacturing method [9]. Briefly, polymers, excipients, plasticizer and APIs were either dissolved or dispersed in water using a Caframo Ultra Torque overhead mixer and an IKA bladed propeller stirrer. Solutions were mixed until homogenous and cast on a heated polyethylene terephthalate (PET) substrate (Amcor Flexibles, Mundelein, IL, USA) secured to an Elcometer® 4340 Automatic Film Applicator using an Elcometer® 3700 Doctor Blade. Films were allowed to dry and were then peeled, cut, and packaged in aluminum foil packaging material.
Disintegration
Visual Disintegration Testing
Visual disintegration tests were performed as previously described [13, 9]. Briefly, a film was placed in 1 mL of water at room temperature and set on an orbital shaker. A timer was started when the film came in contact with the fluid and ended when the film had complete structural loss, which was observed visually. This was used as a control throughout this study.
TA.XTPlus Disintegration Testing
A TA.XTPlus Texture Analyzer (Texture Technologies Corp., Hamilton, MA/Stable Micro Systems, Godalming, Surrey, UK) and associated TA.XTPlus probes and accessories were used for the disintegration testing. The TA-108S5 fixture with five 15 mm openings was used to secure films and the TA-8A: 1/8" diameter rounded end ball probe was used to apply force to the films.
Design Intent
Precision: Precision limit is set initially within a relative standard deviation less than or equal to 15%. Sensitivity: The method will not have a user-defined endpoint, but rather a discriminatory endpoint that can be quantitatively measured by the instrument. Selectivity: The assay can differentiate between films prepared with distinct film forming polymers, thickness or excipients.
Design Selection/ Method
A TA.XTPlus instrument was utilized. Films were secured in the TA-108S5 fixture and the TA-8A: 1/8" diameter rounded end ball probe was affixed to the TA.XTPlus instrument. A “Hold Until Reset” test was developed using the Texture Analyzer software, Exponent. Test parameters were as follows: Test Mode: Compression; Pre-Test: 0.5 mm/sec; Test: 0.2 mm/sec; Post Test: 10 mm/sec; Force: 5–15g; Auto Trigger: 5g; Max tracking Speed: 5 mm/sec; Proportional Gain: 50; Integral Gain: 20; and Differential Gain: 5. Films were secured in the TA-108S5 fixture, the probe applied a constant force to the film product, and a biologically relevant amount of water at room temperature was applied to the film surface where the probe interacts with the film. The probe was able to penetrate the film upon disintegration resulting in an applied force of zero at that point. A curve of force vs. time was plotted, and disintegration time was defined as the time from initial fluid addition until the probe force reached zero. Test parameters such as fluid volume and probe force were optimized in order to reduce error.
Data Analysis
Data was collected and analyzed by Texture Analyzer software, Exponent. For analysis, a specific macro was used to measure the time between fluid addition and zero force application on the film, defined as disintegration time.
Control Definition
As this method is not a standard analytical method, controls for this method were determined through vigorous testing and assessment of various parameters in order to ensure that the intentions set for the method are achieved. These parameters included the impact of crucial testing factors (volume and force) on measured disintegration time, reproducibility and variability of the method through validation with a commercially available film (VCF®), and selectivity of the method through testing with a series of vaginal films with various modifications.
Statistical Analysis
Disintegration times were measured in seconds. Data is represented as average disintegration time, and variability is represented as the standard deviation (SD). Statistical analysis was performed using a one-way ANOVA (GraphPad Prism 6.07). Tukey’s test was used for post-hoc analysis. P values less than 0.05 were considered statistically significant.
