Abstract
Background and Purpose:
Impaired gait, balance, and motor function are common in Parkinson disease (PD) and may lead to falls and injuries. Different forms of exercise improve motor function in PD, but determining which form of exercise is most effective requires a direct comparison of various approaches. In this prospective, controlled trial, we evaluated the impact of tango, treadmill walking, and stretching on gait, balance, motor function, and quality of life. We hypothesized tango and treadmill would improve forward walking and motor severity, and tango also would improve backward walking, balance, and quality of life.
Methods:
Ninety-six participants (age: 67.2±8.9 years, 42% female) with mild to moderate idiopathic PD were serially assigned to tango, treadmill walking, or stretching (active control group) and attended one-hour classes twice weekly for 12 weeks. Assessments occurred off anti-PD medication before and after the intervention and at follow-up 12 weeks after the intervention.
Results:
Forward velocity and backward velocity improved for the treadmill group from baseline to post-test and improvements persisted at follow-up. Backward velocity and motor functioning improved for the stretching group from baseline to post-test but results did not persist at follow-up. There were no significant changes in the tango group across time points.
Discussion and Conclusions:
Contrary to our hypotheses, only treadmill improved forward walking, while backward walking improved with treadmill and stretching. Future research should examine combinations of exercises with a focus on optimizing dosing and examining whether specific characteristics of people with PD correlate with different types of exercise.
Video Abstract available
for more insights from the authors (see Video, Supplemental Digital Content 1)
Introduction
Parkinson disease (PD) is a common neurodegenerative disorder in which people experience deficits in motor function including impaired gait, balance, and mobility.1,2 Decrements in gait velocity occur during forward and backward walking and persist with optimal medication.3 Further, impaired gait, balance, and mobility can be detected early in the disease course, and subsequent decline in gait may indicate the onset of disability.4 These factors highlight the need for complementary approaches, such as exercise, to address these debilitating impairments in PD.1
Group exercise classes using dance, treadmill, and stretching are safe and feasible for individuals with PD, and randomized controlled trials indicate these approaches provide motor benefits.5–9 However, many studies compare exercise programs to non-exercise controls and few studies directly compare the effects of different interventions on motor function in PD to determine whether certain types of exercise better address particular motor impairments. Additionally, limited data examines persistence of benefits after completion of exercise interventions. Unfortunately, variability in outcome measures and exercise programs make it difficult to compare findings across studies.
To address these issues, we aimed to directly compare how tango, treadmill walking, and stretching exercise classes with matching delivery variables affect gait, balance, and motor function in PD immediately following the intervention and after a period without continued intervention. We hypothesized different types of exercise would preferentially improve certain deficits in PD through targeted training, and the effects would persist short term in the absence of continued training. Specifically, for our primary aim, we hypothesized persons in the tango and treadmill groups would improve in forward walking, persons in tango would also improve in backward walking, and no changes in walking velocity in the stretching group would be observed. For our secondary aims, we hypothesized both tango and treadmill would improve motor severity, and dynamic balance and quality of life would improve more among those people in the tango group compared to treadmill and stretching. If different types of exercise best address specific impairments in PD, this could inform the development of personalized or combined programs that may be more beneficial than single mode training.
Materials and Methods
Design Overview
This was a prospective, controlled trial evaluating the effects of three exercise interventions (tango, treadmill, and stretching) on gait and mobility in people with PD. Data were collected between 2013 and 2016. Participants were recruited and serially assigned to the exercise intervention currently enrolling by a research assistant. Serial enrollment, rather than purely random assignment, was required to accommodate limited availability of exercise instructors and the need to complete the trial in a timely fashion. Class sizes ranged from 8–13 participants with three or four waves of classes per intervention. Participants provided written informed consent and the study was approved by the Washington University Human Research Protection Office. All aspects of the study occurred in research facilities on the School of Medicine campus at Washington University in St. Louis.
