Summary
Stem cells regenerate tissues in homeostasis and under stress. By taking cues from their microenvironment or “niche”, they smoothly transition between these states. Immune cells have surfaced as prominent members of stem cell niches across the body. Here, we draw parallels between different stem cell niches to explore the context-specific interactions that stem cells have with tissue resident and recruited immune cells. We also highlight stem cells’ innate ability to sense and respond to stress, and the enduring memory that forms from such encounters. This fascinating crosstalk holds great promise for novel therapies in inflammatory diseases and regenerative medicine.
Introduction
Our body’s tissues are in a constant state of flux, perpetually turning over throughout our lifetimes. Fueling this turnover are self-renewing, tissue stem cells, which give rise to shorter-lived progenitors charged with balancing proliferation and differentiation in order to maintain equilibrium between hyperplasia and atrophy (Figure 1). The rate of cellular replacement during homeostasis is tissue and context specific. It is perpetual in blood, epidermis and intestine, limited in brain and muscle, and episodic in the hair follicle and lactating mammary gland. However, when tissues are damaged, even often-quiescent stem cells can be mobilized into action. Similarly, inflammatory and infectious responses override the normal homeostatic cues in ways that only recently have begun to be appreciated.
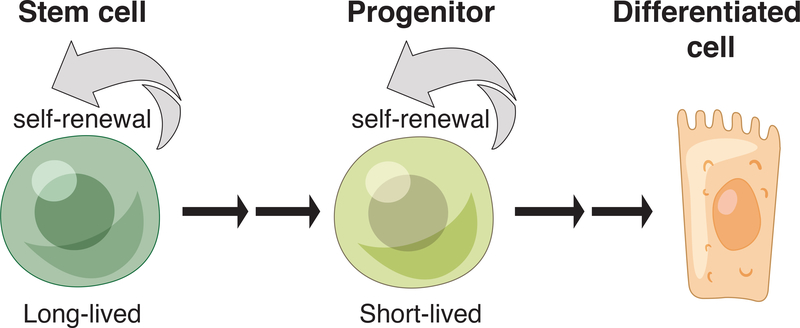
Figure 1: Tissue stem cell hierarchy.
Long-lived stem cells have the ability to self-renew and give rise to short-term progenitors. These short-term progenitors can also replicate themselves and generate differentiated progeny. They are largely responsible for coordinating tissue homeostasis.
Understanding how stem cells adapt to varying physiological and pathological situations requires a close inspection of their local microenvironment or ‘niche’. Each niche is uniquely tailored to suit the particular needs of a tissue, enabling its stem cells to respond to heterogeneous networks of cellular and extracellular inputs. Dynamic niche signals facilitate change in stem cell behavior, e.g. from quiescence to active tissue regeneration. In part, the stem cells’ own progenies become important niche constituents: some progeny signal back to their predecessors to fuel tissue growth, while others signal to stem cells to restore homeostasis (Hsu et al., 2014a; 2014b). Heterologous niche components include extracellular matrix, nerves, vasculature, stromal, and adipose tissue.
Given the crucial duties of stem cells in maintaining tissue integrity and driving regeneration under stress, it is not surprising that immune cells have recently emerged as key components of the niche microcosm and prominent effectors of stem cell behaviors. Indeed, tissues are armed with sophisticated local immune surveillance systems to monitor their health and integrity. Resident and recirculating immune cell populations include cells of the innate immune system such as macrophages and dendritic cells, as well as adaptive immune T cells (reviewed by Fan and Rudensky, 2016) (Figure 2).
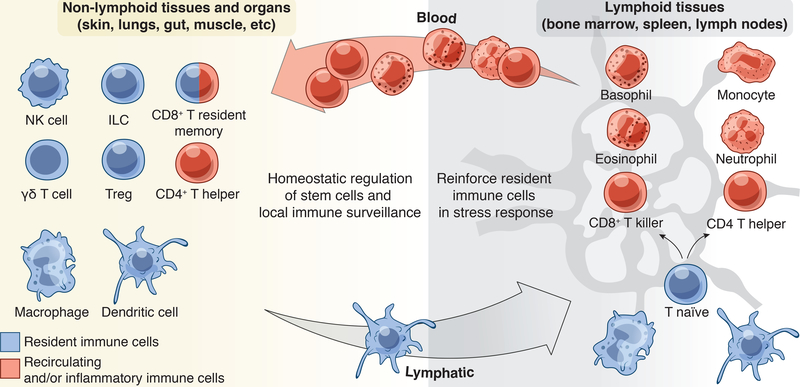
Figure 2: Tissue resident, recirculating, and inflammatory immune cells.
A constellation of immune cells inhabits tissues, the composition of which varies by tissue site and the inflammatory status of the tissue. Extra-lymphoid tissues and in particular epithelial barrier tissues such as the skin, lungs and gut, house the greatest number of resident immune sentinels. This includes dendritic cells, macrophages, innate lymphoid cell (ILC) subsets, γδ T cells and regulatory T cells (Tregs) that seed tissues early in life. With age and exposure to commensals and pathogens, tissues also acquire CD8+ T resident memory cells (TRM) and recirculating CD4+ T helper subsets. During an acute stress response, inflammatory macrophages/monocytes, neutrophils, basophils, and eosinophils are recruited to the damage to reinforce the function of resident cells. Lymphoid organs such as the lymph node and spleen are epicenters for naïve or unprimed T cells. These cells are primed by dendritic cells to differentiate into effectors and migrate into tissues via blood where they enact their effector functions.
The composition and function of resident immune cells varies among tissues. The greatest immune activity is found in the epithelial tissues of skin, lung, and gut, which not only continuously turn over, but also routinely endure the physical, noxious, and pathogenic traumas of our external environment. During these assaults, stem cells communicate with the frontline of resident immune sentinels, to orchestrate the systemic dissemination of distress commands. Responding immune effectors quickly enter from circulation, infiltrating the stressed tissue to clear invading pathogens, aid in repair, and reinstate homeostasis (Figure 2).
Here we review the intricate and vital dialogue between immune cells and stem cells and the consequences of this crosstalk for tissue fitness and function. We discuss increasing evidence that stem cells sense, communicate with and co-opt resident immune cells to aid in tissue homeostasis. In addition, we discuss recent findings underscoring the remarkable capacity of stem cells to sense damage and recruit infiltrating immune cells to help them cope with stress. Stem cells also have intrinsic immune modulatory capabilities and intriguing ways to shield themselves from infections and inflammatory pathology. We summarize how stem cells learn from their inflammatory encounters and adapt their responses to subsequent stressors. Finally, we end with a discussion of the clinical implications and the therapeutic potential of targeting immune-tissue stem cell interactions in inflammatory diseases and regenerative medicine.
Immune cell regulation of tissue stem cell behavior
In recent years, there has been increasing appreciation for the non-canonical functions of immune cells beyond immunity to pathogens (Burzyn et al., 2013a; Davies et al., 2013). A myriad of innate and adaptive immune cells reside in tissues and perform tasks that are essential for organ homeostasis. Included in this new mantle is their proactive role in regulating stem cells and consequently tissue function. Although most studies to date have focused on the crosstalk between immune cells and stem cells within the context of tissue repair, recent findings support a role for resident immune cells in the homeostatic regulation of stem cells. In particular, two tissue-resident immune cell types, macrophages and regulatory T cells (Tregs), have emerged as potent regulators of stem cells under normal physiological conditions (Burzyn et al., 2013a; Davies et al., 2013).
Macrophages in Homeostasis: More Than Just Professional Phagocytes
Macrophages are found in all tissues of the body and show remarkable functional diversity based on their ontogeny and the inflammatory status of the tissue (Epelman et al., 2014; Lavin et al., 2014). Thus, the term macrophage is used to define a broad diversity of cell types. Classically known as professional phagocytes for their ability to devour dead and dying cells, macrophages were among the first identified immune cell types capable of modulating stem cells.
In the bone marrow, macrophages govern hematopoietic stem cell (HSC) retention (Chow et al., 2011)(Figure 3A). CD169+ tissue resident macrophages augment expression of the chemokine CXCL12 by niche mesenchymal stem cells (MSCs) to limit HSC egress into the blood. In addition to their impact on HSCs, this subset of CD169+ macrophages in the bone marrow also supports the expansion of HSC progeny, specifically developing erythroblasts. They do so by forming “erythroblastic islands,” composed of erythroblast progenitors tethered to a central macrophage through multiple receptor-ligand interactions (Chow et al., 2013) (Figure 3A). These intriguing structures act as seeds of erythropoiesis, and when the micro-niches are disrupted by CD169+ macrophage depletion, recovery from hematopoietic stress is hampered. Although, the precise communication circuitry between macrophages and erythroblasts remains unknown, these micro-niches govern erythroblast maturation (Chow et al., 2013).
