Abstract
Training and immobilization are powerful drivers of use-dependent plasticity in human primary motor hand area (M1HAND). In young right-handed volunteers, corticomotor representations of the left first dorsal interosseus and abductor digiti minimi muscles were mapped with neuronavigated transcranial magnetic stimulation (TMS) to elucidate how finger-specific training and immobilization interact within M1HAND. A first group of volunteers trained to track a moving target on a smartphone with the left index or little finger for one week. Linear sulcus shape-informed TMS mapping revealed that the tracking skill acquired with the trained finger was transferred to the nontrained finger of the same hand. The cortical representations of the trained and nontrained finger muscle converged in proportion with skill transfer. In a second group, the index or little finger were immobilized for one week. Immobilization alone attenuated the corticomotor representation and pre-existing tracking skill of the immobilized finger. In a third group, the detrimental effects of finger immobilization were blocked by concurrent training of the nonimmobilized finger. Conversely, immobilization of the nontrained fingers accelerated learning in the adjacent trained finger during the first 2 days of training. Together, the results provide novel insight into use-dependent cortical plasticity, revealing synergistic rather than competitive interaction patterns within M1HAND.
Keywords: immobilization, learning transfer, plasticity, training, transcranial magnetic stimulation
Introduction
Use-dependent plasticity of motor representations in the primary motor hand area (M1HAND) plays a critical role for learning dexterous movements (Lemon 1999; Plautz et al. 2000; Mawase et al. 2017). In humans, motor representations within M1HAND are dynamically shaped by sensorimotor experience (Classen, Knorr et al. 1998; Siebner and Rothwell 2003). Use-dependent representational plasticity has been extensively studied in rodents (Kleim et al. 1998; Alaverdashvili and Paterson 2017) and monkeys (Nudo and Milliken 1996; Nudo et al. 1996; Schieber and Deuel 1997), suggesting a use-dependent competition between cortical motor representations. In monkeys, trained representations in M1 expanded at the expense of the representational zones of the adjacent body parts (Nudo et al. 1996). In contrast, long-term sensorimotor immobilization led to shrinkage of the “restricted” corticomotor representations, boosting the adjacent representations in monkeys and rodents (e.g., Milliken et al. 2013).
Plastic changes in corticomotor representations can be mapped noninvasively in human M1HAND with focal transcranial magnetic stimulation (TMS) (Wassermann et al. 1992; Wilson et al. 1993; Thickbroom et al. 1999; Kleim et al. 2007). Classically, a figure-of-eight-shaped coil is discharged over a grid of scalp positions and the mean amplitude of the motor-evoked potentials (MEPs) is calculated for each grid site. This enables the construction of a corticomotor map, reflecting the corticomotor output to a specific muscle. TMS-based corticomotor mapping revealed “use-dependent representational plasticity of single muscle representations” in M1HAND. Echoing the results obtained in animals, trained cortical muscle representations increased after repeated practice of simple or complex sequential movements (Pascual-Leone et al. 1994; Classen, Liepert et al. 1998; Muellbacher et al. 2001), whereas forced immobilization attenuated corticomotor representation of the immobilized muscles (Liepert et al. 1995). These studies provided converging evidence that training and immobilization are powerful drivers for plasticity in M1HAND, but it remains to be clarified how experience-driven changes of distinct motor representations within M1HAND interact and determine within-area plasticity of human M1HAND.
Here, we investigated how finger-specific visuomotor training and immobilization of the adjacent fingers applied either alone or combined, shape muscle-specific corticomotor representations in human M1HAND. We hypothesized that finger-specific training or finger-specific immobilization would impact on the skill level and cortical representation of the finger that was not targeted by the intervention (i.e., nontrained or nonimmobilized finger). We further anticipated that concurrent training of a finger while immobilizing another finger would shed new light onto the dynamic interactions of increased and reduced use of different motor representations in human M1HAND.
Despite widespread and intermingled motor representations in primate M1HAND (Georgopoulos et al. 1999), there is a consistent lateromedial somatotopic gradient of the abductor digiti minimi (ADM) and first dorsal interosseus (FDI) muscle (Beisteiner et al. 2001, 2004; Gentner and Classen 2006; Quandt et al. 2012). We have recently introduced a novel neuronavigated TMS mapping approach, which readily reveals the somatotopic arrangement of the ADM and FDI representations within M1HAND (Raffin et al. 2015; Dubbioso et al. 2017). Here, we exploited this TMS mapping approach to probe within-area somatotopic rearrangement of motor finger representations in response to training or immobilization of specific fingers.
Our experimental approach was tailored to test how much within-area plasticity in M1HAND is characterized by competition or cooperation. Training-induced strengthening of one motor representation may occur at the expense of the nontrained motor representations. This competition may be particularly expressed when one motor representation is strengthened by training and the other is concurrently weakened by constraining the ability to move. Concurrent immobilization of the nontrained motor representation should boost both, skill acquisition and strengthen the trained motor representation. This hypothesis is supported by the clinical evidence that constraint-induced movement therapy in stroke patients, consisting in constraining the movements made with the intact hand, is currently considered the most effective treatment regimens in physical therapy to improve the outcome of the upper paretic limb (Kwakkel et al. 2015). Here, we tested whether this would apply to a within-hemisphere within-limb representation model.
An alternative account is that experience-dependent plasticity, as induced by changes in sensorimotor experience, may be mutually synergistic. A cooperative and synergistic mode of interaction implies that training of one motor representation would not benefit from concurrently weakening another one by constraining the peripheral movements. A “synergistic” account would rather predict that finger-specific training will benefit other motor representations that are not directly targeted by training. Specifically, training-induced strengthening of the trained motor representation should stabilize other “constrained” motor representations or boost other “nonconstrained” motor representations within the M1HAND, even though these representations were not engaged in the training.
Research on interlimb learning transfer suggests the existence of reciprocal interactions between body part representations in the primary motor cortex through which newly acquired skills can be shared between cortical motor representations. For instance, within-limb transfer from the shoulder–elbow joint to the wrist–finger joint and vice versa has been shown for drawing skill (Vangheluwe et al. 2004, 2005). Vangheluwe and colleagues suggested a dual-layer representation of movements in which the effector-independent component (coding the general movement goal of the task) could be shared within M1 while effector-dependent component (coding the lower level muscle synergies) remains local and specific (Vangheluwe et al. 2005). Synergistic cross-representational interactions have also been evidenced in the somatosensory cortex (Muret et al. 2014). Muret and colleagues (2014) reported perceptual improvements cross the hand–face border after an increase in somatosensory input. Transfer of skill also occurs interhemispherically, between homologous body parts (Gabitov et al. 2015). Coactivation of the trained body part representation during movements of the nontrained body part may contribute to intermanual transfer by “educating” the untrained motor representation (Gabitov et al. 2015).
Methods
Participants
Based on an a priori power analysis, which indicated sample sizes of 23 subjects per group, we recruited 69 healthy individuals (29 females, age range: 19–48 years). Four participants dropped out and one was an extreme outlier in the learning performances, resulting in 63 datasets entered in the group analyses. Participants had no history of neurological or psychiatric illness and took no centrally acting drugs. Only individuals with little (<2 years) or no formal music training were included. All participants were strongly right handed according to the Edinburgh Handedness Inventory (Oldfield 1971). Prior to the study, all participants gave written informed consent according to a protocol approved by the Ethical Committees of the Capital Region of Denmark (H-4-2012-106).