Results and Discussion
The goal of this study was to develop a test that was independent of user control of the endpoint therefore eliminating user bias, and which produced a quantitative and reproducible measurement of disintegration. Furthermore, it was imperative that the test results could distinguish between subtle film formulation changes. This method was developed and tested following a QbD framework, by establishing control definitions and design intent with precision, sensitivity, and selectivity limits. The design intent of this method was to develop a quantitative disintegration method which can be used as standard testing method for polymeric films. The Texture Analyzer instrument was selected because it can be configured to have a discriminatory, non-user defined endpoint for the disintegration time of solid dosage forms. It can provide quantitative measures (up to 2 decimal places) and has a desirable data acquisition rate (up to 500 points per second (pps)). Previous disintegration methods, as discussed, introduce user bias and large variability. Ham et al., when developing a topical microbicide product using the solvent cast method of film manufacture, used visual disintegration methods to evaluate disintegration time of a range of polyvinyl alcohol based formulations. Disintegration times of these films ranged up to 36% RSD demonstrating that visual methods used to evaluate solvent cast films have a wide range of variability [13]. Another group, Garsuch et al., compared different film forming polymers and evaluated disintegration using the slide frame method of film disintegration. Variability was also present in this method and RSD values ranged up to 50% [18]. Therefore, selecting a 15% RSD precision limit is seen as a significantly improved variability target compared to current disintegration methods.
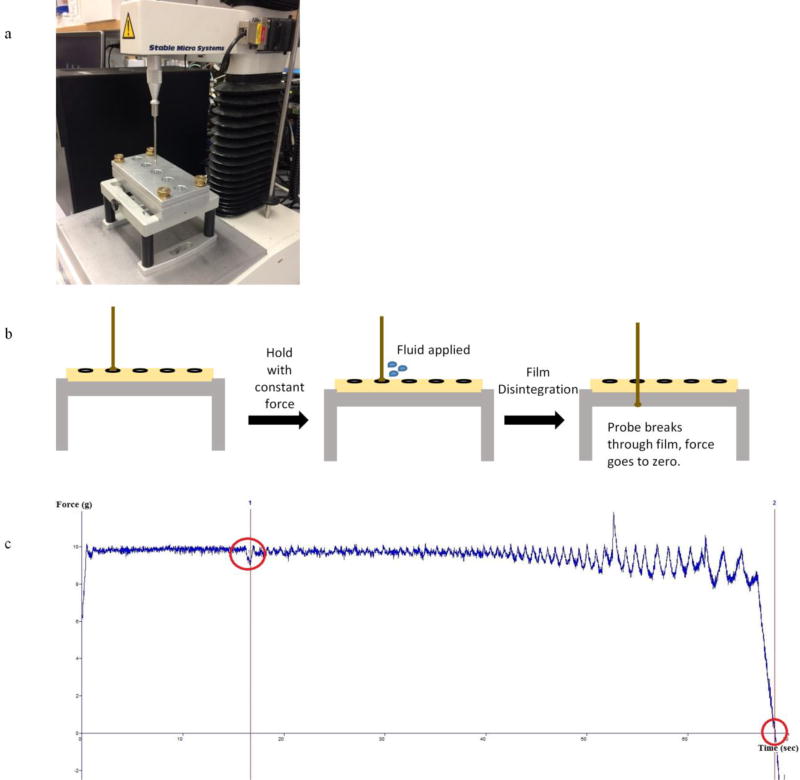
The method was created using the standard template provided by the Textual Analyzer software, Exponent, using a “Hold Until Reset” test. This test applied a small amount of force constantly to the film in the presence of a biologically relevant volume of media, and the time required for the probe to penetrate the film completely, reaching zero force, was measured. The test was reset manually and repeated as necessary. The disintegration time was measured from the time the fluid was added to the film until the force reached zero value. The probe force alone, without fluid addition, was not significant enough to break through the film. Further, the time between when the probe first comes into contact with the film until fluid addition, was controlled for. The parameters that impact the disintegration time were identified and optimized. These included probe force and the volume of fluid applied to the film. The TA-108S5 fixture and the TA-8A: 1/8" diameter rounded ball were selected for set up because this fixture securely holds the film for testing and the ball probe fits within the 15 mm opening. Fig.1 shows the actual setup of the Texture Analyzer instument (a), a graphic of the test setup (b), and a typical disintegration plot produced with the Exponent software (c).
Fig. 1. Texture Analyzer Instrument Setup.