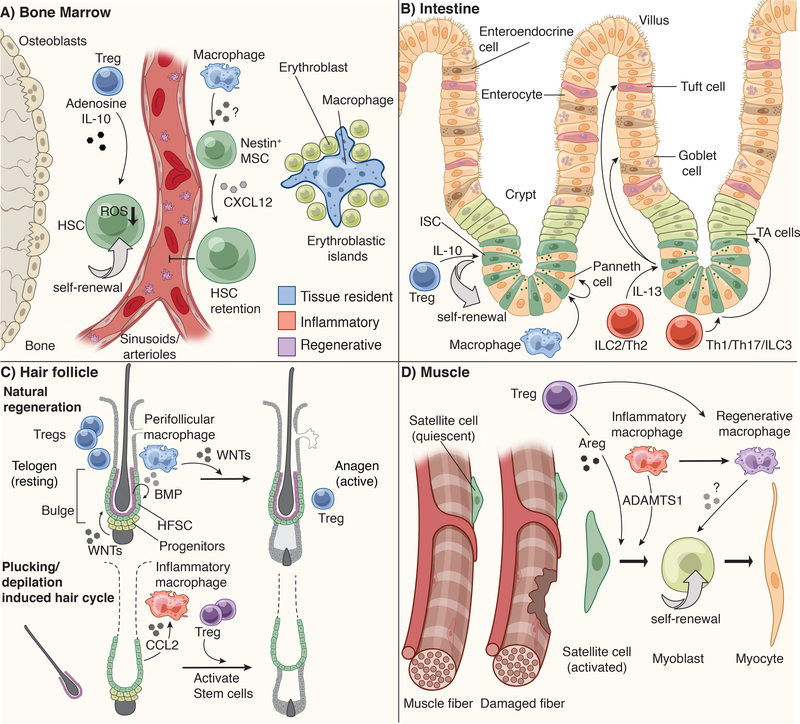
Figure 3: Niche specific immune-stem cell interactions.
A) Hematopoietic stem cells (HSCs) reside in the bone marrow (BM) in close apposition of sinusoids and arterioles. They are retained in this niche by Nestin+ mesenchymal stem cell (MSC)-derived CXCL12. Bone marrow macrophages are critical for maintaining the production of CXCL12 and retaining HSCs in this niche. Bone marrow Tregs are also localized close to HSCs and produce IL-10 and Adenosine to control HSC quiescence and pool size. In the absence of Tregs, HSCs produce more reactive oxygen species (ROS) and consequently increase their numbers. Erythroblast progenitors in the BM aggregate around a central macrophage, which provides cues to direct their maturation into erythrocytes.
B) Intestinal stem cells (ISCs) reside in crypt structures at the base of intestinal villi and are flanked by Paneth cells and transit amplifying (TA) cells. ISCs give rise to all intestinal lineages, including goblet cells, enteroendocrine cells, tuft cells, enterocytes and Paneth cells. However, when ISCs at the base of the crypt are ablated, these progenitors can replenish niche vacancies. Intestinal macrophages promote the differentiation of ISCs to Paneth cells, while resident Tregs promote ISC self-renewal via IL-10. CD4+ T helper (Th)1 and Th17 cells and innate lymphoid cells (ILC) type 3 promote the production of transit amplifying progeny, while Th2 and ILC2 promote differentiation of tuft and goblet cells via IL-13.
C) The hair follicle is one of the few systems in mammals where tissue regeneration happens in natural bursts (the hair cycle) and in the absence of injury. The bulge region of the hair follicle is home to hair follicle stem cells (HFSCs). At the bulge base (hair germ), ‘primed stem cells’ give rise to the short-term progenitors that undergo natural cyclical bouts of active tissue regeneration (anagen). Growth inhibitory signals such as bone morphogenic proteins (BMPs), supplied by both the dermis and the inner bulge niche layer, keep stem cells in quiescence throughout most of the hair cycle. In the resting phase of the hair cycle (telogen), WNT signals and BMP inhibitor levels in the hair germ accumulate until stem cells become activated to launch a new tissue regenerative cycle.
Perifollicular macrophages are a crucial source of WNTs and tip the balance in favor of regeneration. By contrast, Tregs are highest in telogen when stem cells are at the height of quiescence; Tregs reach a low point at the height of the hair growth phase. Hair plucking removes the inner bulge and its quiescence signals, thereby precociously inducing anagen. During this injury response, inflammatory macrophages are also found near the bulge, where they appear to promote hair cycling and disseminate distress signals to adjacent unplucked follicles. Regenerative Tregs also appear to facilitate the progression to anagen following hair depilation.
D) Muscle stem cells (satellite cells) are quiescent at steady state and only activate following injury. Regenerative Tregs and inflammatory macrophages (M1) support satellite cell self-renewal and activation via amphiregulin (Areg) and ADAMTS1, respectively. As repair progresses, Tregs facilitate the switch from inflammatory M1 to regenerative M2 macrophages, which promote myoblast self-renewal and differentiation.
Stem cells in mammary glands (MaSCs) also require signals from tissue macrophages for ductal morphogenesis (Gyorki et al., 2009). Charakbati and colleagues discovered that MaSCs express a Notch ligand Delta like 1 (Dll1), which activates resident Notch-expressing macrophages (Chakrabarti et al., 2018). This interaction stimulates macrophages to produce Wnt ligands, which in turn triggers MaSC proliferation.
More recently, macrophages have been identified as key components of intestinal stem cell (ISC) crypts, which are responsible for sustaining continuous production of the transit-amplifying (TA) cells and their differentiated epithelial cell progeny, including goblet cells, enterocytes, enteroendocrine and Tuft cells that form the adsorptive villi of the intestine (Tan and Barker, 2014)(Figure 3B). Resident macrophages intimately associate with crypts, and when mice are treated with blocking antibodies against CSF1 (colony stimulating factor 1)-receptor, which in the intestinal crypt, are expressed by macrophages, stem cell survival is diminished and ISC lineage choice is skewed (Sehgal et al., 2018).
Hair follicles are unusual in that they undergo natural cyclical bouts of rest (telogen) and regeneration (anagen). Accordingly, the stem cells that fuel this process shift from quiescent to active states and back as they give rise to the shorter-lived proliferative progenitors (transit amplifying cells) that fuel the periodic regrowth of the hair follicle as well as the 7 additional epithelial cell lineages that form the hair and its channel (Yang et al., 2017) (Figure 3C). The activation of this natural, cyclical regenerative process demands changes within the stem cell niche and involves both the dampening of quiescence signals (BMPs) and an increase in stem cell activation/regenerative signals (WNTs) (Greco et al., 2009; Plikus et al., 2008). While the origins of quiescence signals have been traced to terminally differentiated stem cell progeny that line the “inner bulge” (Hsu et al., 2011) as well as longer-range signals from the dermis (Plikus et al., 2008), skin resident macrophages have been identified as a contributor of stem cell activating signals. During the progression from resting to regenerative phases, macrophages gather around the follicle. Intriguingly, as these perifollicular macrophages die off, they release WNT7b and WNT10a to promote the activation of hair follicle stem cells (HFSCs) (Castellana et al., 2014).
Regulatory T cells: Newcomers to the World of Stem Cell Niches
Defined by their expression of transcription factor FOXP3 (forkhead box P3), regulatory T cells (Tregs), are a subset of CD4+ helper T lymphocytes. Like macrophages, Tregs display functional heterogeneity and plasticity as an apparent means of adaption to varying physiological states (Sakaguchi et al., 2013). Tregs are potent suppressors of the immune response, where they function in preventing catastrophic autoimmunity (Josefowicz et al., 2012) A number of stem cell niches have a paucity of inflammatory cells in their vicinity, leading researchers to wonder whether these immune privileged sites might be possible residences for Treg (Fujisaki et al., 2011; Hirata et al., 2018).
The bone marrow is a known reservoir for Tregs, and their specific depletion pointed to a role in controlling HSC quiescence and pool size (Fujisaki et al., 2011). In the absence of bone marrow Tregs, HSCs were fewer in number and displayed increased sensitivity to oxidative stress (Hirata et al., 2018). Treg-derived adenosine, sensed directly by the HSC’s adenosine receptor, has been implicated in HSC responsiveness to oxidative stress and maintenance of quiescence (Figure 3A). This raises the tantalizing possibility that communication networks might exist between stem cells and tissue resident Tregs.