Experimental Approach
To test which mode of interaction characterizes within-area representational plasticity within human M1HAND, healthy right-handed volunteers performed two sessions of a visuomotor tracking task one week apart (Fig. 1c). At baseline (day 1), we performed a careful multichannel electromyographic (EMG) measurement to ensure that participants were only activating the target muscle during tracking while keeping all other muscles relaxed. We recorded the activity in the two intrinsic hand muscles involved in the task (the left FDI and the left ADM) as well as EMG activity in the left abductor pollicis brevis, the left extensor digitorum, and the left flexor digitorum superficialis. We also recorded EMG activity from the homologous right FDI and ADM to check for mirror movements. Visuomotor tracking performance was assessed in the laboratory at baseline (day 1) and postintervention (day 8) using the same tracking task as for training. Performance was tested at a low difficulty level, which was identical for days 1 and 8 (level 1).
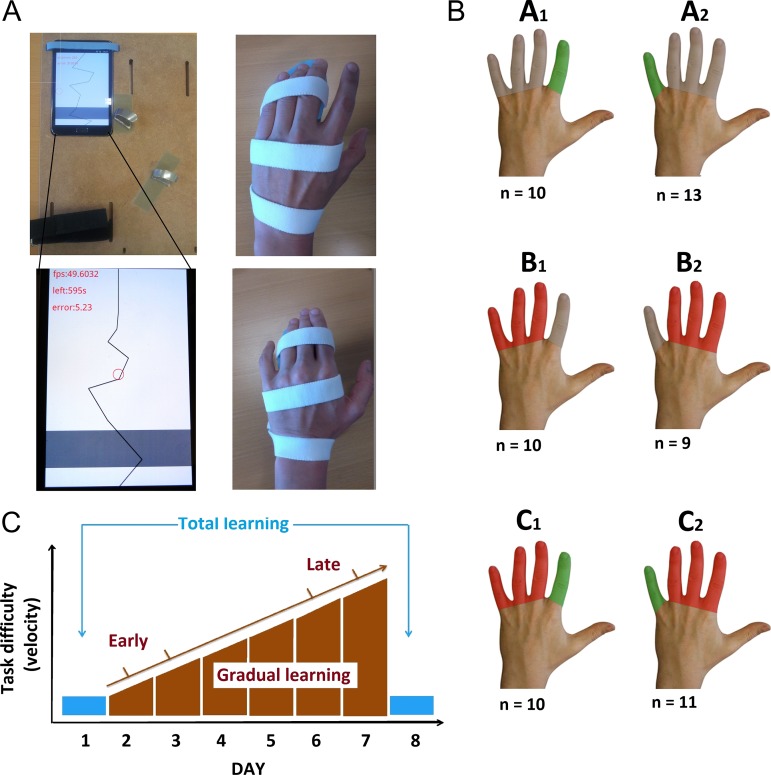
Figure 1.
(A) Smartphone-based finger training using a flexible setup adjustable for training either the left index or little finger (left). The wrist and the nontrained fingers were fixed to the platform with Velcro strap to stabilize their position and to prevent cocontraction during tracking. The tracking task consisted in a moving line going from the top of the screen to the bottom. The red circle reflects the actual position of the subject’s training finger. This red circle was controlled by the index or index placed on the gray line. Feedbacks about the remaining time and online performances were provided. The right pictures display the immobilization procedure of three adjacent fingers (fingers III–V or II–IV) with an individually made splint. (B) Types of interventions: Groups A1 and A2. Selective finger training without immobilization of adjacent fingers; groups B1 and B2: Immobilization of three adjacent fingers without training; groups C1 and C2: Selective finger training with simultaneous immobilization of adjacent fingers. Subgoups 1 and 2 differed in terms of the targeted finger. (C) Assessment of visuomotor tracking skill: Finger tracking with the index and little finger was assessed at days 1 and 8 using exactly the same task settings and during each training session at days 2–7 with a gradual increase in difficulty during consecutive sections.
Using a parallel-group design, participants were randomly assigned to one of the three interventions and were exposed to different sensorimotor experiences during the week between the two experimental sessions (Fig. 1b). Group A (n = 23, 12 females, mean age: 27.4 years) trained the same task with either their left index finger involving the FDI muscle (group A1; n = 10) or their left little finger involving the ADM muscle (group A2; n = 13). They trained three times 10 min a day, while task difficulty gradually increased from session to session. Group B (n = 19, 7 females; mean age: 26.1 years) performed no training, but digits III–V (group B1; n = 10) or digits II–IV (group B2; n = 9) were immobilized. Group C (n = 21, 8 females; mean age: 28.4 years) performed the same training task as group A for one week, but the adjacent fingers were concurrently immobilized. Ten participants (group C1) trained with the index finger, while digits III–V were immobilized (Fig. 1b). Eleven participants (group C2) trained with the little finger, while digits II–IV were immobilized. Learning performances were quantified globally and gradually during the week using the absolute deviation between the target line and the movement performed by the subjects.
Home-Based Finger Tracking Training
Participants assigned to group A or C performed daily visuomotor tracking exercises with a dedicated smartphone for one week. The home-based setting was strictly similar to the laboratory-based setup that was used at baseline and for the final assessment. The smartphone was attached to a wooden platform. The wrist and the nontrained fingers were also fixed to the platform with Velcro strap to stabilize their position and to prevent muscle cocontraction of the nontrained fingers during tracking (Fig. 1a). Participants had to track a moving line with a dot controlled by their index or little finger. Daily training lasted 30 min and was distributed over three separate sessions to avoid fatigue. The difficulty of visuomotor tracking was increased stepwise from days 2 to 7 and tracking performance was recorded on the smartphone without concurrent EMG recording. The velocity and the range of motion on the horizontal axis increased sequentially from level 1 (baseline level) to level 24 (highest level) to allow fair comparison between subjects. Hence, the tracking task became gradually more challenging for all the participants across the training week, starting from really slow movements requiring a maximum of 20 degrees of abduction–adduction to fast tracking requiring 60° abduction–adduction. The time line of visuomotor training is illustrated in Figure 1c.
Finger Immobilization
In group B or C, three adjacent fingers were immobilized in a syndactyly-like position for the entire week (days 1–7) by means of an individually shaped splint. The splint was made up of a rigid plastic form, covered with soft tissue, placed at the level of second phalangeal joint. We took care to ensure that the fingers were immobilized in a physiological position to prevent pain, swelling, or excessive sweating. The device was effective in restricting abduction–adduction and flexion–extension movements of the constrained fingers. Subjects were still able to perform most daily life motor activities with the nonimmobilized fingers of the left hand, but the immobilized fingers could not be moved. Splint-wearing participants were only allowed to remove the splint during their daily washing procedures. In group C, participants performed additional training and were asked to take the splint off for training to match training conditions to group A (training without immobilization). All participants tolerated immobilization without reporting problems. In particular, none of them experienced sustained pain during or after wearing the splint.
Transcranial Magnetic Stimulation
We used a MagPro x100 unit (Magventure, Skovlunde, Denmark) connected to a cool-MC-B35 figure-of-eight coil with windings of 35 mm diameter.
Resting Motor Threshold
First, the site at which a single TMS pulse elicited a maximal motor response was determined for the left FDI muscle. The resting motor threshold (RMTFDI) was then determined at this stimulation site using the parameter estimation by sequential testing (MLS-PEST) approach (Awiszus 2003). Stimulus intensity of TMS was adjusted to individual RMT of the FDI muscle (RMTFDI) and reevaluated at postintervention.
