Opinion statement
Diffuse large B-cell lymphoma (DLBCL) arises from extranodal organs in about 30% of cases. Its prognosis and risk of recurrence in the central nervous system (CNS) vary according to the primary site of origin. Recent studies begin to clarify these differences using molecular classification. Testicular, breast, and uterine DLBCL (as well as possibly primary cutaneous DLBCL, leg-type) share a high prevalence of the non-germinal center B-cell (non-GCB) phenotype and the MYD88/CD79B-mutated (MCD) genotype. These biologic features, which resemble primary CNS lymphoma, may underlie their stage-independent propensity for CNS involvement. Management of these lymphomas should involve CNS prophylaxis, preferably using systemic high-dose methotrexate to prevent intraparenchymal recurrence. Involvement of the kidneys, adrenal glands, ovary, bone marrow, lung, or pleura usually indicates disseminated disease, conferring worse prognosis. Involvement of these sites is often associated with high CNS-International Prognostic Index (IPI), concurrent MYC and BCL2 or BCL6 rearrangements, or intravascular lymphoma—risk factors warranting CNS prophylaxis. In contrast, craniofacial, thyroid, localized bone, or gastric lymphomas have a variable prevalence of the non-GCB phenotype and lack MYD88 mutations. Their outcomes with standard immunochemotherapy are excellent, and the risk of CNS recurrence is low. We recommend individualized consideration of CNS prophylaxis based on the CNS-IPI score and anatomical proximity in cases of epidural, orbital, or skull involvement. Rituximab-containing immunochemotherapy is a standard approach for all extranodal DLBCLs. Surgery is no longer required for any primary site, but routine consolidative radiation therapy is recommended for testicular lymphoma. Radiation therapy also appears to be associated with better progression-free survival in primary bone DLBCL. Future studies should better distinguish primary from secondary sites of extranodal involvement, and investigate the association of newly identified genotypes with the risk of CNS or systemic recurrence.
Keywords: diffuse large B-cell lymphoma, central nervous system prophylaxis, extranodal lymphoma, MYD88, cell of origin, radiation therapy
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous entity comprising intermediate- and high-grade B-cell lymphomas with diverse molecular backgrounds, clinical course, and response to therapy. Up to a third of DLBCLs may arise from extranodal sites, most commonly gastrointestinal tract, skin and soft tissues, bone, or genitourinary tract [1–4]. Additionally, advanced DLBCL can spread to extranodal organs, particularly bone marrow, pleura, peritoneum, liver, and central nervous system (CNS), sometimes obscuring the primary site of origin. Primary extranodal DLBCL is thus more often recognized at early stage (IE/IIE), except when it originates from organs that define stage IV disease. The distinction between primary and secondary involvement makes many studies difficult to interpret, particularly because clinical trials usually report only the number of involved extranodal sites, rather than individual sites of origin [5–8]. The recognition that occurrence of DLBCL in specific organs may be associated with distinct clinical or molecular features, as well as consistent patterns of recurrence, raised interest in examining both extranodal origin and secondary involvement as prognostic factors [3, 2, 4, 9, 10]. Moreover, the risk of CNS recurrence, one of the most devastating complications of DLBCL, appears to be significantly tied to specific extranodal sites [11].
In this review we survey recent evidence regarding prognostic significance, molecular characteristics, and therapeutic implications of extranodal origin in DLBCL. We emphasize the divergent survival outcomes, risk of CNS recurrence, prevalence of subtypes defined by cell of origin or genomic alterations, as well as indications for radiation therapy (RT) for lymphomas arising from specific extranodal sites.
Assessment of prognosis in DLBCL
Prognosis in DLBCL has been historically assessed using clinical features comprising the International Prognostic Index (IPI), but in the era of rituximab-based immunochemotherapy the IPI explains less than 25% of variation in overall survival (OS) and poorly separates the highest-risk subgroups [9, 12]. The enhanced IPI (which improved on the specification of age and lactate dehydrogenase [LDH] as non-binary variables) has identified involvement of CNS, liver/gastrointestinal (GI) tract, lung, or marrow as additional unfavorable prognostic factors [9]. The Spanish Lymphoma Study Group evaluated extranodal origin in addition to the IPI, and found no additive prognostic value, but specific anatomic sites were not distinguished [13]. In contrast, a Danish-Canadian series of 443 patients staged with positron emission tomography (PET) found that 67% had extranodal involvement, that PET uptake in bone/bone marrow, pleura, or female reproductive organs was independently associated with worse progression-free survival (PFS) and OS, and that >2 or >3 involved sites conferred progressively worse prognosis [3]. Another series of 1,221 patients from Japan showed inferior OS with involvement of pleura, peritoneum, small intestine, adrenal glands, testis, bone marrow, or blood [14], while a smaller study from Taiwan listed lung/pleura, liver, lower urinary tract, and bone marrow as high-risk sites [15]. In contrast, a US registry-based analysis of 25,992 cases classified according to the primary site of origin found independently worse OS in DLBCL arising from the GI tract, respiratory system, and liver/pancreas, whereas extranodal lymphomas of head and neck, skin/soft tissue, and bone fared better than nodal disease [2]. Adjusting for stage, there was no significant difference in OS between DLBCL arising from the nodes, genitourinary tract, thyroid gland, or breast.
Considering limited prognostic power of clinical characteristics, recent attention has turned to molecular factors. Gene expression profiling (GEP) can distinguish the prognostically favorable germinal center B-cell (GCB), unfavorable activated B-cell (ABC), and intermediate unclassifiable signatures in DLBCL [16, 17]. Because GEP is not routinely available in clinical practice, the World Health Organization (WHO) allows distinction between GCB and ABC using immunohistochemistry for CD10, BCL2, and BCL6 (Hans algorithm), or similar schemas, despite their limited accuracy [1]. This cell-of-origin designation does not generally correlate with extranodal involvement [17], but the ABC signature is enriched in lymphomas originating from specific extranodal sites (Table 1). Furthermore, next-generation sequencing (NGS) has recently identified subtypes characterized by co-occurring point mutations or rearrangements in MYD/CD79B (MCD subtype), BCL6/NOTCH2 (BN2 subtype), NOTCH1 (N1 subtype), or EZH2/BCL2 (EZB subtype) [18]. The MCD and N1 subtypes showed significantly worse PFS and OS compared with other ABC lymphomas, while the BN2 subtype (mostly of ABC origin) was prognostically favorable. In addition, multiple studies have reported poor prognosis in high-grade B-cell lymphoma with concurrent rearrangements of MYC and BCL2 and/or BCL6 genes (“double-hit” or “triple-hit” lymphoma), characterized by only 25–30% long-term survival after standard chemotherapy using rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) [19–21]. Double-hit lymphomas (predominantly GCB) are separated from DLBCL in the 2016 WHO classification, but remain intermixed in published clinicopathologic observational series [1]. They involve extranodal sites in >60% of cases, predominantly through secondary spread to blood, bone marrow, CNS, or stomach/intestine, whereas their prevalence among primary extranodal lymphomas has not been comprehensively described [22–24]. Additionally, DLBCL expressing both MYC and BCL2 by immunohistochemistry (termed “dual-expresser”) has been associated with worse prognosis [25–29]. More recently, TP53 mutations are increasingly recognized as a characteristic associated with chemotherapy resistance and poor survival, independent of the IPI or cell of origin, and other genomic biomarkers are being investigated [30–33].
Table 1.
Molecular and prognostic features of specific extranodal diffuse large B-cell lymphomas, based on recent observational studies of patients treated with immunochemotherapy.
| Extranodal site | Non-GCB | EFS/PFS | OS | CNS recurrence | Other characteristics |
|---|---|---|---|---|---|
| Craniofacial[68–70] | 82% | 60–79% | 65–87% | 2% |
|
| Thyroid[74, 73] | 22–33% | 79% | 85% | NR |
|
| Mediastinum (PMBLC)[79] | 100%a | 86% | 95% | 3% |
|
| Gl tract[86, 85, 81, 82, 87] | 33–50% | 77–89% | 83–90% | NR |
|
| Kidney[92, 93] | NR | 40–44% | 43–52% | 36–41% |
|
| Testis[101, 94] | 84% | 51–56% | 60–68% | 19–25% |
|
| Breast[110, 107, 111, 108, 109] | 60–90% | 61–66% | 75–82% | 16–24% |
|
| Uterus[112, 110] | 63% | 26% | 33% | 44% |
|
| Skin, leg-type[118, 119] | 100% | NR | 52% | 13% |
|
| Bone[126, 45, 128, 124, 125] | 10–35% | 47–80% | 80–93% | 7% |
|
PMBCL has a specific gene expression profile
DA-EPOCH-R: dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab; EFS: event-free survival; NR: not reported; OS: overall survival; PFS: progression-free survival; PMBCL: primary mediastinal large B-cell lymphoma; RT: radiation therapy; y: year.
