Abstract
Akinesia, a cardinal symptom of Parkinson’s disease, has been linked to abnormal activation in putamen and posterior medial frontal cortex (pMFC). However, little is known whether clinical severity of akinesia is linked to dysfunctional connectivity of these regions. Using a seed-based approach, we here investigated resting-state functional connectivity (RSFC) of putamen, pMFC and primary motor cortex (M1) in 60 patients with Parkinson’s disease on regular medication and 72 healthy controls. We found that in patients putamen featured decreases of connectivity for a number of cortical and subcortical areas engaged in sensorimotor and cognitive processing. In contrast, the pMFC showed reduced connectivity with a more focal cortical network involved in higher-level motor-cognition. Finally, M1 featured a selective disruption of connectivity in a network specifically connected with M1. Correlating clinical impairment with connectivity changes revealed a relationship between akinesia and reduced RSFC between pMFC and left intraparietal lobule (IPL). Together, the present study demonstrated RSFC decreases in networks for motor initiation and execution in Parkinson’s disease. Moreover, results suggest a relationship between pMFC-IPL decoupling and the manifestation of akinetic symptoms.
Keywords: akinesia, functional connectivity, motor initiation, Parkinson’s disease
Introduction
Akinesia, the impairment to initiate spontaneous movements (Hallett et al. 1991), is one of the cardinal symptoms of Parkinson’s disease critically affecting patients’ quality of life (Schrag et al. 2000; Chapuis et al. 2005). James Parkinson described akinesia as the inability of the patient to perform movements “with the promptitude that the will directs” (Parkinson 1817). Later, Kinier Wilson referred to akinesia as a “paralysis of the will”, suspecting its pathomechanism at the “highest level” of motor control (Wilson 1925). Since then, considerable progress has been made in the discovery of the neural mechanisms underlying motor symptoms of Parkinson’s disease at the systems level. Current pathophysiological models of the disease suggest that akinesia arises from dysfunctional cortico-striatal processing, induced by a functional imbalance between direct and indirect basal-ganglia pathways (Wichmann and DeLong 2002; Grafton 2004; Wichmann et al. 2011; Jellinger 2014). Here, the putamen is most prominently affected by the deprivation of dopaminergic input originating from the substantia nigra (Bernheimer et al. 1973; Hornykiewicz and Kish 1987; Morrish et al. 1995; Forno 1996; Rajput et al. 2008). These findings are in line with in vivo neuroimaging studies, showing decreased activation of the putamen in Parkinson patients (for a recent meta-analysis see Herz et al. 2013). At the cortical level, the posterior medial frontal cortex (pMFC) has been suggested to contribute to patients’ impairment in voluntary movement initiation (e.g.MacDonald ; Grafton 2004). This region is considered a central interface mediating cognitive (Ridderinkhof et al. 2004; Dosenbach et al. 2007, 2008; Nachev et al. 2008), motivational, and motor processes (Bush et al. 2002; Rushworth et al. 2004).
In the context of Parkinson’s disease, neuroimaging studies have indicated abnormal activity in the pMFC during self-initiated movements (Playford et al. 1992; Jahanshahi et al. 1995; Haslinger et al. 2001), which ameliorated under dopamine replacement (Jenkins et al. 1992; Rascol et al. 1992; Haslinger et al. 2001) or deep brain stimulation (Davis et al. 1997; Limousin et al. 1997; Fukuda et al. 2001). Moreover, single cell recordings in the macaque pMFC revealed specific neural responses to movement instructions (Escola et al. 2003), which were distorted after inducing akinesia by lesioning this region. Correspondingly, clinical case reports in humans also noted akinetic symptoms resulting from lesions affecting the pMFC (Dick et al. 1986; Meador et al. 1986; Haussermann et al. 2001). Together, these findings suggest that akinesia might arise from a dysfunctional interaction between the pMFC and basal ganglia, especially the putamen (Grafton 2004; Vercruysse et al. 2014).
Of note, both of these regions’ have been described as key nodes in a larger network linked to volitional behavior (Jahanshahi 1998; Hallett 2007; Brass and Haggard 2008; Kranick and Hallett 2013) and self-generated movements (Hoffstaedter et al. 2013, 2014). This cortical network includes lateral and medial prefrontal cortex, mid- and anterior cingulate cortex, insula as well as posterior parietal regions. Recently, an increasing number of fMRI studies have begun to investigate resting-state functional connectivity (RSFC) in Parkinson’s disease (for a systematic overview on RSFC studies in Parkinson’s disease see Tahmasian et al. 2015). However, most of these studies have focused on subcortical seeds, revealing that striatal seeds feature reduced RSFC with the sensorimotor cortex (Helmich et al. 2010; Wu, Long, et al. 2011; Sharman et al. 2013; Luo et al. 2014; Müller-Oehring et al. 2015) and subcortical nuclei including thalamus, subthalamic nucleus, and midbrain (Hacker et al. 2012; Wu et al. 2012). In contrast, the cortical network for movement initiation remains poorly understood with respect to RSFC alterations in Parkinson’s disease.
Hence, the present study aimed at analyzing RSFC of both striatal as well as pMFC seeds and their relation to akinesia in Parkinson’s disease. To this end, 3 seeds in bilateral putamen and pMFC (comprising both hemispheres) were functionally defined based on a coordinate-based meta-analysis of neuroimaging experiments that included the free timing of movements (Hoffstaedter et al. 2014). Furthermore, 2 seeds were placed in bilateral primary motor cortices (M1) to compare RSFC of pMFC and putamen with connectivity in systems related to direct motor execution. All of the aforementioned regions are known to play a crucial role in motor function and were expected to show abnormal connectivity patterns in Parkinson’s disease. However, we hypothesized that akinesia was particularly related to connectivity changes with regions involved in motor initiation, especially the pMFC. To test this hypothesis, we first analyzed RSFC between seeds in Parkinson patients and healthy controls, assessing the configuration of the preselected network in both groups. Next, whole-brain RSFC was computed for each of the 5 seeds in control subjects to identify extended resting-state networks specifically connected to the putamen, M1, and pMFC. These networks, were used to allocate the subsequently assessed aberrations of RSFC in patients. Finally, every seed’s RSFC was tested for correlations with clinical scores of akinesia. In sum, the present study analyzed RSFC of bilateral putamen, bilateral M1, and pMFC to characterize seed-specific resting-state networks in healthy controls, their alterations in Parkinson’s disease, and their potential roles in the manifestation of akinesia.
Materials and Methods
Subjects
Resting-state fMRI data of 60 patients with idiopathic Parkinson’s disease (mean disease duration 6.5 ± 5.5 [SD] years) and 72 healthy participants were included in the analysis. Patients and healthy participants were matched for age, gender, and head movements within the MRI-scanner (please see Table 1 for further sample characteristics). Data were acquired at 2 sites, Aachen University Hospital (31 patients, 32 healthy controls) and Düsseldorf University Hospital (29 patients, 40 healthy controls). Overall disease durations ranged from 0 to 21 years (Düsseldorf: 9.3 ± 5.9, range 0–21 years, Aachen: 3.9 ± 3.5, range 0–15 years). All patients were examined while on their regular dopaminergic medication (mean levodopa equivalent dose 786 ± 517 mg per day, estimated based on Tomlinson et al. 2010). Patients’ general motor impairment was quantified by part III of the Unified Parkinson’s Disease Rating Scale (UPDRS, Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease 2003). Across all patients, the average UPDRS III (on their regular dopaminergic medication) score was 19.6 ± 13.1. As part of this score, item 31 assesses the global spontaneity of movements on a scale from zero (no symptoms) to 4 (severe symptoms). This item was used to rate clinical severity of akinesia and relate it to RSFC alterations in the fMRI analysis. Examinations at all data acquisition sites as well as pooled analysis were approved by the local ethics committees and carried out in accordance with the Declaration of Helsinki. All participants gave informed written consent before entering the study.
Table 1.
Sample characteristics
| HHU Düsseldorf | RWTH Aachen | Overall sample | |||||
|---|---|---|---|---|---|---|---|
| Parkinson patients | Healthy controls | Parkinson patients | Healthy controls | Parkinson patients | Healthy controls | ||
| Age [years] | 59.8 ± 9.2 | 57.1 ± 10.0 | 63.3 ± 10.9 | 62.7 ± 5.6 | 61.6 ± 10.2 | 59.6 ± 8.8 | |
| P (t-test) | 0.262 | 0.749 | 0.216 | ||||
| Gender (m/f)a | (18/11) | (22/18) | (19/12) | (20/12) | (37/23) | (42/30) | |
| P (χ2-test) | 0.557 | 0.921 | 0.697 | ||||
| Movement (FDb) | 0.41 ± 0.18 | 0.36 ± 0.16 | 0.36 ± 0.17 | 0.34 ± 0.15 | 0.39 ± 0.17 | 0.35 ± 0.16 | |
| P (t-test) | 0.226 | 0.597 | 0.242 | ||||
| UPDRS III (ON) | 14.7 ± 7.5 | 24.3 ± 15.5 | 20.0 ± 13.2 | ||||
| L-Dopa equivalent dose | 1113.4 ± 400.1 | 395.9 ± 402.2 | 725.9 ± 532.9 | ||||
| Disease Duration [years] | 9.3 ± 5.9 | 3.9 ± 3.5 | 6.6 ± 5.5 | ||||
| Laterality indexc | −0.03 ± 0.43 | 0.23 ± 0.50 | 0.13 ± 0.47 | ||||
| MMSE | n.a. | 28.6 ± 1.2 | |||||
| MDRS | 136.8 ± 5.8 | n.a. | |||||
| Measurement Parametersd | 3 T / 300 / 2.2 / 30 / 90° / 3.1 × 3.1 × 3.1 mm3 | 3 T / 165 / 2.2 / 30 / 90° / 3.1 × 3.1 × 3.1 mm3 | |||||
HHU, Heinrich Heine University; RWTH, Rheinisch–Westfälische Technische Hochschule.
