Abstract
Objective
We aimed to determine if opioid risk reduction initiatives including dose reduction and risk mitigation strategies for chronic noncancer pain patients receiving chronic opioid therapy (COT) had a differential impact on average daily opioid doses of COT patients at higher risk for opioid-related adverse outcomes compared with lower-risk patients.
Design
Interrupted time series.
Setting
Group Health Cooperative (GH), a health care delivery system and insurance within Washington State, between 2006 and 2014.
Population
GH enrollees on COT defined as receiving a supply of 70 or more days of opioids within 90 days using electronic pharmacy data for filled prescriptions.
Methods
We compared the average daily morphine equivalent doses (MED) of COT patients with and without each of the following higher-risk characteristics: mental disorders, substance use disorders, sedative use, and male gender.
Results
In all four pairwise comparisons, the higher-risk subgroup had a higher average daily MED than the lower-risk subgroup across the study period. Adjusted for covariates, modest differences in the annual rate of reduction in average daily MED were noted between higher- and lower-risk subgroups in three pairwise comparisons: those with mental disorders vs without (–8.2 mg/y vs –5.2 mg/y, P = 0.005), with sedative use vs without (–9.2 mg/y vs –5.8 mg/y, P = 0.004); mg), in men vs women (–8.8 mg/y vs –5.9 mg/y, P = 0.01).
Conclusion
Using clinical policy initiatives in a health care system, dose reductions were achieved among COT patients at higher risk for opioid-related adverse outcomes that were at least as large as those among lower-risk patients.
Keywords: opiates, overdose, risk mitigation, addiction, mental disorders, prescription opioids
Introduction
In 2014, 78 Americans died every day from an opioid overdose, and at least half of these overdose deaths involved prescription opioids [1]. Epidemiologic studies have found a dose-dependent relationship between opioid dose and overdose risk [2–5]. Despite the increased risks associated with high-dose chronic opioid therapy (COT), studies have found that high-dose COT is more common among individuals at higher risk for opioid use disorder (addiction) and overdose [6–11]. Several states and the Centers for Disease Control (CDC) have proposed opioid dosing thresholds to reduce risks of overdose and addiction among COT patients as part of broader efforts to promote guideline-concordant care. Little research has evaluated how efforts to reduce opioid-related risks affect opioid doses of COT patients at higher risk for opioid-related morbidity and mortality, the patients most likely to receive high-dose therapy [12].
Numerous studies have documented prescribing patterns where persons at higher risk for opioid use disorder and overdose are more often prescribed high-dose COT [10,11,13,14]. Characteristics associated with higher risk and higher doses include history of mental disorder, history of alcohol or nonopioid substance use disorders (SUD), concurrent use of sedatives, and male gender [6–11]. Individuals with SUDs and mental disorders are more likely to use opioid prescriptions for nonpain symptoms, such as stress or anxiety, and increase dose without consulting the prescriber [10,11,13,14]. Concurrent use of opioids and sedative medications, such as benzodiazepines, is of concern because they act synergistically to increase respiratory depression [15,16]. Men are also more likely to experience dose escalation despite a higher rate of overdose deaths than women [17].
Sullivan (2010) [11] suggested that one possible reason for these prescribing patterns is that higher-risk patients are distressed and express the greatest demands for pain relief and thus are more likely to be prescribed high-dose COT. Dose escalation may also be a response to increasing tolerance over the course of COT, which may also be related to risk factors for overdose and addiction. The same factors that cause dose escalation in higher-risk patients could also reduce the likelihood for dose reduction. If so, the potential impact of dose reduction initiatives in reducing overdose risk in COT patients could be overestimated if the highest-risk patients were the least likely to reduce dose.
The CDC released recommendations in 2016 aimed at reducing risks associated with COT, which were influenced by earlier state policies, such as the Washington State Agency Medical Directors’ Group (AMDG) Interagency Guideline on Prescribing Opioids for Pain [18,19]. In response to the Washington State guideline, an integrated health care system in Washington State implemented opioid risk reduction initiatives to identify COT patients above a dosing threshold of 120 mg morphine equivalent dose (MED) per day as potentially appropriate candidates for dose reduction with supervision of prescribers with greater numbers of COT patients. Subsequently, the health plan implemented additional risk mitigation strategies and closer monitoring of COT patients, such as a risk-stratified schedule for the frequency of urine drug screening and follow-up visits [20]. Prior research found that these initiatives decreased the average daily MED among COT patients in the health care system receiving both lower and higher doses, with the average daily dose dropped from 75.8 mg to 40.0 mg from 2008 to 2014 [20].