Participants
Participants with idiopathic PD were recruited from the Washington University School of Medicine’s Movement Disorders Center, the Greater St. Louis Area Chapter of the American Parkinson Disease Association, and the surrounding St. Louis community. Inclusion criteria were: 30 years and older, clear benefit from levodopa, Hoehn & Yahr stage I-IV, ability to walk independently with or without an assistive device for at least ten feet, no history of vestibular disease or dementia, and diagnosis of “clinically definite PD.”10 Briefly, a clinically definite PD diagnosis requires a participant to demonstrate either three of the following features, or two symptoms with one of the first three displaying asymmetry: rest tremor, rigidity, bradykinesia, or postural instability; as well as three or more of the following features: unilateral onset, persistent asymmetry, rest tremor, progression, excellent subjective response to levodopa, severe levodopa-induced chorea, levodopa response of at least five years or more, or clinical course of at least ten years. Exclusion criteria included a medical condition for which exercise is contradicted, evidence of abnormality other than PD-related changes on brain imaging, history or evidence of neurological deficit other than PD such as stroke or muscle disease, or orthopedic or muscular problem. Participants were instructed to continue ongoing exercise but not begin new exercise activities until after the follow-up evaluation.
Interventions
Participants completed community-based group exercise classes twice per week for twelve weeks. Class sessions lasted one hour and included a brief warm-up and cool down at the beginning and end, respectively. Warm-up and cool down activities were the same across all three classes, were minimal, and involved deep breathing exercise, trunk rotation, moving neck side-to-side, walking in a circle, side bending stretch, and bending over to touch knees. The same set of three music (tango) CDs was used during all interventions with a new CD introduced every four weeks. All interventions were overseen by a physical therapist knowledgeable of PD. Instructors for the stretching and treadmill groups were laboratory staff trained to work with people who have PD. The tango dance instructors also had training on how to work with people with PD but did not have formal training in tango adapted for people with PD. Two make up classes were offered for all groups, and participants had to attend at least 19/26 classes for inclusion in analyses.
Tango
Participants practiced Argentine tango using an adapted curriculum for PD. Initial classes focused on basic steps; more complex steps and sequences were added over twelve weeks (see Supplementary Digital Content 2, for review of tango syllabus). Participants were asked to change partners and to change roles from leader to follower several times during the course of each session. Dance partners were spouses, caregivers, volunteers, and laboratory staff. The syllabus was standardized and the same instructors taught all tango classes to maintain consistency.
Treadmill
To approximate the intensity of activity in the tango classes, treadmill participants walked at their preferred over ground walking speed. Preferred speed was assessed weekly and treadmill settings were adjusted individually to match over ground walking speeds. Treadmills were arranged in groups of four (two pairs facing each other) to allow for social interactions.
Stretching
This active control group focused on gentle stretching and whole-body flexibility exercises designed for people with PD.11,12 Exercises targeting strength were not included. All exercises were performed seated or standing with support to limit balance challenge (see Supplementary Digital Content 2, for review of stretching syllabus). Social interactions were encouraged through partnered stretching and while holding stretches.
Evaluations
Gender, age, height, weight, Mini-Mental State Exam (MMSE),13 years since PD diagnosis, Levodopa equivalent daily dose (LEDD),14 Physical Activity Scale for the Elderly (PASE)15, and Activities-Specific Balance Confidence Scale (ABC)16 were collected at baseline.
The Mini-Balance Evaluation Systems Test (Mini-BESTest) to measure dynamic balance,17 the Movement Disorders Society Unified Parkinson Disease Rating Scale motor subsection (MDS-UPDRS-III) scores with Hoehn & Yahr staging18 to measure motor severity, and the Parkinson Disease Questionnaire-39 (PDQ-39)19 to measure quality of life were assessed at baseline, after the twelve week intervention (post-test), and follow-up (twelve weeks after post-test). Spatiotemporal gait characteristics were also collected at all three time points using a five-meter GAITRite walkway (CIR Systems Inc., Franklin, NJ). Participants walked at their “normal, comfortable pace” forward (FWD) and backward (BKD). Three trials of each were averaged for analyses. Ground covered (meters) is also reported for the six-minute walk test (SMWT). For the SMWT, participants were asked to “walk for six minutes, covering as much ground as possible” back and forth along a 30-meter path.