Sulcus Shape-Based, Linear TMS Mapping of M1HAND
We applied a novel linear mapping approach, which we have recently developed in our laboratory to study the somatotopic representation of the intrinsic hand muscles in human M1HAND (Raffin et al. 2015). The mapping approach uses neuronavigation to deliver TMS at equidistant sites along a line that follows the individual shape of the central sulcus forming the so-called hand knob (Yousry et al. 1997) to obtain a 1D spatial representation of the corticomuscular excitability profile in M1HAND (Raffin et al. 2015). We stimulated seven targets placed along the bending of the right central sulcus with a coil orientation producing a tissue current perpendicular to the wall of the central sulcus at the target site. Importantly, the middle point of the stimulation grid (i.e., target 4) was individually placed over the center of the hand knob, ensuring comparable mapping condition across all participants (Sun et al. 2016). The order of target stimulation was varied across subjects but maintained constant within subjects. Each of the seven targets was first stimulated with 10 single TMS pulses followed by 10 paired TMS pulses. Single-pulse TMS was applied at an intensity of 120% RMTFDI.
Paired-pulse TMS was used to measure the magnitude and spatial distribution of short-interval intracortical inhibition (SICI) in M1HAND. Paired-pulse TMS was used at an interstimulus interval of 2 ms. The intensity of the conditioning stimulus (CS) was set at 80% and the test stimulus (TS) at 120% of RMTFDI (Roshan et al. 2003). We performed paired-pulse TMS to trace changes in intracortical inhibition, because intracortical inhibition and cortical plasticity are tightly intertwined in M1HAND (Rosenkranz et al. 2007; Cirillo et al. 2011; Coxon et al. 2014; Stavrinos and Coxon 2017).
EMG Recordings
Using a bipolar belly-tendon montage, MEPs were recorded with surface electrodes from the left ADM and FDI muscles during complete muscle relaxation (Ambu Neuroline 700, Ballerup, Copenhagen). The analogic signal was amplified and band-pass filtered (5–600 Hz) with a Digitimer eight-channel amplifier, digitized at a sampling rate of 5000 Hz using a 1201 micro Mk-II unit, and stored on a PC using Signal software (Cambridge Electronic Design, Cambridge, UK).
Data Analyses
Corticomotor Mapping
Individual MEPs were visually inspected to remove trials with significant artefacts or EMG background activity (<1%). The peak-to-peak amplitude of MEPs was extracted using Signal software in the time window between 10 and 40 ms after the TMS stimulus (Cambridge Electronic Design, Cambridge, UK). For the ADM and FDI muscles, we constructed mediolateral corticomotor excitability profiles based on the mean MEP amplitudes for each TMS target site along the central sulcus forming the hand knob. We compared the mediolateral distribution of mean MEP amplitudes in a mixed ANOVA, with the mean “MEP amplitude” evoked by single-pulse TMS at a given stimulation site as dependent variable. The “type of intervention” (group A vs. group B vs. group C) and which “finger received training or immobilization” (subgroup 1 [A1, B1, or C1] vs. subgroup 2 [A2, B2, or C2]) were included as between-subject factors, while the “location of TMS” (target 1–7) and “session” (day 1 vs. day 8) and “muscle” (ADM vs. FDI) as within-subject factors.
We derived two complementary measures from the MEP amplitude profiles to study in more detail dynamic changes in the muscle-specific representations in M1HAND. The area under the curve (AUC) was taken as an index sensitive to a global up or downscaling in corticomotor excitability. The “distance between” the “amplitude-weighted mean position” (WMP) of the FDI and ADM excitability profiles was used to assess changes in spatial proximity of muscle-specific corticomotor representations. The amplitude-WMP was calculated according to the following formula:
The AUC ratio (AUC at day 8/AUC at day 1) and the distance between the WMP of the ADM and FDI muscle representation were analyzed in separate mixed ANOVA models with type of intervention (group A, B, and C) and which finger received training or immobilization (subgroups 1 and 2) as between-subject factor. “Muscle” (FDI vs. ADM) was added as additional within-subject factor to the ANOVA assessing AUC ratio. The factor “session” (days 1 and 8) was only implemented in the ANOVA modeling WMP. The same statistical analysis was applied to the MEP amplitude profiles evoked by paired-pulse stimulation at 2 ms using the normalized MEPs (conditioned/unconditioned MEPs) as a dependent variable.
Visuomotor Tracking
Visuomotor performance was quantified using the mean relative error for each training block. The relative error was defined as the difference in displacement between the tracking finger and a 3-mm target area centered on the target line and calculated for bins of 100 ms throughout the task period, using a custom-made python script. The percentage of time spent in the target area (i.e., being <1.5 mm away from the target line) was then computed for each tracking block (in %).
Learning of visuomotor tracking movements was assessed from two perspectives. To quantify the total amount of learning after the week of training (referred to as “total learning”), we compared the final tracking performance on day 8 with performance at baseline using a tracking task with the same difficulty level. The amount of total learning was determined by dividing the tracking performance measured on day 8 by initial performance on day 1 and expressed in percentage of improvement relative to baseline. We performed a global mixed ANOVA in which the improvement in tracking performance was treated as a dependent variable. The “finger” (index vs. little finger) was treated as within-subject factor and the type of intervention (group A vs. group B vs. group C) and which finger received training or immobilization (i.e., subgroup 1 [A1, B1, and C1] vs. subgroup 2 [A2, B2, and C2]) as between-subject factors. We also computed a more restricted ANOVA model which only included the groups that actually trained for one week, treating finger (index vs. little finger) as within-subject factor and the type of intervention (group A vs. group C) and which finger received training (i.e., subgroup 1 [A1 and C1] vs. subgroup 2 [A2 and C2]) as between-subject factors. Conditional on significant main effects or interactions, we performed follow-up t-tests. In groups A and C, we tested whether total learning in the trained finger would predict the transfer of learning to the nontrained finger, using Pearson's correlation.
To assess the gradual day-to-day improvement in tracking skill (referred to as gradual learning), we quantified the tracking performance at each day of training by subtracting the mean relative error for each day (normalized to the initial score measured at home on day 2) from the maximal possible score (i.e., 100). This value was then scaled to the actual task difficulty using a constant, based on the increase in associated task velocity (e.g., βday3 = 1.05 and βday7 = 1.25). We used the following formula (e.g., for day 3):
This measure was entered into a mixed effects ANOVA model with “day of training” (days 2–7) and the type of intervention (groups A and C) and which finger received training (subgroup 1 [A1 or C1] vs. subgroup 2 [A2 or C2]). We further tested for a correlation between early gradual learning (day 3/day 2) and late gradual learning (day 7/day 6).
Relation Between Representational Plasticity and Visuomotor Learning
We were interested to examine whether changes in AUC or in WMP distance obtained with single- or paired-pulse TMS would predict interindividual differences in visuomotor skill learning of the trained finger in group A (training without immobilization) and group C (training and immobilization of adjacent fingers). To this end, we performed group-specific multiple regression analyses, treating the improvement in tracking performance of the trained finger from days 1 to 8 as a dependent variable. The predictive value of four TMS-derived measures were tested in a stepwise multiple regression model: 1) training-associated change in AUC assessed with single-pulse TMS, 2) training-associated increase in AUC assessed with paired-pulse TMS at an ISI of 2 ms, 3) training-associated change in distance between WMPs of the FDI and ADM excitability profiles assessed with single-pulse TMS and 4) training-associated change in distance between WMPs of the FDI and ADM excitability profiles assessed with paired-pulse TMS at an ISI of 2 ms.
