Abstract
Parkinson’s disease is associated with abnormal oscillatory electrical activities of neurons and neuronal ensembles throughout the basal ganglia-thalamocortical network. It has recently been documented in patients with advanced parkinsonism that the amplitude of gamma-band oscillations (50–200 Hz) in electrocorticogram recordings from the primary motor cortex is abnormally coupled to the phase of beta band oscillations within the same signals. It is not known when in the course of the disease the abnormal phase–amplitude coupling (PAC) arises, and whether it is influenced by arousal or prior exposure to dopaminergic medications. To address these issues, we analyzed the relationship between the severity of parkinsonian motor signs and the extent of PAC in a progressive model of parkinsonism, using primates that were not exposed to levodopa prior to testing. PAC was measured in electrocorticogram signals from the primary motor cortex and the supplementary motor area in 3 monkeys that underwent weekly injections of small doses of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, rendering them progressively parkinsonian. We found that parkinsonism was associated with increased coupling between the phase of low-frequency (4–10 Hz) oscillations and the amplitude of oscillations in the high gamma band (50–150 Hz). These changes only reached significance when the animals became fully parkinsonian. The increased PAC was normalized after levodopa treatment. We also found a similar increase in PAC during sleep, even in normal animals. The identified PAC was independent of concomitant changes in spectral power in the 2.9–9.8Hz or 49.8–150.4 Hz ranges. We conclude that PAC is predominately a sign of advanced parkinsonism, and is, thus, not essential for the development of parkinsonism. However, increased PAC appears to correlate with the severity of fully developed parkinsonism.
Keywords: levodopa, motor cortex, Parkinsonism, phase amplitude coupling, sleep
Introduction
Parkinson’s disease is associated with pathologic oscillatory patterns in the electrical activities of single neurons, and in neuron ensemble activities such as local field potentials or electrocorticograms (ECoGs) in components of the basal ganglia-thalamocortical network (Galvan et al. 2015). The frontal cortex is an important node in this network but the results of spectral analyses of cortical activity patterns in patients with Parkinson’s disease remain contradictory (Babiloni et al. 2011). Abnormal delta and theta band activities have been found in electroencephalographic recordings (EEGs) of parkinsonian patients (Neufeld et al. 1994; Babiloni et al. 2011; but see Soikkeli et al. 1991; Sebban et al. 1999; Serizawa et al. 2008). This emerging low-frequency activity appears to parallel the progression of Parkinson’s disease (Morita et al. 2009), and was shown to be reversed by levodopa treatment (Funfgeld 1995). Recently, a more complex frequency domain measure, that is, the coupling between the phase and the amplitude of the signals at specific frequencies has been analyzed in ECoG signals in parkinsonian patients (Lopez-Azcarate et al. 2010; de Hemptinne et al. 2013). These studies demonstrated that the coupling of the phase of beta band oscillations and the amplitude of gamma band oscillations (50–200 Hz) in the primary motor cortex (MC) of advanced Parkinson’s disease patients are stronger than in patients with craniocervical dystonia or in healthy controls. This increased level of phase–amplitude coupling (PAC) in the parkinsonian patients was specific to the precentral gyrus, and reduced by therapeutic deep brain stimulation. Based on these observations, PAC measurements have been discussed as potentially useful signals for closed-loop control of deep brain stimulation parameters (de Hemptinne et al. 2013, 2015). In a recent study on MPTP-treated monkeys, the cortical PAC has been shown to be dependent of the state of arousal and despite some interesting results in the basal ganglia, no consistent MPTP related changes were found in the MC. In addition, it remains unclear, when these PAC changes develops in the course of the disease, whether these changes are specific to the MC, and how PAC measurements are influenced by levodopa treatment. In this study, we used a progressive model of primate parkinsonism to address these issues. We examined how ECoG PAC changes develops in relation to the development of parkinsonism, studied the impact of dopamine replacement in drug-naïve animals, and examined the relationship between PAC changes and the state of arousal.
Materials and Methods
General Experimental Strategy
We studied ECoG signals from rhesus monkeys that were permanently implanted with ECoG recording electrodes over the MC and the supplementary motor area (SMA). Recordings of ECoG and synchronized video signals were carried out during epochs of wakefulness and sleep, first in the normal state, and then during the gradual development of parkinsonism, induced by repeated small injections of MPTP. In the fully parkinsonian state, we also examined the effects of antiparkinsonian doses of levodopa.
Animals, Surgical Procedures
We report results from 3 adult rhesus monkeys (macaca mulatta; 6–10 kg; monkeys A, B, and C; 2 females and 1 male). Some data from 2 of these animals (monkeys A and B in this report) were also included in an earlier report (Devergnas et al. 2014). The animals were pair-housed with other monkeys, and had ad libitum access to food and water. All experiments were performed in accordance with the United States Public Health Service Policy on the humane care and use of laboratory animals, including the provisions of the “Guide for the Care and Use of Laboratory Animals” (Garber et al. 2011), and approved by the Institutional Biosafety and Animal Care and Use Committees of Emory University.
The animals were first conditioned to accept handling by the experimenters and to sit in a primate chair. They then underwent a surgical procedure, done under aseptic conditions and isoflurane anesthesia (1–3%), to place epidural bone screw electrodes, which were embedded, along with a stainless steel head holder, into an acrylic “cap.” Four epidural screw electrodes (diameter: 0.25 cm, length 0.4 cm) were inserted through small holes drilled in the skull. These electrodes were subsequently used for bipolar ECoG recordings. For each channel, signals were obtained as the differential between 2 electrodes located over the same cortical area (for instance, between the 2 electrodes located over the left primary MC). The electrodes were stereotaxically implanted over the MC and SMA in monkeys A and B, and only over MC in Monkey C. Wires from the electrodes were soldered to a 9-pin connector that was also embedded in the acrylic. The animals were allowed to recover for 1 week after the surgery before recording procedures began.
MPTP Treatment
After completion of the recordings in the normal state, the animals were rendered progressively parkinsonian by weekly administration of small doses of MPTP (0.2–0.6 mg/kg i.m.). Monkey A received 26 injections (10.2 mg/kg total, given over 10 months), monkey B received 19 injections (6.0 mg/kg total, given over 7 months), and monkey C received 18 injections (9.2 mg/kg total, given over 5 months). The animals eventually reached comparable states of stable moderate parkinsonism (defined below). To assess the degree and stability of the MPTP-induced motor disability, the severity of parkinsonism was assessed weekly while the monkey was in an observation cage equipped with infrared beams, allowing us to continuously observe the animal and to automatically register body movements. The same cage was used to evaluate the behavioral effect of levodopa treatment. In the behavioral rating sessions, we used a parkinsonism rating scale to score 10 aspects of motor function: bradykinesia, freezing, extremity posture, trunk posture, action tremor, the frequency of arm and leg movements, finger dexterity, home cage activity, and balance (Galvan et al. 2016; Kammermeier et al. 2016). Each item was scored on a 0-to-3 scale (maximal score: 30). No major differences were observed between the appendicular scores from the right and left sides of the animals (frequency of arm or leg movements, finger dexterity). For the purpose of this study, we report the right-sided scores only, as they were contralateral to the ECoG recordings. The maximal severity of parkinsonism in any animal in this study was 15, corresponding to moderately severe parkinsonism.