Although average daily opioid doses of COT patients were reduced during the years the opioid risk reduction initiatives were implemented, in order to understand the impact these initiatives may have on opioid-related morbidity and mortality, evaluating dosing trends specifically among higher-risk COT patients is critically important. This study aimed to determine if the average daily opioid doses of four higher-risk groups of COT patients in primary care clinics were differentially impacted by opioid risk reduction initiatives compared with their lower-risk counterparts. The characteristics used to define higher-risk groups were a history of mental disorders, history of SUDs, concurrent sedative use, and male gender. We hypothesized that dose reduction would be attenuated in these higher-risk groups compared with the corresponding lower-risk groups.
Methods
Setting
Group Health Cooperative is a consumer-governed, nonprofit integrated health care delivery system in Washington State. Providers in the system deliver care at Group Health’s own facilities, which also house integrated pharmacies. This research was approved by the Group Health Institutional Review Board, which permitted analyses of electronic health care data with a waiver of consent.
Population
Using pharmacy data for filled prescriptions, participants were included in each quarter of the study (three-month calendar window) for which they received ≥70 days’ supply of opioids. This 70-day supply in 90 days threshold for defining COT is consistent with Group Health’s practice guideline [20]. Participants also met the following inclusion criteria for each quarter of the study: age ≥26 years and at least 12 months of continuous enrollment in the health insurance plan prior to and including the current quarter. Patients with cancer pain were excluded and identified by a hospice claim filed within the quarter or by having two or more cancer diagnoses in the prior three years of medical history.
Opioid Dose and Risk Reduction Initiatives
To address the growing epidemic of opioid overdoses, Washington State released an interagency guideline for opioid prescribing for chronic noncancer pain including recommendations to monitor the safety and effectiveness of COT [18,19] which prompted Group Health Cooperative to examine its clinical policies on COT. As a result, from 2008 to 2014, Group Health Cooperative implemented opioid risk reduction initiatives in its clinical facilities initially focused on the opioid prescribing practices of primary care providers (PCPs) for patients with chronic noncancer pain and later multifaceted risk mitigation strategies to increase compliance with updated recommendations from Washington State [21]. A full description of the dose and risk reduction initiatives in the health care system has been published [20,21].
Of key interest were three time periods: 1) baseline period, 2) dose reduction period, and 3) risk-mitigation period.
The baseline time period from 2006 through 2007 predated extensive implementation of the state dose threshold guideline and any implementation of multifaceted opioid risk reduction initiatives.
During the dose reduction period from January 1, 2008, through September 30, 2010, the group practice implemented practice guidelines to discourage high-dose COT, defined as ≥120 mg MED daily. PCPs received lists of the primary care patients they were responsible for treating who were at or above the dosing threshold. PCPs with greater numbers of high-dose COT patients received feedback from medical directors. Lowering COT doses was strongly supported by medical staff leadership and consulting specialists in Physical and Rehabilitation Medicine, including voluntary in-service continuing education sessions regarding chronic pain management attended by many primary care providers.
The risk mitigation period from October 1, 2010, to September 30, 2014, was characterized by a risk-stratified schedule for the frequency of follow-up visits and urine drug screening, compliance with the Washington State new recommendation for pain specialty consultation for patients on ≥120 mg MED daily. Health system pharmacies modified refill processes notifying prescribers of early refills. Practice tools were integrated in the electronic medical record, an online course that had an 87% PCP participation rate [22], and onsite resources for consultation. In 2011, more than half of all COT patients received a yearly urine drug screening compared with 7% in 2009 [23].
Measures
Average Daily Morphine Equivalent Dose
Using methods and conversion factors described elsewhere [2,24–26], we assumed that patients took all prescribed opioids at the maximum dose and on the schedule recommended by their clinicians. The specific daily dose contributed by each opioid prescription fill was determined by dividing the total morphine-equivalent milligrams dispensed in that fill by the number of days supplied. Each patient’s average daily dose was calculated by adding the daily doses of all opioid prescription fills that covered that particular day and dividing by 90 days.
Mental Disorders
We included diagnoses for mental disorders cited in the Washington State guidelines, specifically depression, bipolar, anxiety, conversion disorder, somatization disorders, borderline personality disorder, and post-traumatic stress disorder (PTSD) [19]. Additional diagnoses associated with higher risk for opioid-related adverse outcomes or poor adherence were also included: attention deficit disorder (ADD), autism, schizophrenia spectrum, dementia, or other psychosis [27,28]. For each quarter, we identified COT patients with mental disorders by using electronic administrative data of recorded diagnoses in the prior three years.