We conducted two additional regression analyses to examine whether changes in AUC or in WMP distance obtained with single- or paired-pulse TMS would predict the learning transfer of a visuomotor tracking skill from the trained to the nontrained finger in group A (training without immobilization) and group B (training and immobilization of adjacent fingers). We used the same stepwise multiple regression approach as described above. The only difference was that the total improvement in tracking performance of the nontrained finger from days 1 to 8 as a dependent variable.
Finally, another set of correlational analyses explored whether the changes in AUC or distance in WMP from days 1 to 8 correlated with the amount of incremental learning (early learning score: day 3/day 2 and late learning score: day 7/day 6) and total learning (total learning score: day 8/day 1).
Statistical Considerations
All statistical analyses were performed using SPSS 19 for Windows (IBM). The level of significance was defined as α = 0.05. Bonferroni–Sidak’s procedure was used to correct for multiple comparisons. Data are given as mean ± standard error of the mean (SEM). Normal distribution of the data was confirmed using the Kolmogorov–Smirnov test for all variables. For ANOVA, the Mauchly’s test of sphericity was performed. Greenhouse-Geisser correction method was applied to correct for nonsphericity.
Results
Sixty-three healthy volunteers were either exposed to one week of finger training, finger immobilization, or finger training combined with immobilization of the remaining fingers. One week of finger-specific training or immobilization was sufficient to shape dexterity as well as muscle-specific corticomotor representations in human M1HAND. Critically, each intervention had different effects on manual tracking skill and produced different patterns of within-area reorganization in human M1HAND.
Changes in Visuomotor Tracking Performance
We assessed the cumulative improvement in tracking ability using the percentage change in tracking accuracy at day 8 relative to baseline performance at day 1 (Fig. 2, left panel). Note that the visuomotor tracking tasks performed at days 1 and 8 were matched in difficulty (Fig. 1c). A mixed ANOVA including all three interventional groups revealed a significant effect for the finger targeted by the interventions (F(1,52) = 52.31, P < 0.001). This was due to an overall increase in tracking accuracy for the trained finger (groups A and C) or not immobilized (group B) relative to the nontrained finger (group A) or immobilized finger (groups B and C). The relative improvement in accuracy for the targeted finger depended on the type of intervention (F(2,52) = 10.05, P < 0.001), while there was no systematic difference in the amount of overall learning between the little or index finger (F(1,52) = 1.88, P = 0.18). A mixed ANOVA only including the data obtained in two learning groups (groups A and C) yielded similar results. There was a main effect for the “finger targeted by training” (F(1,38) = 60.01, P < 0.001) and an interaction between type of intervention and “trained finger” (F(1,38) = 33, P < 0.001).
Figure 2.
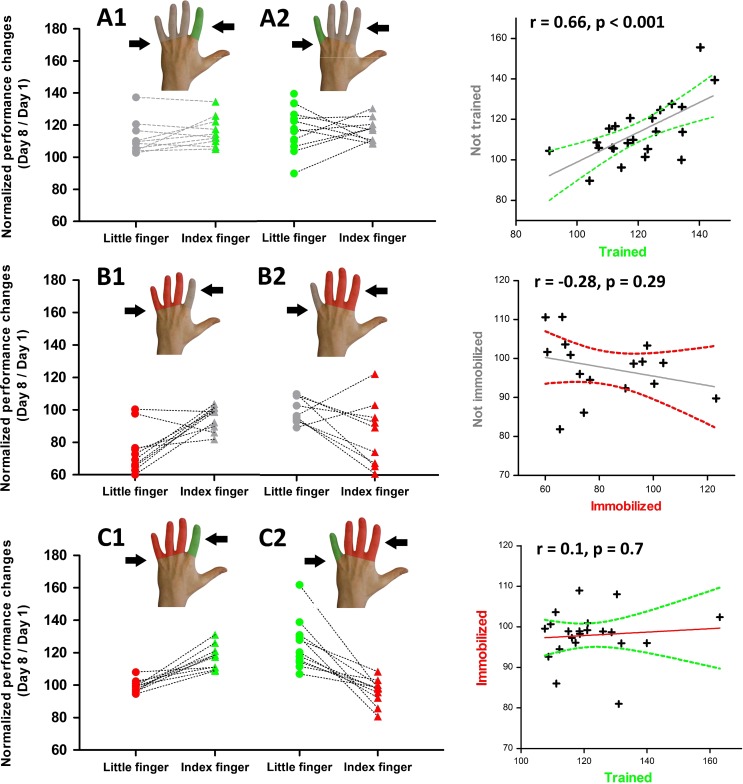
Individual changes in tracking accuracy from days 1 to 8. Left panels: The y values reflect individual tracking accuracy at day 8 expressed as percentage of day 1 for each group. Right panels: The scatter graphs plot the individual performance changes for the two fingers of the same hand separately for each group. The straight gray line reflects the fit of the linear regression and the curved lines represent the 95% confidence interval.
The significant interaction between the type of intervention and the trained finger motivated a follow-up analysis of the overall learning within each interventional group. In the “training-only” group (group A), learning without concurrent immobilization only resulted in a trend advantage in tracking performance for the trained compared with the nontrained fingers (t(22) = 1.94, P = 0.07). At the individual level, the improvement in tracking with the trained fingers correlated with improved tracking performance in the nontrained, nonimmobilized finger (r = 0.66, P < 0.001; Fig. 2, upper right panel). In the training-only group, the nontrained finger showed a significantly higher tracking accuracy at day 8 relative to the nontrained and nonimmobilized finger in the “immobilization-only” group (group B) (t(40) = 4.85, P < 0.001). Together, the data indicate efficient transfer of the learned visuomotor tracking skill to the nontrained finger in the training-only group (Fig. 2, upper panels).
In contrast, no learning transfer occurred, when learning was combined with immobilization (group C). After one week of training, there were significant differences in tracking performances between the learned and the immobilized fingers (t(20) = 7.88, P < 0.001) without any correlation among them (r = 0.1, P = 0.7; Fig. 2, lower panels).
Finger immobilization without concurrent training of the adjacent finger degraded visuomotor tracking ability of the immobilized finger (group B, Fig. 2, middle panels). Pairwise comparison showed a consistent decay in tracking performance at day 8 for the immobilized finger relative to the nonimmobilized nontrained finger (t(18) = 3.59, P = 0.002). The relative decrease in tracking accuracy in the immobilized finger did not correlate with tracking performance in the nonimmobilized, nontrained finger (r = −0.28, P = 0.29), which showed similar tracking performance at days 1 and 8.
Concurrent immobilization of the nontrained fingers failed to boost the acquisition of the tracking skill in the trained finger. Tracking performance was not different for the trained finger in groups A and C (t(42) = 1.14, P = 0.26), showing that overall learning was not enhanced by immobilization of the nontrained fingers in group C. However, concurrent training prevented degradation of tracking skill of the immobilized finger in group C (Fig. 2, lower left panel). The immobilized finger combined with training of the adjacent finger (group C) showed better tracking performance than participants in whom the finger was immobilized without concurrent training of the adjacent finger (group B) (t(38) = 4.33, P < 0.001).