CNS recurrence: risk assessment and prophylaxis
The overall risk of CNS recurrence in DLBCL is 2–5%, and has decreased after introduction of rituximab-based immunochemotherapy [34–36, 11]. Historically, CNS recurrence has been associated with highly proliferative tumors, stage IV or high-IPI disease, involvement of ≥2, or certain specific extranodal sites like the bone marrow, testicle, and paranasal sinuses, though without a clear consensus about who should receive CNS-directed prophylaxis [37–39]. A pooled analysis of 2,164 patients who immunochemotherapy on trials conducted by the German High-Grade Non-Hodgkin Lymphoma Study Group found that age >60, elevated serum lactate dehydrogenase (LDH), poor performance status, advanced-stage disease, and involvement of kidneys or adrenals were independently associated with CNS recurrence [40]. Including involvement of ≥2 extranodal sites as an additional variable, the authors proposed the “CNS-IPI”, with the 2-year risk of CNS recurrence varying from 0.8% (≤1 risk factor), to 2.9% (2–3 risk factors), and to 10.0% (≥4 risk factors). Of note, patients variably received intrathecal prophylaxis—most commonly those with marrow, testicular, epidural, or paranasal sinus involvement. Lack of molecular data and low number of specific extranodal cases limited more granular assessments. The CNS-IPI validation cohort from the British Columbia Cancer Agency (BCCA) additionally identified marrow, testes, orbit, or pericardium as high-risk sites. Importantly, 73% of CNS recurrences were intra-parenchymal, 26% leptomeningeal only, and 1% intraocular [41]. When applied to 984 participants from the randomized United Kingdom National Cancer Research Institute (UK NCRI) trial, the high-risk CNS-IPI group had 2-year CNS recurrence risk of 5.2%, with 81% of intra-parenchymal events [35]. Despite frequent CNS prophylaxis, relapses occurred in lymphomas involving paranasal sinuses (17%), breast (12%), kidneys/adrenals (6%), and bone (5%), but not testis (0%). In the German RICOVER-60 trial, the incidence of CNS recurrence was 4.8%, with 65% of the events being intraparenchymal, 26% leptomeningeal, and 9% involving both compartments [34]. Another large international (UK, Australia, BCCA, Denmark) series of 1,532 patients staged with positron emission tomography (PET) confirmed that multiple extranodal sites and involvement of uterus or testis were associated with CNS recurrence [36]. Therefore, some experts offer CNS prophylaxis to all DLBCLs involving high-risk sites like testes, uterus, breast, or epidural bone lesions [42]. British guidelines recommend prophylaxis for those with elevated LDH and >1 extranodal site, or with testicular, breast, or epidural involvement [43], while the Spanish Lymphoma Group recommends it additionally for high CNS-IPI, double-hit lymphoma, and involvement of kidney or adrenal gland [44]. The US National Comprehensive Cancer Network (NCCN) guidelines acknowledge the uncertain efficacy of intrathecal or systemic prophylaxis, suggesting it in high CNS-IPI, HIV-associated, testicular, breast, or double-hit lymphoma [45].
Considering that high CNS-IPI identifies only 55% of patients who will experience CNS recurrence [40], there is an ongoing need to identify better biomarkers of risk. Two recognized high-risk populations are double-hit (13% risk) [20] and dual-expresser lymphomas (10% risk) [26]. Intrathecal prophylaxis is included in high-intensity protocols applied in double-hit lymphoma, like the dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R), but its value in preventing intraparenchymal recurrences is uncertain. In a multi-institutional retrospective series of 223 patients treated with DA-EPOCH-R, only 39% received CNS prophylaxis [46]. CNS relapses occurred in 5.8%, without any significant difference according to the administration of intrathecal therapy. Researchers from MD Anderson Cancer Center reported a higher rate of CNS recurrence after DA-EPOCH-R in CD5+ disease (33% versus 16%), which in 50% was associated with extranodal involvement at diagnosis, most commonly in the bone marrow (35%) [47]. CD5 positivity may thus be another marker of disseminated disease with high risk of CNS recurrence, reported as 8–11% in three other series [48–50]. Nevertheless, an unmet need persists to identify molecular mechanisms that drive dissemination and homing of DLBCL cells to the CNS, and that could better define the population requiring prophylaxis. Some insight has emerged from the study of primary CNS lymphoma, which is characterized by predominance of the ABC subtype [51], presence of MYD88/CD79B mutations, as well as PD-1/PD-2 pathway alterations [52, 51, 53, 54]—features shared with testicular and certain other extranodal lymphomas. Preliminary data suggest that expression of molecules involved in B-cell adhesion and migration, including CXCR5/CXCL13 axis, CD44, and ITGA10, may also help predict the risk of CNS recurrence [55–57].
The optimal CNS prophylaxis approach remains to be prospectively delineated, and clinical practice largely relies on observational studies. Common strategies include intrathecal methotrexate (with or without cytarabine and/or hydrocortisone), or intravenous high-dose methotrexate [43]. Because of predominance of intra-parenchymal CNS recurrences, and lack of benefit to intrathecal treatment in several studies [34, 58–60], systemic approaches are increasingly favored [44, 61]. Published data suggest potential efficacy of methotrexate at 3–3.5 g/m2 administered either concurrently with R-CHOP (on day 15 of cycles 2, 4, and 6) [62], or after its completion [63]. Some studies used sequential systemic methotrexate and cytarabine-based consolidation [64, 65]. However, further prospective evaluation is needed to determine the true risk/benefit ratio of these approaches.
Prognosis of extranodal DLBCL arising from specific anatomical sites
Craniofacial sites
Historically, DLBCL located in the orbit or paranasal sinuses has been associated with poor prognosis and a high risk of CNS relapse, leading to a recommendation for intrathecal prophylaxis [66, 67]. However, a recent analysis of 290 patients enrolled in German High-Grade Non-Hodgkin Lymphoma Study Group trials (including all craniofacial sites—orbit, paranasal sinuses, and oral cavity, 70% stage IE/IIE) showed a significantly decreased risk of CNS recurrence after rituximab-based chemoimmunotherapy (1.6% versus 4.2%), which was not influenced by prophylaxis (4.2% with versus 2.3% without, P=.98) [68]. Still, 64% of patients with CNS recurrence had paranasal sinus, and 14% orbital involvement, possibly due to anatomic proximity that facilitated direct invasion. Administration of consolidative RT was not associated with better event-free or OS, and overall prognosis was excellent. Consistently, a Korean study showed only one CNS recurrence among 80 cases of in sinonasal DLBCL (with 30% rate of intrathecal prophylaxis), and no difference in survival with RT, confirming that in the rituximab era neither RT nor routine intrathecal therapy are necessary (Table 2) [69]. Little is known about specific molecular features of DLBCL of the head and neck, except that 80% are non-GCB, with frequent gain of 1q31 (containing RGS1, 55%) and loss of 9p21.3 (containing CDKN2A, 64%) [70]. Extranodal head/neck location was associated with a better prognosis than nodal DLBCL in population-derived data, but not in clinicopathologic series [2, 14, 4, 68]. Importantly, Waldeyer’s ring is considered a primary nodal site and does not require special therapeutic considerations [71].
Table 2.