In this regard, Tregs have also been implicated in preserving the integrity of the LGR5+ ISCs in the intestine, where depletion of Tregs leads to a pronounced reduction in ISC numbers (Biton et al., 2017). Consistent with the notion that Tregs can directly influence the stem cells, co-culturing intestinal organoid cultures with Tregs or their effector cytokine IL-10 results in a significant enrichment of LGR5+ ISCs (Figure 3B).
In skin, resident Tregs are found near the stem cell niche of hair follicles (Ali et al., 2017) (Figure 3C). Rosenblum and colleagues recently reported that Treg numbers fluctuate during the normal hair cycle, peaking in telogen when stem cells are quiescent, and then reaching a low during anagen (Ali et al., 2017). Although it remains unclear how Tregs influence hair cycling in their native setting, a role for Tregs in maintaining stemness and quiescence would parallel the role of Tregs in HSCs and ISCs (Ali et al., 2017; Biton et al., 2017; Fujisaki et al., 2011; Hirata et al., 2018).
Macrophages and Tregs in Injury Repair
Over a century ago, Élie Metchnikoff observed the recruitment of macrophages to injured tissues and postulated that these mononuclear cells facilitate repair by clearing debris (Metchnikoff, 1893). Since then, our understanding of the contributions of immune cells to tissue regeneration has improved greatly. Tissue damage resulting from mechanical injury, or exposure to infectious and toxic agents causes the release of damage associated molecular patterns (DAMPS) from dying cells and pathogen associated molecular patterns (PAMPS) from pathogens that activate the immune system (Janeway and Medzhitov, 2002). The ensuing inflammatory response is tightly controlled to ensure efficient healing. Thus, as Metchnikoff predicted, cells of the immune system are indispensable for clearing damage-associated debris and pathogenic or noxious materials that may penetrate tissues upon injury.
Lessons from Damaged Muscle
Recent advances highlight even more sophisticated mechanisms by which macrophages promote regeneration (Aurora and Olson, 2014). This involves a carefully orchestrated, damage-induced, immune response that provides stem cells with temporal signals to guide healing. In skeletal muscle, for instance, quiescent muscle stem cells (satellite cells) are stimulated by damage-induced signals from macrophages, and Tregs to promote their myogenesis and repair of damaged muscle fibers (Arnold et al., 2007; Brack and Rando, 2012; Burzyn et al., 2013b; Du et al., 2017).
During repair, macrophage dynamics remodel satellite cell responses. Inflammation-activated macrophages (M1) promote satellite cell proliferation and expansion by secreting ‘A Disintegrin-Like And Metalloproteinase With Thrombospondin Motifs Type 1’ (ADAMTS1) (Du et al., 2017). ADAMTS1 then targets and suppresses NOTCH function, releasing satellite cells from quiescence and facilitating new tissue growth. As repair proceeds, inflammatory M1 macrophages are replaced with regenerative M2 macrophages, driving the differentiation of the expanded satellite cell pool into myoblasts (Arnold et al., 2007) (Figure 3D). In this way, the two states of macrophages each have distinct roles in the repair process.
As studies on muscle injury show, there is a clear need to halt inflammation-induced stem cell proliferation, while at the same time, promote downstream progenitor (myoblast) proliferation and myocyte differentiation, leading to fused myotubes and new muscle tissue. Interestingly, following injury, clonal Treg populations expand concomitantly with the switch in macrophage state (Burzyn et al., 2013b). These regeneration-associated Tregs secrete the epidermal growth factor (EGF)-like growth factor, amphiregulin (Areg), which in turn stimulates the differentiation of myoblasts from satellite cells (Figure 3D). Further underscoring the importance of this pathway, the accumulation and consequently function of these damage-associated Tregs is driven by the cytokine IL-33, whose loss results in ineffective muscle repair (Kuswanto et al., 2016).
At later stages of healing, Tregs also play a pivotal role in myotube differentiation, as they continue to curb excessive inflammatory T cells responses and allow for M2 macrophage evolution (Burzyn et al., 2013b). These studies raise the enticing possibility that like macrophages, Tregs have a means of sensing and adjusting their function to each stage of repair. If so, this could explain why in some contexts, e.g. intestinal homeostasis, Tregs seem to function in promoting stem cell self-renewal, while in other contexts, e.g. muscle, Tregs promote differentiation.
Eosinophils, which are granulocyte effectors of type 2 immunity, are also rapidly recruited to damaged muscle tissue. There, through their effector cytokine IL-4, they drive the activation of fibroblast/adipocyte progenitors (FAPs), which facilitate myogenesis by clearing necrotic debris. The absence of eosinophil-derived instructive cues, results in atypical FAP differentiation into adipocytes and fatty muscle degeneration (Heredia et al., 2013). Taken together, these findings on the immune responses in muscle repair illustrate beautifully how the coordinated activation of multiple progenitor populations by resident and recruited immune cells is necessary for optimal repair.
Lessons from Injuries to Epidermal Appendages
While studies on injured muscle point to an important role of macrophages and Tregs in orchestrating the behavior of stem cells in normal homeostasis, so too can stem cells act as generals in coordinating the response to injury. One interesting example is hair plucking, which, in contrast to the normal hair cycle, causes local tissue damage to the stem cell niche (bulge) of the hair follicle. Plucking mechanically removes the BMP-producing inner bulge cells, which not only anchor the hair but also participate in maintaining stem cell quiescence during normal telogen (Hsu et al., 2011).
Intriguingly, however, hair plucking not only triggers a new hair cycle, enhancing the regenerative capacity of the stem cells, but it may also activate a damage-sensing mechanism. In this scenario, the chemokine CCL2 is expressed by these hair follicle stem cells, which could explain the associated macrophages that surround the niche (Chen et al., 2015). These macrophages may not just be mere scavengers, as their loss delays the induction of hair cycling and activation of adjacent unplucked follicles.
Chen and colleagues attribute the activation of hair cycling and dissemination of distress signals to the ability of these skin macrophages to secrete the inflammatory cytokine TNFα (Chen et al., 2015).
Curiously, however, TNFα has apoptotic effects on cultured keratinocytes, and mice either lacking TNFα receptor or treated with blocking antibodies to TNFα show accelerated, not delayed, skin regeneration after wounding (Mori et al., 2002). Sifting through the possible indirect mechanisms of action becomes a daunting task, since most tissue cells have receptors for secreted pro-inflammatory factors like TNFα.
Tissue damage is accompanied by an elevation in regeneration-inducing WNTs (Clevers et al., 2014). In zebrafish, tailfin injury results in enhanced WNT-signaling, and interestingly, when WNTs are diminished, fewer macrophages are recruited to the wound site (Petrie et al., 2014). If wound-induced WNTs also elevate levels of stem cell-derived CCL2 and other putative macrophage chemoattractants, this would explain the link between elevated WNTs and macrophage numbers seen in zebrafish skin appendage injuries. It is also possible that in mammalian skin, recruited inflammatory macrophages augment WNT levels in the wound, as seen in the natural hair cycle (Castellana et al., 2014). If so, this could begin to shed light on the current conundrum of why macrophages have such positive effects on regeneration despite their production of inflammatory cytokines such as TNFα.
Similar to macrophage depletion, Treg deficiency also derails new hair growth stimulated by hair depilation (Ali et al., 2017). It is presently still unclear whether depilation elicits a injury response by immune cells. However, the regeneration-associated Treg activity observed upon hair depilation is in line with the role of Tregs in muscle injury. Based upon these comparisons, it is tempting to speculate that depilation-induced injury to the HF niche may induce a transition from homeostatic Tregs to “regenerative” Tregs to stimulate repair (Gerriets et al., 2016; Nosbaum et al., 2016).
Indeed, while many issues await future exploration, it is becoming increasingly clear that through mechanisms distinct from regenerative processes in homeostasis, injury-induced changes to the tissue microenvironment impact not only stem cells and/or their proliferative progeny, but also resident immune cells. For the skin, the underlying mechanisms involved could have important clinical relevance, as alopecia areata, an autoimmune disorder resulting in inflammation and hair loss, has been linked through genome wide association studies to certain genes involved in Treg activation and proliferation (Petukhova et al., 2010).