(a) Instrument setup with TA-108S5 fixture and the TA-8A: 1/8" diameter rounded end ball probe. (b) Graphical schematic of setup and test positions of the Texture Analyzer disintegration technique. (c) Typical plot of force vs. time graph produced with Exponent software. Event at 15 seconds and force to zero (disintegration test end) marked in red
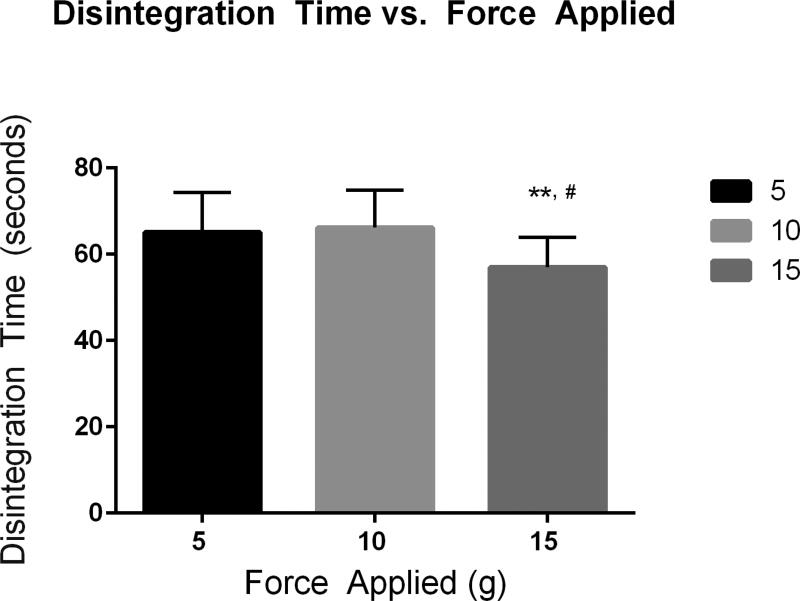
As part of the control section of QbD, three different parameters were evaluated: the impact of crucial testing parameters on outcome, reproducibility and variability of the method through validation with a commercial film, and selectivity of the method through testing with a series of vaginal films with various modifications. The goal of the test was to use a minimal, consistent force that can puncture the film only when the integrity of the film structure is lost in the presence of fluid. It is to be noted that there is no external, consistent force applied in vivo, but squeezing forces within the vagina exist which could aid in product disintegration. For initial volume and force experiments, the Vaginal Contraceptive Film (VCF®, Apothecus Pharmaceutical Corporation) was used. VCF® is a commercially manufactured product therefore less product variability exists when compared to film products manufactured through hand-poured methods on the laboratory scale. Three initial forces of 5, 10 and 15 grams were tested on the instrument when applied to a film. The disintegration test was performed using 15 µL of fluid. Disintegration times between the 5 and 10 gram forces showed no statistically significant differences, but there were significant differences between the 5 and 15 gram and 10 and 15 gram disintegration times (Fig. 2). All deviations were within the set precision limit. Due to the goal in developing this method, the 10 gram force was chosen. The average vaginal squeezing force is estimated to be 4.45 N to 44.5 N over a surface area of approximately 100 cm2 [27]. Based off the area of the film exposed to the fluid (1.767 cm2) and the conversion of N to grams, the resultant force for that area would be approximately 8 grams. The 5 gram force may be more applicable for oral applications, as this force more closely mimics the force which a human tongue applies when licking a probe [21]. Volume of fluid added to the film was the other critical parameter assessed. Based on the probe size and the 15 mm opening of the film holder, the following volumes were selected for testing; 5, 10, 15, 20, 30, 45, and 135 µL (Table 1). Post-hoc analysis using a Tukey test of multiple comparisons for results from volume testing showed there were statistically significant differences between disintegration times when using the following volumes; 5 µL and 15 µL (p value = 0.0040), 15 µL and 30 µL (p value = 0.0498) and 15 µL and 135 µL (p value = 0.0001). These tests show that fluid volume impacted disintegration time and that at higher volumes, 45 µL and above, the relative standard deviation exceeded 15% (Table 1). The mean surface area of the vagina is 87.46 cm2 [2] and average amount of fluid in the vagina is 0.5–0.75 mL [28]. Based off of this, and the area of film exposed to fluid in the holder, 15 µL was selected for our testing of films formulated for vaginal use. Other testing parameters that are specified in the method were varied within the method development stage but did not produce any significant changes in disintegration time outcomes.