Mini-Mental State Examination; MDRS, Mattis Dementia Rating Scale.
am = male, f = female.
bFD: Framewise displacement (within scanner movement).
cLaterality index: Right minus left UPDRS III scores, divided by the sum of right and left scores (Tomer et al. 1993).
dMeasurement parameters: Magnetic field strength/volumes/repetition time [s]/echo time [ms]/flip angle/voxel size.
Regions of Interest
Five spherical regions of interest (ROI, 5 mm radius) were chosen for the RSFC analysis, centered around Montreal Neurological Institute (MNI x y z) coordinates based on the previous literature. The goal of the present study was to examine ROIs consistently associated with motor initiation with respect to Parkinson’s disease. Therefore, coordinates in the pMFC (0 14 48) and bilateral centromedial putamen (left: −28 −2 2; right: 24 2 −2) were derived from local maxima of an activation likelihood estimation meta-analysis examining 103 neuroimaging experiments on volitional hand movements (Hoffstaedter et al. 2014). These seeds were motivated by the observation that movement initiation tasks (i.e., tasks with a free choice of movement initiation) are associated with increased activation in the aforementioned cortico-striatal network formed by the putamen and pMFC. Importantly, the pMFC was not only associated with self-selected timing, but also choice of movement (e.g., between different movement effectors), indicating the region’s central role in different aspects of movement initiation (Hoffstaedter et al. 2013). For comparison, the 4th and 5th ROIs in bilateral primary motor hand areas (M1, left: −34 −26 56; right: 34 −24 52; cf. Roski et al. 2014) were included as control seeds, since M1 is known to be one of the least affected areas by neurodegenerative processes in Parkinson’s disease (Braak et al. 2004).
Seed-Based Resting-State fMRI Analysis
BOLD signal time series were measured using Siemens MAGNETOM TIM Trio 3 Tesla whole body scanners (Erlangen, Germany) at both study sites. For each participant, whole-brain volumes of gradient-echo EPI pulse sequences were acquired (details for each acquisition site are provided in Table 1). Preprocessing was conducted using the SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm). The first 4 scans served as “dummy” images to allow for magnetic field saturation and were discarded prior to further preprocessing. The remaining EPI images were first corrected for head movement by affine registration using a 2-pass procedure, first creating a mean EPI and then realigning all scans to it. The mean EPI image of each participant was then spatially normalized to the MNI non-linear average FSL template using the “unified segmentation” approach (Ashburner and Friston 2005). The ensuing deformation was then applied to the individual EPI volumes. Then, images were smoothed by a 5-mm FWHM Gaussian kernel to account for residual anatomical variation and to meet the requirements of normal distribution of the residuals for Gaussian random field inference to correct for multiple comparisons. Movement effects in the scanner were corrected via regression of 24 movement parameters including the 6 motion parameters derived from the image realignment and their first derivative from the realignment as first and second order term (Power et al. 2012) before band pass filtering between 0.01 and 0.08 Hz (Cordes et al. 2001).
In order to compare the configuration of the predefined network between patients and healthy controls, pairwise RSFC was estimated between all seeds. Therefore, partial temporal correlations were computed using the FSLNets toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets). For each pairwise connection, Fisher’s z-transformed FC values were submitted to one-sample t-tests. The resulting t values, reflecting connection strength as well as consistency across the sample, were z-transformed (i.e., into units of the standard normal distribution) and then fed into an agglomerative hierarchical cluster analysis. Cluster analysis was based on Ward’s method as implemented in Matlab. Although this method does not reveal a finite parcellation (into a specific number of clusters), it aims at the heuristic identification of functionally interpretable subclusters within a given network. Data from healthy controls and patients were analyzed separately to assess network configurations of each group.
After the between-seed RSFC analysis, whole-brain RSFC was computed for each ROI in both patients and healthy controls to account for connectivity alterations in extended resting-state networks of each region. Accordingly, for each subject, linear (Pearson) correlation coefficients were computed between each seed’s time series and those of all other gray matter voxels in the brain. Voxel-wise correlation coefficients were then transformed into Fisher’s z-scores, and tested for consistency across subjects by a second-level analysis of variance (ANOVA). Therefore each seed’s RSFC was entered in a general linear model (GLM), as implemented in SPM8. Additionally, to assess akinesia-related RSFC changes across the whole-brain for each ROI, UPDRS item 31 was introduced as a covariate for each seed’s RSFC in the GLM. Additionally, measurement site (Aachen, Düsseldorf) was added as a covariate of no interest for all analyses to account for site-related effects.
To identify Parkinson patients’ connectivity changes in the context of physiological resting-state networks, we first examined RSFC of the healthy participants. Based on healthy subjects’ RSFC, conjunction analyses were used to delineate 3 networks specifically connected to bilateral putamen, bilateral M1, and pMFC.
That is, specific RSFC with the pMFC was obtained by contrasting RSFC of this region to the RSFC of all remaining seeds: (RSFCpMFC) ∧ (RSFCpMFC – RSFCbilateral putamen) ∧ (RSFCpMFC – RSFCbilateral M1). Resting-state networks specifically connected with bilateral putamen and M1 were computed in analogous fashion. Similar analyses have been employed in previous studies, aiming at disentangling specific connectivity maps related to different ROIs (i.e., Cieslik et al. 2012; Eickhoff et al. 2016; Genon et al. 2017). In sum, the ensuing networks from these conjunctions comprise those regions that were specifically stronger connected with a particular seed, as opposed to the other ones. It is important to note, however, that each seed may feature RSFC and possibly Parkinson-related changes in all 3 networks, which was tested in the following analysis.
In the next step, the 3 maps derived from healthy controls were used to assign connectivity alterations related to Parkinson’s disease. Altered connectivity in patients tested by comparing RSFC of each seed between patients and controls. For example, patients’ connectivity decreases of the putamen were computed using the conjunction: (healthy RSFCbilateral putamen) ∧ (healthy RSFCbilateral putamen – patients’ RSFCbilateral putamen). In turn, increased RSFC with the putamen in Parkinson’s disease was tested in the conjunction: (healthy RSFCbilateral putamen) ∧ (patients’ RSFCbilateral putamen – healthy RSFCbilateral putamen). These conjunctions were likewise applied to contrast patients’ changes in bilateral M1 and pMFC. Besides testing for altered connectivity in Parkinson’s disease, we probed co-variation between akinesia-scores and every ROI’s RSFC. All of the ensuing disease- and symptom-related connectivity maps were then masked by each of the 3 networks, previously derived from healthy controls. This allowed for dissociating pathological connectivity patterns in putamen-, M1-, or pMFC-specific resting-state networks.
Finally, conjunction analyses were used to test whether altered RSFC patterns of the different seeds were localized in converging brain regions. For example, overlap of RSFC decrease between putamen and pMFC was tested by the conjunction: (healthy RSFCbilateral putamen) ∧ (patients’ RSFCbilateral putamen – healthy RSFCbilateral putamen) ∧ (healthy RSFCpMFC) ∧ (patients’ RSFCpMFC – healthy RSFCpMFC). All results were whole-brain, cluster-level (P < 0.05) corrected for multiple comparisons.
Principal Component Analyses on the UPDRS III
RSFC was further related to the remaining UPDRS III (motor) items, including items which are used to assess bradykinesia, rigor and tremor. In this alternative approach, we aimed to reduce dimensionality of UPDRS III items using a principal component analysis as implemented in Matlab. Patients’ scores of the ensuing components explaining most variance across UPDRS items were then related to each seed’s RSFC, analogous to the connectivity analysis on akinesia, described in the previous paragraph. Methods and results of these analyses are in detail provided in the online Supplementary Material.
Anatomical Localization
The SPM Anatomy Toolbox v2.0 (Eickhoff et al. 2005, 2007) was used to allow for investigator-independent anatomical localization of the results. By means of maximum probability maps, activation clusters were automatically assigned to the most likely cytoarchitectonic area.