Outcomes with or without consolidative radiation therapy in select extranodal diffuse large B-cell lymphomas.
| Extranodal site/reference | N | Rituximab (%) | RT (%) |
EFS/PFS | OS | ||
|---|---|---|---|---|---|---|---|
| Univariate | Adjusted HR(95%CI) | Univariate | Adjusted HR(95%CI) | ||||
| Lee, 2015[69] | 80 | 100% | 26% | 5y PFS 56% w/o RT 65% with RT, P=.34 | 5y 65% w/o RT, 65% with RT, P=.98 | ||
| Sohn, 2012[82] | 93 | 59% | 15% | NR, NS | NR, NS | ||
| Ho, 2017[103] | 120 | 53% | 70% | 5y PFS 30% w/o RT 65% with RT, P=.001 | 0.39 (0.24–0.64) |
5y 52% w/o RT 73% with RT, P=.065 | 0.47 (0.28–0.83) |
| Hosein, 2014[107] | 76 | 69% | 65% | NR, NS | NR, NS | ||
| Bone | |||||||
| Pilorge, 2016[128] | 76 | 100% | 20% | NR, NS | NR, NS | ||
EFS: event-free survival; HR: hazard ratio; NR: not reported; NS: not significant; OS: overall survival; PFS: progression-free survival; RT: radiation therapy; w/o: without; y: year
Thyroid gland
DLBCL constitutes 50% of primary thyroid lymphomas, and like the second common marginal zone (MZL) subtype, it is strongly associated with autoimmune (Hashimoto) thyroiditis [72, 73]. Ninety percent of thyroid DLBCLs present at stage IE/IIE. Consequently, they have favorable prognosis similar to stage-matched nodal DLBCL [2, 14, 10]. Specific molecular data are sparse, but most thyroid lymphomas are of the GCB subtype, with rare MYC, BCL2, or BCL6 rearrangements, and no MYD88 mutations [74]. Thyroid DLBCL can be treated with immunochemotherapy alone, or with the combination of chemotherapy and RT. Surgery is no longer used as a standard modality for thyroid lymphoma, and CNS recurrences are unusual. Historical series have suggested better outcomes after consolidative RT [75], but data from the R-CHOP era are lacking. Patients with thyroid DLBCL have received immunochemotherapy alone on contemporary clinical trials [8].
Lung and mediastinum
Primary pulmonary DLBCL is exceptionally rare, and may occur as transformation from extranodal MZL of the mucosa-associated lymphoid tissue (MALT), the predominant (up to 90%) lymphoma in this organ [76]. Pleural involvement is also typically encountered in disseminated disease. Primary mediastinal (thymic) large B-cell lymphoma (PMBCL) constitutes a separate WHO entity with unique clinical, epidemiologic and molecular features [1]. It is characterized by PD-1 overexpression, constitutional JAK-STAT activation, and subtype-specific GEP [77]. Historically treated with RCHOP and consolidative RT, PMBCL has now shown excellent outcomes after DA-EPOCH-R chemotherapy alone, obviating the need for RT in the predominantly young, female population affected by this disease [78–80]. The low risk of CNS recurrence does not warrant prophylaxis.
GI tract
One third of extranodal DLBCL cases occur in the GI tract, making it the most common primary extranodal site [2, 15]. The enhanced IPI considers it high risk, but does not differentiate primary from secondary involvement, or liver from stomach/intestine [9]. Careful histopathologic evaluation is needed to rule out Burkitt or mantle cell lymphoma, diseases often involving the intestine. The GI tract is also a common site of dissemination for double-hit lymphomas [22, 23]. Meanwhile, survival of localized gastric DLBCL appears to be excellent in recent series, independently of cell of origin or MYC/BCL2 dual-expresser status, with no reported CNS recurrences and no difference in outcomes with the use of surgery or RT [81, 82]. Some provocative studies have reported regression of gastric DLBCL after eradication of Helicobacter pylori (found in about 50% of cases), akin to gastric MALT lymphoma [83–85]. In one analysis, 33% of GI lymphomas were non-GCB, whereas MYD88 or CD79B mutations were identified in only <5% [86]. In an examination of 49 cases from Japan, the non-GCB phenotype was seen in 50%, but MYD88 mutation only in 6% [87]. Another series examining genetic profiles of GI DLBCL found only 3% rate of EZH2Y641 mutation and 17% rate of BCL2 rearrangements in the GCB subgroup (none in the ABC subgroup), suggesting a low prevalence of the EZB genotype [88, 18]. In contrast, liver involvement occurs usually in disseminated cases, thus contributing to poor prognosis [9]. Primary hepatic DLBCL may be associated with hepatitis C infection (25–60%), and may have a better prognosis than in cases of secondary involvement, although data are too sparse to make confident conclusions [89–91].
Kidney and adrenal glands
Kidney involvement is associated with stage IV disease in over 90% and a very poor survival in two recent series [92, 93]. The CNS-IPI designates kidney or adrenal involvement as singularly high-risk [40]. Even when studied as an additional factor in otherwise high-risk subtypes, secondary kidney/adrenal involvement conveys a higher rate of CNS recurrence. For example, in a cohort of testicular lymphomas, the HR for CNS relapse was 17.9 (95% confidence interval [CI], 3.8–84.1) when kidney/adrenal spread was present [94]. Primary adrenal lymphomas are mostly described by case reports owing to their exceptional rarity [95]. They exhibit an aggressive course, with salient features of adrenal insufficiency and frequent CNS involvement [96]. Many cases of adrenal lymphomatous tumors reveal presence of intravascular lymphoma, which has a predilection for dissemination into CNS, skin, or endocrine organs, and often has MYD88 and CD79B mutations [97, 98, 1].
Testis
Primary testicular DLBCL develops in an immune-privileged site behind the blood-testis barrier and has a molecular profile very similar to primary CNS lymphoma, including MYD88L265P (70–80%), CD79B and CDKN2A (88%) mutations, as well as alterations in the PD-1/PD-2 loci (50%) [53, 99, 100]. Most cases exhibit the ABC phenotype, and likely high prevalence of the newly recognized MCD genomic subtype [18, 101]. Because of the inherent risk of CNS or contralateral testicular (20%) relapse, the current standard of care for testicular DLBCL includes routine CNS prophylaxis and contralateral testicular RT, although only about half of patients receive RT in clinical practice [102, 101, 103, 104]. Rituximab has improved survival, yet it has not reduced the rates of CNS recurrence [94, 10]. A recent international retrospective of 280 primary testicular lymphomas found that 77% were stage IE or IIE [101]. Only 34% of patients received CNS prophylaxis, which did not decrease the incidence of CNS recurrence (21% at 10 years). A BCCA cohort reported 25% rate of CNS recurrence at median time of 2.3 years, with half of all relapses involving the CNS [94]. This risk appears independent of clinical stage. Interestingly, patients with early stage were more likely to experience an intra-parenchymal recurrence (90%), whereas those with advanced stage had more leptomeningeal recurrences (83%, of which 42% had concurrent parenchymal involvement) [94].
Breast
Primary breast lymphoma accounts for only 2% of extranodal lymphomas, and presents with a MALT component in 5% of cases [105]. Both clinical retrospectives and population-based studies report >90% prevalence of stage IE/IIE disease, and 5-year OS of 75–80% [106–108]. Clinicopathologic series consistently show predominance of the non-GCB phenotype (60–90%) [109, 107], as well as high prevalence of MYD88 (58–70%) and CD79B (36–40%) mutations [110, 111]. Interestingly, these features resemble testicular lymphoma, and breast lymphomas have recently been also recognized to have a notable predilection for CNS recurrence [107, 108]. These data are highlighted in the current NCCN guidelines and may support CNS prophylaxis [45]. Whether the risk of CNS recurrence correlates with the non-GCB phenotype or the MYD88/CD79B-mutated genotype remains to be investigated.
Uterus
A recent large international study found that 4% (27 of 678) of DLBCLs in women involve the uterus or ovary [112]. All cases of ovarian involvement occurred in the context of disseminated lymphoma, whereas 18% of those with uterine involvement were early-stage. Accounting for the IPI, ovarian involvement was not prognostic for PFS or OS outcomes, whereas uterine DLBCL showed worse PFS (HR, 2.28; 95%CI, 1.23–4.22) and OS (HR, 2.34; 95%CI, 1.21–4.52) than other lymphomas. Moreover, a 44% cumulative incidence of CNS relapse at 4 years was reported in uterine DLBCL (and no recurrences in ovarian lymphoma), which would classify it as a high-risk site. Similarly, in a series of 6 Chinese patients with DLBCL of the female genital tract, 4 (including 3 uterine/cervical) relapsed in the CNS [113]. Five of the 7 studied uterine lymphomas had the non-GCB phenotype and 4 had the MYD88L265P mutation [110]. Although confirmatory research is needed, DLBCL of the uterus may be genetically and clinically similar to breast and testicular lymphoma, including the risk of CNS recurrence.
Skin
Unlike the indolent cutaneous MZL and follicle center lymphomas, primary cutaneous DLBCL, leg-type (PCDLBCL-LT) is aggressive, although rituximab has significantly improved survival outcomes [114, 115]. It classically, though not exclusively, presents as rapidly growing tumors on the legs of women over age 60 [1]. PCDLBCL-LT has a strong IRF4/MUM1 expression, ABC phenotype by GEP, and high prevalence of the MYD88L265Pmutation [116–119]. In a French series of 13 patients who had a PCDLBCL-LT that relapsed in the CNS, 5 out of 6 tested cases had the MYD88 mutation [120]. However, the overall reported incidence of CNS recurrence in PCDLBCL-LT varies between 4 and 13%, limiting the ability to recommend prophylaxis without more data [121, 120, 119]. The unique histopathologic and molecular features can help distinguish PCDLBCL-LT from DLBCL with secondary skin involvement, which is disseminated and may also have an unfavorable clinical course.