Fig. 2. Force Testing.
Disintegration times with varying force for the Texture Analyzer disintegration method (** 15 g vs. 10 g, # 15 g vs. 5 g)
Table 1. Volume Testing.
Disintegration times for VCF ® (GMP product) with different volumes of media
| Volume | Average Disintegration Time (seconds) |
Standard Deviation (seconds) |
% Relative Standard Deviation (RSD) |
|---|---|---|---|
| 5 µL | 51.37 | 7.19 | 14.00 |
| 10 µL | 56.19 | 6.07 | 10.80 |
| 15 µL | 66.19 | 8.61 | 13.02 |
| 20 µL | 56.86 | 6.46 | 11.37 |
| 30 µL | 54.55 | 3.99 | 7.32 |
| 45 µL | 59.26 | 13.47 | 22.73 |
| 135 µL | 47.82 | 20.53 | 42.92 |
Reproducibility was evaluated with a marketed product, the VCF®. Testing with VCF® performed on separate days and by different users to assess reproducibility and variability of the test. As highlighted in the data from Table 2, disintegration times were reproducible with average deviations within the desired QbD precision range. The average disintegration time for the VCF® was found to be 57.88 seconds and on average, regardless of user or time, the average % RSD fell within the set precision limit (≤ 15%).
Table 2. Disintegration Testing for VCF®.
Disintegration times for VCF® (GMP product) evaluated by different users
| User | Trial Number | Disintegration Time (seconds) |
Standard Deviation (seconds) |
% Relative Standard Deviation (RSD) |
|---|---|---|---|---|
| A | 1 | 59.60 | 4.81 | 8.07 |
| A | 2 | 60.77 | 4.36 | 7.18 |
| A | 3 | 59.21 | 6.73 | 11.36 |
| B | 4 | 63.89 | 7.35 | 11.50 |
| B | 5 | 53.82 | 9.38 | 17.42 |
| B | 6 | 46.27 | 2.40 | 5.18 |
| B | 7 | 61.61 | 6.82 | 11.08 |
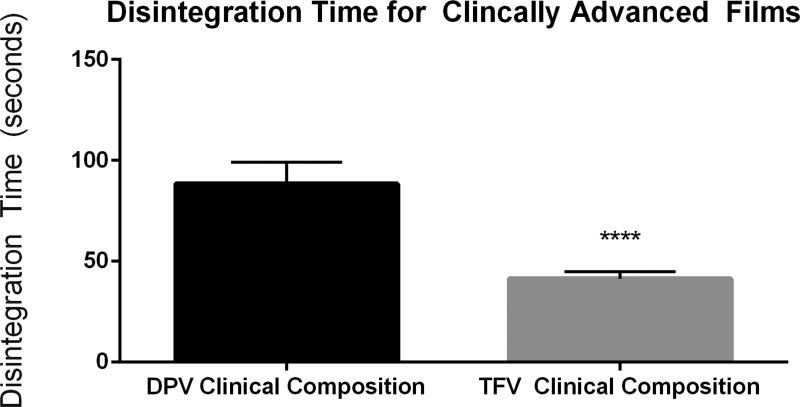
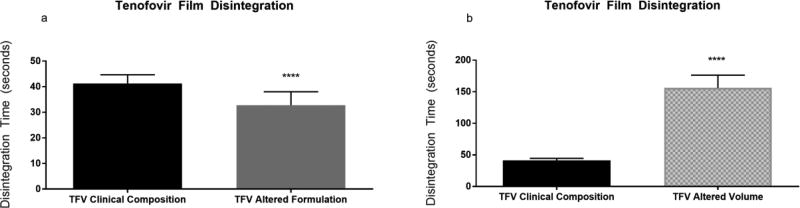
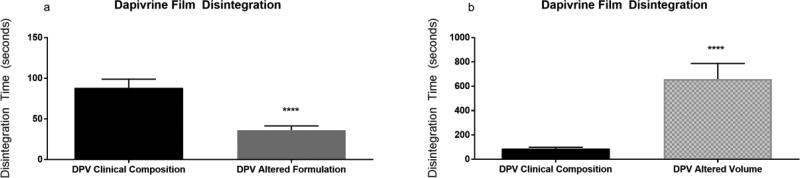
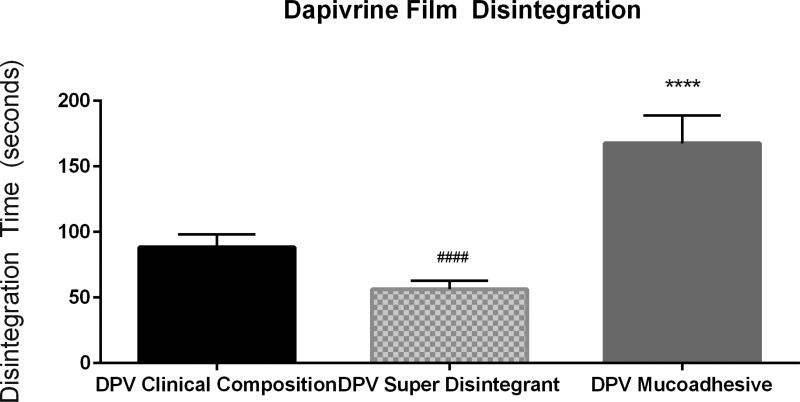
Following this, the selectivity of the method was evaluated with a series of films containing one of two drugs under investigation for HIV prevention. Two clinically advanced antiretroviral compounds, tenofovir (TFV) and dapivirine (DPV), have been formulated into polymeric films for microbicide delivery. These films differ with respect to polymeric base, with the TFV film being a cellulose-based film [6] and the DPV film