Substance Use Disorders
Current or recent history of substance use disorders (SUD) was defined as the presence of a diagnostic code for alcohol or nonopioid abuse/dependence in the prior three years.
Any Sedative Use
Using the AHFS Pharmacologic-Therapeutic Classification system, we identified two classes of sedatives: benzodiazepines and anxiolytics/sedatives/hypnotics [29]. Although benzodiazepines are more commonly prescribed, other sedatives were included because of the increased risk of opioid overdose; for example, zolpidem increases the risk of opioid overdose by as much as 77% [30]. Sedatives were included based on the following criteria: 1) opiate potentiating (additive effect of central nervous system depression with concurrent use of an opiate); 2) likely duration of treatment greater than one week. The following sedatives met inclusion criteria: benzodiazepines, eszopiclone, hydroxyzine, meprobamate, ramelteon, suvorexant, zaleplon, and zolpidem. Sedative use was defined as filling at least one prescription of the aforementioned sedatives within the quarter.
Covariates
Adjustment variables in all models included residence (Eastern vs Western WA), comorbidity (Charlson score of 0, 1, 2, or 3+) [31], age (26–45, 46–64, or 65+ years) and smoking status. Group Health administrative data provided residence and age. For each quarter, electronic administrative data of recorded diagnoses was used to characterize comorbidity using the Charlson score (prior one year) and smoking status (prior three years).
Statistical Analyses
To describe the sample, we presented characteristics of patients overall and by subgroups of their first eligible quarter. For each longitudinal subgroup analysis, we estimated trends in the average daily opioid dose over time using a separate linear regression model that included main effects for the subgroup of interest (e.g., men vs women), adjustment covariates (including other subgroups), and calendar time (measured quarterly and modeled using linear splines [32], with knots at the first quarter of 2008 and fourth quarter of 2010 to permit different trends across the time periods of interest). Potential differential temporal trends by subgroups were estimated by including model interaction terms between the main effects for the subgroup of interest and the linear spline terms for calendar time. Regression model parameters were estimated using generalized estimating equations (GEE) assuming a working correlation matrix; robust standard errors were calculated via the sandwich estimator [33] to account for within-person correlation over time. The adjusted estimates and 95% confidence intervals (CIs) for the change per year in average daily MED (Δ mean) were then provided for each subgroup of interest. Age-stratified analyses were performed, not as part of primary analyses. Sensitivity analyses were performed, truncating the average daily MED at 500 mg to assess the effect of extreme observations.
Secondary analyses were performed estimating the differential impact of the initiative on the proportion of COT patients receiving ≥120 mg MED daily. Modified Poisson regression models were used to estimate relative risks (RRs) and 95% CIs for the relative change per year in proportion of COT patients receiving ≥120 mg average daily MED for each subgroup; models included main effects, interaction terms, and adjustment for covariates in the same manner as the primary analyses. As before, GEE and robust sandwich estimators were used to estimate model parameters and calculate standard errors for these modified Poisson models [34].
Results
There were 23,809 COT patients meeting eligibility criteria over the 8.75-year study, and the average number of person-years of observation over the study period was 2.08; approximately 80–86% of the sample was retained from one quarter to the next. As of their first eligibility quarter, the sample was 63.8% women, 47.1% were age 46 to 64 years, and 87.6% resided in Western Washington (Table 1). The population had a relatively low level of comorbidity, with 66.0% scoring 0–1 on the Charlson Comorbidity Index [31]. About one-fourth had a current or recent history of smoking. Of the high-risk characteristics included, the most common was a mental disorder diagnosis (56.2%), followed by sedative use (32.4%) and SUD (9.9%). Of those with mental disorders, 70.8% were women, 13.7% had an SUD, and 42.8% filled at least one prescription for a sedative in the first eligible quarter. Of those with SUDs, 78.1% had a co-occurring mental disorder, 53.7% were women, and 43.5% used sedatives. Among those with any sedative use, 74.1% had a mental disorder, 70.3% were women, and 13.2% had an SUD.
Table 1.