Results
Hierarchical Clustering Based on RSFC Between Seeds
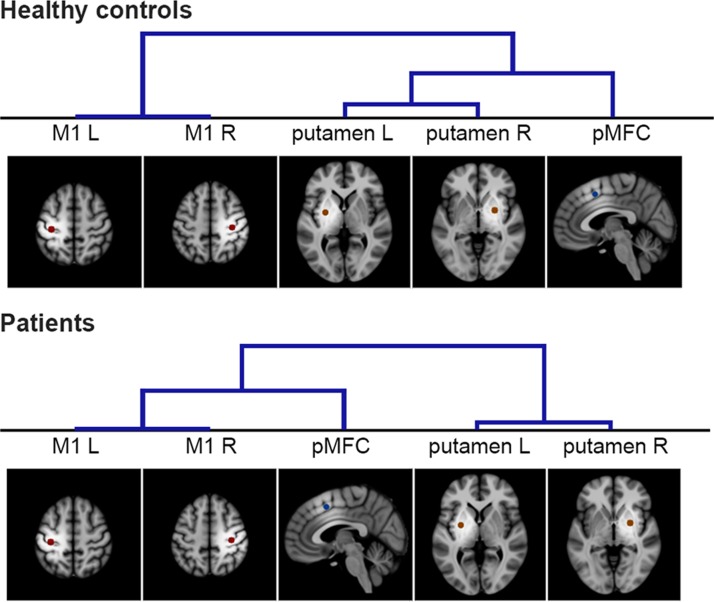
Cross-correlations between all seeds’ time series (bilateral putamen, bilateral M1, pMFC) enabled us to group seeds based on their connectivity strengths between each other. This hierarchical clustering analysis was separately performed for healthy controls and patients (Fig. 1). In both groups, strongest connectivity was found for interhemispheric interactions between anatomically homolog regions, leading to their consistent clustering as pairs. Moreover, in the healthy control group, bilateral putamen and pMFC were clustered in one group, separated from bilateral M1. In contrast, Parkinson patients featured stronger connectivity between pMFC and M1, clearly separated from bilateral putamen. Hence, we observed a distinct hierarchical clustering between regions of movement initiation and execution in patients compared to controls.
Figure 1.
Hierarchical clustering of ROIs. Hierarchical clustering of ROIs based on their RSFC in healthy participants (top) and Parkinson patients (bottom). Using FSLNets, seeds with relatively strong RSFC were grouped first as illustrated by lower branches in the cluster trees. The qualitative comparison of both hierarchies indicates a shift of pMFC connectivity from homolog putamen to homolog M1 ROIs in Parkinson’s disease.
RSFC Networks in Healthy Participants
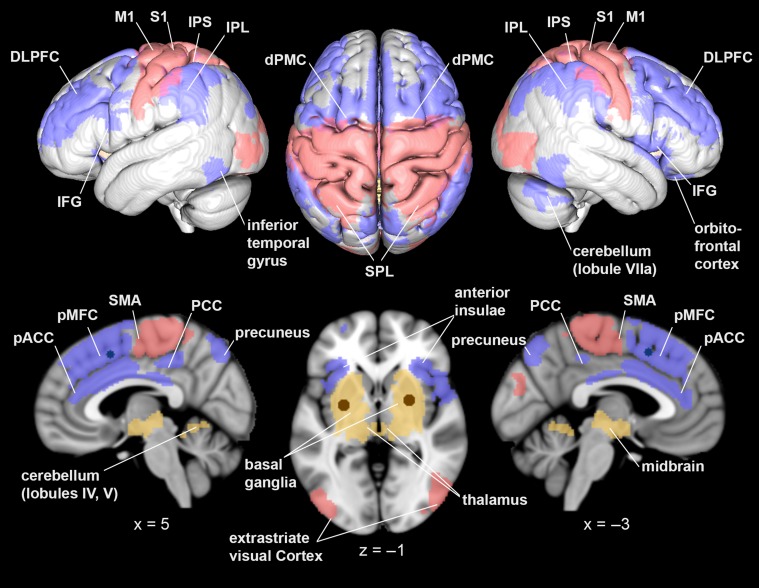
For each ROI, brain regions showing strongest connectivity estimates compared to all other seeds were isolated in healthy controls. For example, the analysis of specific RSFC of bilateral putamen only yielded those brain regions, that were (1) functionally connected to bilateral putamen, and (2) stronger connected to bilateral putamen than to bilateral M1 and pMFC seeds. This putamen-specific network comprised bilateral basal ganglia, midbrain, thalamus, left amygdala, right posterior insula, and bilateral cerebellum (bilateral lobules IV and V; Diedrichsen et al. 2009) (Fig. 2).
Figure 2.
Resting-state functional connectivity networks in healthy controls. Differentiation of 3 resting-state networks in healthy participants: these yielded stronger connectivity to bilateral putamen (yellow), bilateral M1 (red), and pMFC (blue), as contrasted to the respective other seeds. All results were cluster-level corrected at P < 0.05. Images were rendered into a T1-weighted MNI single subject template using mango (http://ric.uthscsa.edu/mango/).
Equivalent contrast analyses identified specific resting-state networks for bilateral M1 (regions stronger connected with bilateral M1 than bilateral putamen and pMFC seeds) and pMFC (regions stronger connected with pMFC than with bilateral putamen and M1 seeds). The M1-specific network comprised the medial and lateral primary motor cortex, the posterior portion of the supplementary motor area (SMA; Geyer 2004), the dorsal premotor cortex (dPMC), primary somatosensory cortex (S1), superior parietal lobule (SPL), the medial bank of the intraparietal sulcus (IPS), extrastriate visual cortex, and right parietal operculum.
Finally, the resting-state network specifically connected with pMFC contained bilateral inferior frontal gyri (IFG; areas 44 and 45), perigenual anterior cingulate cortex (pACC), and posterior cingulate cortex (PCC; Vogt et al. 2004; Palomero-Gallagher et al. 2009), pMFC, dPMC, dorsolateral prefrontal cortex (DLPFC), anterior insulae, inferior parietal lobules (IPL), the lateral bank of the IPS, posterior areas of the SPL (7 P, 5 Ci; Scheperjans et al. 2008), precuneus, inferior temporal gyri, right orbitofrontal cortex, and right cerebellum (lobule VIIa crus I).
In summary, 3 networks specifically connected to putamen, M1 and pMFC were dissected by contrasting seeds’ RSFC (Fig. 2). Importantly, these networks were solely derived from healthy participants’ data. Thus, they provided representative functional connectivity maps to test for pathological connectivity changes.
RSFC Changes in Parkinson’s Disease
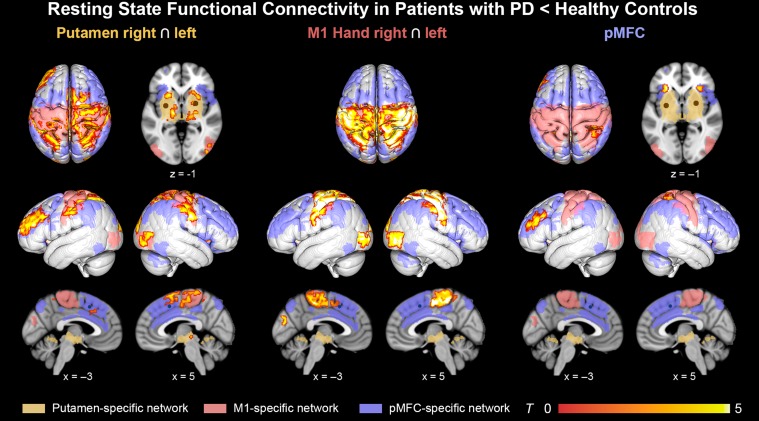
Connectivity changes in Parkinson’s disease were analyzed within the 3 seed-specific networks. There were only connectivity decreases in patients compared to healthy controls (Fig. 3), whereas no significant increases were found. The putamen seeds showed RSFC decreases in all 3 examined networks, that is the putamen-, pMFC-, and M1-specific network. First, in the putamen-specific network, we identified decreased putaminal RSFC with bilateral thalamus, putamen, and pallidum. Second, in the M1-specific network, putamen exhibited reduced connectivity with bilateral SMA, S1, M1, SPL, IPS, extrastriate visual cortex, and right dPMC. Third, in the pMFC-specific network, putamen showed reduced RSFC with bilateral IPS, pMFC, left DLPFC, and left IPL.
Figure 3.
Resting-state functional connectivity decrease in Parkinson’s disease. RSFC decrease in Parkinson patients assigned to the 3 resting-state networks as defined in the healthy group. These networks were rendered in yellow (putamen-specific network), red (M1-specific network), and blue (pMFC-specific network). Left column: RSFC with bilateral putamen was reduced in regions from all 3 networks. Middle column: RSFC with bilateral M1 declined most selectively in the M1-related network. Right column: Focal reductions of RSFC with the pMFC seed were found in pMFC- and M1-related networks. All results were cluster-level corrected at P < 0.05.