Participants were assessed in the practically defined OFF medication state (≥12 hour withdrawal from anti-Parkinson medications) and evaluations occurred at the same time of day for all time points for each participant. Participants were permitted to rest as often as needed during each evaluation session.
Blinded ratings were used in analyses for the MDS-UPDRS-III and Mini-BESTest. Videos were assigned unpatterned ten character alphanumeric codes to ensure the MDS-certified rater was blinded to group, medication status, and time point.
Statistical Analyses
Baseline characteristics were compared between groups using Chi square, ANOVA, or Kruskal-Wallis, as appropriate. Generalized estimating equations (GEE) with time (baseline, post-test, and follow-up), group (tango, treadmill, stretching), and the interaction of time by group as categorical predictors were conducted for each of our outcomes: FWD gait velocity, BKD gait velocity, SMWT, Mini-BESTest, MDS-UPDRS-III, and PDQ-39. GEE models are able to account for within-subject correlations of repeated measures using an autoregressive correlation structure and include all available data points using the all-available-pairs method.20 Normal distributions using the identity link were specified. Residuals from each GEE model were checked for extreme outliers. One participant in the treadmill group had an extreme outlier at follow-up for the Mini-BESTest. As a conservative method, this value was changed to match baseline and post-test to indicate no change. Wald-test statistics for type 3 GEE analysis are reported along with the parameter estimates and robust empirical standard errors estimates. Simple differences of the group by time interactions least square means were examined to determine the significant changes for each outcome. Significance level of p < 0.05 initially set for primary outcome, however, due to multiple comparisons for six outcomes, a probability level less than a corrected p < 0.008 was considered significant for the GEE estimates. SAS 9.4 was used for statistical analyses. A power analysis using the clinically meaningful difference of 0.1 m/s for forward velocity, 0.2 standard deviation,21 and 80% power indicated a total sample size of 42 was needed. To account for 30% attrition, a sample size of 55 was needed for this three-group repeated measure analysis.
Results
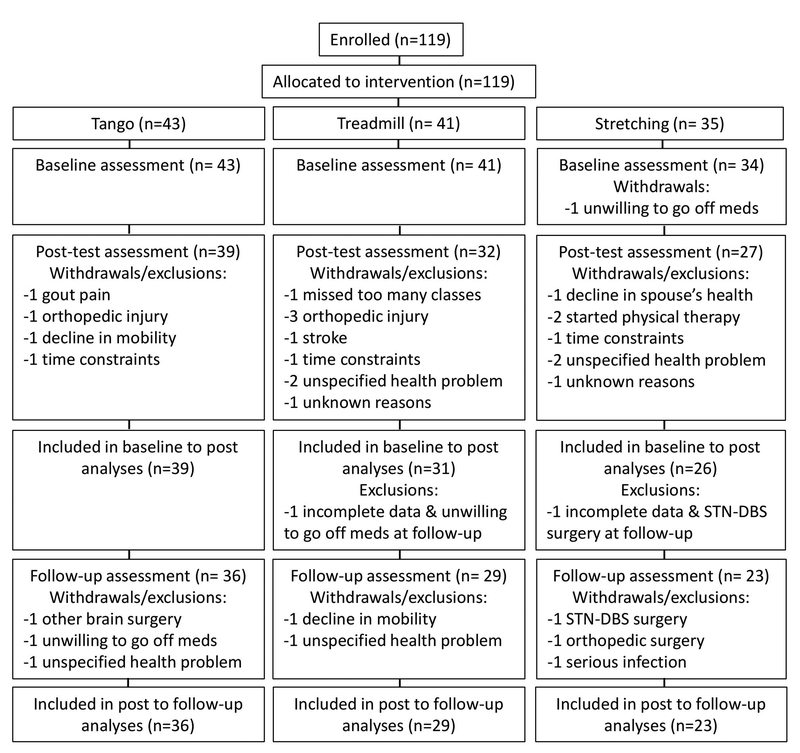
No adverse events were reported. Twenty-two participants who withdrew or had incomplete data were excluded from analyses (Figure 1). Baseline characteristics for the 22 individuals excluded from the analyses (Table 1) were not different than the included participants for gender, age, height, weight, MMSE, years since PD diagnosis, LEDD, PASE, ABC, or baseline MDS-UPDRS-III scores (p > 0.05, Table 1). Spearman correlation between Hoehn and Yahr stage and group assignment was not significant among those who did not finish the study (r = −0.03, p = 0.90). Exercise groups did not differ in any of the aforementioned baseline characteristics (p > 0.05, Table 1) nor did they differ at baseline for any of the outcome measures.