Day-to-day Changes in Finger Tracking Performance
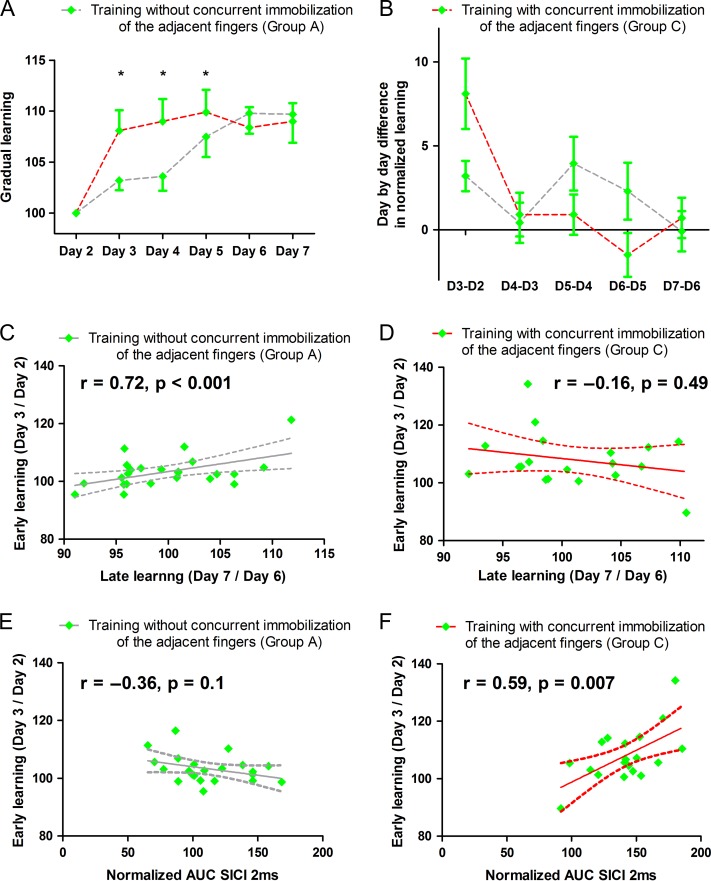
We analyzed the behavioral data that had been recorded on the smartphone during home-based training sessions from days 2 to 7. Note that we only recorded performance of the trained finger in groups A and C and that all subjects reported that they selectively engaged the target finger during the home-based trainings. Tracking accuracy was normalized to the gradual increase in difficulty level of the task from day to day. Daily training resulted in a gradual improvement of tracking skill (Fig. 3a). Mixed effects ANOVA showed a main effect of day of training (F(3.24,37) = 15.6, P < 0.001) which did not differ between training with the index or little finger (F(1,37) = 3.29, P = 0.08). While the total amount of performance improvement from baseline to day 8 was not different in groups A and C, we found differences in the dynamics of day-to-day learning in the trained fingers between groups A and C (Fig. 3a,b). This was confirmed by an interaction between the day of training and type of intervention (F(5,37) = 2.54, P = 0.03). The immobilization of the adjacent fingers accelerated early learning in group C. Group C showed a better tracking accuracy on days 3, 4, and 5 relative to group A in which finger tracking was trained without concurrent immobilization of the adjacent fingers (see Fig. 3a,b for the incremental learning curves for both trained fingers and Table 1 for post hoc t-tests comparisons).
Figure 3.
Day-to-day improvement in tracking accuracy of the trained finger. Data from the index and little fingers are pooled together. (A and B) The panels show day-by-day improvements in visuomotor tracking for the trained fingers depending on the status of the adjacent fingers. Panel A shows the learning rate for each day, representing the mean normalized tracking performance from each day scaled to the actual tracking difficulty. Panel B shows the difference in learning rate compared with the previous day. Training with immobilization shows faster early learning than training without immobilization. For details regarding the calculation of the daily learning rate, see the main text. (C and D). The panels plot early day-to-day learning against late day-to-day learning for learning without (C) or with (D) immobilization of the adjacent fingers. The straight gray line reflects the fit of the linear regression and the curved lines represent the 95% confidence interval. Early learning only scaled linearly with late learning when finger training was performed without concurrent immobilization of the adjacent fingers (the correlation remained significant even without the extreme data point, r = 0.51, P = 0.032). Panels E and F show the linear relationship between the early learning and the amount of cortical disinhibition in M1HAND measured through the relative reduction in SICI from days 1 to 8 in group A and in group C (E and F). This suggests a possible link between the early enhancement of skill acquisition and training-induced reduction of intraortical inhibition in group C where training was combined with immobilization. *p < 0.05 Bonferroni-corrected.
Table 1.
Statistical results of post hoc t-tests comparing the normalized gradual learning across days in the two groups receiving training. Group A: Training without immobilization, group C: Training with immobilization of the adjacent fingers.
| Day | t values | Df | P-values |
|---|---|---|---|
| 2 | −1.29 | 39 | 0.21 |
| 3 | −3.27 | 39 | 0.002 |
| 4 | −3.35 | 39 | 0.002 |
| 5 | −2.65 | 39 | 0.012 |
| 6 | −1.35 | 39 | 0.19 |
| 7 | −1.84 | 39 | 0.07 |
In group A, learning was performed without concurrent immobilization, and the amount of early learning (mean of days 2 and 3) correlated with the magnitude of late learning (mean of days 6 and 7). The consecutive day-to-day recordings revealed a linear increase in skill over consecutive days in group A (r = 0.72, P < 0.001, Fig. 3c). This gradual continuous performance gain was not present in group C, in which learning was combined with immobilization of the adjacent fingers (r = −0.16, P = 0.49, Fig. 3d). Concurrent immobilization of the adjacent fingers modified the day-to-day buildup of skill level from session to session during one week of training. Group C showed an acceleration of early learning and a flattened slope of late learning, showing a comparable overall amount of skill acquisition as group A after one week of training. Post hoc analyses revealed that the rapid early increase in tracking performances (days 2–3) scaled with the amount of cortical disinhibition in M1HAND as reflected by the relative reduction in SICI from days 1 to 8 in group C (r = 0.59, P = 0.007, corrected for multiple comparisons, Fig. 3e,f). No consistent relation between improvement in tracking performance and cortical disinhibition was found in group A (r = −0.36, P = 0.1). Note that this relatively short 1-week period did not allow us to observe a plateau in the learning performances as one would expect after prolonged training of such visuomotor tracking task.
Experience-Dependent Within-Area Plasticity in Right M1HAND
Sulcus shape-based TMS mapping was used to map the corticomotor representations of the left FDI and ADM muscles in each individual. Sulcus shape-based mapping showed that all interventions triggered a reorganization of cortical representations, which involved changes in corticomotor excitability and spatial representation. (Figs 4 and 5). Corticospinal excitability was measured as AUC, representing the mean MEP amplitude for all seven-map positions. The ratio between AUC values obtained at day 8 (post-training) and day 1 (baseline) reflected relative changes in corticomotor excitability from days 1 to 8.
Figure 4.