being a polyvinyl alcohol based film [9]. Both visual and quantitative disintegration testing were performed on a series of TFV and DPV containing films. Disintegration testing using the Texture Analyzer method could distinguish between these two formulations and showed that they differed significantly with a P-value less than 0.0001 (Fig. 3). Initial film formulations of these compounds were modified by either increasing thickness and/or utilization of alternate film-forming polymers. TFV films were formulated with a polymer base of polyvinylpyrrolidone (PVP) (as opposed to hydroxyethyl cellulose) and DPV films were modified from a polyvinyl alcohol base to a cellulose based film. The disintegration results for TFV and DPV are shown in Figs. 4 and 5, respectively. Visual disintegration was conducted for these films by submerging film in 1 mL of water and measuring time until complete structural loss while rotating on an orbital shaker. Compared to visual disintegration for these same films (Table 3), the Texture Analyzer method produced lower deviations (RSD < 15%) and the endpoint was not defined by the user. The Texture Analyzer method was able to distinguish between the volume and formulation modifications (p<0.0001) (Figs. 4 and 5). In order to see if the method could distinguish between minor formulation variations which did not change base polymer, the DPV formulation was altered through the addition of a mucoadhesive polymer to extend disintegration, or through the inclusion of a super-disintegrant, sodium starch glycolate, to enhance disintegration. Comparisons of average disintegration times between these film formulations showed that they both differed significantly from the quick-dissolving clinical DPV film disintegration time and from each other (p < 0.0001) (Fig. 6).
Fig. 3.
TFV and DPV Clinical Film Disintegration Testing Comparison of disintegration times obtained with the Texture Analyzer method for two clinically advanced vaginal films
Fig. 4. Texture Analyzer Disintegration for Tenofovir Films.
Disintegration times for Tenofovir films which have been modified in terms of base (a) or thickness (b)
Fig. 5. Texture Analyzer Disintegration for Dapivirine Films.