Likewise, the pMFC was tested for RSFC abnormalities in Parkinson’s disease. Within the M1-specific network, the pMFC seed showed decreased connectivity to bilateral S1 and right SPL. Additionally, in the pMFC-specific network, the pMFC seed exhibited decreased RSFC with bilateral anterior insulae and left DLPFC.
Furthermore, pathological connectivity changes were tested in M1 as a control region. RSFC decreases with bilateral M1 were mainly found in the M1-specific network including SMA, dPMC, M1, S1, SPL, IPS, and extrastriate visual cortex. In addition, an M1 connectivity decrease was observed in the pre-SMA. As revealed by the RSFC analysis in healthy controls, this region is part of the pMFC-specific network, which includes medial frontal regions rostral to the SMA. In contrast to the M1- and pMFC-specific networks, M1 did not show significantly altered connectivity in the putamen-specific network.
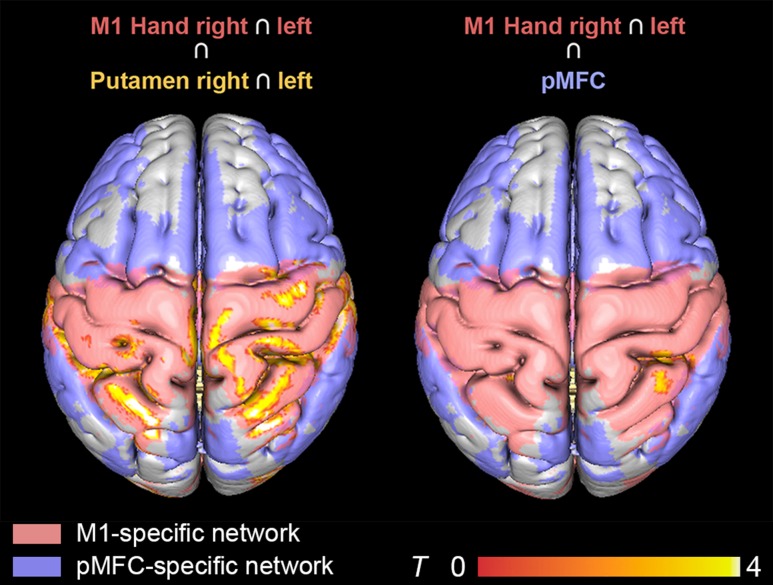
Finally, the aforementioned RSFC alterations in Parkinson’s disease were tested for overlapping effects across seeds (Fig. 4). Convergent connectivity reductions were especially found for putamen and M1. Both regions featured reduced connectivity with the SMA, dPMC, and M1 in the right hemisphere as well as bilateral S1, SPL, IPS, and extrastriate visual cortex. Putamen seeds furthermore showed overlapping RSFC changes with the pMFC seed, namely in bilateral S1 and right SPL. No significant overlap was found when conjoining patients’ RSFC reductions of M1 and the pMFC.
Figure 4.
Overlap in resting-state connectivity decrease across seeds. Overlapping patterns of RSFC decrease were observed between M1 and putamen (left) as well as M1 and pMFC (right). All results were cluster-level corrected at P < 0.05.
Akinesia-Related RSFC Changes in Parkinson’s Disease
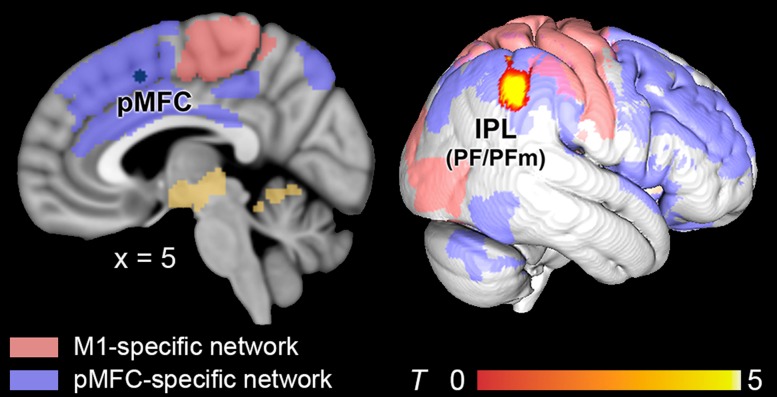
To analyze the relationship of akinesia and RSFC alterations in Parkinson’s disease, akinesia scores were included in the GLM for each seed. Thus, RSFC with every seed was tested for correlations with akinesia. While no association between akinesia and RSFC could be found within the putamen-, or M1-specific networks, connectivity within the pMFC-specific network was related to akinesia in Parkinson’s disease. That is, RSFC between pMFC and right IPL (PF, PFm, Caspers et al. 2006) showed a negative correlation with akinesia scores. That is, higher impairment was correlated with a decline of RSFC between the pMFC seed and right IPL (Fig. 5).
Figure 5.
Connectivity decrease related to patients’ global spontaneity of movements. Decreased RSFC between the pMFC seed and the inferior parietal lobule (IPL, areas PF/PFm) correlated with impaired spontaneity of movement initiation in Parkinson’s disease. Resting-state networks from healthy controls were rendered in yellow (putamen-related regions), red (M1-related regions), and blue (pMFC-related regions). All results were cluster-level corrected at P < 0.05.
Discussion
The pMFC and putamen are considered key regions for the spontaneous initiation of movements, which is critically impaired in Parkinson’s disease. The present study elucidated abnormal RSFC changes of these regions, and 2 control seeds in bilateral M1, in a cohort of 60 Parkinson patients and 72 healthy controls. First, the examination of RSFC between the 5 seeds indicated disturbed connectivity between putamen and pMFC seeds in patients. Alterations of RSFC were further examined in 3 seed-specific resting-state networks, reflecting preferential connections with a given seed (relative to the others) in the healthy control group (Fig. 2). In Parkinson’s disease, all seeds showed reduced RSFC within their respective network. For example, M1 showed a RSFC decrease mainly in the M1-specific sensorimotor network, pMFC lost RSFC with both anterior insulae and left DLPFC (pMFC-specific network), and bilateral putamen showed marked RSFC loss within the putamen-specific subcortical network (Fig. 3). Finally, decreasing RSFC between pMFC and right IPL correlated with higher scores of akinesia in Parkinson’s disease.
Network Hierarchy Amongst Seeds
Based on inter seed correlations, hierarchical clustering analyses yielded different network hierarchies in healthy controls as compared to Parkinson patients (Fig. 1). In controls, pMFC and putamen were clustered in one group, separate from bilateral M1 seeds. This network configuration is consistent with the seeds’ different functional implications: While putamen and pMFC have been associated with the initiation of movements (Goldberg 1985; Deiber et al. 1999; Jenkins et al. 2000; Cunnington et al. 2002; Hoffstaedter et al. 2013, 2014), the primary motor cortex is mainly related to motor execution (Chen et al. 1991; Tanji and Mushiake 1996; Roski et al. 2014). The strong functional connectivity between pMFC and centromedial putamen seeds, as observed during motor initiation tasks, furthermore parallels their structural interconnectivity, indicated by probabilistic tractography in diffusion weighted imaging data (Draganski et al. 2008; Helmich et al. 2010; Tziortzi et al. 2014; Neggers et al. 2015). Interestingly, RSFC across seeds yielded a different network hierarchy in Parkinson’s disease. That is, patients’ pMFC was less connected with putamen than M1. This separation of cortical and subcortical seeds in patients (Fig. 1) indicates dysfunctional cortico-striatal connectivity, which has been frequently observed in functional connectivity studies (Helmich et al. 2010; Kwak et al. 2010; Luo et al. 2014; Michely et al. 2015; Müller-Oehring et al. 2015). In relation to the decreased cortico-striatal connectivity, the hierarchical clustering suggested stronger connectivity between cortical seeds (M1, pMFC) in patients. In line with this observation, neuropathological changes in Parkinson’s disease are known to start subcortically, whereas cortical regions are affected at later stages of the disease (Braak et al. 2004). By quantitatively comparing RSFC between patients and HC, the following paragraphs provide a detailed analysis of altered cortico-striatal and cortico-cortical connectivity in Parkinson’s disease.