For our study of the relationship between the severity of parkinsonism and the extent of changes in parameters describing PAC changes in ECoG signals, we binned the range of parkinsonism scores into 3 groups: stage 1 (scores between 0 and 5), stage 2 (scores between 6 and 10), and stage 3 (scores between 11 and 15). Assessments of symptom severity were made weekly, before, and at least 5 days after each MPTP injection. The series of MPTP injections was terminated once the animal reached scores indicating stage 3 parkinsonism. Recovery after the MPTP injections was assessed on a weekly basis. If no change in the motor stage was observed during the 4 weeks following the last MPTP injections, parkinsonism was considered to be stable.
Cortical Recordings
All video-ECoG recordings were made while the animals sat in a primate chair with their head immobilized, leaving their body and limbs free to move. On each recording day, video images of the face of the animal and ECoG signals from MC and SMA were simultaneously recorded. The cortical electrical signals were amplified, bandpass filtered (0.3−500 Hz), sampled at 1 kHz, and stored on a computer disk with a data acquisition interface (Power 1401; CED, Cambridge, UK) and commercial software (Spike2, CED).
In monkeys A and B, recordings were made weekly throughout the study on the day following the behavioral observations (Fig. 1A, B). In monkey C, only signals from the normal and fully parkinsonian state were available. The recording sessions encompassed epochs of wakefulness and sleep. These were identified during off-line studies (see below), and analyzed separately. The effects of levodopa were examined in separate sessions after the animal had developed stable stage 3 parkinsonism (see following section).
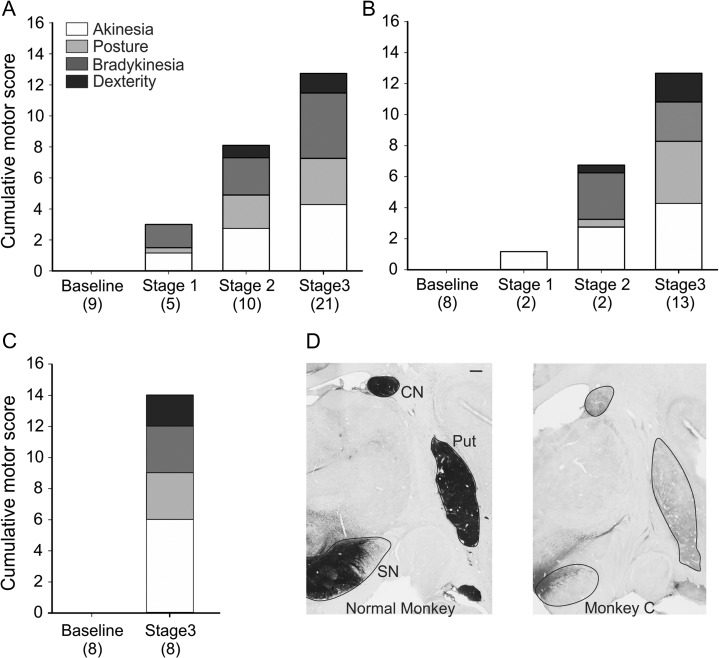
Figure 1.
Progression of parkinsonism and Tyrosine hydroxydase (TH) immunolabeling in the basal ganglia. Plots A, B, and C show parkinsonism rating scores from monkeys A, B, and C, respectively, throughout the development of parkinsonism. The number of behavioral observations across is indicated below each column. Note that we only had baseline and stage 3 scores available for monkey C. The summary scores shown here are averages of groupings of the individual parkinsonism rating scores in each of the severity states. The akinesia summary score (white) includes subscores for freezing and akinesia of the right leg and arm. The posture score (light grey) includes subscores for balance, trunk and extremity postures. The bradykinesia summary score (dark grey) is the sum of the subscores for home cage behavior and bradykinesia. The dexterity score (black) includes the subscores for (kinetic) tremor and finger dexterity. D Data shows TH staining in a normal monkey and in monkey C treated with MPTP, demonstrating the substantial reduction of TH staining in the treated animal. TH labeling results from monkeys A and B were similar, and shown in a previous publication (Devergnas et al. 2014). CN, caudate nucleus; Put, putamen; SN, substantia nigra. Scale bar (1 mm) in the left image applies to both images.
Drug Treatment
The effect of systemic levodopa treatment on the animals’ behavior and the electrical signals was studied in monkeys A and B. We administered intramuscular injections of a 4:1 mixture of levodopa and the dopa-decarboxylase inhibitor benserazide (both from Sigma-Aldrich, St. Louis, MO), at a levodopa dose of 30 and 20 mg/kg, respectively, in monkeys A and B. For each animal, we first determined the latency and peak of the antiparkinsonian levodopa effect, using the observation techniques described above. After a 20-min baseline observation period, the monkeys received levodopa/benserazide, followed by continuous monitoring for 120 additional minutes. The animals did not develop involuntary movements in response to the drug treatments. The behavioral observation sessions were followed by (separate) ECoG recording sessions during which the animals received the same doses of levodopa, and were examined for the same amount of time. Individual levodopa injections were separated by at least 2 days. We carried out 8 levodopa injections in monkey A (4 each for behavioral observation and electrophysiological recordings), and 6 in monkey B (3 each for behavioral observation and electrophysiological recordings). In addition, 4 control injections (saline) were carried out in monkey B (2 each for behavioral assessments and electrophysiological recordings). The peak behavioral effect of levodopa was found 30 min after the injection, based on blinded evaluation of cage activity in 5 min increments (data previously published in Devergnas et al. 2014). We then compared PAC measures obtained during the 5 min epoch immediately preceding the injections to those obtained during the 5 min peak effect period on each day, using paired statistics.
Termination of the Experiment
After completion of the experiment, the monkeys were euthanized with an overdose of pentobarbital sodium (100 mg/kg, i.v.) and then transcardially perfused with cold oxygenated Ringer’s solution, followed by perfusion with a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffer. The brains were then removed from the skull, cut coronally into 10 mm thick blocks, and postfixed overnight in 4% paraformaldehyde. The blocks were later cut into 60 μm thick coronal sections using a vibrating microtome. Sections were immunostained for tyrosine hydroxylase (TH) to assess the degree of loss of dopaminergic fibers in the striatum and dopaminergic neurons in the substantia nigra pars compacta (SNc) (Fig. 1C, data from monkeys A and B previously published in Devergnas et al. 2014).