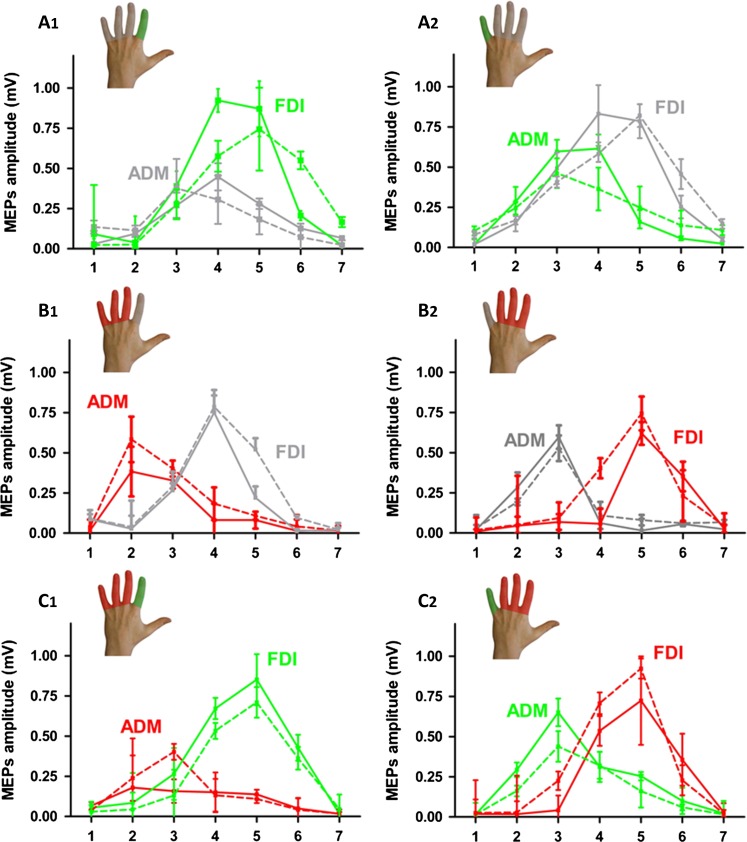
Mediolateral cortical excitability profiles of the FDI and ADM muscle obtained with neuronavigated single-pulse TMS in the three experimental groups (A1/A2, B1/B2, and C1/C2). The color of the lines indicates whether the muscle was trained (green), immobilized (red), or neither immobilized nor trained (gray) on day 1 (dotted line) and day 8 (full line). Data points represent the mean value of each group. Error bars equal SEM.
Figure 5.
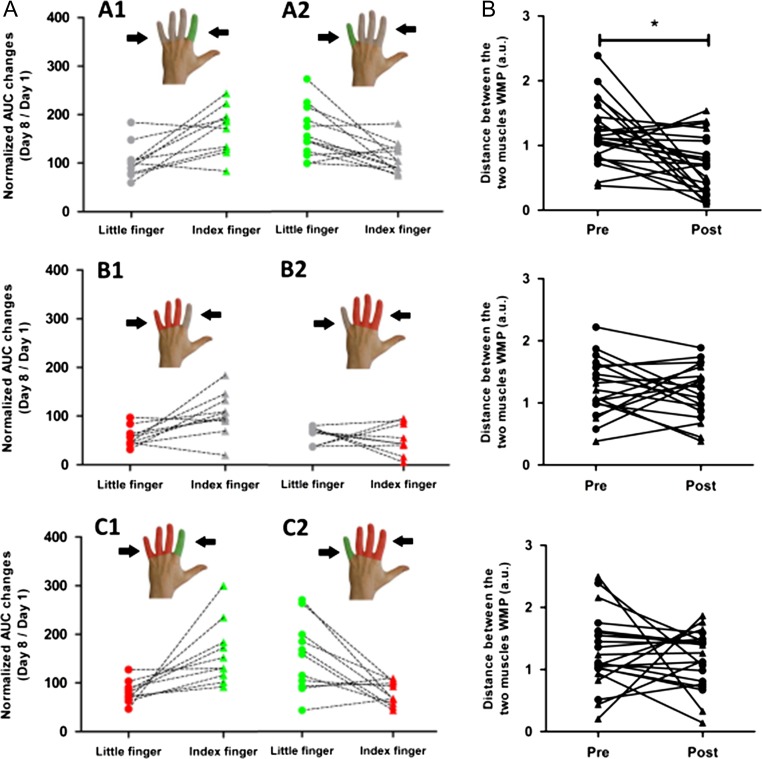
Individual changes in mediolateral corticomotor representations of the left FDI and ADM muscles in right M1HAND following finger-specific training or immobilization. Corticomotor representations were probed with sulcus shape-based single-pulse TMS mapping. Left panels: Relative changes in the AUC from days 1 to 8 given as percentage of baseline values. The color of the lines indicates whether the muscle was trained (green), immobilized (red), or neither immobilized nor trained (gray); Right panels: Distance between the average mediolateral position of the muscle profiles (DWMP) before and after the intervention. Triangles symbolize the index finger and circles symbolize the little finger. *p < 0.05 Bonferroni-corrected.
Changes in Regional Corticospinal Excitability
Visuomotor tracking training increased regional corticospinal excitability in the trained muscles regardless of which finger was trained (Figs 4 and 5, panels A and C). Conversely, immobilization alone attenuated corticospinal excitability of the immobilized muscle (Figs 4 and 5, panel A). The opposite effects of training and immobilization were reflected by a statistical interaction between the type of intervention and muscle for the AUC ratio (F(2,55) = 3.81, P = 0.03). The bidirectional use-dependent change in corticospinal excitability did not differ between the FDI or ADM muscle (F(1,55) = 0.16, P = 0.69). There was also a main effect of muscle caused by larger AUC values for FDI relative to ADM muscle across all conditions (F(1,55) = 40.63, P < 0.001), presumably reflecting the higher relevance of the FDI muscle for dexterous movements during everyday life.
Follow-up comparisons revealed that one week of finger tracking training produced excitability increases in the training muscle. This increase in corticomotor excitability of the trained finger was comparable between groups whether or not the nontrained finger was immobilized (group A vs. group C: t(42) = 0.75, P = 0.45). In group B, immobilization alone induced a reduction in AUC, but this reduction in corticospinal excitability of the immobilized muscle was prevented by concurrent training of the nonimmobilized finger in group C (group C vs. group B, t(36) = 3,07, P = 0.004). Moreover, the “training-only” group (group A) showed larger AUCs of the nontrained finger muscle compared with the nontrained, nonimmobilized finger muscle in the “immobilization-only” group (group B) (t(38) = 7,7, P < 0.001).
Within-Area Reorganization in Right M1HAND
Sulcus shape-based TMS mapping confirmed the well-known somatotopic arrangement of cortical finger representations in the M1HAND with the FDI muscle being represented more laterally than the ADM muscle (Fig. 4). Accordingly, statistical comparison of mean MEP amplitudes at each stimulation position showed an interaction between location of TMS and muscle (F(6300) = 34.25, P < 0.001).
Fingers' movements partially share the same motor cortical areas (Sanes et al. 1995; Beisteiner et al. 2001; Dechent and Frahm 2003), but in our study, we were able to show that selective finger training resulted in a partial convergence of cortical muscle representations (i.e., further increasing the existing overlap) only when the nontrained fingers were mobile. The spatial representations of the FDI and ADM muscle in M1HAND moved toward each other, showing more overlap in group A after training, but not in groups B and C. This pattern was confirmed by mixed effects ANOVA, which tested how the various interventions altered the distance between finger representations. We used the distance between the amplitude-weighted mean position (DWMP) of the FDI and ADM excitability profiles as an index of spatial proximity between finger representations (see Methods section and Fig. 5b). Mixed effects ANOVA revealed a change in spatial proximity between the FDI and ADM representation after one week relative to preinterventional baseline (main effect of session: F(1,57) = 6.7, P = 0.011). The spatial shift critically depended on the type of intervention, as indicated by an interaction between session and type of intervention (F(2,55) = 3.32, P = 0.043). In the “training-only” group (group A), pairwise post hoc t-tests showed that the mean position of the trained and nontrained muscle profiles shifted toward each other, resulting in smaller DWMP values (group A; t(22) = 3.45, P = 0.002, paired t-test). In contrast, mean DWMP did not change in groups B and C in which immobilization was applied (P > 0.5).