Comparing Roles of Macrophages and Tregs in Homeostasis and Injury.
Restoring tissue integrity during and after an active immune response is paramount for limiting pathology and promoting inflammatory resolution. Thus, it is reasonable to expect that macrophages and Tregs serve the dual functions of modulating immunity and promoting regeneration. Consistent with this notion, macrophages and Tregs seem to either repurpose their existing immune pathways and/or adopt novel pro-regenerative functions to engage stem cells and short-lived progenitors (Figure 3). Given their burgeoning role in controlling stem cells, conversations between stem cells and tissue resident immune cells seem likely to become unhinged in disease states. This hypothesis is consistent with a number of pathological conditions are associated with loss of immune regulation, inflammation, and tissue hyperplasia (Gersemann et al., 2011; Lowes et al., 2007; Naik et al., 2017; Ordovas-Montanes et al., 2018a).
Many fundamental questions remain. Are macrophages and Tregs obligatory residents of all stem cell niches? Like stem cells, do macrophages and Tregs play universal roles in regeneration in homeostasis and/or wound repair? How much of their participation emanates from macrophage and/or Treg autonomous signaling, as opposed to taking their cues from the stem cells themselves or from other cells within the niche microenvironment? Given the broad if not universal role of WNT signaling in tissue regeneration, how many cell types besides stem cells and macrophages are impacted by regenerative WNT signaling at a wound site? Do stem cells directly communicate with immune cells? If so, what molecular language to they employ in these conversations and how does this change in response to injury? As these questions are answered, it will be interesting to see whether a unifying principle of stem cell-immune crosstalk can be assigned to tissue injuries, while still permitting tailoring according to the type of stem cell and wound involved.
Stem cell crosstalk with other niche resident immune cells
Whether direct or indirect, stem cell-immune cell interactions include not only macrophages and Tregs, but also other tissue resident immune cells, such as innate lymphoid cells. Indeed, multiple subsets of innate lymphoid cells can play context-specific roles in directing regeneration and differentiation of stem cells (Lindemans et al., 2015; Moltke et al., 2016). Probing stem cell interactions with other tissue resident cells including dendritic cells, natural killer cells, and effector and memory T cells will reveal if and how these functionally distinct populations contribute to niches (Fan and Rudensky, 2016; Mueller and Mackay, 2016) (Figure 2).
Granulocytes, such as neutrophils, are typically reserved for active immune responses and not traditionally considered “tissue resident” in non-lymphoid tissues at steady state (Figure 2). However, in the bone marrow where these immune effectors are generated, they modulate the HSC niche and exert their influence on HSC retention and regeneration (Casanova-Acebes et al., 2013; Bowers et al., 2018). Since neutrophils are lineage-derived from HSCs, this could reflect a feedback mechanism employed by neutrophils to control their predecessors (Hsu et al., 2014a).
Resident immune cells seed extra-lymphoid tissues early in life and continue to elaborate with age. Characterizing the immune cells that reside within stem cell niches of healthy tissues and analyzing their phenotypic consequences when systematically ablated will shed light on their contributions to stem cell functions. Of additional importance will be elucidating how different immune cells home to stem cell niches. Is this process initiated by stem cells themselves, or do other members of the niche control immune localization in their ecosystem? The density and activity of immune cells differs markedly across tissues, as exemplified by the varied repertoire of immune cells that patrol epithelial barrier tissues such as the skin and gut (Fan and Rudensky, 2016; Mueller and Mackay, 2016). Do stem cells in these tissues more readily take their cues from immune cells under physiological conditions, while other less immunologically active tissues call upon their immune partners only under duress? Addressing these questions will require dissection of the immune-stem cell crosstalk within individual stem cell niches as well as at the macro level comparatively across organ systems.
Stem Cells and Immune Cells in Inflammation
The dialogue between immune cells and stem cells in wound repair has ancient roots. When a tissue barrier is breached, stem cells must repair the damage as quickly as possible to limit pathogen entry. It is tempting to speculate that damage-sensing stem cells might place the emergency call that recruits an immune response. If so, this could provide an immediate means of focusing immune reactions towards the wound site and possibly also amplifying the regenerative response. Glimpses of highly targeted immune responses during wound-repair can be gleaned by eliminating either cellular constituents of the inflammatory immune repertoire or the individual factors they express. Because epithelial tissues bear the brunt of inflammation-inducing noxious agents and pathogens, they are particularly relevant models to study the intertwined relationship between immunity and regeneration.
Stem Cells Adapt to Their Inflammatory Milieu
Sustained inflammatory challenges are associated with specific modules of immunity that can be broadly classified into T helper (Th) 17, 1, and 2 responses, each of which engages a unique set of effector mechanisms intended to mitigate the posed threat. For instance, a Th17 response results in the recruitment of neutrophils and eradication of extracellular pathogens; a Th1 response activates macrophages to cope with intracellular pathogens; and Th2 responses are critical for worm clearance (Vahedi et al., 2013). Guided by these threat-specific milieus, stem cells tailor their behavior to reinforce tissue integrity and contribute to host defense.
Xavier and colleagues examined the differentiation trajectory of ISCs in the presence of Th1, Th2, and Th17 in vitro (Biton et al., 2017). Co-culturing intestinal organoids with different classes of T cells or their prototypic effector cytokines resulted in a striking loss of ISCs and the enrichment of short-lived progeny, so-called ‘transit amplifying’ (TA) progenitors. Notably, each co-culture system resulted in unique subsets of lineage-specific, terminally differentiated cells. Th1 co-cultures increased the proportion of Paneth and goblet cells; Th17 signals promoted generation of enterocytes; and Th2 signals shifted the organoid toward an enteroendocrine fate.
Interestingly, these differentiated lineages in turn are known to enact effector functions necessary for mitigating the posed threat. For instance, parasite infections result in the enrichment of Th2 cytokine IL-13, which signals to ISCs to drive differentiation of tuft and goblet cells that in turn, secrete mucus to aide in parasite expulsion and act to reinforce Th2 responses (Moltke et al., 2016). Moreover, during an active response, immune effectors localize to the vicinity of the stem cells to form the “inflamed niche” and instruct stem cell behavior (Biton et al., 2017; Naik et al., 2017). Accordingly, ISCs express receptors for a number of inflammatory mediators, enabling them to adjust to their specific inflammatory milieu (Biton et al., 2017; Lindemans et al., 2015; Moltke et al., 2016).
It is unclear how stem cells revert back to their homeostatic functions once inflammation resolves. However, their ability to do so is vital for tissue health. Indeed, the absence of appropriate spatial and temporal resolution of an active immune response leads to chronic inflammation. This dire condition appears to spare no tissue stem cell and is central to a number of immune-mediated diseases including psoriasis, atopic dermatitis, asthma, rhinosinusitis and inflammatory bowel disease (IBD) (Gersemann et al., 2011; Lowes et al., 2007; Ordovas-Montanes et al., 2018). Moreover, in these chronic states, prolonged stem cell dysregulation can result in a defective barrier and antimicrobial function, which in turn can permit microbial penetration and further intensify the inflammatory response (Belkaid and Hand, 2014). Not surprisingly, chronic inflammation in tissues results in activation of aberrant wound repair programs (Lowes et al., 2007; Rieder et al., 2007).
Age-Associated Inflammation and Stem Cells
Age-associated decline in tissue function is usually marked by increased levels of pro-inflammatory mediators, low-grade systemic inflammation and impaired wound healing (Blau et al., 2015; Goldberg and Dixit, 2015). Recent studies suggest that some of these age-related defects result from miscommunication between immune cells and tissue stem cells and/or an accumulation of proinflammatory mediators in the tissue.
Mouse skin offers an interesting ecosystem to examine this failed conversation between resident immune cells and aged-stem cells, as its epidermis houses a unique population of γδ T cells, called dendritic epidermal T cells (DETCs), that orchestrate wound repair (Jameson et al., 2002). Upon wounding, epidermal progenitors upregulate expression of Skint genes that are essential for alerting DETCs (Keyes et al., 2016). In turn, DETCs secrete keratinocyte growth factor and insulin growth factors to stimulate epidermal progenitor proliferation and accelerate healing (Jameson et al., 2002) (MacLeod et al., 2013). In aging skin, this communication network breaks down, and as a result, epidermal progenitors are left on their own to re-epithelialize the wound bed, without the help of DETCs (Keyes et al., 2016). Although human epidermis lacks DETCs, similar impairments in the dialogue between other tissue resident T cells and stem cells could underlie chronic wounds in aged individuals.