Data Analysis
Arousal Scoring
Prior to PAC analysis, we scored the state of arousal of the animal throughout the recordings, using a manual scoring method (Devergnas et al. 2014). For this, the synchronously recorded ECoG signals and face video footage were split into consecutive 10 s epochs. Each of these epochs were scored as representing either wakefulness, drowsiness, or sleep, using the “sleep score” Spike2 script (CED). The stage of the animal was classified as being “awake” if it was attentive to its surroundings with eyes open, and showed low-amplitude EEG. “Sleep” episodes were defined as periods during which the monkey was quiet with its eyes closed while the EEG exhibited high amplitude slow activity (characteristic of non-REM sleep, Fig. 5B) (Hsieh et al. 2008; Barraud et al. 2009; Devergnas et al. 2014; Galvan et al. 2016). Episodes with ambiguous findings were classified as representing “drowsiness.” Epochs with movement artifact were classified as “artifact.” “Drowsiness” and “artifact” epochs were not further analyzed.
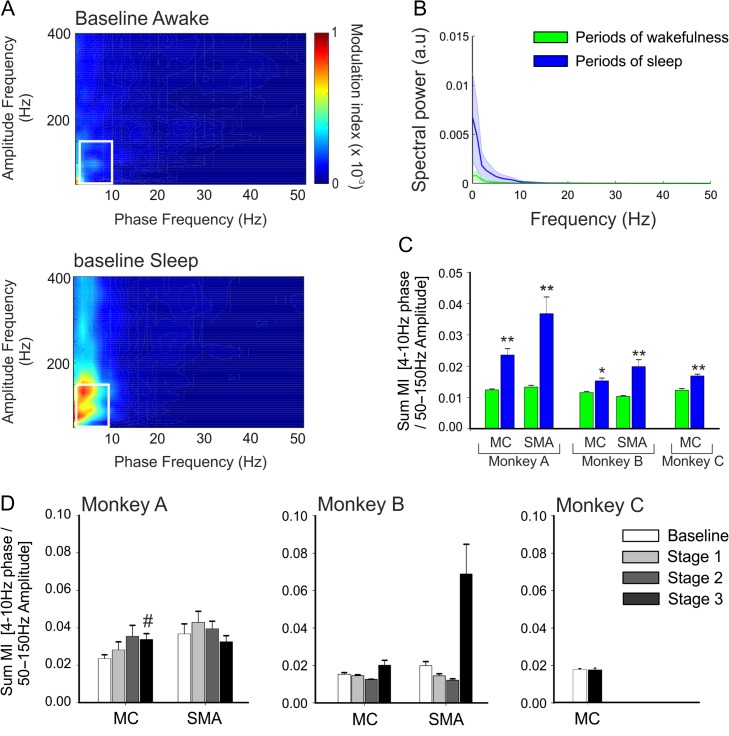
Figure 5.
Effects of sleep on cortical PAC. (A) Examples of MI maps observed in the SMA of monkey A, during periods of wakefulness and sleep (from the same day) at baseline. (B) Spectral analysis of the SMA of monkey A, during periods of wakefulness (blue) and sleep (green) in the normal stage. (C) Sum MI values during sleep in the pre-MPTP phase (means + SEM; *P < 0.05; **P < 0.01; Wilcoxon signed rank test). (D) Sum MI values during periods of sleep across baseline and stages 1–3 of parkinsonism in monkeys A and B, and in baseline and stage 3 parkinsonism in monkey C. Comparisons between the baseline and the 3 stages of parkinsonism with a nonparametric one-way ANOVA on rank did not yield differences. #P < 0.05; Mann–Whitney test for significant difference between the normal state and stage 3 parkinsonism.
Signal Preparation
All subsequent data analysis steps were done with custom-written MATLAB scripts (MATLAB 8.4; The Mathworks, Natick, MA, USA). After importing data into the Matlab environment, the identified epochs of wakefulness or sleep were extracted separately from each data record and concatenated to create files of a standard length of 60 s. All data were mean-corrected, and line noise artifacts were removed using a 58–62 Hz fourth order Butterworth notch filter. In our experience, the intensity of PAC is not entirely stable over time, resulting in noisy PAC estimates when using short data segment, and overly smooth estimates when using very long estimates. As a compromise between these extremes, we always used the average of the first 5 “awake” or sleep periods, each lasting 60 s to assign PAC values on a given recording day. To study levodopa-induced changes, we compared the averaged 5 periods of 60 s of wakefulness preceding the injection to the 60 periods at the peak of the behavioral levodopa effect, 30 min after the injection.
Calculation of Modulation Index Values (PAC Analysis)
Modulation indices (MIs) were calculated using an entropy-based Kullback–Leibler (KL) distance approach (Tort et al. 2008,2010). This method defines an MI for individual phase/amplitude pairs (the processing steps are described in Supplementary Fig. 1). To accomplish this, the signal (x(t)) was separately filtered to generate phase and amplitude signals. To generate phase data, the signal was bandpass filtered in 2 Hz steps covering the 2–52 Hz range, each with a bandwidth of 4 Hz (2 Hz in front and 2 Hz behind), resulting in 26, overlapping, filtered phase signals, (). To compute the amplitude data, x was bandpass filtered in 5 Hz steps between 50 and 400 Hz, each with a bandwidth of 10 Hz (5 Hz in front and 5 Hz behind), resulting in 71 separate amplitude signals (). The binned signals were then Hilbert-transformed, resulting in their respective analytic signals, and . This analytic signal is of the form:
where, H is the Hilbert function. Using Matlab’s angle function on each we obtained the phase of each signal . The amplitude of each signal was calculated as For each pair of and , we binned into 20° bins, covering the entire 0–360° range, and calculated the mean amplitude in each bin k, denoted as . These mean amplitudes were then normalized by dividing each by the sum of the mean amplitudes across all phase angle bins,
Finally, the KL-distance was used to obtain the MI for each phase amplitude pair, such that,
(Tort et al. 2010). scores describe whether the distribution of mean amplitudes across bins is uniform. High values identify frequency pairs at which the distribution is not uniform. MI values for the entire range of frequencies were used to construct color-coded “phase–amplitude comodulograms.”
In our sample of recordings, we visually identified prominent parkinsonism-associated changes in the PAC (MI values) between the 4–10 Hz and 50–150 Hz bands (Fig. 2). For statistical comparisons, we summed the MI values in the 4–10 Hz phase/50–150 Hz amplitude ranges (see, e.g., black box in Fig. 2) on each experimental day. In the following, we refer to this sum as “sum MI.” Since treatments may not only alter the magnitude of PAC but also the phase or the frequencies at which the coupling occurs (Lopez-Azcarate et al. 2010; Ozkurt et al. 2011), the frequencies and phase pairs representing the maximal coupling were also determined for each recordings. We obtained similar results using an alternative PAC method using a nonconstant bin sizes for the amplitudes (Supplementary Fig. 2).