Figure 1.
Participant flow diagram.
Table 1.
Participant demographics at baseline.
| Alla (n = 96) | Tango (n = 39) | Treadmill (n = 31) | Stretching (n = 26) | Drop/Incomplete (n = 22) | |
|---|---|---|---|---|---|
| Mean (SD) | |||||
| ABC | 78.97 (20.67) | 79.47 (20.80) | 76.05 (21.46) | 81.71 (19.85) | 67.89 (25.78) |
| Age | 67.16 (8.94) | 66.73 (9.52) | 68.52 (9.54) | 66.18 (7.30) | 65.71 (10.43) |
| Disease duration (yrs) | 5.47 (4.59) | 6.10 (4.82) | 5.59 (3.81) | 4.40 (5.04) | 6.28 (4.98) |
| Height (cm) | 171.75 (10.93) | 170.96 (12.31) | 172.31 (9.32) | 172.28 (10.85) | 172.37 (13.33) |
| LEDD | 788.91 (548.15) | 886.08 (548.88) | 778.58 (532.51) | 655.48 (556.93) | 888.00 (543.58) |
| MMSE | 28.65 (1.35) | 28.79 (0.92) | 28.90 (1.19) | 28.12 (1.88) | 28.09 (1.63) |
| MDS-UPDRS III | 36.69 (11.19) | 36.92 (12.17) | 35.39 (11.31) | 37.88 (9.68) | 40.82 (12.97) |
| PASE | 133.62 (71.46) | 145.84 (66.32) | 133.05 (80.43) | 115.97 (66.31) | 116.43 (81.24) |
| Weight (lbs) | 176.59 (38.40) | 175.38 (40.84) | 176.19 (31.85) | 178.88 (42.93) | 181.55 (48.19) |
| N (%) | |||||
| Hoehn & Yahr | |||||
| Sex (female) | 40 (41.7) | 14 (35.9) | 14 (45.2) | 12 (46.2) | 7 (31.8) |
ABC, Activities-Specific Balance Confidence Scale; CM, centimeter; LEDD, Levodopa Equivalent Daily Dose; LBS, pounds; MDS-UPDRS-III, Movement Disorders Society Unified Parkinson Disease Rating Scale; M, Mean; MMSE, Mini-Mental Status Examination; N, Number; PASE, Physical Activity Scale for the Elderly; SD, Standard deviation; Yrs, Years
All – does not include participants counted in the dropped out/incomplete column.
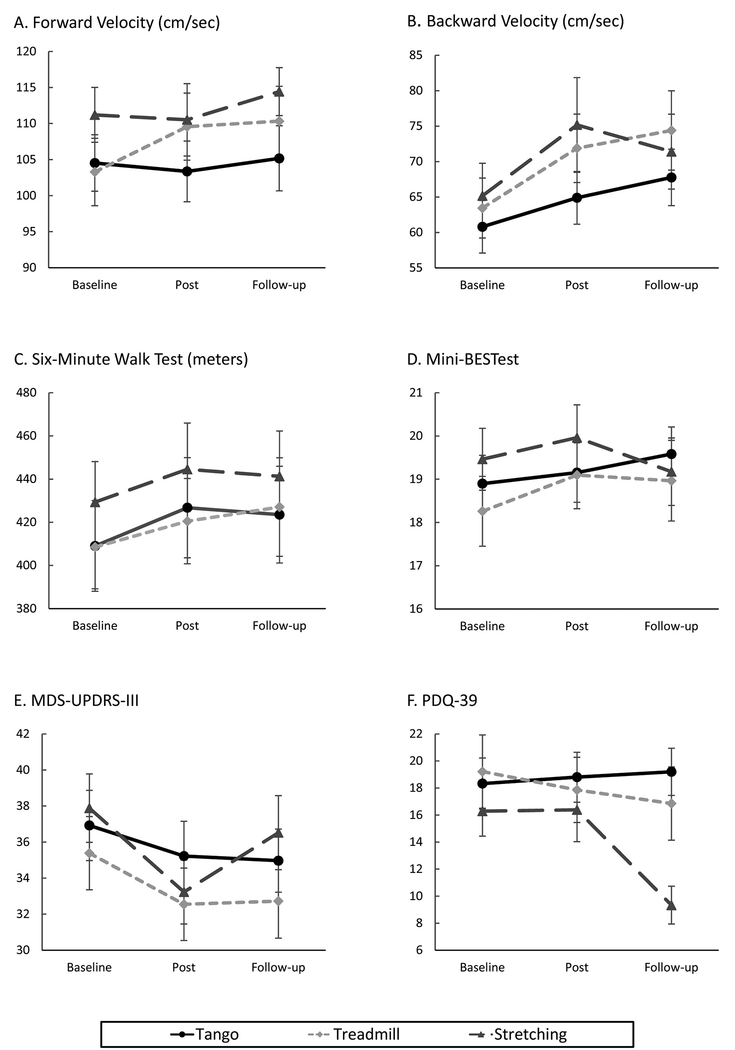
Wald estimates for the overall models are presented in Table 2. Analysis of GEE parameter estimates with empirical standard errors and simple differences for the interaction terms with confidence intervals are included in Supplemental Digital Content 3. Means and standard errors are illustrated in Figure 2 (also see Supplemental Digital Content 3).
Table 2.
Wald Statistics for Generalized Estimating Equation Analysis