Disintegration times for Dapivirine films which have been modified in terms of base (a) or thickness (b)
Table 3. TFV and DPV Clinical Film Visual Disintegration Testing.
Comparison of disintegration times obtained with the visual disintegration in 1 mL of fluid for two clinically advanced vaginal films
| Film | Average Disintegration Time (seconds) |
Standard Deviation (seconds) |
% Relative Standard Deviation (RSD) |
|---|---|---|---|
| TFV Clinical Composition | 124.50 | 23.95 | 19.24 |
| DPV Clinical Composition | 227.00 | 40.88 | 18.01 |
Fig. 6. Texture Analyzer Disintegration for Modified Dapivirine Films.
Disintegration times for Dapivirine films which have formulation modifications hypothesized to impact release profile
Direct comparisons with previously published vaginal film disintegration results were difficult to draw, regardless of the fact that these films were manufactured through the same method of solvent casting and set to similar thickness values. Akil et al. reported disintegration times less than ten minutes [9], while Ham et al. reported values ranging from 5–35 minutes depending on the formulation [29]. Statistical analysis of these results (standard deviations, %RSD) were not provided. It was also difficult to evaluate the reproducibility of other methods; either no raw data was given or the standard deviations were not included. Visual disintegration tests were conducted with films used in these studies and produced results with %RSD values that ranged up to 35% and in general showed a greater amount of variability than the current method.
There is a clear need for a standard, quantitative method to measure disintegration of the versatile film dosing platform. The Texture Analyzer disintegration method described here provides such a test. Using a focused set of experiments, this test was developed to provide a reproducible and robust method which can distinguish between products and product attribute alterations. Utilization of a Texture Analyzer instrument enabled the design of a method that provided the sensitivity, selectivity and precision set forth before establishing this method. Control definitions (force and volume) were set and the reproducibility using the marketed product VCF® was measured. Forces of 5, 10, and 15 grams can be used based on film-specific indication if needed, though the 10 gram force was most suitable for these tests. Fluid amount applied to films can impact disintegration time, and at higher volumes of fluid used, the variation of the test increased. This shows the need to keep volume low (≤ 30 µL). The repeatability and reproducibility of the method was confirmed through 7 different trials done by two different users, confirming minimal user bias introduced. Specificity of the method was evaluated using a range of clinically advanced antiretroviral drug containing films, with alterations in base polymer, excipient, and volume. Specific parameters of this test, such as force applied and volume and type of fluid applied, can be tailored to fit the exact application for oral, vaginal, or other topical films. This quantitative test is essential to understand ultimate in vivo functionality and efficacy and it could be included as a product target specification for film products.
Conclusions
Both oral and vaginal films have been shown to accommodate pharmaceuticals with a wide range of physiochemical properties for various applications. Specialized equipment such as contact angle and mechanical and surface testing equipment have led to the development of standardized testing methods which provide quantitative measurements of this dosage form’s physical and mechanical attributes. One significantly important area that has yet to be standardized is in vitro disintegration for films. Current laboratory methods used are based on methods developed for other dosage forms or employ visual methods resulting in methods with high variability and user bias. The endpoint for most visual tests is defined when the film shows complete structural loss [12, 30, 13], which can be demarcated differently by each user. These tests also differ in setup and fluid amount [31], making it difficult to draw any comparisons between film products. To minimize user bias and standardize testing, a quantitative Texture Analyzer method was developed to measure the time it takes for a minimal amount of force to penetrate a film after the addition of a known amount of fluid. This method was designed to accurately and reproducibly measure the disintegration time of vaginal films but can be modified for application to other film types.
Acknowledgments
The current work was funded through the National Institute of Allergy and Infectious Diseases (grant number: 5U19AI082639) and the Bill & Melinda Gates Foundation. Tenofovir was graciously provided by CONRAD (Arlington, Virginia, USA) and dapivirine was graciously provided by the International Partnership for Microbicides (IPM, Silver Spring, MD, USA). We would like to acknowledge Jeanne Held of Texture Technologies for guidance in the establishment of this method. We would also like to acknowledge Sravan Patel for his help in the preparation of this manuscript.