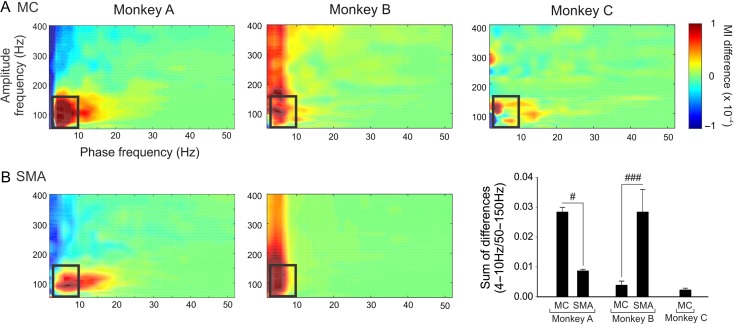
Figure 2.
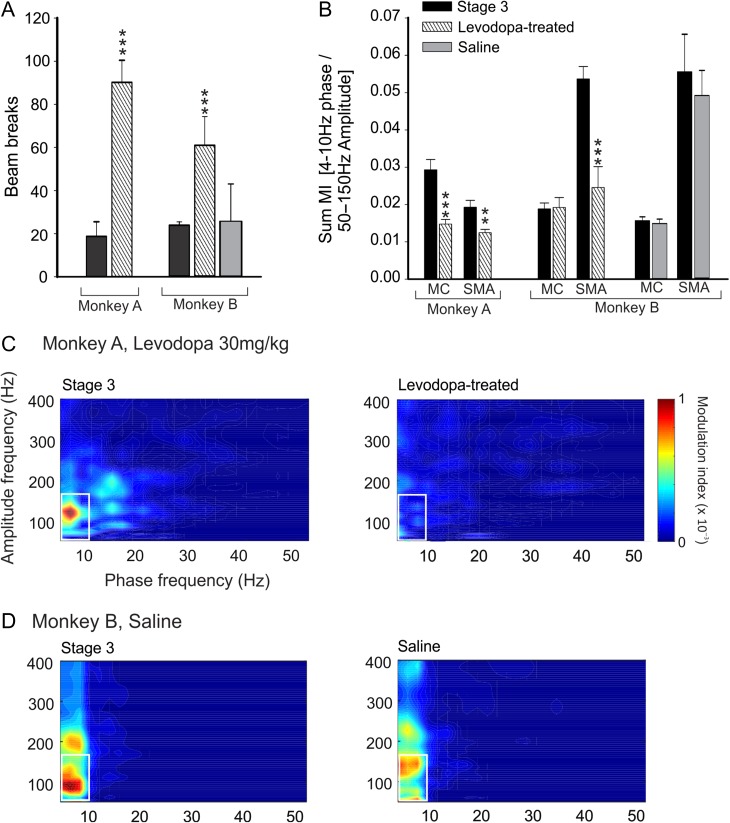
Change in cortical PAC associated with stage 3 parkinsonism. The figure shows color-coded parkinsonism-associated differences in MI values during wakefulness in MC (A) and SMA (B) for monkeys A, B and C. The black boxes on the maps show the ranges of frequencies (4–10 Hz for phase and 50–150 Hz for amplitude) that were used for statistical analyses of MI differences. (C) Comparison of the sum of the differences (mean ± standard error of measurement [SEM]) between the MI values obtained in stage 3 and baseline, in the range of frequencies represented by the black box in A and B. Comparison between SMA and MC were done with Mann–Whitney tests, #P < 0.05, ###P < 0.001.
Spectral Analysis
An analysis of power spectral densities was carried out, using the same data segments that were used for the aforementioned PAC analysis. We used the Welch technique, with Hamming windowing, and a fast Fourier transform segment length of 1024 samples with no overlap, resulting in a final spectral resolution of almost 1 Hz. Changes in power were analyzed in the 3.9–9.8 Hz and the 49.8–150.4 Hz ranges, each normalized to the total power (1–150.4 Hz) of the respective data.
Statistics
Because of differences between the results from individual monkeys (see below), statistics were done separately for each animal. To visualize the effect of parkinsonism on the cortical PAC, for each animal, we subtracted the mean PAC values obtained in the normal stage (baseline) from the corresponding PAC values obtained in stage 3 of parkinsonism. This analysis was performed separately for periods of wakefulness (Fig. 2) and sleep (Fig. 5). Comparison between the sum MI values of MC and SMA were done for monkeys A and B with a nonparametric Mann–Whitney test.
To analyze the changes through the stages of progressive parkinsonism in monkeys A and B, we used a nonparametric Kruskal–Wallis one-way-ANOVA, followed by the use of Dunn’s post hoc test for the comparison with the baseline values, if appropriate. We used a nonparametric Mann–Whitney test to compare data from the normal and stage 3 parkinsonism in each of the animals. A Pearson’s correlation test was used to test the (linear) relation between the sum MIs and the corresponding motor scores, and between the sum MIs and the corresponding integrated normalized power for the 3.9–9.8 and 49.8–150.4 Hz ranges. Finally, to analyze the effects of levodopa, we compared the sum MIs obtained before the injections to values obtained 30 min after the injections (see above), using a Wilcoxon signed rank paired test. For all tests, P < 0.05 indicated statistical significance.
Results
Behavioral Effects of MPTP
The monkeys started to show the first motor abnormalities after the first or second MPTP injections (stage 1). In stage 2, all the animals exhibited some akinesia, posture, bradykinesia and dexterity impairments (Fig. 1A–C). The subsequent progression from stage 2 to 3 involved a worsening of the existing disability, without the development of new signs. The composition of parkinsonian signs at the different stages of parkinsonism differed slightly between the animals (Fig. 1), but all monkeys developed marked bradykinesia, arm and leg akinesia, and a stooped posture. At the end of the MPTP treatment, monkeys A, B, and C reached parkinsonism rating scores of 16, 16, and 14, respectively, and no recovery was seen after this point. As expected, postmortem TH staining revealed a substantial loss of dopamine in striatum and SNc. This is shown for monkey C in Figure 1D; similar results for the other 2 animals were previously reported (Devergnas et al. 2014).
Change Induced by Parkinsonism in MC and SMA PAC Maps
We restricted this analysis to the segments recorded while the monkey was awake. To visualize changes induced by parkinsonism on the PAC maps, we subtracted the average of the baseline comodulograms from the average of the stage 3 comodulograms (shown in Fig. 2A, B). While the extent of cortical PAC changes differed substantially between the animals, for all animals, the sum MI values (as indicated by the black rectangle in Fig. 2) were higher in the parkinsonian state compared with the nonparkinsonian (Mann–Whitney test: P ranging from <0.001 to 0.041; Fig. 3). Parkinsonism-related changes of the sum MI values in MC and SMA did not differ consistently in the 2 animals for which we had ECoG data from both regions (Fig. 2C). In monkey A, the parkinsonism-induced increase was stronger in the MC than in SMA (0.028 ± 0.002 and 0.0009 ± 0.0005 P = 0.016, respectively, for MC and SMA), while the opposite was the case in monkey B (0.004 ± 0.001 and 0.0028 ± 0.007 P < 0.001, respectively, for MC and SMA).