Characteristics of COT patients (as of each patient’s first COT eligibility-quarter during the study period)
| Gender |
Substance Use Disorders |
Mental Disorders |
Any Sedative Use |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Female | Male | No | Yes | No | Yes | No | Yes | |
| N = 23, 809 | N = 15, 197 | N = 8,612 | N = 21,458 | N = 2,351 | N = 10,440 | N = 13,369 | N = 16,089 | N = 7,720 | |
| % | % | % | % | % | % | % | % | % | |
| Western Washington | 87.6 | 87.8 | 87.2 | 87.8 | 85.9 | 87.8 | 87.5 | 88.0 | 86.8 |
| Female | 63.8 | 100.0 | 0.0 | 64.9 | 53.7 | 54.9 | 70.8 | 60.8 | 70.3 |
| Age, y | |||||||||
| 26–45 | 24.3 | 24.7 | 23.7 | 23.3 | 33.4 | 20.9 | 26.9 | 22.7 | 27.5 |
| 46–64 | 47.1 | 43.7 | 53.0 | 47.1 | 46.2 | 49.2 | 45.4 | 45.7 | 50.0 |
| 65+ | 28.7 | 31.7 | 23.4 | 29.6 | 20.4 | 29.9 | 27.7 | 31.6 | 22.5 |
| Substance use disorders | 9.9 | 8.3 | 12.7 | 0.0 | 100.0 | 4.9 | 13.7 | 8.3 | 13.2 |
| Mental disorders | 56.2 | 62.3 | 45.3 | 53.8 | 78.1 | 0.0 | 100.0 | 47.5 | 74.1 |
| Any sedative use | 32.4 | 35.7 | 26.7 | 31.2 | 43.5 | 19.1 | 42.8 | 0.0 | 100.0 |
| Comorbidity score | |||||||||
| 0 | 57.4 | 57.9 | 56.4 | 58.0 | 51.6 | 61.6 | 54.0 | 57.2 | 57.7 |
| 1 | 8.6 | 7.8 | 10.0 | 8.8 | 7.4 | 8.9 | 8.4 | 8.7 | 8.5 |
| 2 | 14.8 | 15.2 | 13.9 | 14.5 | 17.2 | 13.8 | 15.5 | 14.2 | 15.9 |
| 3+ | 19.3 | 19.1 | 19.6 | 18.8 | 23.9 | 15.7 | 22.1 | 19.9 | 18.0 |
| Smoking | 26.7 | 25.9 | 28.2 | 24.5 | 47.5 | 23.0 | 29.7 | 25.5 | 29.4 |
COT = chronic opioid therapy.
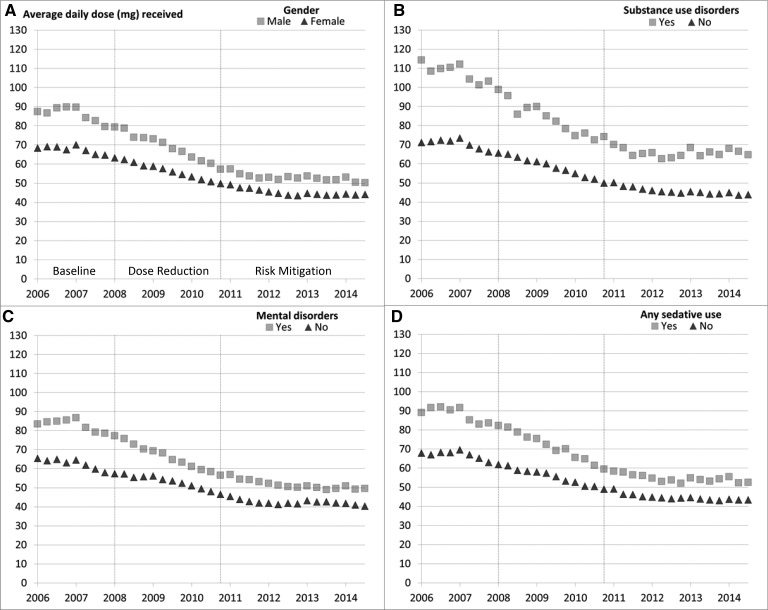
Figure 1 demonstrates the unadjusted trends in average daily MED among higher- and lower-risk subgroups for each pairwise comparison across all three study periods. In all four pairwise comparisons of higher- and lower-risk subgroups, the higher-risk subgroup had a higher average daily MED than the lower-risk subgroup across all three periods of the study (Figure 1).
Figure 1.
Unadjusted average daily opioid morphine equivalent dose received among chronic opioid therapy patients stratified by higher- and lower-risk subgroups across three study periods.