Bone
DLBCL involving bones as the only site of disease has a different prognosis from cases with a secondary bone or bone marrow dissemination. In a series of 161 patients with stage I/II primary bone DLBCL from the International Extranodal Lymphoma Study Group (IELSG), predating the use of PET or rituximab, 87% had stage I disease, and outcomes were similar with or without consolidative RT after chemotherapy (5-year PFS 74% versus 67%, P=.47) [122]. The risk of CNS relapse was low (2.5%), despite only 6 patients receiving prophylaxis. A separate analysis of multifocal (polyostotic) bone lymphoma showed better PFS (56% versus 34%) and OS (74% versus 36%) compared with stage IV DLBCL with secondary skeletal dissemination, and suggested better survival with the use of RT [123]. Researchers from MD Anderson described 102 patients with primary bone DLBCL (56% stage I/II), who had 5-year PFS of 80% after RCHOP chemotherapy, also improved by consolidative RT, at least in stage I/II disease [124]. Similarly, an analysis of prospective trials from Germany suggested better PFS with RT [125]. The overall favorable prognosis of bone lymphoma can be partly ascribed to a GCB, centrocyte-like GEP signature [126]. Of note, MYD88 mutations are absent in primary bone lymphoma [127]. French investigators reported 80% OS at 4 years in bone lymphoma, classifying 65% of cases as GCB [128]. In contrast, a series from the Moffitt Cancer Center reported 5-year PFS of 75% for unifocal, but only 14% for multifocal bone DLBCL (P=.002), with OS of 92% and 47% (P<.001), respectively [45]. Four out of 53 patients (3 with multifocal lesions, 2 with spinal involvement) experienced a CNS recurrence, highlighting the need for further research distinguishing unifocal, multifocal, and secondary bone DLBCL, as well as the differential CNS risk associated with craniospinal or long-bone lesions.
Summary
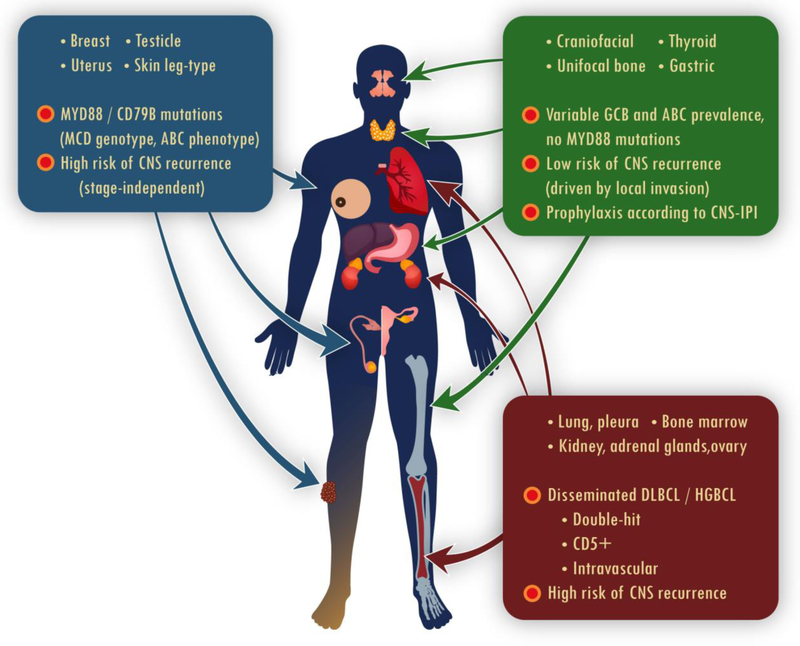
Primary extranodal large B-cell lymphomas are common and very heterogeneous in their features and outcomes. Recent molecular data have begun to clarify their divergent clinical behavior (Figure 1). The peculiar, stage-independent risk of CNS spread in testicular, breast, uterine, and possibly PCDBLCL-LT, may be related to prevalent MCD (MYD88/CD79B-mutated) genomic subtype in these lymphomas. It remains to be seen how this genotype might facilitate invasion of the CNS parenchyma, and whether therapies targeting the B-cell receptor or NF-κB signaling pathways could lower the risk. Some sites of extranodal involvement, like kidney/adrenals, lung/pleura, or ovary, almost always indicate disseminated disease with a high propensity to invade the bone marrow and leptomeningeal compartments, particularly in double-hit lymphoma. Conversely, unifocal bone, craniofacial, thyroid, or gastric DLBCL show a relatively favorable prognosis with standard immunochemotherapy. Their risk of CNS recurrence might be largely driven by potential local invasion due to anatomic proximity when epidural, orbital, or skull involvement is present, thus requiring a case-by-case approach to prophylaxis. Future studies can help clarify the relationship between extranodal DLBCLs and their indolent MALT counterparts, and whether the favorable behavior of some ABC-like lymphomas (e.g. in the stomach or craniofacial sites) might be hypothetically explained by less aggressive genotypes (e.g. the BCL6/NOTCH2 subtype). Finally, prospective clinical studies are needed to establish consistent, biologically-driven strategy for CNS prophylaxis in DLBCL that could effectively prevent parenchymal recurrences.
Figure 1.
Mechanisms associated with variable prognosis and risk of CNS recurrence in extranodal DLBCL of different anatomical origin.
ABC: activated B-cell subtype; CNS: central nervous system; DLBCL: diffuse large B-cell lymphoma; GCB: germinal center B-cell subtype; HGBCL: high-grade B-cell lymphoma
Acknowledgements
Supported by 128608-RSGI-15–211-01-CPHPS from the American Cancer Society, and U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health. The authors thank Peter Green for assistance with medical illustration.
Footnotes
Conflict of Interest
Thomas A. Ollila and Adam J. Olszewski declare they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Swerdlow SH, World Health Organization, International Agency for Research on Cancer WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition ed. World Health Organization classification of tumours. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 2.Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: an analysis of the Surveillance, Epidemiology and End Results database. Am J Hematol 2014;89(3):310–4. doi:10.1002/ajh.23638. [DOI] [PubMed] [Google Scholar]
- 3.El-Galaly TC, Villa D, Alzahrani M, Hansen JW, Sehn LH, Wilson D et al. Outcome prediction by extranodal involvement, IPI, R-IPI, and NCCN-IPI in the PET/CT and rituximab era: A Danish-Canadian study of 443 patients with diffuse-large B-cell lymphoma. Am J Hematol. 2015;90(11):1041–6. doi:10.1002/ajh.24169. [DOI] [PubMed] [Google Scholar]
- 4.Hui D, Proctor B, Donaldson J, Shenkier T, Hoskins P, Klasa R et al. Prognostic implications of extranodal involvement in patients with diffuse large B-cell lymphoma treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Lymphoma. 2010;51(9):1658–67. doi:10.3109/10428194.2010.504872. [DOI] [PubMed] [Google Scholar]
- 5.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117–26. doi:10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 6.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105–16. doi:10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817–26. doi:10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- 8.Lamy T, Damaj G, Soubeyran P, Gyan E, Cartron G, Bouabdallah K et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood. 2018;131(2):174–81. doi:10.1182/blood-2017-07-793984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–42. doi:10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olszewski AJ, Winer ES, Castillo JJ. Improved survival with rituximab-based chemoimmunotherapy in older patients with extranodal diffuse large B-cell lymphoma. Leuk Res. 2014;38(8):866–73. doi:10.1016/j.leukres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Ferreri AJ. Risk of CNS dissemination in extranodal lymphomas. Lancet Oncol. 2014;15(4):e159–69. doi:10.1016/S1470-2045(13)70568-0. [DOI] [PubMed] [Google Scholar]
- 12.Olszewski AJ, Winer ES, Castillo JJ. Validation of clinical prognostic indices for diffuse large B-cell lymphoma in the National Cancer Data Base. Cancer Causes Control. 2015;26(8):1163–72. doi:10.1007/s10552-015-0610-8. [DOI] [PubMed] [Google Scholar]
- 13.Montalban C, Diaz-Lopez A, Dlouhy I, Rovira J, Lopez-Guillermo A, Alonso S et al. Validation of the NCCN-IPI for diffuse large B-cell lymphoma (DLBCL): the addition of beta2 -microglobulin yields a more accurate GELTAMO-IPI. Br J Haematol. 2017;176(6):918–28. doi:10.1111/bjh.14489. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Tomita N, Yokoyama M, Tsunoda S, Yano T, Murayama K et al. Prognostic impact of extranodal involvement in diffuse large B-cell lymphoma in the rituximab era. Cancer. 2012;118(17):4166–72. doi:10.1002/cncr.27381. [DOI] [PubMed] [Google Scholar]
- 15.Lu CS, Chen JH, Huang TC, Wu YY, Chang PY, Dai MS et al. Diffuse large B-cell lymphoma: sites of extranodal involvement are a stronger prognostic indicator than number of extranodal sites in the rituximab era. Leuk Lymphoma. 2015;56(7):2047–55. doi:10.3109/10428194.2014.982636. [DOI] [PubMed] [Google Scholar]
- 16.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11. doi:10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 17.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–47. doi:10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018;378(15):1396–407. doi:10.1056/NEJMoa1801445.* Large translational study identifying DLBCL genotype categories associated with disparate survival outcomes. It identfied the MYD88/CD79B-mutated genotype particularly prevalent in certain extranodal lymphomas.