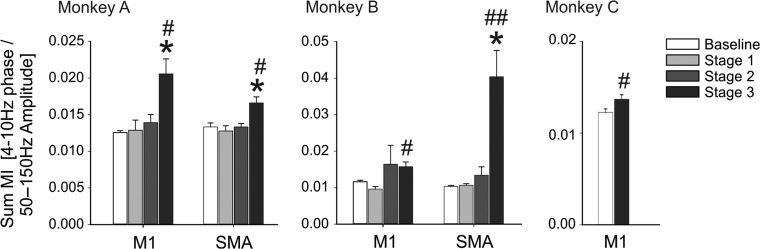
Figure 3.
Progressive changes in cortical PAC. Data shows the progressive changes in the sum MI values in monkeys A and B, and change between the baseline and stage 3 values in monkey C. Means ± SEM. Comparisons between baseline and the 3 stages of parkinsonism were done with a nonparametric one-way ANOVA on rank, followed by Dunn’s post hoc testing (*P < 0.05). Comparisons between the baseline and stage 3 parkinsonism were done with the nonparametric Mann–Whitney test (#P < 0.05 and ##P < 0.01).
Late Increase of Cortical PAC in the Progression of the Disease
We examined the ECoG PAC changes at different severities of parkinsonism in monkeys A and B. There were no significant PAC changes (compared with control) in the mildly affected parkinsonian states (stages 1 and 2). The increase of the sum MI values reached significance in stage 3 parkinsonism (Fig. 3). A similar significant increase was found in MC of Monkey C when comparing baseline and stage 3. Despite the absence of significant increase of the sum MI in stages 1 and 2, the sum MI was positively correlated with the global motor score and the subgroups (akinesia, bradykinesia, posture, dexterity, Table 1). This result was likely driven by the strong differences between the low values of the PAC index in the baseline compared with the high values in stage 3 parkinsonism (Supplementary Fig. 3). The frequency pairs (for phase or amplitudes) at which PAC values were maximal in MC or SMA did not differ between the different stages of parkinsonism, suggesting that worsening parkinsonism is associated with increasing PAC at the same phase/amplitude frequency pair rather than associated with the emergence of new coupling pairs (Supplementary Fig. 4).
Table 1.
Correlation between global or right-sided parkinsonism rating scores and sum MI values, using data from all stages of parkinsonism in each monkey
| Monkey A | Monkey B | |||
|---|---|---|---|---|
| MC | SMA | MC | SMA | |
| Global motor score | 0.48** | 0.49*** | 0.52** | 0.66*** |
| Akinesia | 0.52*** | 0.27 | 0.44* | 0.51** |
| Posture | 0.42** | 0.68*** | 0.56** | 0.77** |
| Bradykinesia | 0.53*** | 0.17 | 0.45* | 0.45* |
| Dexterity | 0.21 | 0.52** | 0.38 | 0.66*** |
The akinesia summary score includes subscores for freezing and akinesia of legs and arms. The posture score includes subscores for balance, trunk and extremity postures. The bradykinesia subscore includes scores for home cage behavior and the score for “bradykinesia.” The dexterity score includes the subscores for movement tremor and finger dexterity. The table shows coefficients of correlation. *P < 0.05; **P < 0.01; ***P < 0.001 (Pearson test). Bold values indicate P < 0.05.
Levodopa Treatment Induced Changes in PAC
Treatment with levodopa increased each animal’s locomotor activity, peaking 30 min after the drug administration, as documented by counting infrared beam breaks in our observation cage (Fig. 6A; Devergnas et al. 2014). As shown in Fig. 4B, levodopa injections induced a decrease in MI values. At a time when the behavioral levodopa effects were maximal, the MI values were significantly reduced in the SMA of both animals, and in MC of monkey A. Saline injections (only done in monkey B) did not alter locomotion or PAC values (Fig. 4B–D). The frequencies and phase involved in the maximal phase amplitude coupling before the injection and at the maximal effect of levodopa (30 min after the levodopa injection) were not significantly different (Supplementary Fig. 4).
Figure 6.
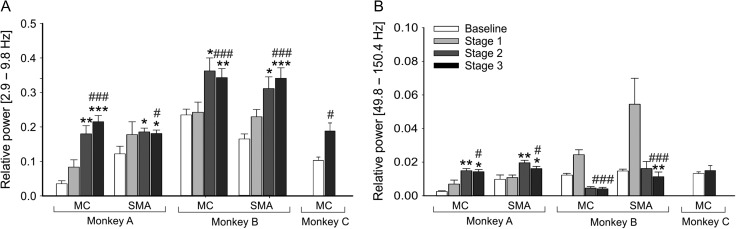
Changes in spectral power in MC and SMA. (A) Spectral power in the 3.9–9.8 Hz range (normalized to the power in the 1–150.4 Hz range) across different stages of parkinsonism. (B) Spectral power in the 49.8–150.4 Hz range (normalized to the total power in the 1–150.4 Hz range) across different stages of parkinsonism. Means + SEM. Comparisons between baseline and the 3 stage of parkinsonism were done with a nonparametric one-way ANOVA on rank, followed by Dunn’s post hoc testing (*P < 0.05, **P < 0.01, and ***P < 0.001). Comparisons between the baseline and stage 3 parkinsonism were done with the nonparametric Mann–Whitney test (#P < 0.05 and ###P < 0.001).
Figure 4.
Levodopa-induced changes in behavior and cortical PAC. (A) Increase of spontaneous movement 30 minutes after levodopa injection (30 and 20 mg/kg, respectively, for monkeys A and B), and absence of behavioral effect of saline injections (monkey B only). (B) Levodopa- and saline-induced changes of the sum MI values (means + SEM; *P < 0.05; **P < 0.01; ***P < 0.001; comparisons between sum MI values obtained preinjection and 30 min after injection, Wilcoxon signed rank test). (C) Examples of MI maps observed in the SMA of monkey A before levodopa injection (right) and 30 min following levodopa injection (left). (D) Examples of PAC maps observed in SMA of monkey B before and 30 min after saline injection.
Sleep-Related Changes in ECoG PAC Maps From MC and SMA are Independent of Parkinsonism
As shown in Figure 5A, comodulograms based on data recorded while the monkeys were asleep differed from those obtained during wakefulness. In the normal stage, the sum MI values were increased during periods of sleep compared with periods of wakefulness (Fig. 5C), but the phase and amplitude at which PAC values were maximal were not consistently different during the period of sleep and wakefulness (Supplementary Fig. 4). In contrast to the findings during wakefulness (Fig. 3), the sum-MI values did not consistently track the stage of parkinsonism during sleep (Fig. 5D).