There was no involvement from any pharmaceutical companies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the grant sources.
Footnotes
Declaration of interest
The authors declare that they have no conflicts of interest.
References
- 1.Bala R, Pawar P, Khanna S, Arora S. Orally dissolving strips: A new approach to oral drug delivery system. International journal of pharmaceutical investigation. 2013;3(2):67–76. doi: 10.4103/2230-973X.114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson LM, Rohan LC. The importance of the vaginal delivery route for antiretrovirals in HIV prevention. Therapeutic delivery. 2011;2(12):1535–50. doi: 10.4155/tde.11.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunge KE, Dezzutti CS, Rohan LC, Hendrix CW, Marzinke MA, Richardson-Harman N, et al. A phase 1 trial to assess the safety, acceptability, pharmacokinetics, and pharmacodynamics of a novel dapivirine vaginal film. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016;71(5):498–505. doi: 10.1097/QAI.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson JA, Marzinke MA, Bakshi RP, Fuchs EJ, Radebaugh CL, Aung W, et al. Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B) AIDS research and human retroviruses. 2017;33(4):339–46. doi: 10.1089/AID.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunge K, Dezzutti C, Macio I, Hendrix C, Lisa C, Rohan L, editors. FAME-02: a phase I trial to assess safety, PK, and PD of gel and film formulations of dapivirine; Conference on Retroviruses and Opportunistic Infections (CROI); 2014. [Google Scholar]
- 6.Bunge KE, editor. Conference on Retroviruses and Opportunistic Infections (CROI) Boston: Massachusetts; 2016. Phase I Trial to Assess Safety, PK, and PD of Film and Gel Formulations of Tenofovir. [Google Scholar]
- 7.Fernández-Romero JA, Deal C, Herold BC, Schiller J, Patton D, Zydowsky T, et al. Multipurpose prevention technologies: the future of HIV and STI protection. Trends in microbiology. 2015;23(7):429–36. doi: 10.1016/j.tim.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson DJ. Monoclonal Antibody-Based Multipurpose Microbicides. [Accessed March 2 2018];National Institute of Allergy and Infectious Diseases. 2011 https://projectreporter.nih.gov/project_info_description.cfm?aid=8209659&icde=0.
- 9.Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M, et al. Development and Characterization of a Vaginal Film Containing Dapivirine, a Non- nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug delivery and translational research. 2011;1(3):209–22. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arya A, Chandra A, Sharma V, Pathak K. Fast dissolving oral films: an innovative drug delivery system and dosage form. International Journal of Chem Tech Research. 2010;2(1):576–83. [Google Scholar]
- 11.Kalyan S, Bansal M. Recent trends in the development of oral dissolving film. Int J PharmTech Res. 2012;4(2):725–33. [Google Scholar]
- 12.Akil A, Agashe H, Dezzutti CS, Moncla BJ, Hillier SL, Devlin B, et al. Formulation and characterization of polymeric films containing combinations of antiretrovirals (ARVs) for HIV prevention. Pharmaceutical research. 2015;32(2):458–68. doi: 10.1007/s11095-014-1474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ham AS, Rohan LC, Boczar A, Yang L, K WB, Buckheit RW., Jr Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharmaceutical research. 2012;29(7):1897–907. doi: 10.1007/s11095-012-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Administration UFD. Guidance for Industry Orally Disintegrating Tablets. Silver Spring, MD: Center for Drug Evaluation and Research (CDER), US Dept of Health and Human Services; 2008. [Google Scholar]
- 15.Convention TUSP. Chapter <701> Disintegration. Rockville, MD: The United States Pharmacopeia Convention; 2009. The United States Pharmacopeia Convention. [Google Scholar]