In addition to grappling with breakdowns in their communications with resident immune cells, aging stem cells also experience an accumulation of inflammatory mediators that leads to their functional decline. In the skin, the proliferative capacity of hair follicle stem cells diminishes, as does their capacity for hair regeneration (Matsumura et al., 2016; Doles et al., 2012). Notably, blocking the inflammatory response is sufficient to reverse the proliferative defects in these stem cells. Similarly, in the intestine, aged macrophages increase expression of TNFα, resulting in diminished ISC function, breaches in the epithelial barrier and increased intestinal permeability. This breakdown in tissue integrity creates a feed-forward circuit of inflammation, further perpetuating the aged phenotype (Thevaranjan et al., 2017).
The link between inflammation and aging stem cells is conserved across species, even those with different lifespans. In Drosophila intestine, for instance, ISCs are regulated by macrophage-like hemocytes, which are recruited upon tissue damage (Ayyaz et al., 2015). In young flies, the interaction with hemocytes promotes ISC proliferation and infection resistance, but in aging flies, it leads to intestinal dysplasia. Whether in insects or in mammals, blurring the physical lines of defense across an epithelial barrier causes bacteria translocation into the underlying tissue. These penetrating microbes further stimulate macrophages and other immune cells thereby perpetuating the age-driven inflammatory circuit.
A common theme emerging across aging tissues is that perturbations in the inflammatory mediators combine with intrinsic alterations in the stem cells to disrupt tissue architecture. Indeed, in a number of in vitro and in vivo settings, aged stem cells exhibit altered proliferative or differentiation capacity (Oh et al., 2014). Hyperproliferation of aged Drosophila ISCs leads to aberrant assembly and the formation of polyploid clusters. This deviation in stem cell behavior radically alters intestinal morphology, and can be recapitulated in young flies exposed to paraquat, an oxidative stress agent (Biteau et al., 2008). Aged and paraquat-exposed ISCs maintain high levels of JNK activity, suggesting a common underlying stress response in both settings (Biteau et al., 2008).
These are just a few of the many emerging examples that draw remarkable parallels between inflammatory diseases and age-related tissue dysfunction. In these situations, a buildup of proinflammatory factors invariably leads to tissue dysmorphia and functional decline. Thus, the keys to reversing the aging process in stem cells and rejuvenating tissues will be therapies that not only stimulate tissue stem cell self-renewal but also target inflammatory mediators that accumulate with age.
Immune Privilege: Protection from Friendly Fire
Tissues have evolved a number of strategies to preserve their precious pools of stem cells in the face of harm. These include flooding the niche with anti-inflammatory mediators and upregulating the expression of immune suppressive molecules. Bone marrow Tregs, for instance, produce the immune suppressive cytokine IL-10 in the vicinity of HSCs to limit inflammatory stress in their niche (Fujisaki et al., 2011) (Figure 3A). This permissive environment facilitates the transplantation of genetically nonidentical allo-HSCs, which are rapidly lost upon depletion of Tregs.
The epithelial tissues that line our body have developed particularly sophisticated means of protecting their stem cells. The hair follicle stem cell niche has long been described as a site of immune privilege, expressing low levels of immune-activating molecules and high numbers of Tregs (Ali et al., 2017; Christoph et al., 2000). Working smoothly in normal homeostasis, this inflammation-repelling sanctuary can become overwhelmed by exuberant immune responses (Petukhova et al., 2010). In such inflammatory settings, immune signals can also be protective. ISCs for instance, rely on signals from type 3 innate lymphoid cells (ILC3) for protection from inflammatory and injurious damage and stimulate their activation via IL-22 to promote regeneration (Aparicio-Domingo et al., 2015; Hanash et al., 2012). Intriguingly, IL-22 and its downstream effector STAT3 bypass the need for Paneth cell-derived signals to activate ISCs and promote regeneration (Lindemans et al., 2015). Obviating requirements for homeostatic niche signals such as Wnts, and instead co-opting inflammatory cues to guide their behavior, may represent a stem cell adaptation to persevere and preserve tissue integrity under inflammatory stress.
Agudo and colleagues recently uncovered that a stem cell’s immune privilege status is linked to its level of activity (Agudo et al., 2018). Like most tissue cells, cycling stem cells of the intestine, ovary and mammary gland display major histocompatibility class I (MHC-I) as well as an array of cytokine receptors on their surface. Thus, as stem cells acquire mutations that are displayed as surface neoantigens complexed with MHC-I, they are likely to be expeditiously cleared from the tissue by killer T cells. This feature could serve as a check on hyperplasia and tumor formation. By comparison, quiescent stem cells such as those of the hair follicle and muscle, downregulate their antigen presenting machinery, which allows them to stay under the radar of immune surveillance and maintain tissue production long-term, albeit while increasing the mutational burden of these long-lived cells (Zindl and Chaplin, 2010). How cancerous cells similarly co-opt immune modulatory pathways such as PD-L1 to escape from immune surveillance and contribute tumor relapse following therapy is an intense avenue of study, which is still unfolding.
Thus, immune privilege can be both extrinsically imposed on stem cells (i.e. by Tregs) and at least in the cancerous state, intrinsically tuned by upregulating the expression of immune suppressive molecules (Barber et al., 2006). This ability to maneuver the immune system may have profound implications for stem cell clonal selection and tissue fitness.
Stem Cell Intrinsic Immunity and Innate Pathogen Sensing Pathways.
In addition to engaging immune cells, stem cells themselves have the ability to protect themselves against stressful situations, including pathogen encounters. In a recent study, Wu and colleagues discovered that stem cells are hardwired to express antiviral interferon-stimulated genes (ISGs), which help them fight viral infections (Wu et al., 2018) (Figure 4A). Although the precise means by which stem cells maintain high ISG expression is unclear, they express an array of PAMP and DAMP receptors, which could be responsible for upregulating ISGs. In the case of active viral infections, mesenchymal stem cells can activate ISGs by sensing infection through the cytosolic double stranded (ds) viral DNA sensor cGAS (Yang et al., 2015). Because many of these receptors have endogenous ligands that are available even in the absence of infections, stem cells have installed regulatory machineries to avoid aberrant activation by innate sensors. Dormant HSCs for example, express the circular RNA cis-GAS, with higher affinity for cGAS than its activating ligand, enabling calibration of HSC sensitivity to endogenous dsDNA and thereby preventing exhaustion (Xia et al., 2018) (Figure 4A).
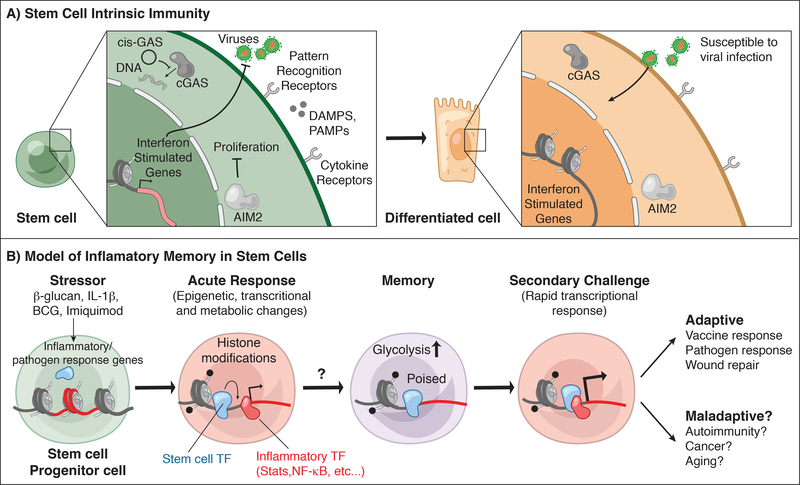
Figure 4: Stem cell intrinsic immunity and memory.
A) Stem cells express a variety of cytokine and pattern recognition receptors that can sense damage associated and pathogen associated molecular patterns (DAMPs, PAMPs) and cytokine signals from immune cells. These include both surface associated receptors and intracellular sensors such as cGAS and absent in melanoma 2 (AIM2) which bind double stranded DNA. AIM2 limits intestinal stem cell proliferation by modulating AKT. Hematopoietic stem cells express a circular RNA “cis-GAS” to regulate cGAS activation and limit exhaustion. SCs also express high levels of interferon-stimulated genes (ISGs) in contrast to differentiated progeny, and these baseline ISGs protect SCs from viral infections.