Link Between Changes in Spectral Power and PAC Analysis
In monkeys A and B, the spectral power in the 3.9–10 Hz frequency range increased in MC and SMA with the development of parkinsonism. In the SMA and MC, this reached significance in stage 2 parkinsonism for both animals (Fig. 6A). This result is consistent with our previous report (Devergnas et al. 2014). No consistent results were found across the monkeys for the changes in the power in the 49.8–150.4 Hz range through the progression of the motor symptom (Fig. 6B). As shown in Table 2, the sum MI were not consistently correlated with the power in the 3.9–9.8 or 49.8–150.4 Hz ranges in baseline or in stage 3 parkinsonism.
Table 2.
Correlation between sum MI and spectral power in the 3.9–9.8 Hz and 49.8–150.4 Hz range in MC and SMA in normal and stage 3
| Monkey A | Monkey B | Monkey C | |||
|---|---|---|---|---|---|
| MC | SMA | MC | SMA | MC | |
| Spectral power [3.9–9.8 Hz] | |||||
| Normal | 0.39 | 0.31 | −0.43 | −0.08 | 0.7 |
| Stage 3 | 0.14 | −0.35 | −0.28 | 0.64* | 0.26 |
| Spectral power [49.8–150.4 Hz] | |||||
| Normal | 0.09 | −0.34 | −0.20 | 0.81** | 0.34 |
| Stage 3 | 0.03 | −0.25 | −0.66* | −0.77** | −0.43 |
Shown are coefficients of correlation, calculated separately for each frequency range and stage of parkinsonism in each animal. *P < 0.05, ***P < 0.001. Pearson’s test. Bold values indicate P < 0.05.
Discussion
This study confirms that parkinsonism is associated with an increase of the coupling between the phase of low-frequency oscillations (in this case, 4–10 Hz) and the amplitude of high gamma band oscillations (50–150 Hz) in ECoG signals. We also found that the parkinsonism-associated increase in coupling was reduced by levodopa treatment, and that there was no consistent correlation between the PAC changes and changes in spectral power in these specific bands. The abnormal coupling of cortical activities was only detectable in the presence of prominent parkinsonian signs, while changes in the low-frequency spectral components were already detectable in less severely affected animals. We also found that the state of wakefulness affected cortical PAC in the same ranges of frequencies than the parkinsonism-related changes.
Parkinsonism-Related Changes in Cortical Oscillatory Activity
Parkinsonism was characterized by an increase in the coupling of the phase frequency in the 4–10 Hz band and the amplitude in the 50–150 Hz band. These PAC components differ from those previously found in Parkinson’s disease patients where abnormal PAC was identified between the phase of the beta band (12–30 Hz) and the amplitude of the gamma band (50–200 Hz) (de Hemptinne et al. 2013, 2015). Similar differences in parkinsonism-associated spectral frequency alterations between human and monkey have previously been found in LFP recordings (Devergnas et al. 2014; Connolly et al. 2015). Thus, pathologic brain oscillatory activity tends to affect lower frequencies in monkeys than in humans.
In previous studies of PAC in local field potential signals from the STN, levodopa treatment was found to affect the magnitude and the specific frequency ranges at which the coupling occurs (Lopez-Azcarate et al. 2010; Ozkurt et al. 2011). However, this was not the case in our study of the effects of levodopa treatment on ECoG signals in monkeys. We also did not find that increasing dopamine loss (as we assume occurred during the course of MPTP treatment) affected the dominant PAC frequencies. Similarly, it has been shown that the frequencies showing maximal PAC values were not affected by high frequency stimulation of the STN in patients with Parkinson’s disease (de Hemptinne et al. 2015).
While a significant correlation between PAC measurements and beta band power was observed in the cortical activity of Parkinson’s disease patients, therapeutic STN stimulation only lowered PAC values (de Hemptinne et al. 2015), seemingly uncoupling gamma and beta band activities. In our case, even though both PAC values involving the 4–10 Hz range, and spectral power of the same band increased in the parkinsonian state, they were not significantly correlated. As shown in our previous report (Devergnas et al. 2014), the increase of spectral power in the 3.9–9.8 Hz range already reached significance in stage 2, thus being more sensitive to parkinsonism than the sum MI values reported here.
Changes of Cortical Activity in the Early Stage of the Disease
Parkinsonian motor signs are associated with the appearance of abnormal cortical oscillatory activity that occur before changes in the basal ganglia in both 6-ODHA treated rodents (Viaro et al. 2011; Javor-Duray et al. 2015) and MPTP-treated monkeys (Goldberg et al. 2002; Escola et al. 2003; Devergnas et al. 2014). While some of these changes may be pathophysiologically causal, they have also been speculated to be compensatory in nature (Quiroga-Varela et al. 2013). In our study, neither spectral nor PAC changes were detectable in the mildly parkinsonian state (stage 1), rendering it unlikely that such changes cause or compensate for early parkinsonism. This finding also suggests that these markers of oscillatory activities would be poor biomarkers for the early detection of parkinsonism. Of course, it needs to be remembered that our study (as most other studies in the literature) focused on spontaneous oscillatory activity. Oscillatory changes associated with active movements may occur earlier in the course of the development of parkinsonism (Escola et al. 2003). Cortical PAC has been proposed as a control signal for closed-loop therapeutic deep brain stimulation in patients with advance parkinsonism (de Hemptinne et al. 2015). The use of cortical source signals would have the major advantage of using a signal with minimal artifact compared with signals from the STN (Little et al. 2013). However, the influence of other natural physiological phenomenon on cortical PAC, like movement or sleep, needs to be explored.
Interaction Between the State of Arousal and Cortical PAC and Parkinsonism
Changes in arousal occur in parkinsonian patients, either associated with the parkinsonian state itself (Rye et al. 2000; Najafi et al. 2013), or with exposure to dopaminergic medications (Bliwise et al. 2012). Arousal deficits have also been documented in MPTP-treated monkeys (Daley et al. 2002; Barraud et al. 2009). While the identification of the different stage of sleep was not possible with our assessment method, we found in all animals an increase of the sum MI values during sleep. Unlike the findings in the awake state, the sum MI values did not significantly differ across the stages of parkinsonism. Our analysis of arousal-related PAC changes is obviously limited by the fact that only relatively few data segments representing sleep were available, and that we were not able to identify different stages of sleep. Given that the sleep-related PAC changes affected the same ranges of frequencies as parkinsonism-related changes and that parkinsonian animals (and patients) develop prominent sleepiness, sleep and parkinsonism-related changes may compound one another. Further analysis of this topic would be interesting as it may help to determine whether the increased in PAC is a pathologic finding specifically associated with parkinsonism or a finding that similarly occurs under some physiologic circumstances. More globally, our results are a reminder that assessments of the electrophysiologic abnormalities that accompany parkinsonism is incomplete without considering the state of wakefulness of the individual tested. Findings of parkinsonism-like changes during physiologic sleep would obviously complicate the use of PAC changes as a control signal for closed-loop DBS.