The dosing reduction period was marked by the highest rates of decline per year in average daily MED for the COT patient population for the four comparisons of higher- and lower-risk subgroups. Adjusted for covariates, the differences in the rate of decline in average daily MED per year during the dose reduction period between the higher- and lower-risk groups were modest but statistically significant for those with and without mental disorders, with and without any sedative use, and in men vs women, with the higher-risk groups having slightly higher decrements in dose than the lower-risk groups. For those with and without SUDs, there was modest evidence (P = 0.064) of a difference in the adjusted rate of decline in average daily MED during the dose reduction period. Minimal further dose reductions occurred during the risk mitigation period for all groups, and the average annual decline was not significantly different between lower- and higher-risk groups in the other periods (Table 2).
Table 2.
Estimated change (Δ) per year in average daily opioid dose by high-risk subgroup
|
Gender |
Substance Use Disorders |
||||
| Time Period |
Female |
Male |
No |
Yes |
|
| ΔMean (95% CI) | ΔMean (95% CI) | ΔMean (95% CI) | ΔMean (95% CI) | ||
| 1: Baseline Jan 2006–Jan 2008 | −3.7 (−5.6 to − 1.9) | −5.3 (−9.4 to − 1.3) | −3.8 (−5.8 to − 1.8) | −8.8 (−17.0 to − 0.6) | |
| P* | 0.479 | 0.263 | |||
| 2: Dose reduction Jan 2008–Oct 2010 | −5.9 (−7.0 to − 4.8) | −8.8 (−10.8 to − 6.9) | −6.5 (−7.6 to − 5.5) | −10.7 (−14.9 to − 6.5) | |
| P* | 0.010 | 0.064 | |||
| 3: Risk mitigation Oct 2010–Sep 2014 | −1.3 (−2.1 to − 0.6) | −1.5 (−2.7 to − 0.3) | −1.5 (−2.1 to − 0.8) | −0.8 (−3.4 to 1.9) | |
| P* | 0.851 | 0.635 | |||
|
Mental Disorders |
Any Sedative Use |
||||
| Time period |
No |
Yes |
No |
Yes |
|
| ΔMean (95% CI) | ΔMean (95% CI) | ΔMean (95% CI) | ΔMean (95% CI) | ||
| 1: Baseline Jan 2006–Jan 2008 | −3.7 (−6.5 to − 1.0) | −5.0 (−7.7 to − 2.3) | −3.8 (−6.0 to − 1.6) | −5.3 (−8.6 to − 2.0) | |
| P* | 0.539 | 0.456 | |||
| 2: Dose reduction Jan 2008–Oct 2010 | −5.2 (−6.6 to − 3.8) | −8.2 (−9.7 to − 6.7) | −5.8 (−7.0 to − 4.6) | −9.2 (−11.1 to − 7.2) | |
| P* | 0.005 | 0.004 | |||
| 3: Risk mitigation Oct 2010–Sep 2014 | −1.1 (−2.2 to 0.0) | −1.5 (−2.4 to − 0.7) | −1.3 (−2.1 to − 0.6) | −1.6 (−2.7 to − 0.5) | |
| P* | 0.605 | 0.716 | |||
CI = confidence interval.
Each P value is based on a 1-degree-of-freedom Wald chi-square test for the difference in rates of change between the two groups within the given time period. Boldface indicates statistical significance (P < 0.05).
Secondary analyses examining the proportion of COT patients on ≥120 mg average daily MED found a similar decline across all three periods of the study for all four pairwise comparisons (Supplementary Data). No significant differences were noted comparing higher-risk with lower-risk patients in relative change per year in the proportion of COT patients on ≥120 mg average daily MED in any of the three study periods (Supplementary Data).
Sensitivity analyses truncating the average daily MED at 500 mg did not differ substantially from the primary analyses. Age-stratified analyses did not yield substantial differences in average daily dose or rate of dose decline for those age 26 to 45 years compared with the age 46–64 group (Supplementary Data). Although COT patients age ≥65 years could potentially be at higher risk for drug toxicity at higher doses, the average daily dose for those age ≥65 years was 54.9 mg MED at the beginning and dropped to 38.2 mg MED by the end of the study, so most older patients were at relatively low doses.