| Outcome | Effect/Interaction | Chi-Square | Pr > ChiSq |
|---|---|---|---|
| Forward velocity | Time | 3.20 | 0.2018 |
| Group | 2.47 | 0.2909 | |
| Time × Group | 9.15 | 0.0574 | |
| Backward velocity | Time | 26.27 | < 00.0001* |
| Group | 1.50 | 0.4716 | |
| Time × Group | 4.27 | 0.3703 | |
| SMWT | Time | 9.81 | 0.0074* |
| Group | 0.98 | 0.6112 | |
| Time × Group | 3.26 | 0.5158 | |
| Mini-BESTest | Time | 5.09 | 0.0783 |
| Group | 0.59 | 0.7452 | |
| Time × Group | 5.26 | 0.2616 | |
| MDS-UPDRS-III | Time | 17.45 | 0.0002* |
| Group | 0.90 | 0.6366 | |
| Time × Group | 3.40 | 0.4926 | |
| PDQ-39 | Time | 11.78 | 0.0028* |
| Group | 4.47 | 0.1069 | |
| Time × Group | 22.76 | 0.0001* |
Mini-BESTest, Mini-Balance Evaluation Systems Test; MDS-UPDRS-III, Movement Disorders Society Unified Parkinson Disease Rating Scale; PDQ-39, Parkinson Disease Questionnaire-39; SMWT, Six-minute walk test;
p < 0.008
Figure 2.
Means and standard errors for tango, treadmill, and stretching groups at baseline, post-test (after 12 week intervention), and follow-up (12 weeks after post-test). Estimates of significance from simple differences of group by time least square means generated from the generalized estimating equation models (also see Supplemental Digital Content 3).
A. Forward velocity: Significant improvements for treadmill group between baseline and post-test (p = 0.0007). B. Backward velocity: Significant improvement for treadmill group between baseline and post-test (p < 0.0001) and baseline and follow-up (p = 0.0002). Significant improvement for stretching group between baseline and post-test (p = 0.0047). C. SMWT: A trend towards improvement for tango between baseline and post-test (p = 0.026). Participants in the tango group significantly declined between post-test and follow-up (p = 0.0043). D. Mini-BESTest: No significant effects. E. MDS-UPDRS-III: Significant improvement between baseline and post-test for the stretching group (p = 0.0004). F. PDQ-39: Significant improvement for stretching group between post-test and follow-up (p = 0.0002).