Parkinsonism Induced Changes of PAC are Similar in MC or SMA
MC and SMA play different roles in voluntary movement, and may be differentially affected by parkinsonism. In primates, MC is involved in the execution of movement, while SMA may play a greater role in motor planning (Romo and Schultz 1987; Kurata and Wise 1988; Nachev et al. 2008), although similar patterns of recruitment of MC and SMA cells can be seen in some motor tasks (Kurata and Wise 1988; Chen et al. 1991). Both structures are linked via reciprocal connections (Luppino et al. 1993; Lu et al. 1994). MC and SMA are similarly innervated by dopamine, either directly through the mesocortical network (Thierry et al. 1973; Tassin et al. 1977; Simon et al. 1979; Williams and Goldman-Rakic 1993), and receive inputs from the basal ganglia via the ventral motor thalamus (Alexander and Crutcher 1990; Holsapple et al. 1991; Anderson et al. 2001; Schultz et al. 1998). In agreement with previous studies that had shown increased coherence between SMA and MC in Parkinson’s disease patients (Pollok et al. 2013), we found that the parkinsonism-associated differences in PAC was similar in MC and SMA.
Potential Limitations
There are several limitations to this study. The small number of animals is the most significant shortcoming of this and other primate studies, caused by obvious technical and ethical constraints. The small number of animals is a shortcoming of this study, caused by obvious technical and ethical constraints. To mitigate the inevitable intersubject variability, statistical analyses were performed in individual animals, and the discussion was focused on those findings that were consistent across animals. However, we recognize that there were interindividual differences, specifically with regard to the absolute values of PAC and differences between M1 and SMA (Fig. 2). The regional differences found between the animal could be explained by differences in the placement of the ECoG electrodes (for instance, the posterior SMA electrode may have sampled rostral portions of MC in 1 animal, but not the other). Of course, differences may also reflect true biological differences between animals. It has recently been proposed that a subject-specific configuration should be completed to efficiently use the changes in PAC as a biomarker for PD (Escobar et al. 2017). This point is important and should be explored in future studies, using more closely spaced electrodes. In addition, it would have been interesting to separately analyze cortical PAC during REM and non-REM sleep, because REM sleep abnormalities are prevalent in PD patients and MPTP-treated monkeys (Barraud et al. 2009; Al-Qassabi et al. 2017; Bliwise et al. 2013). However, our recordings were done in animals sitting in a primate chair, with their head fixed which likely prevents the animals from reaching deep stages of sleep. Another potential limitation of this study is that the motoric states were not quantified during the recordings. It has been shown that movement can affect PAC in motor cortical areas, independent of parkinsonism (Yanagisawa et al. 2012), generally resulting in a reduction of PAC. The altered number of spontaneous movements in our animals may have contributed to the extent and patterns of the observed PAC changes. This is an important issue that deserves further study.
Conclusion
Our results confirm the presence of strong PAC in the frontal motor cortical areas in the parkinsonian state that is dependent on the state of arousal. The observed coupling in the awake parkinsonian state decreased with levodopa treatment. The abnormal coupling was only detectable when the animals were showing at least moderately severe motor signs, suggesting that the disturbances underlying PAC may not be essential for the development of motor symptoms. The relatively late appearance of PAC changes suggest that these changes would not be sensitive enough to allow it to be used an early biomarker for Parkinson’s disease.
Supplementary Material
Notes
The authors thank Dr. Olivier Darbin for his work in the initial phases of the experiment. We also thank Susan Jenkins for her expert technical help with the histologic studies. Conflict of Interest: The authors declare no competing financial interests.
Funding
Grants from the NIH/National Institute of Neurological Disorder and Stroke (NINDS) (P50-NS071669, P50-NS098685, R01-NS054976), and the NIH/Office of Research Infrastructure Programs (ORIP) to the Yerkes Center (P51-OD011132).
References
- Al-Qassabi A, Fereshtehnejad SM, Postuma RB. 2017. Sleep disturbances in the prodromal stage of Parkinson disease. Curr Treat Options Neurol. 19:22. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. 1990. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13:266–271. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Ruffo M, Buford JA, Inase M. 2001. Pallidal and cortical determinants of thalamic activity In: Kultas-Ilinsky K, Ilinsky IA, editors. Basal ganglia and thalamus in health and movement disorders. Austria: Springer; p. 93–104. [Google Scholar]
- Babiloni C, De Pandis MF, Vecchio F, Buffo P, Sorpresi F, Frisoni GB, Rossini PM. 2011. Cortical sources of resting state electroencephalographic rhythms in Parkinson’s disease related dementia and Alzheimer’s disease. Clin Neurophysiol. 122:2355–2364. [DOI] [PubMed] [Google Scholar]
- Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, Balzamo E, Bezard E, Tison F, Ghorayeb I. 2009. Sleep disorders in Parkinson’s disease: the contribution of the MPTP non-human primate model. Exp Neurol. 219:574–582. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Trotti LM, Wilson AG, Greer SA, Wood-Siverio C, Juncos JJ, Factor SA, Freeman A, Rye DB. 2012. Daytime alertness in Parkinson’s disease: potentially dose-dependent, divergent effects by drug class. Mov Disord. 27:1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL, Trotti LM, Juncos JJ, Factor SA, Freeman A, Rye DB. 2013. Daytime REM sleep in Parkinson’s disease. Parkinsonism Relat Disord. 19:101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DF, Hyland B, Maier V, Palmeri A, Wiesendanger M. 1991. Comparison of neural activity in the supplementary motor area and in the primary motor cortex in monkeys. Somatosens Mot Res. 8:27–44. [DOI] [PubMed] [Google Scholar]
- Connolly AT, Connolly AT, Jensen AL, Bello EM, Netoff TI, Baker KB, Johnson MD, Vitek JL. 2015. Modulations in oscillatory frequency and coupling in globus pallidus with increasing parkinsonian severity. J Neurosci. 35:6231–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley J, Turner R, Bliwise D, Rye D. 2002. Excessive daytime sleepiness following MPTP-induced dopamine depletion in Rhesus monkeys. J Sleep Res. 11(s1):44–45. [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. 2013. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci USA. 110:4780–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, Starr PA. 2015. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 18:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergnas A, Pittard D, Bliwise D, Wichmann T. 2014. Relationship between oscillatory activity in the cortico-basal ganglia network and parkinsonism in MPTP-treated monkeys. Neurobiol Dis. 68:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar D, Johnson LA, Nebeck SD, Zhang J, Johnson MD, Baker KB, Molnar GF, Vitek JL. 2017. Parkinsonism and vigilance: alteration in neural oscillatory activity and phase-amplitude coupling in the basal ganglia and motor cortex. J Neurophysiol. 118(5):2654–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escola L, Michelet T, Macia F, Guehl D, Bioulac B, Burbaud P. 2003. Disruption of information processing in the supplementary motor area of the MPTP-treated monkey: a clue to the pathophysiology of akinesia? Brain. 126:95–114. [DOI] [PubMed] [Google Scholar]