Discussion
Contrary to our hypotheses, we did not find attenuated dose reduction for COT patients in higher-risk subgroups. Only modest differences were noted in the annual rate of dose reduction among COT patients at higher risk compared with their lower-risk counterparts, with higher-risk patients achieving slightly larger dose reductions than lower-risk patients, albeit these differences were not consistently statistically significant. Consistent with prior research, higher-risk COT patients were found to be receiving higher doses across all study periods. There could be several reasons for our findings. First, COT patients at higher risk were on higher doses at the beginning of the study and were more likely identified on providers’ lists of COT patients on ≥120 mg MED daily during the dose reduction period. Similarly, because of the greater starting doses, higher-risk COT patients had larger potential for dose reduction compared with those at lower risk who faced a floor effect at very low doses, where therapeutic effects are not likely maintained. The fact that COT dose reductions were observed among individuals more likely to experience opioid use disorders and overdose during implementation of the opioid risk reduction initiatives is a key finding because of the dose-dependent relationship between opioid dose and risk for opioid use disorder and overdose [26,35–37].
Randomized controlled trials are rarely possible to assess the impact of policy changes. The interrupted time series design allowed us to visually display the dynamic response of opioid dosing trends to clinical policy initiatives and to compare trends before and during implementation of different components of the initiatives introduced at different time points, providing an intuitive graphical display of dosing trends [38,39]. Stratified analyses were conducted in order to evaluate the differential impact of the initiatives on subpopulations of individuals such as those at higher risk for opioid-related adverse outcomes [40]. The use of the lower-risk comparison group from the same population of COT patients diminishes time-varying confounding such as media coverage of the opioid epidemic or other secular trends that could affect providers’ prescribing practices [40]. Notably, the greatest dose decline occurred during the period when PCPs were alerted to their COT patients above the 120 mg MED dosing threshold in combination with supervisory guidance from medical staff leaders and relevant consulting specialists. Supervisory guidance to avert high-risk prescribing was described in another study where a physician organization referred physicians prescribing large quantities of opioids to an educational course, which led to a decline in opioid prescribing, compared with self-referred physicians and matched controls [41].
We utilized an open cohort design to capture the target population of patients who would be affected by the initiatives, such as more recent enrollees of the health plan on high-dose therapy who would be eligible for risk reduction initiatives and potentially in higher-risk subgroups. Following a fixed cohort of COT patients over time based on use in the early years of the study would include many persons in the analyses who had either discontinued opioid use or who used opioids infrequently, who were not a target of the initiatives intended to reduce risks among persons using opioids on a daily or near-daily basis. Because the initiatives were specifically targeted at risk reduction and not increasing discontinuation of chronic opioid therapy, the composition of our sample remained relatively stable across the study period in that 80–86% of the sample was retained from one quarter to the next.
Provider diagnoses in the prior three years were used to ascertain SUDs and mental disorders, which is longer than the minimum one year of enrollment required for inclusion in the study. Although there could be possible differential capture between one year-vs more-than-one-year enrollees, in order to avoid missing capture of appropriate diagnoses, we used three-year look-back periods for these diagnoses but still recruited individuals with a minimum of one year of enrollment history who could potentially be eligible for risk reduction initiatives. Furthermore, the prevalence of these conditions is likely underestimated due to under-recognition of these diagnoses by clinicians and reticence to document them in medical records, thus potentially warranting longer look-back periods [42]. Still, more than half of COT patients had a diagnosis of a mental disorder in the prior three years. Although COT patients with SUDs and sedative use are most commonly cited as those at higher risk for opioid use disorder and overdose [12], the prevalence of those factors was relatively low in comparison with mental disorders in this sample. Also, the prevalence of comorbid mental disorders among those with an SUD was 78.1%. Similar prevalence rates for mental disorders have been reported by other studies of COT patients with SUDs and sedative use [8,13,16,35,6]. The only high-risk subgroup that did not have a majority of patients with diagnosed mental disorder was men (45%).
This study had a number of limitations. The main outcome, average daily MED, is timely and relevant because the harms associated with long-term opioid therapy are largely dose dependent; however we do not address the effect of these initiatives on patient-reported outcomes. Instead, this study addresses whether patients with risk factors for opioid use disorder and overdose were differentially affected by initiatives to reduce opioid doses. Also, we were unable to capture data on those age 18 to 25 years because individuals had to be at least 18 years of age at the start of the study in 2006 in order to meet institutional review board requirements for waiver of informed consent. Results could be less generalizable to other states without prescribing guidelines, though more states are promoting opioid dose guidelines as a result of public concern [12].