For forward velocity, the Wald estimates for time and group were not significant, nor was the interaction. However, the GEE parameter estimates indicated a trend towards the treadmill group improving between baseline and post (Figure 2A) compared to the stretching group (b = 6.98, SE = 3.3, p = 0.03) and this improvement was maintained through follow-up. For backward velocity, the Wald estimates and GEE parameter estimates indicated a significant time effect. Simple differences indicate the treadmill (p < 0.0001) and stretching (p = 0.005) groups significantly improved between baseline and post, and the increase in backward velocity was maintained at follow-up for the treadmill group (Figure 2B). The Wald estimate for time also was significant for the SMWT; the tango group showed the most improvement among the three groups between baseline and post-test (p = 0.026), but they also declined more than the other two groups between post and follow-up (Figure 2C). None of the effects were significant for the Mini-BESTest (Figure 2D). Both Wald and GEE estimates indicated significant effects of time for the MDS-UPDRS-III; the stretching group significantly improved between baseline and post-test (p = 0.0004), however, the improvement was not maintained from post-test to follow-up (Figure 2E). The PDQ-39 measure showed significant time and time by group effects; simple differences indicated no improvement between baseline and post for any group, but the stretching group improved between post and follow-up (Figure 2F).
Discussion
This study is the first to directly compare tango, treadmill, and stretching interventions with similar delivery variables in people with PD. While our results indicate different exercises may differentially improve outcomes relating to gait, motor functioning, balance, and quality of life, few of our hypotheses were confirmed. It is worth noting that our results may have been different if we measured participants in the ‘on’ state that more closely corresponds to their everyday living. We conducted assessments in the ‘off’ state to reduce variance associated with Parkinson-related medications.
For our outcomes relating to gait, we anticipated improvements for both the tango and treadmill groups for forward velocity, but this was only true for participants in the treadmill group. We also expected improvement among the tango group in backward velocity considering the dance includes backward stepping. However, while the tango group showed some improvement, the stretching and treadmill groups both significantly improved their backward walking velocity between baseline and post-test. Moreover, this improvement persisted for the treadmill group. With regard to walking endurance, all three groups improved on the SMWT with tango improving the most, though these changes were not significant.
We speculate the lack of improvement on the gait assessments for the tango group may be related to more time spent observing and learning dance steps compared to the treadmill group engaging in continuous walking during the classes, or related to the training of the instructors for the tango classes. Previously, our tango instructors were physical therapists, certified personal trainers, or movement scientists with training in dance, whereas for this intervention the dance instructors were not trained in physical therapy or movement science.
Our results do suggest treadmill exercise may be beneficial for forward and backward walking. Our findings are similar to a systematic review that reported treadmill training in PD increased gait speed and stride length, but walking distance did not consistently improve6, and an independent study showed increases on the SMWT for people with PD in a low-intensity treadmill intervention as well as in a stretching and resistance intervention.8 In regards to stretching, a previous intervention designed to increase spinal flexibility and improve mobility reported favorable outcomes in functional axial rotation.22 Similarly, it is possible the stretching group may have improved in backward walking because of enhanced flexibility and freedom of movement.
Next, we predicted the tango and treadmill groups would experience improvements on the MDS-UPDRS-III, a measure of motor sign severity. While all three groups improved, the improvement was only significant for the stretching group. This is in keeping with a previous study that found improvement on the MDS-UPDRS-III among people in a stretching group compared to treadmill training.8 In addition, Schenkman and colleagues found less change on this measure among people with PD in a high-intensity treadmill exercise group compared to a moderate-intensity exercise group or usual care.23
Lastly, we expected the tango group would also experience benefits in balance and quality of life. Changes in balance, as tested with the Mini-BESTest, were unremarkable. We may have seen changes in balance with a more sensitive posturographic assessment. Quality-of-life scores also did not change between baseline and post-test for any of our groups. This may be related to the length of our study, as a sensitivity to change analyses noted four months may be too brief of a period to observe noticeable changes on the PDQ-39.24 A six-month study comparing a progressive resistance exercise intervention to a stretching, balance, and strengthening exercise program among people with PD found improvement in PDQ-39 scores among the former intervention only.25
We also considered if our findings indicated any improvements that were clinically meaningful. The treadmill group changed the most in forward gait velocity (6.3 cm/sec). The stretching group changed the most in backward velocity (10 cm/sec) and motor severity (4.65 points). The tango group changed the most in timed walking distance (4.4%). None of these differences are of a magnitude that would make them clinically meaningful.