Subcortical and Cortico-Striatal Connectivity in Parkinson’s Disease
In healthy subjects, a subcortical network was identified by its specific RSFC to bilateral putamen, in contrast to M1 and pMFC (Fig. 2). This putamen-specific network included bilateral caudate nuclei, pallidum, midbrain, thalamus and subthalamic nuclei, which are known to be also structurally interconnected (Kalil 1978; Albin et al. 1989; Nakano et al. 1990; Redgrave et al. 2010). Within this subcortical network, Parkinson patients showed reduced RSFC of bilateral putamen with pallidum and thalamus. Moreover, subcortical connectivity reductions were related to symptoms of bradykinesia as shown in detail in the online Supplementary Material of this article (Supplementary Fig. 4S). This is in line with previous studies reporting decreased RSFC between subcortical nodes, for example between putamen and thalamus (Hacker et al. 2012; Wu et al. 2012). Notably, these regions are predominantly involved in cortico-striatal circuits, which play a crucial role in pathophysiological models of Parkinson’s disease (Alexander et al. 1986; Redgrave et al. 2010). Accordingly, dopaminergic depletion in the putamen arises from the degeneration of dopaminergic neurons in the substantia nigra pars compacta (Hornykiewicz and Kish 1987; Morrish et al. 1995; Forno 1996). This, in turn, affects intrinsic basal-ganglia processing but importantly also its interaction with cortical regions (DeLong 1983; Jahanshahi 1998; Wichmann and DeLong 2002; Grafton 2004; Wichmann et al. 2011). Well in line, the present results not only show reduced putamen RSFC with subcortical nodes, but also extensive decoupling across cortical regions that are functionally stronger connected with M1 or pMFC (Fig. 3), including the primary and secondary motor- and somatosensory cortex, SMA, pre-SMA, right dPMC, extrastriate visual cortex, and left DLPFC. The former regions participate in cortico-striatal loops, whose dysfunction may explain sensorimotor and habitual dysfunction in Parkinson’s disease (Abbruzzese and Berardelli 2003; Graybiel 2008; Redgrave et al. 2010). Correspondingly, previous RSFC studies in Parkinson patients have reported cortico-striatal decoupling mainly with sensorimotor regions, especially when selecting seeds in the posterior putamen (Helmich et al. 2010; Luo et al. 2014). The centromedial putamen examined in the present study is located between posterior and anterior aspects of the putamen, as it was defined by its involvement in voluntary motor initiation (Hoffstaedter et al. 2014). As opposed to posterior putamen seeds, central regions of the putamen feature stronger structural connections to frontal premotor regions and more rostral putamen seeds have been shown to connect with prefrontal areas in humans (Lehericy, Ducros, Krainik, et al. 2004; Lehericy, Ducros, Van de Moortele, et al. 2004; Draganski et al. 2008; Tziortzi et al. 2014). In agreement with the subregional organization of the putamen, the presently used seeds in the putamen show RSFC reductions with bilateral pre-SMA, dPMC, and left DLPFC. This network has been associated with cognitive motor functions such as action selection (Rowe et al. 2010) and planning (Nachev et al. 2005; Tanji and Hoshi 2008). Thereby, the observed dysconnectivity between central parts of the putamen and prefrontal systems for motor-cognition complements previous analyses on posterior versus anterior putamen in Parkinson’s disease (Helmich et al. 2010; Luo et al. 2014). In sum, the centromedial putamen shows a widespread decoupling from subcortical and cortical regions involved in both movement initiation and execution in patients. In sum, the present findings outline dysfunctional circuits between aspects of the putamen usually involved in motor initiation, and cortical networks, associated with sensory, motor, and cognitive processing.
pMFC-Related Connectivity in Parkinson’s Disease
In healthy participants, the pMFC seed featured strongest RSFC with the DLPFC, IFG, anterior insulae, pre-SMA, anterior midcingulate cortex, IPS, and IPL (Fig. 2). These regions have been consistently related to cognitive, i.e., higher motor control (Jenkins et al. 2000; Boecker et al. 2008; Brass and Haggard 2008; François-Brosseau et al. 2009; Rowe and Siebner 2012; Hoffstaedter et al. 2013) as well as to non-motor tasks, and motivational processing (Niendam et al. 2012; Rottschy et al. 2012, Chong et al. 2017). Within this network, the pMFC has been suggested to play a key role in movement initiation (Deiber et al. 1999; Cunnington et al. 2002; Hoffstaedter et al. 2013). Correspondingly, subdural stimulation of this region has been reported to trigger motor responses, often with the experience of an “urge” to move (Fried et al. 1991). Moreover, clinical case reports have described akinetic symptoms after pMFC lesions (Dick et al. 1986; Meador et al. 1986; Haussermann et al. 2001). Importantly, functional neuroimaging studies on voluntary movements performed by Parkinson patients have identified abnormal activation not solely in the pMFC, but also areas like the DLPFC, insulae, and IPL (Playford et al. 1992; Jahanshahi et al. 1995; Samuel et al. 1997; Kikuchi et al. 2001). Yet, it is unclear whether—and how—pathological interactions between the pMFC and these regions relate to akinesia. Interestingly, although the present findings show a relatively preserved pMFC connectivity, focal reductions were found within the pMFC-specific network. In this network, the pMFC showed focal reductions of RSFC with bilateral anterior insulae as well as the left DLPFC. This suggests dysfunctional processing in a core network for multiple cognitive demands, including working memory, attention, and inhibition (Alvarez and Emory 2006; Duncan 2010; Müller et al. 2015). Atrophy within this network, specifically in the pMFC and anterior insulae, has been commonly observed across several neuropsychiatric disorders (Goodkind et al. 2015). With respect to Parkinson’s disease, these regions are known to be amongst the first neocortical regions showing disease-specific neurodegeneration (Braak et al. 1996, 2004, 2006), and neurotransmitter dysfunction (Pavese et al. 2010; Christopher et al. 2014, 2015). Recently, PET studies have increasingly linked reduced dopamine receptor binding in anterior insulae with Parkinson patients’ cognitive symptoms (Christopher et al. 2014, 2015). Moreover, reduced cerebral blood flow in the right insula has been associated with higher UPDRS scores (Hsu et al. 2007).
Besides motor initiation, pMFC, DLPFC, and insula have also been linked to motivational processing, motor vigor and the integration of effort costs and rewards during decision making (Walton et al. 2003; Schweimer and Hauber 2006; Croxson et al. 2009; Chong et al. 2017). Both motivational and motor deficits have been shown to respond to dopaminergic modulation in PD, indicating an overlapping neural system underlying apathy and akinesia (Chong et al. 2015; Le Bouc et al. 2016; Le Heron et al. 2018). Although the present study did not assess patients’ motivational deficits, it suggests decreased connectivity in systems associated with higher-level motivational processing.
Further, DLPFC dysfunction in Parkinson patients has been associated with deficits in attention demanding tasks (Brown et al. 1998; Dirnberger et al. 2005; Zgaljardic et al. 2006; Trujillo et al. 2015), as well as compromised cognitive motor control (Rowe et al. 2002). Rowe et al. (2002) observed that during action monitoring healthy individuals’ effective connectivity between DLPFC and pMFC increases, whereas patients failed to show this attention-modulated increase. Dopaminergic medication, however, led to an increase of DLPFC-pMFC connectivity, which correlated with the improvement of motor performance (Michely et al. 2015). Based on resting-state fMRI data, the present study suggests a decoupling between DLPFC and pMFC in Parkinson patients, also in the absence of a task. In sum, the pMFC shows reduced RSFC with regions that are commonly involved in both higher cognitive, motivational and basic motor functions and their decline.
Interestingly, reduction of pMFC connectivity with the right IPL (areas PF, PFm, Fig. 5) was related to the degree of akinesia in the patient group. Evidence from functional neuroimaging studies in humans (Corbetta et al. 2008; Seghier 2013) and single cell recordings in monkeys (Fogassi 2005) has consistently highlighted the IPL’s role in integrating multimodal perceptual information and higher-order conceptual representations (Caspers et al. 2012). Further, fMRI (Farrer et al. 2008) and lesion studies (Sirigu et al. 2004) in humans suggest that the IPL strongly contributes to the awareness of self-generated actions. While healthy individuals are able to report their intention to move about 250 ms before movement onset, patients with IPL lesions were impaired in this ability (Sirigu et al. 2004). This suggests a key role of the IPL in the subjective experience of voluntary movement initiation before its onset. This suggestion is supported by electrophysiological work by Desmurget et al. (2009), who showed that direct electrical stimulation of the IPL triggered the conscious sense of “wanting to move” (Desmurget et al. 2009). However, in striking contrast to pMFC stimulation (Fried et al. 1991), IPL stimulation did not result in muscular responses, even at stronger stimulation intensities. A recent review of electrophysiological, clinical, and behavioral studies therefore indicated that the IPL may process earlier stages of intentional movement generation, whereas the pMFC regulates responses shortly before their execution (Desmurget and Sirigu 2012). Interestingly, akinesia in Parkinson’s disease has been suggested to arise from an impaired conversion from intentional motor plans into “overt actions” (Jahanshahi 1998). The present results suggest that this impairment is associated with a reduction of RSFC between right IPL and pMFC, thereby highlighting the importance of functional interaction between these regions with respect to the manifestation of akinesia in Parkinson’s disease.