B) Stem cells are trained by a variety of acute inflammatory encounters: directly by β-glucan, IL-1β, Bacillus Calmette–Guérin (BCG) vaccine for Mycobacterium tuberculosis, and indirectly by Toll like receptor 7 (TLR7) agonist (Imiquimod) induced inflammation. These stimuli activate inflammatory transcription factors (STATs, NF-κB), which likely facilitate the remodeling of chromatin and acquisition of histone modifications at inflammatory stress response genes resulting in dramatic transcriptional and metabolic changes to the SCs and altered activation and cellular output. Upon resolution, stem cells retain changes to a subset of chromatin loci including altered accessibility and histone modifications. These changes may be maintained by homeostatic transcription factors in the absence of overt inflammation. Inflammation-experienced stem cells also exhibit heightened glycolytic activity and in the case of HSCs exhibit bias towards the myeloid lineage. During a secondary challenge, stem cells and their progeny exhibit heighted responses at genes corresponding with memory chromatin domains. This adaptive behavior promotes vaccine and pathogen responses and in the case of epithelial stem cells augmented wound healing. On the other hand, a negative consequence of such memory may be increased predisposition to autoimmunity, cancer or aging.
Another cytosolic DNA sensor, absent in melanoma 2 (AIM2), also seems to function in stem cell biology, as when AIM2 is lost, gut microbiota become dysbiotic and ISCs proliferate uncontrollably. In a manner that appears to be independent of its traditional role in governing the inflammasome pathway, AIM2-deficient mice display an increased susceptibility to develop colon cancer (Man et al., 2015; Wilson et al., 2015) (Figure 4A).
Neuronal stem/ precursor cells in the brain express an array of toll like receptors (TLRs), which sense a broad range of microbial and damage signals through an equally complex array of ligands. Studies in mice that lack different TLRs highlight their broad roles in controlling proliferation, differentiation, and migration of neural stem cells (Alvarado and Lathia, 2016). Not surprisingly, however, different TLRs and ligands can have markedly distinct and varied effects on stem cell behavior. Notably, a number of neurodevelopmental disorders are linked to early pathogen exposure, suggesting that inappropriate TLR activation on neural progenitors has grave consequences for disease (Atladóttir et al., 2010; Jiang et al., 2016; Patterson, 2009).
HSCs are perhaps the most stress-sensitive tissue stem cells whose rapid response is vital for organismal survival. These stem cells are therefore highly in tune with their macro and microenvironment, and express a myriad of TLRs whose engagement is capable of rapidly skewing fate choices from the normal differentiation program (Nagai et al., 2006). By increasing innate immune effectors such as macrophages, HSCs can bolster systemic immunity to swiftly cope with threats. As part of a motile organ system, HSCs patrol the body and also reside in niches outside the bone marrow such as the lung (Lefrançais et al., 2017; Massberg et al., 2007; Wright et al., 2001). These circulating and peripherally localized HSCs may be the first to sense danger, rapidly respond, or serve as an alternate reservoir when the primary niche is compromised.
Together, these studies point to a binary role for innate sensing by stem cells, first as a primitive pathogen detection system and second, as an intrinsic rheostat to guide their own behavior. One reason for this may be the presence of signals emanating from the damaged tissue necessitating the cooperation of anti-pathogen and regenerative responses within stem cells. Although, the relative contribution of innate receptors on stem cells versus their accessory cells is still unclear, it seems increasingly likely that synergistic stimulation of innate sensors on stem cells during an active pathogen or damage response will be essential to trigger the immune-stem cell cross-talk that is necessary to ensure rapid and efficient restoration of tissue homeostasis.
Stem cells remember inflammation
Owing to their longevity, stem cells encounter a number of inflammatory pressures. Their participation in inflammatory reactions poises stem cells to remember assaults long after they resolve. Recent studies have shed light on the remarkable capacity of stem cells to document inflammatory encounters by altering their chromatin landscape and subsequently their function.
The paradigm of inflammatory imprinting or “training” has been well documented in innate immune cells, which in contrast to antigen-specific T and B lymphocytes, alters the behavior of innate effectors to non-specific secondary stimuli (Netea et al., 2016). A series of elegant experiments have recently chronicled inflammatory training in parental HSCs. Kaufman and colleagues discovered a dramatic rewiring of HSCs following administration of the Mycobacterium tuberculosis (Mtb) vaccine. Intriguingly, HSCs then pass on this knowledge to their macrophage and monocyte progeny, which by elevating inflammatory mediators are more efficient at killing Mtb. These effects appeared to be rooted in changes to chromatin, as macrophages from vaccinated mice retained activating histone modifications (H3K4me3 and H3K27ac) at genes associated with pathogen responses (Figure 4B). Importantly, adoptive transfer and parabiosis studies revealed that vaccine-induced memory is long lasting and autonomous to HSCs (Kaufmann et al., 2018).
In a complementary study, β-glucan, a bacterial and fungal cell wall component induced IL-1β release, which was capable of training both HSCs and myeloid progenitors. These trained HSCs and myeloid progenitors were able to more efficiently ward off inflammatory challenges when compared to naïve HSCs. Intriguingly, IL-1β-trained HSCs exhibited dramatic changes in their energy metabolism, displaying augmented glycolysis and cholesterol biosynthesis, adjustments that turned out to be critical for conferring downstream functional changes in β-glucan-dependent HSC training (Figure 4B) (Mitroulis et al., 2018).
Microbial stimuli are not the only environmental stressors that can induce inflammation and reprogram progenitors. Calorically rich “Western” diets are associated with type II diabetes, obesity, and cardiovascular disease, all of which have an inflammatory component (Hotamisligil, 2006). Remarkably, mice fed even transiently (4 weeks) with a western diet displayed HSC proliferation, skewing of HSC lineages and exaggerated responses to a variety of inflammatory assaults. HSC chromatin accessibility was altered in a manner that was traced to the inflammasome activator NLRP3 (NLR Family Pyrin Domain Containing 3 NLR Family Pyrin Domain Containing 3), which in response to a “Western” diet metabolite, became activated to process and release the pro-inflammatory cytokine IL-1β (Christ et al., 2018). Exactly how IL1β sets off its downstream effectors to elicit sustained changes in chromatin accessibility remain unclear.
Unexpectedly, the link between inflammation, the inflammasome, IL-1β and epigenetic reprogramming of stem cells also has its reach outside the hematopoietic system, in this case, in the skin epithelial stem cells that experience an acute inflammation (Naik et al., 2017). While inflammatory memory has long been thought to be a phenomenon unique to hematopoietic lineages, surface epithelia bear the brunt of inflammation and ultimately are responsible for expeditious repair of tissue damage. By possessing a memory of an acute inflammatory assault, epithelial stem cells are better able to cope with subsequent barrier breaches. Epidermal stem cells remodel their chromatin following acute inflammation and although most of these changes in chromatin accessibility resolve following restoration of tissue homeostasis, a number of genomic loci associated with inflammation and the stress response remain accessible. Upon a secondary inflammatory challenge, e.g. wounding, these experienced stem cells rapidly upregulate transcripts governed by accessible chromatin domains. A key mediator of this memory is the inflammasome activator AIM2, which augments IL-1β to promote the regenerative process following injury (Naik et al., 2017).
Ordovas-Montanes and colleagues recently extended the findings of non-hematopoietic stem cell memory to respiratory epithelial progenitors in human allergic inflammatory disease (Ordovas-Montanes et al., 2018b). Airway epithelia from chronic rhinitis polyps displayed an enrichment for basal progenitor programs. Secondary stimulation of cultured polyp cells with type 2 cytokines IL-4 and IL-13 revealed a heightened transcriptional response and reinforcement of Wnt/β-catenin pathways that drive basal cell programs compared to non-polyp tissue. Although the basis of this memory remains unexplored, it likely contributes to epithelial pathology and dysfunction in allergic disease. If so, by bearing an inflammatory memory to adapt rapidly to injury, stem cells may face deleterious maladaptations in chronic inflammatory conditions. Exploring the relative contribution of immune and non-immune memory to disease onset and pathology in recurrent inflammatory diseases will be critical for devising therapeutic strategies with long-term efficacy.