Conclusions
In summary, we found that higher-risk COT patients experienced comparable reductions in opioid dose to lower-risk patients subsequent to implementation of initiatives intended to reduce use of high-dose COT. This study provides evidence for the impact of policy initiatives aiming to promote COT guideline-concordant care and reduce COT-related risks for overdose and addiction consistent with the CDC recommendations. Washington State legislation played a key role in motivating the health care system to address high-dose COT prescribing. Further study is needed to determine whether policy initiatives at the institutional level can reduce opioid prescribing without state-wide guidance.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contributions of members of the Patient Advisory Committee guiding this research, including Catherine Cartwright, Penny Cowen, David Duhrkoop (chairperson), Mariann Farrell, Ada Giudice-Tompson, Kathryn Guthrie, Catherine Lippincott, Max Sokolnicki, and Betts Tully.
Funding sources: This research was supported by a grant the Patient-Centered Outcomes Research Institute (R-IHS-1306-02198, Von Korff). The contribution from Dr. Thakral was supported by the National Institute on Aging (grant number: T32-AG-0276-7709). The study sponsors had no role in the study design; the collection, analysis, or interpretation of the data; the writing of the report; or the decision to submit the report for publication.
Disclosures and conflicts of interest: In the past three years, Dr. Von Korff was the Principal Investigator of grants to Group Health Research Institute (GHRI) from Pfizer Inc. that concerned opioids. These grants also supported work on opioids by Dr. Shortreed, Ms. Saunders, and Mr. Walker. Drs. Von Korff and Shortreed are co-investigators on grants to GHRI from the Campbell Alliance for US Food and Drug Administration–mandated postmarketing surveillance studies of extended-release opioids. Dr. Shortreed has also received funding from research grants awarded to GHRI by Bristol-Myers Squibb. Ms. Saunders owns stock in Merck. The remaining authors report no conflicts.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
References
- 1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM.. Increases in drug and opioid overdose deaths-United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2016;64:1378–82.http://dx.doi.org/10.15585/mmwr.mm6450a3 [DOI] [PubMed] [Google Scholar]
- 2. Dasgupta N, Funk MJ, Proescholdbell S, et al. Cohort study of the impact of high‐dose opioid analgesics on overdose mortality. Pain Med 2016;17:85–98. [DOI] [PubMed] [Google Scholar]
- 3. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid‐related toxicity or overdose among veterans health administration patients. Pain Med 2014;15(11):1911–29. [DOI] [PubMed] [Google Scholar]
- 4. Liang Y, Turner BJ.. Assessing risk for drug overdose in a national cohort: Role for both daily and total opioid dose? J Pain 2015;16(4):318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bohnert AS, Logan JE, Ganoczy D, Dowell D.. A detailed exploration into the association of prescribed opioid dosage and overdose deaths among patients with chronic pain. Med Care 2016;54(5):435–41.http://dx.doi.org/10.1097/MLR.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M.. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain 2007;129(3):355–62. [DOI] [PubMed] [Google Scholar]
- 7. Rogers KD, Kemp A, McLachlan AJ, Blyth F, Landau R.. Adverse selection? A multi-dimensional profile of people dispensed opioid analgesics for persistent non-cancer pain. PLoS One 2013;8(12):e80095.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howe CQ, Sullivan MD.. The missing ‘P’in pain management: How the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry 2014;36(1):99–104. [DOI] [PubMed] [Google Scholar]
- 9. Morasco BJ, Duckart JP, Dobscha SK.. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med 2011;26(9):965–71.http://dx.doi.org/10.1007/s11606-011-1734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weisner CM, Campbell CI, Ray GT, et al. Trends in prescribed opioid therapy for non-cancer pain for individuals with prior substance use disorders. Pain 2009;145(3):287–93.http://dx.doi.org/10.1016/j.pain.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sullivan MD. Who gets high‐dose opioid therapy for chronic non‐cancer pain? Pain 2010;151(3):567–8. [DOI] [PubMed] [Google Scholar]
- 12. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 13. Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK.. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain 2010;151(3):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR.. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med 2012;10(4):304–11.http://dx.doi.org/10.1370/afm.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fulton-Kehoe D, Garg RK, Turner JA, et al. Opioid poisonings and opioid adverse effects in workers in Washington State. Am J Ind Med 2013;56(12):1452–62.http://dx.doi.org/10.1002/ajim.22266 [DOI] [PubMed] [Google Scholar]
- 16. Jones CM, McAninch JK.. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med 2015;49(4):493–501.http://dx.doi.org/10.1016/j.amepre.2015.03.040 [DOI] [PubMed] [Google Scholar]
- 17. Kaplovitch E, Gomes T, Camacho X, et al. Sex differences in dose escalation and overdose death during chronic opioid therapy: A population-based cohort study. PLoS One 2015;10(8):e0134550.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016;315:1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Washington State Agency Medical Directors’ Group. Interagency Guideline on Opioid Dosing for Chronic Non-cancer Pain: An Educational Pilot to Improve Care and Safety with Opioid Treatment. Olympia, Washington: Agency Directors' Medical Group; 2007.