M1-Related Connectivity in Parkinson’s Disease
The M1-specific resting-state network was delineated by contrasting M1 seeds’ RSFC to all other seeds’ connectivity in the healthy control group. The resulting network comprised the primary and secondary sensorimotor cortex, SMA, and dPMC, IPS, SPL, as well as extrastriate visual areas (Fig. 2). Especially the former regions are well known as a structurally interconnected network (Cavada and Goldman-Rakic 1989; Krubitzer and Kaas 1990; Dum and Strick 2005) engaged in sensorimotor integration (Johnson et al. 1993; Caminiti et al. 1996; Corbetta et al. 2000; Caspers et al. 2012) and motor execution (Kurata and Tanji 1986; He et al. 1993; Dum and Strick 1996; Grèzes and Decety 2001). Strikingly, M1 connectivity with all of these regions was significantly reduced in Parkinson patients compared to controls (Fig. 3). In contrast, patients’ M1 connectivity with the subcortical, putamen-specific network was not significantly different. M1 also featured normal connectivity with most regions of the frontoparietal, pMFC-specific network, except for the pre-SMA (Fig. 3). This finding agrees with previous studies (Wu, Long, et al. 2011; Michely et al. 2015), which did not find significantly altered M1 connectivity with prefrontal regions in Parkinson’s disease, compared with healthy individuals (but see Wu, Wang, et al. 2011). Hence, the present results suggest a selective decline of M1 connectivity with a broader sensorimotor network, which normally features strong RSFC with M1 in the healthy brain (Fig. 2). This finding might be linked to previous behavioral (Abbruzzese and Berardelli 2003) and electrophysiological (Lefaucheur 2005) evidence for dysfunctional sensorimotor processing in Parkinson’s disease. For example, hand movements (Rickards and Cody 1997; Khudados et al. 1999) and transcranial magnetic stimulation (TMS)-induced motor responses (Sailer et al. 2003; Wagle Shukla et al. 2013) were less amenable to somatosensory input in Parkinson patients. Such indicators for deficient sensorimotor processing are in line with the marked RSFC decrease between M1 and the adjacent sensorimotor cortex. Yet, disturbed RSFC in these regions is in striking contrast to the histopathological distribution of Parkinson’s disease-related Lewy-body accumulation, which are rarely observed in the primary motor cortex even during late stages of Parkinson’s disease (Braak et al. 2003, 2004). It is therefore unlikely that the decreased connectivity with M1 arises from this region’s neurodegeneration per se. Rather, functional connectivity with M1 may be affected by distant regions that degenerate earlier in the course of Parkinson’s disease, such as the putamen. Indeed, conjunction analyses revealed that M1 and putamen share dysconnectivity in almost all regions of the M1-specific network, including the right SMA and dPMC as well as bilateral primary and secondary sensorimotor cortex, IPS, SPL, and extrastriate visual areas (Fig. 4). Assuming that these regions are primarily affected by an insufficient striatal facilitation in Parkinson’s disease (DeLong 1983; Jahanshahi 1998; Wichmann and DeLong 2002; Grafton 2004; Wichmann et al. 2011), their decoupling from M1 might develop as a secondary effect. In sum, the present study demonstrates a selective RSFC loss between M1 and a functionally related sensorimotor network, possibly reflecting an extension of network pathology from cortico-striatal to cortico-cortical connectivity in Parkinson’s disease.
Limitations
A limitation of the present study is that all patients were assessed under regular dopaminergic medication. Thus, drug effects cannot be distinguished from neuropathological alterations in patients’ functional connectivity. Unfortunately, a differentiation of the latter underlies practical challenges especially in later disease stages, since dopamine replacement therapies are known to induce long term effects, persisting under dopamine withdrawal (Lesser et al. 1979; Chase 1998). While some of the effects observed in the present study, such as the reduction of cortico-striatal RSFC, has also been observed in drug-naïve patients (Luo et al. 2014), longitudinal monitoring of RSFC before and after dopaminergic treatment may further disentangle effects induced by drugs and degeneration, respectively. Moreover, the clinical assessment of akinesia was limited to the UPDRS. Given the results of the present study, a more detailed examination of akinetic symptoms appears promising for future studies, allowing a more detailed analysis of clinical deficits in relation to functional connectivity in Parkinson’s disease.
Conclusion
The present study investigated altered connectivity in parkinson’s disease, analyzing seed-based RSFC of bilateral putamen and pMFC, functionally defined by motor initiation tasks, as well as M1, defined by motor execution. While, hierarchical clustering based on inter seed connectivity indicated a decoupling between regions involved in motor initiation (putamen and pMFC), an extended RSFC analysis in seed-specific networks revealed Parkinson-related connectivity alterations on the subcortical and cortical level. In line with pathophysiological models of Parkinson’s disease, putamen showed reduced connectivity with basal ganglia, sensorimotor areas, but also regions involved in higher-level cognitive functions such as the left DLPFC. In contrast, altered connectivity with M1 was restricted to a cortical sensorymotor network. Of note, pMFC showed reduced connectivity in a core network for higher cognitive demands and its decreased connectivity with the right IPL was related to patients’ impairment of spontaneous movement generation. We thereby provide evidence that cortical networks associated with cognitive motor control are involved in the manifestation of akinesia in Parkinson’s disease.
Supplementary Material
Notes
Conflict of Interest: None declared.
Funding
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, EI 816/11-1), the National Institute of Mental Health (R01-MH074457), the Helmholtz Association of German Research Centres (Portfolio Theme “Supercomputing and Modeling for the Human Brain”) and the European Union’s Horizon 2020 Research and Innovation Programme (Grant Agreements No. 720270, HBP SGA1 and 785907, HBP SGA2). J.M., G.R.F., and C.G. are supported by the Deutsche Forschungsgemeinschaft (Clinical Research Group KFO219 “Basal-Ganglia-Cortex-Loops: Mechanisms of Pathological Interactions and Therapeutic Modulation”; GR 3285/5–1). G.R.F and C.G. received additional funding from the University of Cologne Emerging Groups Initiative (CONNECT group) implemented into the Institutional Strategy of the University of Cologne and the German Excellence Initiative.
References
- Abbruzzese G, Berardelli A. 2003. Sensorimotor integration in movement disorders. Mov Disord. 18:231–240. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12:366–375. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 9:357–381. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. 2006. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 16:17–42. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2005. Unified segmentation. Neuroimage. 26:839–851. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. 1973. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 20:415–455. [DOI] [PubMed] [Google Scholar]
- Boecker H, Jankowski J, Ditter P, Scheef L. 2008. A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. Neuroimage. 39:1356–1369. [DOI] [PubMed] [Google Scholar]
- Braak H, Bohl JR, Müller CM, Rüb U, de Vos RAI, Del Tredici K. 2006. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 21:2042–2051. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J. 1996. Pattern of brain destruction in Parkinson“s and Alzheimer’s diseases. J Neural Transm. 103:455–490. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos R. 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Ageing. 24:197–211. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. 2004. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 318:121–134. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P. 2008. The what, when, whether model of intentional action. Neuroscientist. 14:319–325. [DOI] [PubMed] [Google Scholar]
- Brown RG, Soliveri P, Jahanshahi M. 1998. Executive processes in Parkinson’s disease—random number generation and response suppression. Neuropsychologia. 36:1355–1362. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. 2002. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 99:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti R, Ferraina S, Johnson PB. 1996. The sources of visual information to the primate frontal lobe: a novel role for the superior parietal lobule. Cereb Cortex. 6:319–328. [DOI] [PubMed] [Google Scholar]
- Caspers S, Amunts K, Zilles K. 2012. Posterior parietal cortex: multimodal association cortex. In: Paxinos G, Mai JK, editors. The human nervous system. 3rd ed. Amsterdam, NL: Elsevier; p. 1036–1055. [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. 2006. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 33:430–448. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. 1989. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 287:422–445. [DOI] [PubMed] [Google Scholar]
- Chapuis S, Ouchchane L, Metz O, Gerbaud L, Durif F. 2005. Impact of the motor complications of Parkinson’s disease on the quality of life. Mov Disord. 20:224–230. [DOI] [PubMed] [Google Scholar]
- Chase TN. 1998. Levodopa therapy: consequences of the nonphysiologic replacement of dopamine. Neurology. 50:S17–S25. [DOI] [PubMed] [Google Scholar]
- Chen DF, Hyland B, Maier V, Palmeri A, Wiesendanger M. 1991. Comparison of neural activity in the supplementary motor area and in the primary motor cortex in monkeys. Somatosens Mot Res. 8:27–44. [DOI] [PubMed] [Google Scholar]