While still in its infancy, uncovering commonalities in mechanisms underlying stem cell memory across different tissues will shape our understanding of inflammatory training and its contribution to tissue fitness. One area begging for insights is how epigenetic memory works, and how chromatin information can be propagated to offspring when stem cells divide. Inflammation-associated transcription factors such as STATs and NF-κB are activated rapidly by post-translational mechanisms, and the ensuing chromatin changes they elicit are notable in that they contain many accessible regions with sequence motifs not just for STATs and NF-κB but also for stem cell transcription factors (Naik et al., 2017). These newly accessible domains also contain binding sites for stem cell transcription factors, suggesting that they may remain open by binding these factors even when inflammation-activated TFs are no longer present. If so, this would explain why some chromatin domains remain poised long after the inflammation has resolved (Figure 4B).
Another factor contributing to the stabilization of this state could be DNA modifications, i.e. methylation (Hofmeister et al., 2017). Although histone modifications are less stable in their inheritance than DNA methylation, many stem cells divide infrequently, and this may permit more dynamic modifications to be sustained over time. At present, the molecular details underlying inflammatory memory are obscure, but offer a plethora of interesting avenues to explore in the future to add to our growing understanding of recurrent inflammatory disorders that often manifest in a single location.
Translating the Knowledge of Immune-Stem Cell Cross-Talk to the Clinic
Throughout this review, we have touched upon the key signals that activate immune-stem cell crosstalk to drive homeostasis and wound repair, but that when dysregulated can lead to disease. In this regard, there are three obvious means of leveraging these interactions for therapeutic benefit: (1) boosting immune mediators to promote regeneration, (2) correcting immune abnormalities that underlie tissue degeneration, and (3) interrupting this dialogue to curb pathologies in autoimmune conditions and aging (Karin and Clevers, 2016; Mass et al., 2017).
A number of immune-derived cytokines and growth factors such as amphiregulin, IL-6 and IL-22 are known to directly promote healing (Ali et al., 2017; Keyes et al., 2016; Lindemans et al., 2015). Infusing damaged tissues with recombinant versions of these factors and/or identifying agonists for their downstream targets in stem cells could greatly enhance repair. For instance, in muscular dystrophies, characterized by progressive skeletal muscle loss, augmenting Tregs or regenerative M2 macrophages, or alternatively, supplying the tissue with their effector cytokines, might help to restore muscle function (Arnold et al., 2007; Burzyn et al., 2013b). In cases such as chronic non-healing wounds, where stem cells fail to emit or respond to signals in their microenvironment, proregenerative signals will need to be complemented with a rewiring of the inflammatory circuitry in order to promote healing. A precise calibration of the inflammatory responses that enable repair will be necessary to kick-start healing in these situations (Zhao et al., 2016).
Proper resolution of inflammation demands repair, regeneration, and clearance of damaged tissue. For instance, in recurrent IBD, ISCs from inflamed intestines may be less capable of orchestrating efficient tissue repair. Indeed, single nucleotide polymorphisms (SNPs) in genes involved in epithelial restitution have a strong association with IBD pathology (McCole, 2014). In vitro-generated organoids with corrected SNP variants is one way to overcome this issue and restore the intestinal barrier. Such genetically-engineered organoids can be transplanted into inflamed intestines to promote healthy tissue restoration (Holmberg et al., 2017). As researchers continue to explore this exponentially expanding field of immune-stem cell interactions, they will be armed with a better understanding of the specific factors that can be therapeutically targeted to promote regeneration or curb inflammation-associated tissue dysfunction.
Acknowledgments
We thank Sigrid Knemeyer for illustrating our figures. We are also grateful to the many friends and colleagues in the stem cell and immunology fields whose work inspired us to write this review. Given the reference constraints, we regret that we could not cite all of the worthy papers on this topic. However, we have tried to present a balanced view of the field, attempting to clarify areas of seeming discrepancies and presenting the exciting areas of research that await us all. S.N. is a recipient of the Damon Runyon Dale F. Frey Award for Breakthrough Scientists (DFS-30–18). S.B.L. is the recipient of a Ruth L Kirschstein Predoctoral Individual NRSA Fellowship (1F31AR068920–01A1). E.F. is an Investigator of the Howard Hughes Medical Institute. This work was supported by grants from the NIH (R01-AR050452 and R01-AR31737, E.F.) and a L’Oreal For Women in Science Fellowship (S.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A, Merad M, et al. (2018). Quiescent Tissue Stem Cells Evade Immune Surveillance. Immunity 48, 271–285.e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong H-A, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, et al. (2017). Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 169, 1119–1129.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado AG, and Lathia JD (2016). Taking a Toll on Self-Renewal: TLR-Mediated Innate Immune Signaling in Stem Cells. Trends in Neurosciences 39, 463–471. [DOI] [PubMed] [Google Scholar]
- Aparicio-Domingo P, Romera-Hernandez M, Karrich JJ, Cornelissen F, Papazian N, Lindenbergh-Kortleve DJ, Butler JA, Boon L, Coles MC, Samsom JN, et al. (2015). Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. The Journal of Experimental Medicine 212, 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, and Chazaud B (2007). Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of Experimental Medicine 204, 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, and Parner ET (2010). Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders 40, 1423–1430. [DOI] [PubMed] [Google Scholar]
- Aurora AB, and Olson EN (2014). Immune modulation of stem cells and regeneration. Cell Stem Cell 15, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyaz A, Li H, and Jasper H (2015). Haemocytes control stem cell activity in the Drosophila intestine. Nature Cell Biology 17, 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, and Ahmed R (2006). Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, and Hand TW (2014). Role of the microbiota in immunity and inflammation. Cell 157, 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, and Jasper H (2008). JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3, 442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton M, Haber A, Beyaz S, Rogel N, Smillie C, Shekhar K, Schnell A, Chen Z, Wu C, Ordovas-Montanes J, et al. (2017). T helper cells modulate intestinal stem cell renewal and differentiation. bioRxiv. [DOI] [PMC free article] [PubMed]
- Blau HM, Cosgrove BD, and Ho ATV (2015). The central role of muscle stem cells in regenerative failure with aging. Nature Medicine 21, 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers E, Slaughter A, Frenette PS, Kuick R, Pello OM, and Lucas D (2018). Granulocyte-derived TNFα promotes vascular and hematopoietic regeneration in the bone marrow. Nature Medicine 24, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, and Rando TA (2012). Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell 10, 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Benoist C, and Mathis D (2013a). Regulatory T cells in nonlymphoid tissues. Nature Immunology 14, 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. (2013b). A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, et al. (2013). Rhythmic Modulation of the Hematopoietic Niche through Neutrophil Clearance. Cell 153, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana D, Paus R, and Perez-Moreno M (2014). Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biology 12, e1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Celià-Terrassa T, Kumar S, Hang X, Wei Y, Choudhury A, Hwang J, Peng J, Nixon B, Grady JJ, et al. (2018). Notch ligand Dll1 mediates cross-talk between mammary stem cells and the macrophageal niche. Science (New York, N.Y.) 360, eaan4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, et al. (2015). Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 161, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, Pinho S, Leboeuf M, Noizat C, van Rooijen N, et al. (2013). CD169⁺ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature Medicine 19, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. (2011). Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. The Journal of Experimental Medicine 208, 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, et al. (2018). Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 172, 162–175.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph T, Müller-Röver S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, Rückert R, and Paus R (2000). The human hair follicle immune system: cellular composition and immune privilege. The British Journal of Dermatology 142, 862–873. [DOI] [PubMed] [Google Scholar]