Strengths of our exercise interventions include keeping the warm-ups and cool downs, session length, sessions per week, intervention duration, class environment, and class location consistent across classes. However, further investigation of the components and variables necessary and sufficient for a successful exercise program may facilitate design of optimal combined or tailored programs for PD. Components may relate to the physical activity performed (intensity, duration, type), class environment (groups size, instructor interaction, social/emotional support), participant characteristics (baseline activity levels, disease severity), and/or participant education/expectations (knowledge of exercise benefits, motivation, belief in effectiveness). For example, participants in our treadmill training group walked at their preferred over ground walking speed to approximate the intensity of the Tango group whereas other researchers have found beneficial effects of high-intensity treadmill training compared to moderate-intensity.23 Thus, future studies should consider maximizing an individual’s response to exercise and tailoring the program to match each individual’s baseline function and ability to progress.
Likewise, considering the impact of personalized medicine approaches for exercise compared to group programs is important for this population. King et al., reported individualized physical therapy focusing on function and balance combined with group therapy to improve gait may be more efficacious than unsupervised exercise among people with PD.26 Future work also should investigate the differential effects of interventions and retention of benefits for active versus sedentary individuals and investigate what other factors may be predictive of responsiveness to exercise. Additional studies are needed before definitive recommendations can be made.
Conclusions
To our surprise, the improvements noted in all three groups across three months of exercise were modest at best and not of sufficient magnitude to be clinically meaningful. Nonetheless, the improvements suggest group exercise, regardless of modality, may convey some benefit to individuals with PD. This is in keeping with prior studies and suggests that patients have a variety of potentially beneficial options to pursue when considering community-based group exercise approaches. Regardless of the form of exercise, the maintenance of participation over the course of the disease is likely a critical factor. As such, patient preference and motivation, in addition to feasibility and effectiveness of different exercise approaches, should be taken into account when determining which activities will be useful for a given person. Future research should examine what factors predict who will benefit the most and who will remain engaged over the long-term, in various exercise approaches. There is also a need for studies examining optimization of exercise dosing and whether multi-faceted approaches (e.g., employing stretching, treadmill, and tango in combination) are more effective than single mode approaches.
Supplementary Material
Conflicts of Interest and Sources of Funding.
We have no conflicts of interest to disclose. Funding was provided by NIH NINDS R01NS077959, 2T32HD007434, NIH/NCMRR/NICHD/NINDS K12 HD055931, Greater St. Louis Chapter of the American Parkinson Disease Association (APDA), Parkinson Study Group and the Parkinson’s Disease Foundation’s Advancing Parkinson’s Treatments Innovations Grant, Barnes Jewish Hospital Foundation (Elliot Stein Family Fund and Parkinson Disease Research Fund), and APDA Advanced Research Center at Washington University in St. Louis.