- Chong TT-J, Apps M, Giehl K, Sillence A, Grima LL, Husain M. 2017. Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol. 15:e1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong TT-J, Bonnelle V, Manohar S, Veromann K-R, Muhammed K, Tofaris GK, Hu M, Husain M. 2015. Dopamine enhances willingness to exert effort for reward in Parkinson’s disease. Cortex. 69:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher L, Duff-Canning S, Koshimori Y, Segura B, Boileau I, Chen R, Lang AE, Houle S, Rusjan P, Strafella AP. 2015. Salience network and parahippocampal dopamine dysfunction in memory-impaired Parkinson disease. Ann Neurol. 77:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher L, Marras C, Duff-Canning S, Koshimori Y, Chen R, Boileau I, Segura B, Monchi O, Lang AE, Rusjan P, et al. . 2014. Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson’s disease with mild cognitive impairment. Brain. 137:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB. 2012. Is there “One” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 23:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. 2000. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 3:292–297. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron. 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. 2001. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TEJ, Rushworth MFS. 2009. Effort-based cost-benefit valuation and the human brain. J Neurosci. 29:4531–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. 2002. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 15:373–385. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taub E, Houle S, Lang AE, Dostrovsky JO, Tasker RR, Lozano AM. 1997. Globus pallidus stimulation activates the cortical motor system during alleviation of parkinsonian symptoms. Nat Med. 3:671–674. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. 1999. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol. 81:3065–3077. [DOI] [PubMed] [Google Scholar]
- DeLong MR. 1983. The neurophysiologic basis of abnormal movements in basal ganglia disorders. Neurobehav Toxicol Teratol. 5:611–616. [PubMed] [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. 2009. Movement intention after parietal cortex stimulation in humans. Science. 324:811–813. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Sirigu A. 2012. Conscious motor intention emerges in the inferior parietal lobule. Curr Opin Neurobiol. 22:1004–1011. [DOI] [PubMed] [Google Scholar]
- Dick JP, Benecke R, Rothwell JC, Day BL, Marsden CD. 1986. Simple and complex movements in a patient with infarction of the right supplementary motor area. Mov Disord. 1:255–266. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. 2009. A probabilistic MR atlas of the human cerebellum. Neuroimage. 46:39–46. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Frith CD, Jahanshahi M. 2005. Executive dysfunction in Parkinson’s disease is associated with altered pallidal-frontal processing. Neuroimage. 25:588–599. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. 2008. A dual-networks architecture of top-down control. Trends Cognitive Sci. 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. . 2007. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci. 104:11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJM, Deichmann R, Ashburner J, Frackowiak RSJ. 2008. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 28:7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. 1996. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 16:6513–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. 2005. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 25:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. 2010. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 14:172–179. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. 2016. Functional segregation of the human dorsomedial prefrontal cortex. Cereb Cortex. 26:304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras M-H, Evans AC, Zilles K, Amunts K. 2007. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 36:511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Escola L, Michelet T, Macia F, Guehl D, Bioulac B, Burbaud P. 2003. Disruption of information processing in the supplementary motor area of the MPTP-treated monkey: a clue to the pathophysiology of akinesia? Brain. 126:95–114. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frey SH, Van Horn JD, Tunik E, Turk D, Inati S, Grafton ST. 2008. The angular gyrus computes action awareness representations. Cereb Cortex. 18:254–261. [DOI] [PubMed] [Google Scholar]
- Fogassi L. 2005. Parietal lobe: from action organization to intention understanding. Science. 308:662–667. [DOI] [PubMed] [Google Scholar]
- Forno LS. 1996. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 55:259–272. [DOI] [PubMed] [Google Scholar]
- François-Brosseau F-E, Martinu K, Strafella AP, Petrides M, Simard F, Monchi O. 2009. Basal ganglia and frontal involvement in self-generated and externally-triggered finger movements in the dominant and non-dominant hand. Eur J Neurosci. 29:1277–1286. [DOI] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. 1991. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 11:3656–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Mentis MJ, Ma Y, Dhawan V, Antonini A, Lang AE, Lozano AM, Hammerstad J, Lyons K, Koller WC, et al. . 2001. Networks mediating the clinical effects of pallidal brain stimulation for Parkinson’s disease: a PET study of resting-state glucose metabolism. Brain. 124:1601–1609. [DOI] [PubMed] [Google Scholar]
- Genon S, Li H, Fan L, Müller VI, Cieslik EC, Hoffstaedter F, Reid AT, Langner R, Grefkes C, Fox PT, et al. . 2017. The right dorsal premotor mosaic: organization, functions, and connectivity. Cereb Cortex. 27:2095–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S. 2004. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol. 174:I–VIII–1–89. [DOI] [PubMed] [Google Scholar]
- Goldberg G. 1985. Supplementary motor area structure and function—review and hypotheses. Behav Brain Sci. 8:567–588. [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, et al. . 2015. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST. 2004. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol. 14:715–719. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. 2008. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 31:359–387. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Decety J. 2001. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Mapp. 12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ. 2012. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain. 135:3699–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. 2007. Volitional control of movement: the physiology of free will. Clin Neurophysiol. 118:1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, Cohen LG, Bierner SM. 1991. Studies of sensory and motor cortex physiology: with observations on akinesia in Parkinson’s disease. Electroencephalogr Clin Neurophysiol Suppl. 43:76–85. [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO. 2001. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain. 124:558–570. [DOI] [PubMed] [Google Scholar]
- Haussermann P, Wilhelm T, Keinath S, Stölzle C, Conrad B, Ceballos-Baumann A. 2001. Primary central nervous system lymphoma in the SMA presenting as rapidly progressive parkinsonism. Mov Disord. 16:962–965. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. 1993. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 13:952–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. 2010. Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb Cortex. 20:1175–1186. [DOI] [PubMed] [Google Scholar]
- Herz DM, Eickhoff SB, Løkkegaard A, Siebner HR. 2013. Functional neuroimaging of motor control in parkinson’s disease: A meta-analysis. Hum Brain Mapp. 35:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB. 2013. The “What” and “When” of self-initiated movements. Cereb Cortex. 23:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Langerner R, Benders A, Grefkes C, Eickhoff SB (2014), ‘Dissecting sub-components of the neural network for volitional hand movements - an ALE meta-analysis’, OHBM Annual Meeting.
- Hornykiewicz O, Kish SJ. 1987. Biochemical pathophysiology of Parkinson’s disease. Adv Neurol. 45:19–34. [PubMed] [Google Scholar]
- Hsu J-L, Jung T-P, Hsu C-Y, Hsu W-C, Chen Y-K, Duann J-R, Wang H-C, Makeig S. 2007. Regional CBF changes in Parkinson’s disease: a correlation with motor dysfunction. Eur J Nucl Med Mol Imaging. 34:1458–1466. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M. 1998. Willed action and its impairments. PCGN. 15:483–533. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins H, Brown RG, Marsden CD, Passingham RE, Brooks DJ. 1995. Self-initiated versus externally triggered movements .1. An investigation using measurement of regional cerebral blood-flow with pet and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 118:913–933. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. 2014. The pathomechanisms underlying Parkinson’s disease. Expert Rev Neurother. 14:199–215. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, Brooks DJ. 1992. Impaired activation of the supplementary motor area in Parkinson’s disease is reversed when akinesia is treated with apomorphine. Ann Neurol. 32:749–757. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. 2000. Self-initiated versus externally triggered movements II. The effect of movement predictability on regional cerebral blood flow. Brain. 123:1216–1228. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Caminiti R. 1993. Cortical networks for visual reaching. Exp Brain Res. 97:361–365. [DOI] [PubMed] [Google Scholar]
- Kalil K. 1978. Patch-like termination of thalamic fibers in the putamen of the rhesus monkey: an autoradiographic study. Brain Res. 140:333–339. [DOI] [PubMed] [Google Scholar]
- Khudados E, Cody FW, O’Boyle DJ. 1999. Proprioceptive regulation of voluntary ankle movements, demonstrated using muscle vibration, is impaired by Parkinson’s disease. J Neurol Neurosurg Psychiatr. 67:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Takeda A, Kimpara T, Nakagawa M, Kawashima R, Sugiura M, Kinomura S, Fukuda H, Chida K, Okita N, et al. . 2001. Hypoperfusion in the supplementary motor area, dorsolateral prefrontal cortex and insular cortex in Parkinson’s disease. J Neurol Sci. 193:29–36. [DOI] [PubMed] [Google Scholar]
- Kranick SM, Hallett M. 2013. Neurology of volition. Exp Brain Res. 229:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. 1990. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 10:952–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Tanji J. 1986. Premotor cortex neurons in macaques: activity before distal and proximal forelimb movements. J Neurosci. 6:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Müller MLTM, Dayalu P, Seidler RD. 2010. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson’s disease. Front Syst Neurosci. 4:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouc R, Rigoux L, Schmidt L, Degos B, Welter M-L, Vidailhet M, Daunizeau J, Pessiglione M. 2016. Computational dissection of dopamine motor and motivational functions in humans. J Neurosci. 36:6623–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron C, Plant O, Manohar S, Ang Y-S, Jackson M, Lennox G, Hu MT, Husain M. 2018. Distinct effects of apathy and dopamine on effort-based decision-making in Parkinson’s disease. Brain. 141:1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J-P. 2005. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson’s disease: influence of antiparkinsonian treatment and cortical stimulation. Clin Neurophysiol. 116:244–253. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Krainik A, Francois C, Van de Moortele P-F, Ugurbil K, Kim D-S. 2004. a. 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex. 14:1302–1309. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele P-F, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim D-S. 2004. b. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 55:522–529. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Fahn S, Snider SR, Cote LJ, Isgreen WP, Barrett RE. 1979. Analysis of the clinical problems in parkinsonism and the complications of long-term levodopa therapy. Neurology. 29:1253–1260. [DOI] [PubMed] [Google Scholar]
- Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. 1997. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Ann Neurol. 42:283–291. [DOI] [PubMed] [Google Scholar]
- Luo C, Song W, Chen Q, Zheng Z, Chen K, Cao B, Yang J, Li J, Huang X, Gong Q, et al. . 2014. Reduced functional connectivity in early-stage drug-naive Parkinson’s disease: a resting-state fMRI study. Neurobiol Aging. 35:431–441. [DOI] [PubMed] [Google Scholar]
- MacDonald V, Halliday GM. 2002. Selective loss of pyramidal neurons in the pre-supplementary motor cortex in Parkinson’s disease. Mov Disord. 17:1166–1173. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Watson RT, Bowers D, Heilman KM. 1986. Hypometria with hemispatial and limb motor neglect. Brain. 109(Pt 2):293–305. [DOI] [PubMed] [Google Scholar]
- Michely J, Volz LJ, Barbe MT, Hoffstaedter F, Viswanathan S, Timmermann L, Eickhoff SB, Fink GR, Grefkes C. 2015. Dopaminergic modulation of motor network dynamics in Parkinson’s disease. Brain. 138:664–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrish PK, Sawle GV, Brooks DJ. 1995. Clinical and [F-18] dopa pet findings in early Parkinson’s disease. J Neurol Neurosurg Psychiatr. 59:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s 2003. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 18:738–750. [DOI] [PubMed] [Google Scholar]
- Müller VI, Langner R, Cieslik EC, Rottschy C, Eickhoff SB. 2015. Interindividual differences in cognitive flexibility: influence of gray matter volume, functional connectivity and trait impulsivity. Brain Struct Funct. 220:2401–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Sullivan EV, Pfefferbaum A, Huang NC, Poston KL, Bronte-Stewart HM, Schulte T. 2015. Task-rest modulation of basal ganglia connectivity in mild to moderate Parkinson’s disease. Brain Imaging Behav. 9:619–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. 2008. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 9:856–869. [DOI] [PubMed] [Google Scholar]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. 2005. Volition and conflict in human medial frontal cortex. Curr Biol. 15:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Hasegawa Y, Tokushige A, Nakagawa S, Kayahara T, Mizuno N. 1990. Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in the Japanese monkey, Macaca fuscata. Brain Res. 537:54–68. [DOI] [PubMed] [Google Scholar]
- Neggers SFW, Zandbelt BB, Schall MS, Schall JD. 2015. Comparative diffusion tractography of corticostriatal motor pathways reveals differences between humans and macaques. J Neurophysiol. 113:2164–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. 2012. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. 2009. Receptor architecture of human cingulate cortex: evaluation of the four‐region neurobiological model. Hum Brain Mapp. 30:2336–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. 1817. An Essay on the Shaking Palsy. [DOI] [PubMed]
- Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ. 2010. Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain. 133:3434–3443. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. 1992. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol. 32:151–161. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O. 2008. Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology. 70:1403–1410. [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Chollet F, Celsis P, Montastruc JL, Marc-Vergnes JP, Rascol A. 1992. Supplementary and primary sensory motor area activity in Parkinson’s disease. Regional cerebral blood flow changes during finger movements and effects of apomorphine. Arch Neurol. 49:144–148. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. 2010. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 11:760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickards C, Cody FW. 1997. Proprioceptive control of wrist movements in Parkinson’s disease. Reduced muscle vibration-induced errors. Brain. 120(Pt 6):977–990. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. 2004. The role of the medial frontal cortex in cognitive control. Science. 306:443–447. [DOI] [PubMed] [Google Scholar]
- Roski C, Caspers S, Lux S, Hoffstaedter F, Bergs R, Amunts K, Eickhoff SB. 2014. Activation shift in elderly subjects across functional systems: an fMRI study. Brain Struct Funct. 219:707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. 2012. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 60:830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Nimmo-Smith I. 2010. Action selection: a race model for selected and non-selected actions distinguishes the contribution of premotor and prefrontal areas. Neuroimage. 51:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Siebner HR. 2012. The motor system and its disorders. Neuroimage. 61:464–477. [DOI] [PubMed] [Google Scholar]
- Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. 2002. Attention to action in Parkinson’s disease: impaired effective connectivity among frontal cortical regions. Brain. 125:276–289. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. 2004. Action sets and decisions in the medial frontal cortex. Trends Cognitive Sci. 8:410–417. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. 2003. Short and long latency afferent inhibition in Parkinson’s disease. Brain. 126:1883–1894. [DOI] [PubMed] [Google Scholar]
- Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, Brooks DJ. 1997. Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain. 120(Pt 6):963–976. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. 2008. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 18:846–867. [DOI] [PubMed] [Google Scholar]
- Schrag A, Jahanshahi M, Quinn N. 2000. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatr. 69:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J, Hauber W. 2006. Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learn Mem. 13:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML. 2013. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman M, Valabregue R, Perlbarg V, Marrakchi-Kacem L, Vidailhet M, Benali H, Brice A, Lehericy S. 2013. Parkinson’s disease patients show reduced cortical-subcortical sensorimotor connectivity. Mov Disord. 28:447–454. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Daprati E, Ciancia S, Giraux P, Nighoghossian N, Posada A, Haggard P. 2004. Altered awareness of voluntary action after damage to the parietal cortex. Nat Neurosci. 7:80–84. [DOI] [PubMed] [Google Scholar]
- Tahmasian M, Bettray LM, van Eimeren T, Drzezga A, Timmermann L, Eickhoff CR, Eickhoff SB, Eggers C. 2015. A systematic review on the applications of resting-state fMRI in Parkinson’s disease: Does dopamine replacement therapy play a role? Cortex. 73:80–105. [DOI] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. 2008. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 88:37–57. [DOI] [PubMed] [Google Scholar]
- Tanji J, Mushiake H. 1996. Comparison of neuronal activity in the supplementary motor area and primary motor cortex. Brain Res Cogn Brain Res. 3:143–150. [DOI] [PubMed] [Google Scholar]
- Tomer R, Levin BE, Weiner WJ. 1993. Side of onset of motor symptoms influences cognition in Parkinson’s disease. Ann Neurol. 34:579–584. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. 2010. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 25:2649–2653. [DOI] [PubMed] [Google Scholar]
- Trujillo JP, Gerrits NJHM, Vriend C, Berendse HW, van den Heuvel OA, van der Werf YD. 2015. Impaired planning in Parkinson’s disease is reflected by reduced brain activation and connectivity. Hum Brain Mapp. 36:3703–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Haber SN, Searle GE, Tsoumpas C, Long CJ, Shotbolt P, Douaud G, Jbabdi S, Behrens TEJ, Rabiner EA, et al. . 2014. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb Cortex. 24:1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse S, Gilat M, Shine JM, Heremans E, Lewis S, Nieuwboer A. 2014. Freezing beyond gait in Parkinson’s disease: a review of current neurobehavioral evidence. Neurosci Biobehav Rev. 43:213–227. [DOI] [PubMed] [Google Scholar]
- Visser M, Marinus J, Stiggelbout AM, van Hilten JJ. 2006. Responsiveness of impairments and disabilities in Parkinson’s disease. Parkinsonism Relat Disord. 12:314–318. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Vogt LJ. 2004. Cingulate Gyrus In: Paxinos G, Mai JK, editors. The Human Nervous System Amsterdam, NL: Elsevier, p. 915–49. [Google Scholar]
- Wagle Shukla A, Moro E, Gunraj C, Lozano A, Hodaie M, Lang A, Chen R. 2013. Long-term subthalamic nucleus stimulation improves sensorimotor integration and proprioception. J Neurol Neurosurg Psychiatr. 84:1020–1028. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MFS. 2003. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. 2002. Neurocircuitry of Parkinson’s disease In: Davis K, Charney D, Coyle J, Nemeroff C, editors. Neuropharmacology: the fifth generation of progress. Philadelphia, PA: Lippincott; p. 1761–79. [Google Scholar]
- Wichmann T, DeLong MR, Guridi J, Obeso JA. 2011. Milestones in research on the pathophysiology of Parkinson’s disease. Mov Disord. 26:1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. 1925. Disorders of motility and of muscle tone. Lancet. 206:53–62. [Google Scholar]
- Wu T, Long X, Wang L, Hallett M, Zang Y, Li K, Chan P. 2011. a. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp. 32:1443–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. 2011. b. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage. 55:204–215. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang J, Wang C, Hallett M, Zang Y, Wu X, Chan P. 2012. Basal ganglia circuits changes in Parkinson’s disease patients. Neurosci Lett. 524:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Feigin A, Eidelberg D. 2006. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson’s disease. J Clin Exp Neuropsychol. 28:1127–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.