- 19.Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–9. doi:10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oki Y, Noorani M, Lin P, Davis RE, Neelapu SS, Ma L et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166(6):891–901. doi:10.1111/bjh.12982. [DOI] [PubMed] [Google Scholar]
- 21.Petrich AM, Gandhi M, Jovanovic B, Castillo JJ, Rajguru S, Yang DT et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124(15):2354–61. doi:10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 22.Niitsu N, Okamoto M, Miura I, Hirano M. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia. 2009;23(4):777–83. doi:10.1038/leu.2008.344. [DOI] [PubMed] [Google Scholar]
- 23.Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114(17):3533–7. doi:10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 24.Tomita N, Tokunaka M, Nakamura N, Takeuchi K, Koike J, Motomura S et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica. 2009;94(7):935–43. doi:10.3324/haematol.2008.005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021–31; quiz 250. doi:10.1182/blood-2012-10-460063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savage KJ, Slack GW, Mottok A, Sehn LH, Villa D, Kansara R et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127(18):2182–8. doi:10.1182/blood-2015-10-676700. [DOI] [PubMed] [Google Scholar]
- 27.Horn H, Ziepert M, Becher C, Barth TF, Bernd HW, Feller AC et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121(12):2253–63. doi:10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 28.Staiger AM, Ziepert M, Horn H, Scott DW, Barth TFE, Bernd HW et al. Clinical Impact of the Cell-of-Origin Classification and the MYC/ BCL2 Dual Expresser Status in Diffuse Large B-Cell Lymphoma Treated Within Prospective Clinical Trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2017;35(22):2515–26. doi:10.1200/JCO.2016.70.3660. [DOI] [PubMed] [Google Scholar]
- 29.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460–7. doi:10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 30.Xu-Monette ZY, Wu L, Visco C, Tai YC, Tzankov A, Liu WM et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120(19):3986–96. doi:10.1182/blood-2012-05-433334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang XJ, Medeiros LJ, Bueso-Ramos CE, Tang G, Wang S, Oki Y et al. P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma. Mod Pathol. 2017;30(2):194–203. doi:10.1038/modpathol.2016.178. [DOI] [PubMed] [Google Scholar]
- 32.Karube K, Enjuanes A, Dlouhy I, Jares P, Martin-Garcia D, Nadeu F et al. Integrating genomic alterations in diffuse large B-cell lymphoma identifies new relevant pathways and potential therapeutic targets. Leukemia. 2018;32(3):675–84. doi:10.1038/leu.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy A, Zhang J, Davis NS, Moffitt AB, Love CL, Waldrop A et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell. 2017;171(2):481–94 e15. doi:10.1016/j.cell.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113(17):3896–902. doi:10.1182/blood-2008-10-182253. [DOI] [PubMed] [Google Scholar]
- 35.Gleeson M, Counsell N, Cunningham D, Chadwick N, Lawrie A, Hawkes EA et al. Central nervous system relapse of diffuse large B-cell lymphoma in the rituximab era: results of the UK NCRI R-CHOP-14 versus 21 trial. Ann Oncol. 2017;28(10):2511–6. doi:10.1093/annonc/mdx353.* Re-analysis of a randomized trial, identifying risk factors for CNS recurrence, and its association with certain extranodal sites of involvement. It also confirms that parenchymal brain recurrences predominate in the RCHOP era.
- 36.El-Galaly TC, Villa D, Michaelsen TY, Hutchings M, Mikhaeel NG, Savage KJ et al. The number of extranodal sites assessed by PET/CT scan is a powerful predictor of CNS relapse for patients with diffuse large B-cell lymphoma: An international multicenter study of 1532 patients treated with chemoimmunotherapy. Eur J Cancer. 2017;75:195–203. doi:10.1016/j.ejca.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 37.van Besien K, Ha CS, Murphy S, McLaughlin P, Rodriguez A, Amin K et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91(4):1178–84. [PubMed] [Google Scholar]
- 38.Bos GM, van Putten WL, van der Holt B, van den Bent M, Verdonck LF, Hagenbeek A. For which patients with aggressive non-Hodgkin’s lymphoma is prophylaxis for central nervous system disease mandatory? Dutch HOVON Group. Ann Oncol. 1998;9(2):191–4. [DOI] [PubMed] [Google Scholar]
- 39.Hollender A, Kvaloy S, Nome O, Skovlund E, Lote K, Holte H. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol. 2002;13(7):1099–107. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz N, Zeynalova S, Nickelsen M, Kansara R, Villa D, Sehn LH et al. CNS International Prognostic Index: A Risk Model for CNS Relapse in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP. J Clin Oncol. 2016;34(26):3150–6. doi:10.1200/JCO.2015.65.6520.** Large observational study describing development of the CNS-IPI in a cohort of clinical trial participants. The index was then validated in an independent population-based cohort from BCCA.
- 41.Kansara R, Villa D, Gerrie AS, Klasa R, Shenkier T, Scott DW et al. Site of central nervous system (CNS) relapse in patients with diffuse large B-cell lymphoma (DLBCL) by the CNS-IPI risk model. Br J Haematol. 2017;179(3):508–10. doi:10.1111/bjh.14229. [DOI] [PubMed] [Google Scholar]
- 42.Chin CK, Cheah CY. How I treat patients with aggressive lymphoma at high risk of CNS relapse. Blood. 2017;130(7):867–74. doi:10.1182/blood-2017-03-737460. [DOI] [PubMed] [Google Scholar]
- 43.McMillan A, Ardeshna KM, Cwynarski K, Lyttelton M, McKay P, Montoto S et al. Guideline on the prevention of secondary central nervous system lymphoma: British Committee for Standards in Haematology. Br J Haematol. 2013;163(2):168–81. doi:10.1111/bjh.12509. [DOI] [PubMed] [Google Scholar]
- 44.Penalver FJ, Sancho JM, de la Fuente A, Olave MT, Martin A, Panizo C et al. Guidelines for diagnosis, prevention and management of central nervous system involvement in diffuse large B-cell lymphoma patients by the Spanish Lymphoma Group (GELTAMO). Haematologica. 2017;102(2):235–45. doi:10.3324/haematol.2016.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu H, Zhang L, Shao H, Sokol L, Sotomayor E, Letson D et al. Prognostic significance of soft tissue extension, international prognostic index, and multifocality in primary bone lymphoma: a single institutional experience. Br J Haematol. 2014;166(1):60–8. doi:10.1111/bjh.12841. [DOI] [PubMed] [Google Scholar]
- 46.Malecek MK, Petrich AM, Rozell S, Chu B, Trifilio S, Galanina N et al. Frequency, risk factors, and outcomes of central nervous system relapse in lymphoma patients treated with dose-adjusted EPOCH plus rituximab. Am J Hematol. 2017;92(11):1156–62. doi:10.1002/ajh.24864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thakral B, Medeiros LJ, Desai P, Lin P, Yin CC, Tang G et al. Prognostic impact of CD5 expression in diffuse large B-cell lymphoma in patients treated with rituximab-EPOCH. Eur J Haematol. 2017;98(4):415–21. doi:10.1111/ejh.12847. [DOI] [PubMed] [Google Scholar]
- 48.Alinari L, Gru A, Quinion C, Huang Y, Lozanski A, Lozanski G et al. De novo CD5+ diffuse large B-cell lymphoma: Adverse outcomes with and without stem cell transplantation in a large, multicenter, rituximab treated cohort. Am J Hematol. 2016;91(4):395–9. doi:10.1002/ajh.24299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu-Monette ZY, Tu M, Jabbar KJ, Cao X, Tzankov A, Visco C et al. Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget. 2015;6(8):5615–33. doi:10.18632/oncotarget.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyazaki K, Yamaguchi M, Suzuki R, Kobayashi Y, Maeshima AM, Niitsu N et al. CD5-positive diffuse large B-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol. 2011;22(7):1601–7. doi:10.1093/annonc/mdq627. [DOI] [PubMed] [Google Scholar]
- 51.Camilleri-Broet S, Criniere E, Broet P, Delwail V, Mokhtari K, Moreau A et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190–6. doi:10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Aguilar A, Idbaih A, Boisselier B, Habbita N, Rossetto M, Laurenge A et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res. 2012;18(19):5203–11. doi:10.1158/1078-0432.CCR-12-0845. [DOI] [PubMed] [Google Scholar]
- 53.Chapuy B, Roemer MG, Stewart C, Tan Y, Abo RP, Zhang L et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–81. doi:10.1182/blood-2015-10-673236.** Translational study identifying common genomic features of primary CNS and testicular lymphoma. These features include prevalent MYD88 and CD79B mutations, as well as copy number alterations involving the PD-L1 / PD-L2 genes.