Experience-Dependent Changes in Intracortical Inhibition
Paired-pulse TMS mapping at an interstimulus interval of 2 ms was used to examine the magnitude or spatial distribution of short-latency intracortical inhibition (SICI). The overall strength of SICI, as reflected by the AUC of SICI across all stimulation sites (AUCSICI), was modified depending on the type of intervention. Only participants who had been practicing visuomotor tracking movements for a week showed reduced SICI in the trained muscle representation as revealed the mean AUCSICI (Fig. 6). Mean AUCSICI showed an interaction between the type of intervention and session for SICI in the trained finger muscle (F(2,56) = 1.4, P = 0.037). We calculated the ratio between AUCSICI on day 8 and AUCSICI on day 1, expressed as percentage of day 1 to quantify the relative change of overall SICI in each participant. An AUC ratio above 100% reflects a postinterventional decrease in SICI (i.e., disinhibition) relative to baseline. Using this variable, follow-up comparisons confirmed less SICI for the trained finger muscle representation in both training groups (groups A and C) relative to the nontrained and nonimmobilized muscle in group B which only underwent immobilization (group A vs. group B: t(42) = 2.9, P = 0.006; group C vs. group B: t(36) = 5.22, P < 0.001). No difference in AUCSICI was found between the two training groups (group A vs. group C: t(38) = 0.18, P = 0.86).
Figure 6.
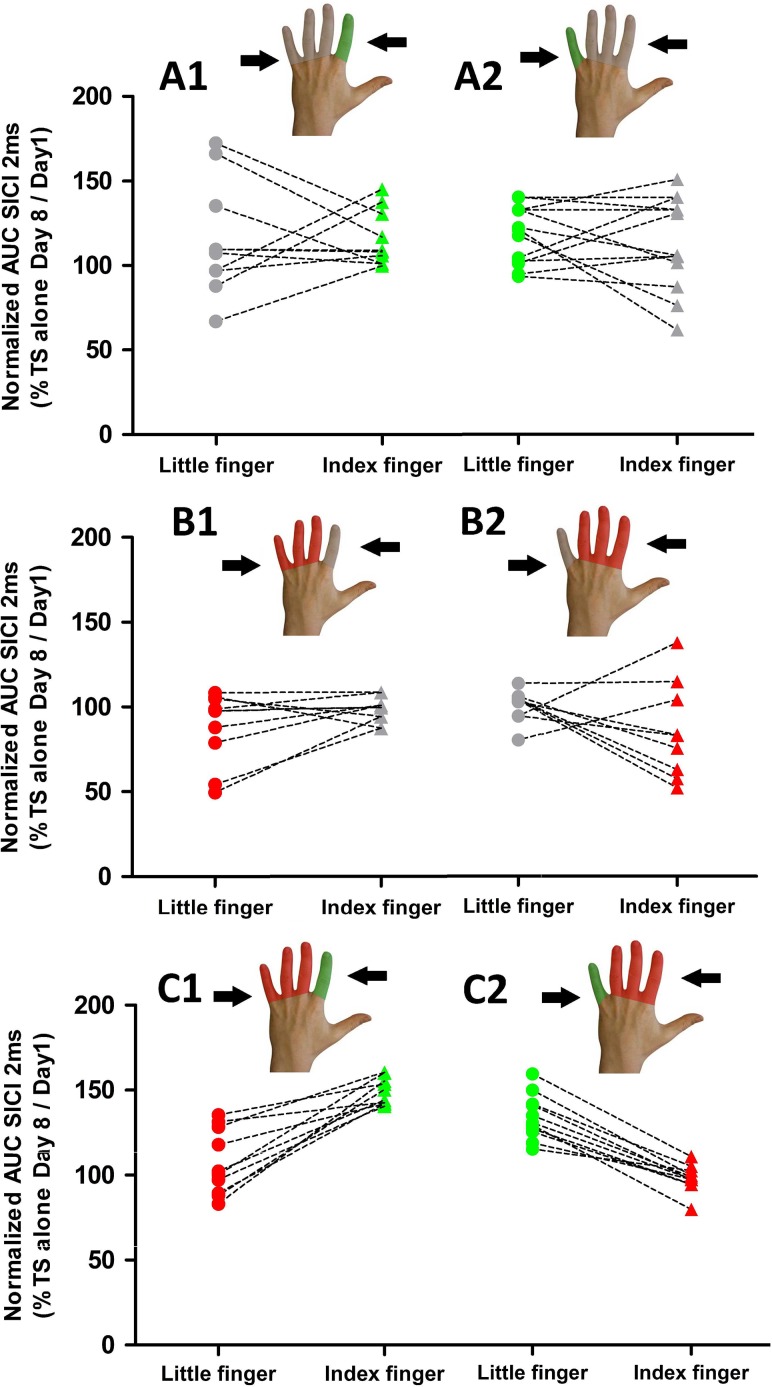
Effects of finger-specific training or immobilization on mediolateral representations of short-latency intracortical inhibition (SICI) in M1HAND probed with sulcus shape-based, dual-pulse TMS. The AUC(SICI) at day 7 were expressed as percentage of AUC(SICI) at baseline to capture relative changes in overall SICI after immobilization and training. Left panels. Individual AUC(SICI) ratios for the FDI and ADM muscle representations for the three types of interventions. An AUC ratio above the 100% line reflects a postinterventional decrease in SICI (i.e., disinhibition) relative to baseline. The color of the lines indicates whether the muscle was trained (green), immobilized (red), or neither immobilized nor trained (gray); Right panels. Distances between the average mediolateral position of the SICI profiles (DWMP) are displayed before and after the intervention for the three main types of interventions. Triangles symbolize the index finger and circles symbolize the little finger.
While both training interventions reduced the level of intracortical inhibition in the cortical representation of the trained muscle, they differed in terms of their impact on intracortical inhibition of the nontrained muscle representation (Fig. 6). When finger training was not combined with immobilization, training-related disinhibition occurred in the cortical representations of both, the trained and nontrained muscles (group A). In contrast, it remained restricted to the cortical representation of the trained muscle in individuals, in whom finger training was combined with immobilization (group C). Considering only the two groups in which training was performed, ANOVA of SICI revealed an interaction between the type of intervention and “muscle targeted by training” (F(1,36) = 6.9, P = 0.012) and a main effect for the trained muscle (F(1,36) = 24,96, P < 0.001). Post hoc analyses showed a difference between AUCSICI of the trained and immobilized muscle in the group, in which training and immobilization were combined (group C, t(20) = 7.34, P < 0.0001). In contrast, there was no difference in AUCSICI between the trained and nontrained muscles after training in the training-only group (group A, t(22) = 0.96, P = 0.35).
Immobilization alone increased the level of intracortical inhibition in contralateral M1HAND. In the immobilized muscle, SICI increased from baseline to day 8 in individuals who underwent immobilization without any training (group B; Fig. 6). Immobilization caused a relative decrease in AUC(SICI) ratio, while the AUCSICI ratio did not change in the nonimmobilized, nontrained muscle, resulting in a significant difference between immobilized and nonimmobilized muscle at day 8 (t(18) = 2.33, P = 0.032).
In terms of spatial expression of SICI in M1HAND, the spatial profile of conditioned MEP amplitudes (i.e., SICI profiles) followed those of the unconditioned MEPs evoked by the single pulse alone, showing that the relative magnitude of SICI was comparable across stimulation sites. Accordingly, ANOVA revealed no interaction between location of TMS and muscle for SICI (F(6336) = 1.79, P = 0.1). None of the interventions had a consistent effect on the spatial arrangement of muscle-specific SICI profiles. Using the DWMP values for the SICI excitability profiles as a dependent variable, the mixed ANOVA revealed neither main effects nor interactions between the type of intervention and session (P > 0.54).