- 16.Bhyan B, Jangra S, Kaur M, Singh H. Orally fast dissolving films: innovations in formulation and technology. Int J Pharm Sci Rev Res. 2011;9(2):9–15. [Google Scholar]
- 17.Low A, Kok SL, Khong Y, Chan SY, Gokhale R. Pharmaceutics, Drug Delivery and Pharmaceutical Technology: A New Test Unit for Disintegration End-Point Determination of Orodispersible Films. Journal of pharmaceutical sciences. 2015;104(11):3893–903. doi: 10.1002/jps.24609. http://dx.doi.org/10.1002/jps.24609 [DOI] [PubMed] [Google Scholar]
- 18.Garsuch V, Breitkreutz J. Comparative investigations on different polymers for the preparation of fast-dissolving oral films. Journal of Pharmacy and Pharmacology. 2010;62(4):539–45. doi: 10.1211/jpp.62.04.0018. [DOI] [PubMed] [Google Scholar]
- 19.Preis M, Woertz C, Schneider K, Kukawka J, Broscheit J, Roewer N, et al. Design and evaluation of bilayered buccal film preparations for local administration of lidocaine hydrochloride. European Journal of Pharmaceutics and Biopharmaceutics. 2014;86(3):552–61. doi: 10.1016/j.ejpb.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Irfan M, Rabel S, Bukhtar Q, Qadir MI, Jabeen F, Khan A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharmaceutical Journal. 2016;24(5):537–46. doi: 10.1016/j.jsps.2015.02.024. https://doi.org/10.1016/j.jsps.2015.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preis M, Gronkowsky D, Grytzan D, Breitkreutz J. Comparative study on novel test systems to determine disintegration time of orodispersible films. Journal of Pharmacy and Pharmacology. 2014;66(8):1102–11. doi: 10.1111/jphp.12246. [DOI] [PubMed] [Google Scholar]
- 22.Rathore AS, Winkle H. Quality by design for biopharmaceuticals. Nat Biotech. 2009;27(1):26–34. doi: 10.1038/nbt0109-26. [DOI] [PubMed] [Google Scholar]
- 23.Winkle H, editor. Implementing quality by design; PDA/FDA Joint Regulatory Conference; 2007. [Google Scholar]
- 24.Borman P, Chatfield M, Nethercote P, Thompson D, Truman K. The application of quality by design to analytical methods. Pharmaceutical Technology. 2007;31(12):142–52. [Google Scholar]
- 25.Puertollano M, Cartwright T, Aylott M, Kaye N. Assessing an analytical method for the dissolution profile of an extended-release tablet in accordance with QbD. Tablets and Capsules. 2009;7(1):30–9. [Google Scholar]
- 26.Robinson JA, Marzinke MA, Fuchs EJ, Bakshi RP, Spiegel HML, Coleman JS, et al. Comparison Of The Pharmacokinetics And Pharmacodynamics Of Single-Dose Tenofovir Vaginal Film And Gel Formulation (Fame-05) Journal of acquired immune deficiency syndromes. 2017 doi: 10.1097/qai.0000000000001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieweg SL, Katz DF. Interpreting properties of microbicide drug delivery gels: analyzing deployment kinetics due to squeezing. Journal of pharmaceutical sciences. 2007;96(4):835–50. doi: 10.1002/jps.20774. [DOI] [PubMed] [Google Scholar]
- 28.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–5. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 29.Ham AS, Rohan LC, Boczar A, Yang L, Buckheit KW, Buckheit RW., Jr Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharmaceutical research. 2012;29(7):1897–907. doi: 10.1007/s11095-012-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machado RM, Palmeira-De-Oliveira A, Martinez-De-Oliveira J, Palmeira-De-Oliveira R. Vaginal films for drug delivery. Journal of pharmaceutical sciences. 2013;102(7):2069–81. doi: 10.1002/jps.23577. [DOI] [PubMed] [Google Scholar]
- 31.Grammen C, Van den Mooter G, Appeltans B, Michiels J, Crucitti T, Ariën KK, et al. Development and characterization of a solid dispersion film for the vaginal application of the anti-HIV microbicide UAMC01398. International journal of pharmaceutics. 2014;475(1–2):238–44. doi: 10.1016/j.ijpharm.2014.08.054. http://dx.doi.org/10.1016/j.ijpharm.2014.08.054 [DOI] [PubMed] [Google Scholar]