- 54.Braggio E, Van Wier S, Ojha J, McPhail E, Asmann YW, Egan J et al. Genome-Wide Analysis Uncovers Novel Recurrent Alterations in Primary Central Nervous System Lymphomas. Clin Cancer Res. 2015;21(17):3986–94. doi:10.1158/1078-0432.CCR-14-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer L, Korfel A, Pfeiffer S, Kiewe P, Volk HD, Cakiroglu H et al. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res. 2009;15(19):5968–73. doi:10.1158/1078-0432.CCR-09-0108. [DOI] [PubMed] [Google Scholar]
- 56.Lemma SA, Kuusisto M, Haapasaari KM, Sormunen R, Lehtinen T, Klaavuniemi T et al. Integrin alpha 10, CD44, PTEN, cadherin-11 and lactoferrin expressions are potential biomarkers for selecting patients in need of central nervous system prophylaxis in diffuse large B-cell lymphoma. Carcinogenesis. 2017;38(8):812–20. doi:10.1093/carcin/bgx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemma SA, Pasanen AK, Haapasaari KM, Sippola A, Sormunen R, Soini Y et al. Similar chemokine receptor profiles in lymphomas with central nervous system involvement - possible biomarkers for patient selection for central nervous system prophylaxis, a retrospective study. Eur J Haematol. 2016;96(5):492–501. doi:10.1111/ejh.12626. [DOI] [PubMed] [Google Scholar]
- 58.Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. J Clin Oncol. 2009;27(1):114–9. doi:10.1200/JCO.2008.16.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar A, Vanderplas A, LaCasce AS, Rodriguez MA, Crosby AL, Lepisto E et al. Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: findings from a large national database. Cancer. 2012;118(11):2944–51. doi:10.1002/cncr.26588. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Chen B, Xu X. Impact of rituximab on incidence of and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: a systematic review and meta-analysis. Leuk Lymphoma. 2014;55(3):509–14. doi:10.3109/10428194.2013.811239. [DOI] [PubMed] [Google Scholar]
- 61.Zahid MF, Khan N, Hashmi SK, Kizilbash SH, Barta SK. Central nervous system prophylaxis in diffuse large B-cell lymphoma. Eur J Haematol. 2016;97(2):108–20. doi:10.1111/ejh.12763. [DOI] [PubMed] [Google Scholar]
- 62.Abramson JS, Hellmann M, Barnes JA, Hammerman P, Toomey C, Takvorian T et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116(18):4283–90. doi:10.1002/cncr.25278. [DOI] [PubMed] [Google Scholar]
- 63.Ferreri AJ, Bruno-Ventre M, Donadoni G, Ponzoni M, Citterio G, Foppoli M et al. Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br J Haematol. 2015;168(5):654–62. doi:10.1111/bjh.13194. [DOI] [PubMed] [Google Scholar]
- 64.Holte H, Leppa S, Bjorkholm M, Fluge O, Jyrkkio S, Delabie J et al. Dose-densified chemoimmunotherapy followed by systemic central nervous system prophylaxis for younger high-risk diffuse large B-cell/follicular grade 3 lymphoma patients: results of a phase II Nordic Lymphoma Group study. Ann Oncol. 2013;24(5):1385–92. doi:10.1093/annonc/mds621. [DOI] [PubMed] [Google Scholar]
- 65.Recher C, Coiffier B, Haioun C, Molina TJ, Ferme C, Casasnovas O et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03–2B): an open-label randomised phase 3 trial. Lancet. 2011;378(9806):1858–67. doi:10.1016/S0140-6736(11)61040-4. [DOI] [PubMed] [Google Scholar]
- 66.Laskin JJ, Savage KJ, Voss N, Gascoyne RD, Connors JM. Primary paranasal sinus lymphoma: natural history and improved outcome with central nervous system chemoprophylaxis. Leuk Lymphoma. 2005;46(12):1721–7. doi:10.1080/17402520500182345. [DOI] [PubMed] [Google Scholar]
- 67.Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol. 2004;124(2):151–9. doi:10.1046/j.1365-2141.2003.04749.x. [DOI] [PubMed] [Google Scholar]
- 68.Murawski N, Held G, Ziepert M, Kempf B, Viardot A, Hanel M et al. The role of radiotherapy and intrathecal CNS prophylaxis in extralymphatic craniofacial aggressive B-cell lymphomas. Blood. 2014;124(5):720–8. doi:10.1182/blood-2013-10-535021. [DOI] [PubMed] [Google Scholar]
- 69.Lee GW, Go SI, Kim SH, Hong J, Kim YR, Oh S et al. Clinical outcome and prognosis of patients with primary sinonasal tract diffuse large B-cell lymphoma treated with rituximab-cyclophosphamide, doxorubicin, vincristine and prednisone chemotherapy: a study by the Consortium for Improving Survival of Lymphoma. Leuk Lymphoma. 2015;56(4):1020–6. doi:10.3109/10428194.2014.946027. [DOI] [PubMed] [Google Scholar]
- 70.Carreras J, Kikuti YY, Bea S, Miyaoka M, Hiraiwa S, Ikoma H et al. Clinicopathological characteristics and genomic profile of primary sinonasal tract diffuse large B cell lymphoma (DLBCL) reveals gain at 1q31 and RGS1 encoding protein; high RGS1 immunohistochemical expression associates with poor overall survival in DLBCL not otherwise specified (NOS). Histopathology. 2017;70(4):595–621. doi:10.1111/his.13106. [DOI] [PubMed] [Google Scholar]
- 71.de Leval L, Bonnet C, Copie-Bergman C, Seidel L, Baia M, Briere J et al. Diffuse large B-cell lymphoma of Waldeyer’s ring has distinct clinicopathologic features: a GELA study. Ann Oncol. 2012;23(12):3143–51. doi:10.1093/annonc/mds150. [DOI] [PubMed] [Google Scholar]
- 72.Stein SA, Wartofsky L. Primary thyroid lymphoma: a clinical review. J Clin Endocrinol Metab. 2013;98(8):3131–8. doi:10.1210/jc.2013-1428. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe N, Noh JY, Narimatsu H, Takeuchi K, Yamaguchi T, Kameyama K et al. Clinicopathological features of 171 cases of primary thyroid lymphoma: a long-term study involving 24553 patients with Hashimoto’s disease. Br J Haematol. 2011;153(2):236–43. doi:10.1111/j.1365-2141.2011.08606.x. [DOI] [PubMed] [Google Scholar]
- 74.Knief J, Gebauer N, Bernard V, Schemme J, Reddemann K, Gebauer J et al. Oncogenic mutations and chromosomal aberrations in primary extranodal diffuse large B-cell lymphomas of the thyroid--a study of 21 cases. J Clin Endocrinol Metab. 2015;100(2):754–62. doi:10.1210/jc.2014-3250. [DOI] [PubMed] [Google Scholar]
- 75.Onal C, Li YX, Miller RC, Poortmans P, Constantinou N, Weber DC et al. Treatment results and prognostic factors in primary thyroid lymphoma patients: a rare cancer network study. Ann Oncol. 2011;22(1):156–64. doi:10.1093/annonc/mdq310. [DOI] [PubMed] [Google Scholar]
- 76.Cardenas-Garcia J, Talwar A, Shah R, Fein A. Update in primary pulmonary lymphomas. Curr Opin Pulm Med. 2015;21(4):333–7. doi:10.1097/MCP.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 77.Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood. 2011;118(10):2659–69. doi:10.1182/blood-2011-05-326538. [DOI] [PubMed] [Google Scholar]