- Funfgeld EW. 1995. Computerised brain electrical activity findings of parkinson patients suffering from hyperkinetic side effects (hypersensitive dopamine syndrome) and a review of possible sources. J Neural Transm Suppl. 46:351–365. [PubMed] [Google Scholar]
- Galvan A, Devergnas A, Wichmann T. 2015. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front Neuroanat. 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Devergnas A, Pittard D, Masilamoni G, Vuong J, Daniels JS, Morrison RD, Lindsley CW, Wichmann T. 2016. Lack of antiparkinsonian effects of systemic injections of the specific T-type calcium channel blocker ML218 in MPTP-treated monkeys. ACS Chem Neurosci. 7:1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, Kohn DF, Lipman NS, Locke PA, Melcher J, et al. 2011. Guide for the care and use of laboratory animals. 8th ed.Washington, DC: National Academies Press (US). [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. 2002. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson’s disease. J Neurosci. 22:4639–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsapple JW, Preston JB, Strick PL. 1991. The origin of thalamic inputs to the “hand” representation in the primary motor cortex. J Neurosci. 11:2644–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh KC, Robinson EL, Fuller CA. 2008. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep. 31:1239–1250. [PMC free article] [PubMed] [Google Scholar]
- Javor-Duray BN, Vinck M, van der Roest M, Mulder AB, Stam CJ, Berendse HW, Voorn P. 2015. Early-onset cortico-cortical synchronization in the hemiparkinsonian rat model. J Neurophysiol. 113:925–936. [DOI] [PubMed] [Google Scholar]
- Kammermeier S, Pittard D, Hamada I, Wichmann T. 2016. Effects of high-frequency stimulation of the internal pallidal segment on neuronal activity in the thalamus in parkinsonian monkeys. J Neurophysiol. 116:2869–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Wise SP. 1988. Premotor and supplementary motor cortex in rhesus monkeys: neuronal activity during externally- and internally-instructed motor tasks. Exp Brain Res. 72:237–248. [DOI] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, Foltynie T, Limousin P, Ashkan K, Fitz Gerald J, et al. 2013. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 74:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Azcarate J, Tainta M, Rodríguez-Oroz MC, Valencia M, González R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre M. 2010. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci. 30:6667–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL. 1994. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol. 341:375–392. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. 1993. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 338:114–140. [DOI] [PubMed] [Google Scholar]
- Morita A, Kamei S, Serizawa K, Mizutani T. 2009. The relationship between slowing EEGs and the progression of Parkinson’s disease. J Clin Neurophysiol. 26:426–429. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. 2008. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 9:856–869. [DOI] [PubMed] [Google Scholar]
- Najafi MR, Chitsaz A, Askarian Z, Najafi MA. 2013. Quality of sleep in patients with Parkinson’s disease. Int J Prev Med. 4:S229–S233. [PMC free article] [PubMed] [Google Scholar]
- Neufeld MY, Blumen S, Aitkin I, Parmet Y, Korczyn AD. 1994. EEG frequency analysis in demented and nondemented parkinsonian patients. Dementia. 5:23–28. [DOI] [PubMed] [Google Scholar]
- Ozkurt TE, Butz M, Homburger M, Elben S, Vesper J, Wojtecki L, Schnitzler A. 2011. High frequency oscillations in the subthalamic nucleus: a neurophysiological marker of the motor state in Parkinson’s disease. Exp Neurol. 229:324–331. [DOI] [PubMed] [Google Scholar]
- Pollok B, Kamp D, Butz M, Wojtecki L, Timmermann L, Südmeyer M, Krause V, Schnitzler A. 2013. Increased SMA-M1 coherence in Parkinson’s disease—pathophysiology or compensation? Exp Neurol. 247:178–181. [DOI] [PubMed] [Google Scholar]
- Quiroga-Varela A, Walters JR, Brazhnik E, Marin C, Obeso JA. 2013. What basal ganglia changes underlie the parkinsonian state? The significance of neuronal oscillatory activity. Neurobiol Dis. 58:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Schultz W. 1987. Neuronal activity preceding self-initiated or externally timed arm movements in area 6 of monkey cortex. Exp Brain Res. 67:656–662. [DOI] [PubMed] [Google Scholar]
- Rye DB, Bliwise DL, Dihenia B, Gurecki P. 2000. FAST TRACK: daytime sleepiness in Parkinson’s disease. J Sleep Res. 9:63–69. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. 1998. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 37:421–429. [DOI] [PubMed] [Google Scholar]
- Sebban C, Zhang XQ, Tesolin-Decros B, Millan MJ, Spedding M. 1999. Changes in EEG spectral power in the prefrontal cortex of conscious rats elicited by drugs interacting with dopaminergic and noradrenergic transmission. Br J Pharmacol. 128:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa K, Kamei S, Morita A, Hara M, Mizutani T, Yoshihashi H, Yamaguchi M, Takeshita J, Hirayanagi K. 2008. Comparison of quantitative EEGs between Parkinson disease and age-adjusted normal controls. J Clin Neurophysiol. 25:361–366. [DOI] [PubMed] [Google Scholar]
- Simon H, Stinus L, Tassin JP, Lavielle S, Blanc G, Thierry AM, Glowinski J, Le Moal M. 1979. Is the dopaminergic mesocorticolimbic system necessary for intracranial self-stimulation? Biochemical and behavioral studies from A10 cell bodies and terminals. Behav Neural Biol. 27:125–145. [DOI] [PubMed] [Google Scholar]
- Soikkeli R, Partanen J, Soininen H, Pääkkönen A, Riekkinen P Sr. 1991. Slowing of EEG in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 79:159–165. [DOI] [PubMed] [Google Scholar]
- Tassin JP, Stinus L, Simon H, Blanc G, Thierry AM, Le Moral M, Cardo B, Glowinski J. 1977. Distribution of dopaminergic terminals in rat cerebral cortex: role of dopaminergic mesocortical system in ventral tegmental area syndrome. Adv Biochem Psychopharmacol. 16:21–28. [PubMed] [Google Scholar]
- Thierry AM, Blanc G, Sobel A, Stinus L, Glowinski J. 1973. Dopaminergic terminals in the rat cortex. Science. 182:499–501. [DOI] [PubMed] [Google Scholar]
- Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. 2008. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci USA. 105:20517–20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski R, Eichenbaum H, Kopell N. 2010. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 104:1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaro R, Morari M, Franchi G. 2011. Progressive motor cortex functional reorganization following 6-hydroxydopamine lesioning in rats. J Neurosci. 31:4544–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. 1993. Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine-specific antibody. Cereb Cortex. 3:199–222. [DOI] [PubMed] [Google Scholar]
- Yanagisawa T, Yamashita O, Hirata M, Kishima H, Saitoh Y, Goto T, Yoshimine T, Kamitani Y. 2012. Regulation of motor representation by phase-amplitude coupling in the sensorimotor cortex. J Neurosci. 32:15467–15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.