- 20. Von Korff M, Dublin S, Walker RL, et al. The impact of opioid risk reduction initiatives on high-dose opioid prescribing for patients on chronic opioid therapy. J Pain 2016;17(1):101–10.http://dx.doi.org/10.1016/j.jpain.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trescott CE, Beck RM, Seelig MD, Von Korff MR.. Group Health’s initiative to avert opioid misuse and overdose among patients with chronic noncancer pain. Health Aff (Millwood) 2011;30(8):1420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saunders KW, Shortreed S, Thielke S, et al. Evaluation of health plan interventions to influence chronic opioid therapy prescribing. Clin J Pain 2015;31:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turner JA, Saunders K, Shortreed SM, et al. Chronic opioid therapy risk reduction initiative: Impact on urine drug testing rates and results. J Gen Intern Med 2014;29(2):305–11.http://dx.doi.org/10.1007/s11606-013-2651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Von Korff M, Saunders K, Ray GT, et al. Defacto long-term opioid therapy for non-cancer pain. Clin J Pain 2008;24:521.http://dx.doi.org/10.1097/AJP.0b013e318169d03b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valenstein M, Taylor KK, Austin K, et al. Benzodiazepine use among depressed patients treated in mental health settings. Am J Psychiatry 2004;161(4):654–61.http://dx.doi.org/10.1176/appi.ajp.161.4.654 [DOI] [PubMed] [Google Scholar]
- 26. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305(13):1315–21.http://dx.doi.org/10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 27. Sullivan MD, Edlund MJ, Steffick D, Unützer J.. Regular use of prescribed opioids: Association with common psychiatric disorders. Pain 2005;119(1–3):95–103. [DOI] [PubMed] [Google Scholar]
- 28. Washington State Agency Medical Direcotrs’ Group. Interagency Guideline on Opioid Dosing for Chronic Non-cancer Pain: An Educational Aid to Improve Care and Safety with Opioid Therapy. Olympia, Washington: Agency Directors' Medical Group; 2010.
- 29. McEvoy GK, ed. AHFS: Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2015. [Google Scholar]
- 30. Turner BJ, Liang Y.. Drug overdose in a retrospective cohort with non-cancer pain treated with opioids, antidepressants, and/or sedative-hypnotics: Interactions with mental health disorders. J Gen Intern Med 2015;30:1081–96.http://dx.doi.org/10.1007/s11606-015-3199-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40(5):373–83.http://dx.doi.org/10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 32. Hastie T, Tibshirani R, Friedman J.. The Elements of Statisitical Learning: Data Mining, Inference, and Predection, 2nd edition.New York: Springer; 2009. [Google Scholar]
- 33. Zeger SL, Liang KY.. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42(1):121–30.http://dx.doi.org/10.2307/2531248 [PubMed] [Google Scholar]
- 34. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159(7):702..http://dx.doi.org/10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 35. Braden JB, Sullivan MD, Ray GT, et al. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry 2009;31(6):564–70.http://dx.doi.org/10.1016/j.genhosppsych.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med 2010;152(2):85–92.http://dx.doi.org/10.7326/0003-4819-152-2-201001190-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN.. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011;171(7):686–91. [DOI] [PubMed] [Google Scholar]
- 38. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D.. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27(4):299–309. [DOI] [PubMed] [Google Scholar]
- 39. Jandoc R, Burden AM, Mamdani M, Lévesque LE, Cadarette SM.. Interrupted time series analysis in drug utilization research is increasing: Systematic review and recommendations. J Clin Epidemiol 2015;68(8):950–6. [DOI] [PubMed] [Google Scholar]
- 40. Penfold RB, Zhang F.. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediat 2013;13(6):S38–44. [DOI] [PubMed] [Google Scholar]
- 41. Kahan M, Gomes T, Juurlink DN, et al. Effect of a course-based intervention and effect of medical regulation on physicians’ opioid prescribing. Can Fam Physician 2013;59:e231–39. [PMC free article] [PubMed] [Google Scholar]
- 42. Tyree PT, Lind BK, Lafferty WE.. Challenges of using medical insurance claims data for utilization analysis. Am J Med Qual 2006;21(4):269–75.http://dx.doi.org/10.1177/1062860606288774 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.