- 78.Dunleavy K, Pittaluga S, Maeda LS, Advani R, Chen CC, Hessler J et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–16. doi:10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giulino-Roth L, O’Donohue T, Chen Z, Bartlett NL, LaCasce A, Martin-Doyle W et al. Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol. 2017;179(5):739–47. doi:10.1111/bjh.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah NN, Szabo A, Huntington SF, Epperla N, Reddy N, Ganguly S et al. R-CHOP versus dose-adjusted R-EPOCH in frontline management of primary mediastinal B-cell lymphoma: a multi-centre analysis. Br J Haematol. 2018;180(4):534–44. doi:10.1111/bjh.15051. [DOI] [PubMed] [Google Scholar]
- 81.Kawajiri A, Maruyama D, Maeshima AM, Nomoto J, Makita S, Kitahara H et al. Impact of the double expression of MYC and BCL2 on outcomes of localized primary gastric diffuse large B-cell lymphoma patients in the rituximab era. Blood Cancer J. 2016;6(9):e477. doi:10.1038/bcj.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sohn BS, Kim SM, Yoon DH, Kim S, Lee DH, Kim JH et al. The comparison between CHOP and R-CHOP in primary gastric diffuse large B cell lymphoma. Ann Hematol. 2012;91(11):1731–9. doi:10.1007/s00277-012-1512-4. [DOI] [PubMed] [Google Scholar]
- 83.Kuo SH, Yeh KH, Wu MS, Lin CW, Hsu PN, Wang HP et al. Helicobacter pylori eradication therapy is effective in the treatment of early-stage H pylori-positive gastric diffuse large B-cell lymphomas. Blood. 2012;119(21):4838–44; quiz 5057. doi:10.1182/blood-2012-01-404194. [DOI] [PubMed] [Google Scholar]
- 84.Ferreri AJ, Govi S, Raderer M, Mule A, Andriani A, Caracciolo D et al. Helicobacter pylori eradication as exclusive treatment for limited-stage gastric diffuse large B-cell lymphoma: results of a multicenter phase 2 trial. Blood. 2012;120(18):3858–60. doi:10.1182/blood-2012-06-438424. [DOI] [PubMed] [Google Scholar]
- 85.Kuo SH, Yeh KH, Chen LT, Lin CW, Hsu PN, Hsu C et al. Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: a distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J. 2014;4:e220. doi:10.1038/bcj.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frick M, Bettstetter M, Bertz S, Schwarz-Furlan S, Hartmann A, Richter T et al. Mutational frequencies of CD79B and MYD88 vary greatly between primary testicular DLBCL and gastrointestinal DLBCL. Leuk Lymphoma. 2018;59(5):1260–3. doi:10.1080/10428194.2017.1370546. [DOI] [PubMed] [Google Scholar]
- 87.Nagakita K, Takata K, Taniguchi K, Miyata-Takata T, Sato Y, Tari A et al. Clinicopathological features of 49 primary gastrointestinal diffuse large B-cell lymphoma cases; comparison with location, cell-of-origin, and frequency of MYD88 L265P. Pathol Int. 2016;66(8):444–52. doi:10.1111/pin.12439. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Yu K, Li M, Zeng K, Wei J, Li X et al. EZH2 overexpression in primary gastrointestinal diffuse large B-cell lymphoma and its association with the clinicopathological features. Hum Pathol. 2017;64:213–21. doi:10.1016/j.humpath.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 89.Bronowicki JP, Bineau C, Feugier P, Hermine O, Brousse N, Oberti F et al. Primary lymphoma of the liver: clinical-pathological features and relationship with HCV infection in French patients. Hepatology. 2003;37(4):781–7. doi:10.1053/jhep.2003.50121. [DOI] [PubMed] [Google Scholar]
- 90.Peng Y, Qing AC, Cai J, Yue C, French SW, Qing X. Lymphoma of the liver: Clinicopathological features of 19 patients. Exp Mol Pathol. 2016;100(2):276–80. doi:10.1016/j.yexmp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Page RD, Romaguera JE, Osborne B, Medeiros LJ, Rodriguez J, North L et al. Primary hepatic lymphoma: favorable outcome after combination chemotherapy. Cancer. 2001;92(8):2023–9. [DOI] [PubMed] [Google Scholar]
- 92.Villa D, Connors JM, Sehn LH, Gascoyne RD, Savage KJ. Diffuse large B-cell lymphoma with involvement of the kidney: outcome and risk of central nervous system relapse. Haematologica. 2011;96(7):1002–7. doi:10.3324/haematol.2011.041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lehners N, Kramer I, Schwarzbich MA, Ho AD, Witzens-Harig M. Analysis of clinical characteristics and outcome of patients with previously untreated diffuse large B-cell lymphoma and renal involvement in the rituximab era. Leuk Lymphoma. 2016;57(11):2619–25. doi:10.3109/10428194.2016.1157869. [DOI] [PubMed] [Google Scholar]
- 94.Kridel R, Telio D, Villa D, Sehn LH, Gerrie AS, Shenkier T et al. Diffuse large B-cell lymphoma with testicular involvement: outcome and risk of CNS relapse in the rituximab era. Br J Haematol. 2017;176(2):210–21. doi:10.1111/bjh.14392. [DOI] [PubMed] [Google Scholar]
- 95.Rashidi A, Fisher SI. Primary adrenal lymphoma: a systematic review. Ann Hematol. 2013;92(12):1583–93. doi:10.1007/s00277-013-1812-3. [DOI] [PubMed] [Google Scholar]
- 96.Kim YR, Kim JS, Min YH, Hyunyoon D, Shin HJ, Mun YC et al. Prognostic factors in primary diffuse large B-cell lymphoma of adrenal gland treated with rituximab-CHOP chemotherapy from the Consortium for Improving Survival of Lymphoma (CISL). J Hematol Oncol. 2012;5:49. doi:10.1186/1756-8722-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hussain S, Hallam S, Beltran L, Haroon A, Majumdar K, Shamash J et al. Intravascular large B-cell lymphoma presenting as a pituitary mass with bilateral adrenal enlargement and haemophagocytic lymphohistiocytosis. Br J Haematol. 2017. doi:10.1111/bjh.14715. [DOI] [PubMed] [Google Scholar]
- 98.Schrader AMR, Jansen PM, Willemze R, Vermeer MH, Cleton-Jansen AM, Somers SF et al. High prevalence of MYD88 and CD79B mutations in intravascular large B-cell lymphoma. Blood. 2018. doi:10.1182/blood-2017-12-822817. [DOI] [PubMed] [Google Scholar]
- 99.Kraan W, van Keimpema M, Horlings HM, Schilder-Tol EJ, Oud ME, Noorduyn LA et al. High prevalence of oncogenic MYD88 and CD79B mutations in primary testicular diffuse large B-cell lymphoma. Leukemia. 2014;28(3):719–20. doi:10.1038/leu.2013.348. [DOI] [PubMed] [Google Scholar]
- 100.Oishi N, Kondo T, Nakazawa T, Mochizuki K, Tanioka F, Oyama T et al. High prevalence of the MYD88 mutation in testicular lymphoma: Immunohistochemical and genetic analyses. Pathol Int. 2015;65(10):528–35. doi:10.1111/pin.12336. [DOI] [PubMed] [Google Scholar]
- 101.Deng L, Xu-Monette ZY, Loghavi S, Manyam GC, Xia Y, Visco C et al. Primary testicular diffuse large B-cell lymphoma displays distinct clinical and biological features for treatment failure in rituximab era: a report from the International PTL Consortium. Leukemia. 2016;30(2):361–72. doi:10.1038/leu.2015.237.* A multi-institutional observational series describing therapy and outcomes of primary testicular lymphoma. It suggests a benefit of prophylactic intrathecal therapy and radiation therapy, which were associated with lower risk of recurrence.