- Clevers H, Loh KM, and Nusse R (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science (New York, N.Y.) 346, 1248012–1248012. [DOI] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, and Taylor PR (2013). Tissue-resident macrophages. Nature Immunology 14, 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doles J, Storer M, Cozzuto L, Roma G, and Keyes WM (2012). Age-associated inflammation inhibits epidermal stem cell function. Genes & Development 26, 2144–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Shih C-H, Wosczyna MN, Mueller AA, Cho J, Aggarwal A, Rando TA, and Feldman BJ (2017). Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nature Communications 8, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, and Randolph GJ (2014). Origin and functions of tissue macrophages. Immunity 41, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, and Rudensky AY (2016). Hallmarks of Tissue-Resident Lymphocytes. Cell 164, 1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Celso, Lo C, Tsuyuzaki H, et al. (2011). In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474, 216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ, Locasale JW, et al. (2016). Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nature Immunology 17, 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersemann M, Stange EF, and Wehkamp J (2011). From intestinal stem cells to inflammatory bowel diseases. World Journal of Gastroenterology 17, 3198–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EL, and Dixit VD (2015). Drivers of age-related inflammation and strategies for healthspan extension. Immunological Reviews 265, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Cruz-Racelis, Dela J, and Fuchs E (2009). A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorki DE, Asselin-Labat M-L, van Rooijen N, Lindeman GJ, and Visvader JE (2009). Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Research : BCR 11, R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, et al. (2012). Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, and Chawla A (2013). Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Furuhashi K, Ishii H, Li HW, Pinho S, Ding L, Robson SC, Frenette PS, and Fujisaki J (2018). CD150high Bone Marrow Tregs Maintain Hematopoietic Stem Cell Quiescence and Immune Privilege via Adenosine. Cell Stem Cell 22, 445–453.e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister BT, Lee K, Rohr NA, Hall DW, and Schmitz RJ (2017). Stable inheritance of DNA methylation allows creation of epigenotype maps and the study of epiallele inheritance patterns in the absence of genetic variation. 18, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg FE, Seidelin JB, Yin X, Mead BE, Tong Z, Li Y, Karp JM, and Nielsen OH (2017). Culturing human intestinal stem cells for regenerative applications in the treatment of inflammatory bowel disease. EMBO Molecular Medicine 9, 558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2006). Inflammation and metabolic disorders. Nature 444, 860–867. [DOI] [PubMed] [Google Scholar]
- Hsu Y-C, Li L, and Fuchs E (2014a). Emerging interactions between skin stem cells and their niches. Nature Medicine 20, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y-C, Li L, and Fuchs E (2014b). Transit-Amplifying Cells Orchestrate Stem Cell Activity and Tissue Regeneration. Cell 157, 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, and Havran WL (2002). A role for skin gammadelta T cells in wound repair. Science (New York, N.Y.) 296, 747–749. [DOI] [PubMed] [Google Scholar]
- Janeway CA, and Medzhitov R (2002). Innate immune recognition. Annual Review of Immunology 20, 197–216. [DOI] [PubMed] [Google Scholar]
- Jiang H-Y, Xu L-L, Shao L, Xia R-M, Yu Z-H, Ling Z-X, Yang F, Deng M, and Ruan B (2016). Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain, Behavior, and Immunity 58, 165–172. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu L-F, and Rudensky AY (2012). Regulatory T cells: mechanisms of differentiation and function. Annual Review of Immunology 30, 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, and Clevers H (2016). Reparative inflammation takes charge of tissue regeneration. Nature 529, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier J-C, et al. (2018). BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 172, 176–190.e19. [DOI] [PubMed] [Google Scholar]
- Keyes BE, Liu S, Asare A, Naik S, Levorse J, Polak L, Lu CP, Nikolova M, Pasolli HA, and Fuchs E (2016). Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 167, 1323–1338.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, and Mathis D (2016). Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 44, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, and Amit I (2014). Tissue-Resident Macrophage Enhancer Landscapes Are Shaped by the Local Microenvironment. Cell 159, 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, et al. (2017). The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. (2015). Interleukin-22 promotes intestinalstem-cell-mediated epithelial regeneration. Nature 528, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, and Krueger JG (2007). Pathogenesis and therapy of psoriasis. Nature 445, 866–873. [DOI] [PubMed] [Google Scholar]
- MacLeod AS, Hemmers S, Garijo O, Chabod M, Mowen K, Witherden DA, and Havran WL (2013). Dendritic epidermal T cells regulate skin antimicrobial barrier function. The Journal of Clinical Investigation 123, 4364–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RKS, Gurung P, et al. (2015). Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell 162, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass E, Jacome-Galarza CE, Blank T, Lazarov T, Durham BH, Ozkaya N, Pastore A, Schwabenland M, Chung YR, Rosenblum MK, et al. (2017). A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease. Nature 549, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. (2007). Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131, 994–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Mohri Y, Binh NT, Morinaga H, Fukuda M, Ito M, Kurata S, Hoeijmakers J, and Nishimura EK (2016). Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science (New York, N.Y.) 351, aad4395–aad4395. [DOI] [PubMed] [Google Scholar]
- McCole DF (2014). IBD candidate genes and intestinal barrier regulation. Inflammatory Bowel Diseases 20, 1829–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metchnikoff E (1893). Lectures on the Comparative Pathology of Inflammation.
- Mitroulis I, Ruppova K, Wang B, Chen L-S, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, et al. (2018). Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 172, 147–161.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltke, von J, Ji M, Liang H-E, and Locksley RM (2016). Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R, Kondo T, Ohshima T, Ishida Y, and Mukaida N (2002). Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology 16, 963–974. [DOI] [PubMed] [Google Scholar]
- Mueller SN, and Mackay LK (2016). Tissue-resident memory T cells: local specialists in immune defence. Nature Reviews. Immunology 16, 79–89. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, and Kincade PW (2006). Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24, 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, Polak L, Kulukian A, Chai S, and Fuchs E (2017). Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O’Neill LAJ, and Xavier RJ (2016). Trained immunity: A program of innate immune memory in health and disease. Science (New York, N.Y.) 352, aaf1098–aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosbaum A, Prevel N, Truong H-A, Mehta P, Ettinger M, Scharschmidt TC, Ali NH, Pauli ML, Abbas AK, and Rosenblum MD (2016). Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. Journal of Immunology (Baltimore, Md. : 1950) 196, 2010–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Lee YD, and Wagers AJ (2014). Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nature Medicine 20, 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, Wadsworth MH, Hughes TK, Kazer SW, Yoshimoto E, et al. (2018a). Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, Wadsworth MH, Hughes TK, Kazer SW, Yoshimoto E, et al. (2018b). Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature 139, 1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH (2009). Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behavioural Brain Research 204, 313–321. [DOI] [PubMed] [Google Scholar]
- Petrie TA, Strand NS, Yang C-T, Tsung-Yang C, Rabinowitz JS, and Moon RT (2014). Macrophages modulate adult zebrafish tail fin regeneration. Development 141, 2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, Kim H, Singh P, Lee A, Chen WV, et al. (2010). Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 466, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, la Cruz, de D, Baker RE, Maini PK, Maxson R, and Chuong CM (2008). Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451, 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder F, Brenmoehl J, Leeb S, Schölmerich J, and Rogler G (2007). Wound healing and fibrosis in intestinal disease. Gut 56, 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Vignali DAA, Rudensky AY, Niec RE, and Waldmann H (2013). The plasticity and stability of regulatory T cells. Nature Reviews. Immunology 13, 461–467. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Donaldson DS, Pridans C, Sauter KA, Hume DA, and Mabbott NA (2018). The role of CSF1R-dependent macrophages in control of the intestinal stem-cell niche. Nature Communications 9, 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DW-M, and Barker N (2014). Intestinal stem cells and their defining niche. Current Topics in Developmental Biology 107, 77–107. [DOI] [PubMed] [Google Scholar]
- Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, et al. (2017). Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host & Microbe 21, 455–466.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi G, C Poholek A, Hand TW, Laurence A, Kanno Y, O’Shea JJ, and Hirahara K (2013). Helper T-cell identity and evolution of differential transcriptomes and epigenomes. Immunological Reviews 252, 24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, et al. (2015). Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nature Medicine 21, 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, and Weissman IL (2001). Physiological migration of hematopoietic stem and progenitor cells. Science (New York, N.Y.) 294, 1933–1936. [DOI] [PubMed] [Google Scholar]
- Wu X, Dao Thi VL, Huang Y, Billerbeck E, Saha D, Hoffmann H-H, Wang Y, Silva LAV, Sarbanes S, Sun T, et al. (2018). Intrinsic Immunity Shapes Viral Resistance of Stem Cells. Cell 172, 423–438.e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Wang S, Ye B, Du Y, Li C, Xiong Z, Qu Y, and Fan Z (2018). A Circular RNA Protects Dormant Hematopoietic Stem Cells from DNA Sensor cGAS-Mediated Exhaustion. Immunity 48, 688–701.e7. [DOI] [PubMed] [Google Scholar]
- Yang H, Adam RC, Ge Y, Hua ZL, and Fuchs E (2017). Epithelial-Mesenchymal Micro-niches Govern Stem Cell Lineage Choices. Cell 169, 483–496.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Wang J, Wu M, Li M, Wang Y, and Huang X (2015). Mesenchymal stem cells detect and defend against gammaherpesvirus infection via the cGAS-STING pathway. Scientific Reports 5, 7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Liang H, Clarke E, Jackson C, and Xue M (2016). Inflammation in Chronic Wounds. International Journal of Molecular Sciences 17, 2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindl CL, and Chaplin DD (2010). Immunology. Tumor immune evasion. Science (New York, N.Y.) 328, 697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]