Footnotes
ClinicalTrials.gov Identifier: NCT01768832
Protocol: https://www.ncbi.nlm.nih.gov/pubmed/25652002?dopt=Abstract
References
- 1.Shen X, Wong-Yu ISK, Mak MKY. Effects of exercise on falls, balance, and gait ability in Parkinson’s disease: A meta-analysis. Neurorehabil Neural Repair. 2016;30(6):512–527. doi:10.1177/1545968315613447 [DOI] [PubMed] [Google Scholar]
- 2.Fahn S Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci 2003;991(1):1–14. doi:10.1111/j.1749-6632.2003.tb07458.x [DOI] [PubMed] [Google Scholar]
- 3.McNeely ME, Duncan RP, Earhart GM. Medication improves balance and complex gait performance in Parkinson disease. Gait Posture. 2012;36(1):144–148. doi:10.1016/j.gaitpost.2012.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The evolution of disability in Parkinson disease. Mov Disord 2008;23(6):790–796. doi:10.1002/mds.21879 [DOI] [PubMed] [Google Scholar]
- 5.Sharma NK, Robbins K, Wagner K, Colgrove YM. A randomized controlled pilot study of the therapeutic effects of yoga in people with Parkinson’s disease. Int J Yoga. 2015;8(1):74–79. doi:10.4103/0973-6131.146070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrholz J, Kugler J, Storch A, Pohl M, Hirsch K, Elsner B. Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst Rev 2015;(9):CD007830. doi:10.1002/14651858.CD007830.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lötzke D, Ostermann T, Büssing A. Argentine tango in Parkinson disease – a systematic review and meta-analysis. BMC Neurol 2015;15:226. doi:10.1186/s12883-015-0484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shulman LM, Katzel LI, Ivey FM, et al. Randomized clinical trial of 3 types of physical exercise for patients with parkinson disease. JAMA Neurol 2013;70(2):183–190. doi:10.1001/jamaneurol.2013.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132–143. doi:10.1177/1545968311421614 [DOI] [PubMed] [Google Scholar]
- 10.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet 1999;88(5):539–543. doi:10.1002/(SICI)1096-8628(19991015)88:5<539::AID-AJMG19>3.0.CO;2-S [PubMed] [Google Scholar]
- 11.Ellis T, Rork T, Dalton D. Be Active! An Exercise Program for People with Parkinson’s Disease Staten Island, NY: The American Parkinson Disease Association; 2008. https://www.apdaparkinson.org/uploads/files/BeActive_Feb2008-enw.pdf. [Google Scholar]
- 12.Cianci H Parkinson’s Disease: Fitness Counts. Miami, FL: National Parkinson Foundation; 2006. http://www.parkinson.org/sites/default/files/Fitness_Counts.pdf. [Google Scholar]
- 13.Cockrell JR, Folstein MF. Mini-mental state examination (MMSE). Psychopharmacol Bull 1988;24(4):689–692. [PubMed] [Google Scholar]
- 14.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 2010;25(15):2649–2653. doi:10.1002/mds.23429 [DOI] [PubMed] [Google Scholar]
- 15.Washburn RA, Ficker JL. Physical activity scale for the elderly (PASE): The relationship with activity measured by a portable accelerometer. J Sports Med Phys Fit Turin 1999;39(4):336–340. [PubMed] [Google Scholar]
- 16.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol Ser A 1995;50A(1):M28–M34. doi:10.1093/gerona/50A.1.M28 [DOI] [PubMed] [Google Scholar]
- 17.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J Rehabil Med 2010;42(4):323–331. doi:10.2340/16501977-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. doi:10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997;26(5):353–357. doi:10.1093/ageing/26.5.353 [DOI] [PubMed] [Google Scholar]
- 20.Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. 2004;7(2):127–150. doi:10.1177/1094428104263672 [Google Scholar]
- 21.Hackney ME, Earhart GM. Short duration, intensive tango dancing for Parkinson disease: An uncontrolled pilot study. Complement Ther Med. 2009;17(4):203–207. doi:10.1016/j.ctim.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenkman M, Cutson TM, Kuchibhatla M, et al. Exercise to improve spinal flexibility and function for people with Parkinson’s disease: A randomized, controlled trial. J Am Geriatr Soc 1998;46(10):1207–1216. doi:10.1111/j.1532-5415.1998.tb04535.x [DOI] [PubMed] [Google Scholar]
- 23.Schenkman M, Moore CG, Kohrt WM, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol 2018;75(2):219–226. doi:10.1001/jamaneurol.2017.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkinson C, Fitzpatrick R, Peto V. Sensitivity to change of the PDQ-39 In: The Parkinson’s Disease Questionnaire: User Manual for the PDQ-39, PDQ-8, and PDQ Summary Index. UK: University of Oxford; 1998:34–37. [Google Scholar]
- 25.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord 2013;28(9):1230–1240. doi:10.1002/mds.25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King LA, Wilhelm J, Chen Y, et al. Effects of group, individual, and home exercise in persons with Parkinson disease: A randomized clinical trial. J Neurol Phys Ther 2015;39(4):204–212. doi:10.1097/NPT.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.