- 102.Vitolo U, Chiappella A, Ferreri AJ, Martelli M, Baldi I, Balzarotti M et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol. 2011;29(20):2766–72. doi:10.1200/JCO.2010.31.4187. [DOI] [PubMed] [Google Scholar]
- 103.Ho JC, Dabaja BS, Milgrom SA, Smith GL, Reddy JP, Mazloom A et al. Radiation therapy improves survival in patients with testicular diffuse large B-cell lymphoma(). Leuk Lymphoma. 2017;58(12):2833–44. doi:10.1080/10428194.2017.1312381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ollila TA, Olszewski AJ. Radiation therapy in primary testicular lymphoma (PTL): Does practice match the standard of care? ASCO Annual Meeting. 2018. Abs. 7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ryan G, Martinelli G, Kuper-Hommel M, Tsang R, Pruneri G, Yuen K et al. Primary diffuse large B-cell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol. 2008;19(2):233–41. doi:10.1093/annonc/mdm471. [DOI] [PubMed] [Google Scholar]
- 106.Thomas A, Link BK, Altekruse S, Romitti PA, Schroeder MC. Primary Breast Lymphoma in the United States: 1975–2013. J Natl Cancer Inst. 2017;109(6). doi:10.1093/jnci/djw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hosein PJ, Maragulia JC, Salzberg MP, Press OW, Habermann TM, Vose JM et al. A multicentre study of primary breast diffuse large B-cell lymphoma in the rituximab era. Br J Haematol. 2014;165(3):358–63. doi:10.1111/bjh.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yhim HY, Kim JS, Kang HJ, Kim SJ, Kim WS, Choi CW et al. Matched-pair analysis comparing the outcomes of primary breast and nodal diffuse large B-cell lymphoma in patients treated with rituximab plus chemotherapy. Int J Cancer. 2012;131(1):235–43. doi:10.1002/ijc.26352. [DOI] [PubMed] [Google Scholar]
- 109.Aviles A, Neri N, Nambo MJ. The role of genotype in 104 cases of diffuse large B-cell lymphoma primary of breast. Am J Clin Oncol. 2012;35(2):126–9. doi:10.1097/COC.0b013e318209aa12. [DOI] [PubMed] [Google Scholar]
- 110.Cao XX, Li J, Cai H, Zhang W, Duan MH, Zhou DB. Patients with primary breast and primary female genital tract diffuse large B cell lymphoma have a high frequency of MYD88 and CD79B mutations. Ann Hematol. 2017;96(11):1867–71. doi:10.1007/s00277-017-3094-7. [DOI] [PubMed] [Google Scholar]
- 111.Taniguchi K, Takata K, Chuang SS, Miyata-Takata T, Sato Y, Satou A et al. Frequent MYD88 L265P and CD79B Mutations in Primary Breast Diffuse Large B-Cell Lymphoma. Am J Surg Pathol. 2016;40(3):324–34. doi:10.1097/PAS.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 112.El-Galaly TC, Cheah CY, Hutchings M, Mikhaeel NG, Savage KJ, Sehn LH et al. Uterine, but not ovarian, female reproductive organ involvement at presentation by diffuse large B-cell lymphoma is associated with poor outcomes and a high frequency of secondary CNS involvement. Br J Haematol. 2016;175(5):876–83. doi:10.1111/bjh.14325.* An intrenational observational study demonstrating high risk of CNS recurrence in DLBCL involving the uterus.
- 113.Cao XX, Li J, Zhang W, Duan MH, Shen T, Zhou DB. Patients with primary diffuse large B-cell lymphoma of female genital tract have high risk of central nervous system relapse. Ann Hematol. 2014;93(6):1001–5. doi:10.1007/s00277-013-2003-y. [DOI] [PubMed] [Google Scholar]
- 114.Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(10):1052–5. doi:10.1002/ajh.24462. [DOI] [PubMed] [Google Scholar]
- 115.Grange F, Joly P, Barbe C, Bagot M, Dalle S, Ingen-Housz-Oro S et al. Improvement of survival in patients with primary cutaneous diffuse large B-cell lymphoma, leg type, in France. JAMA Dermatol. 2014;150(5):535–41. doi:10.1001/jamadermatol.2013.7452. [DOI] [PubMed] [Google Scholar]
- 116.Pham-Ledard A, Prochazkova-Carlotti M, Andrique L, Cappellen D, Vergier B, Martinez F et al. Multiple genetic alterations in primary cutaneous large B-cell lymphoma, leg type support a common lymphomagenesis with activated B-cell-like diffuse large B-cell lymphoma. Mod Pathol. 2014;27(3):402–11. doi:10.1038/modpathol.2013.156. [DOI] [PubMed] [Google Scholar]
- 117.Hoefnagel JJ, Dijkman R, Basso K, Jansen PM, Hallermann C, Willemze R et al. Distinct types of primary cutaneous large B-cell lymphoma identified by gene expression profiling. Blood. 2005;105(9):3671–8. doi:10.1182/blood-2004-04-1594. [DOI] [PubMed] [Google Scholar]
- 118.Pham-Ledard A, Beylot-Barry M, Barbe C, Leduc M, Petrella T, Vergier B et al. High frequency and clinical prognostic value of MYD88 L265P mutation in primary cutaneous diffuse large B-cell lymphoma, leg-type. JAMA Dermatol. 2014;150(11):1173–9. doi:10.1001/jamadermatol.2014.821. [DOI] [PubMed] [Google Scholar]
- 119.Pham-Ledard A, Prochazkova-Carlotti M, Deveza M, Laforet MP, Beylot-Barry M, Vergier B et al. Molecular analysis of immunoglobulin variable genes supports a germinal center experienced normal counterpart in primary cutaneous diffuse large B-cell lymphoma, leg-type. J Dermatol Sci. 2017;88(2):238–46. doi:10.1016/j.jdermsci.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 120.Gardette E, Maraval A, Brunet-Possenti F, Quereux G, Beltraminelli H, Templier I et al. Central nervous system involvement of primary cutaneous diffuse large B-cell lymphoma, leg type: 13 cases. J Eur Acad Dermatol Venereol. 2017;31(11):e498–e501. doi:10.1111/jdv.14358. [DOI] [PubMed] [Google Scholar]
- 121.Bekkenk MW, Postma TJ, Meijer CJ, Willemze R. Frequency of central nervous system involvement in primary cutaneous B-cell lymphoma. Cancer. 2000;89(4):913–9. [DOI] [PubMed] [Google Scholar]
- 122.Bruno Ventre M, Ferreri AJ, Gospodarowicz M, Govi S, Messina C, Porter D et al. Clinical features, management, and prognosis of an international series of 161 patients with limited-stage diffuse large B-cell lymphoma of the bone (the IELSG-14 study). Oncologist. 2014;19(3):291–8. doi:10.1634/theoncologist.2013-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Messina C, Ferreri AJ, Govi S, Bruno-Ventre M, Gracia Medina EA, Porter D et al. Clinical features, management and prognosis of multifocal primary bone lymphoma: a retrospective study of the international extranodal lymphoma study group (the IELSG 14 study). Br J Haematol. 2014;164(6):834–40. doi:10.1111/bjh.12714. [DOI] [PubMed] [Google Scholar]
- 124.Tao R, Allen PK, Rodriguez A, Shihadeh F, Pinnix CC, Arzu I et al. Benefit of consolidative radiation therapy for primary bone diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2015;92(1):122–9. doi:10.1016/j.ijrobp.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 125.Held G, Zeynalova S, Murawski N, Ziepert M, Kempf B, Viardot A et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol. 2013;31(32):4115–22. doi:10.1200/JCO.2012.48.0467. [DOI] [PubMed] [Google Scholar]
- 126.Li X, Xu-Monette ZY, Yi S, Dabaja BS, Manyam GC, Westin J et al. Primary Bone Lymphoma Exhibits a Favorable Prognosis and Distinct Gene Expression Signatures Resembling Diffuse Large B-Cell Lymphoma Derived From Centrocytes in the Germinal Center. Am J Surg Pathol. 2017;41(10):1309–21. doi:10.1097/PAS.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 127.Xu Y, Li J, Ouyang J, Li J, Xu J, Zhang Q et al. Prognostic relevance of protein expression, clinical factors, and MYD88 mutation in primary bone lymphoma. Oncotarget. 2017;8(39):65609–19. doi:10.18632/oncotarget.19936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pilorge S, Harel S, Ribrag V, Larousserie F, Willems L, Franchi P et al. Primary bone diffuse large B-cell lymphoma: a retrospective evaluation on 76 cases from French institutional and LYSA studies. Leuk Lymphoma. 2016;57(12):2820–6. doi:10.1080/10428194.2016.1177180. [DOI] [PubMed] [Google Scholar]