Relation Between Representational Plasticity and Visuomotor Learning
We were interested to see whether our TMS-derived measures of representational plasticity would predict interindividual differences in visuomotor skill learning of the trained finger or in learning transfer to the nontrained finger. To this end, we performed separate forward stepwise multiple regression analyses for the two training groups (groups A and C) treating the total learning scores as a dependent variable. The DWMP and AUC ratios of both finger muscles (FDI and ADM muscles) acquired with single-pulse and paired-pulse TMS were entered as potential predictors.
We first report the results regarding visuomotor learning of the trained finger. In the learning-only group (group A), the only TMS-based marker of representational plasticity that predicted the individual amount of training-induced visuomotor learning was the AUC increase for single-pulse MEPs elicited in the trained muscle (Beta: 0.5, P = 0.014; Table 2). The forward stepwise multiple regression model was significant (F(1,21) = 7.14, P = 0.014) and explained approximately 20% of the variance in overall finger tracking learning. For exploratory purposes, we also performed Pearson’s correlation analyses, which showed a positive correlation between learning from days 1 to 8 and the relative AUC increase in the trained muscle (r = 0.5, P = 0.014, for all the other correlations: P > 0.05, corrected for multiple comparisons).
Table 2.
Regression analyses and predictive models for the learning transfer: Separate models were computed for groups A and C. The following predictors were entered into the regressions as independent variables using a backward stepwise technique: Total learning scores obtained by the adjacent finger, the distance of amplitude-weighted mean position (DWMP) on the spTMS profiles, and the AUC ratios acquired with single-pulse and paired-pulse TMS (AUCSP and AUCPP).
| Models | Significant predictors | ||||||
|---|---|---|---|---|---|---|---|
| Adj. R2 | F-value | Df | P-value | Variable | Beta | P | |
| Trained fingers | Group A (training without immobilization of the adjacent fingers) | ||||||
| 0.22 | 7.14 | 21 | 0.014 | AUCsp | 0.5 | 0.014 | |
| Group C (training with immobilization of the adjacent fingers) | |||||||
| Not significant | — | ||||||
| Nontrained fingers | Group A (training without immobilization of the adjacent fingers) | ||||||
| 0.23 | 7.48 | 21 | 0.012 | DWMP | −0.51 | 0.012 | |
| Group C (training with immobilization of the adjacent fingers) | |||||||
| Not significant | — | ||||||
In the group in which training and immobilization were combined (group C), the forward stepwise multiple regression model was not significant (Table 2). However, in line with the finding in group A, group C displayed a trendwise positive correlation between the total learning and the AUC increase in the trained muscle (r = 0.42, P = 0.05).
We also tested which TMS-derived measure of representational plasticity predicts improvement in tracking skill in the nontrained muscle. In the learning-only group (group A), regression analysis revealed that the increasing proximity of the corticomotor representations of the FDI and ADM muscle predicted individual acquisition of visuomotor tracking skill with the nontrained finger (Beta: −0.51, P = 0.012, Table 2). The forward stepwise multiple regression model on the total learning was significant (F(1,21) = 7.48, P = 0.012) and explained approximately 20% of total variance. The more the two muscle representations converged, the stronger was the amount of learning transfer to the nontrained muscle (r = −0.47, P = 0.023, for all the other correlations: P > 0.05). This was not the case in group C, the forward stepwise multiple regression model was nonsignificant (see Table 2), indicating that the prevention of immobilization-induced skill degradation by concurrent training was not explained by any of the four TMS-derived measures of representation plasticity.
Discussion
To the best of our knowledge, this is the first study to combine finger-specific skill training and immobilization with novel TMS-based mapping procedures to study the experience-induced representational plasticity in the intact human M1HAND. Our results are at variance with the hypothesis that constraining the adjacent fingers will boost skill acquisition of the trained, nonconstrained finger. Our results rather provide evidence for synergistic interactions with respect to experience-induced plasticity and skill changes in human M1HAND.
In the following, we first discuss the cortical reorganization produced by visuomotor learning alone and then elaborate on how the learning-induced reorganization pattern was modified by concurrent immobilization of the adjacent fingers.
Visuomotor Finger Training Alone
Visuomotor finger training shaped motor representational of the entire M1HAND area and led to a transfer of the learned skill to the nontrained finger (Fig. 7). One week of finger tracking training boosted the representation strength of the trained muscle representation, increased the spatial overlap, and attenuated intracortical inhibition of the trained and nontrained finger muscle of the same hand (Fig. 7). This pattern was expressed regardless of which finger was trained (groups A1 and A2). The overall increase in corticomotor excitability of the trained muscle predicted the individual amount of practice-induced visuomotor learning. This finding is in good agreement with previous animal studies (Nudo et al. 1996; Kleim et al. 1998; Molina-Luna et al. 2008; Pruitt et al. 2016) or grid-based TMS mapping (Pascual-Leone et al. 1995; Svensson et al. 2003; Kleim et al. 2006; Tyc and Boyadjian 2006; Boudreau et al. 2013) showing an expansion of the cortical representational maps of the trained body part. Likewise, there is consistent evidence showing that learning-induced upscaling of corticomotor excitability in the trained muscle supports the acquisition of novel motor skills (Koeneke et al. 2006; Bagce et al. 2013).
Figure 7.
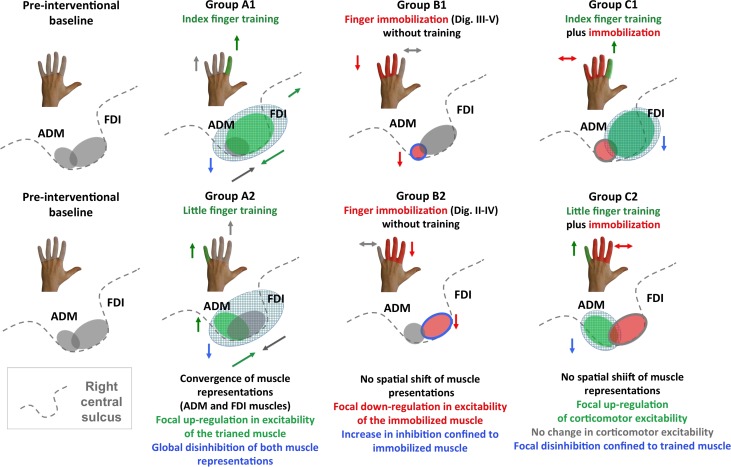
Synopsis of within-area reorganization in right M1HAND observed in groups A1, B1, C1 and A2, B2 and C2. The left panels illustrate the preinterventional state with the gray areas reflecting the cortical representations of the FDI and ADM muscle in the right M1HAND. The arrows close to the schematic drawings of the hand summarize changes in learning performances for the trained and nontrained fingers. The gray shading illustrates “absence of intervention,” the green shading illustrates “training,” and the red shading illustrates “immobilization.” The arrows close to the schematic drawing of the central sulcus illustrate the direction of intervention-specific changes in muscle representations and intracortical inhibition.
In addition to an overall strengthening of the trained corticomotor representation, a spatial reorganization within M1HAND emerged over the course of one week (Fig. 7, groups A1 and A2). Finger tracking training shortened the distance between the two mean positions of the trained and nontrained cortical motor representations. The relative convergence of corticomotor representations within M1HAND predicted individual transfer of the learned tracking skill to the nontrained finger. The more the cortical representations converged, the higher the learning transfer to the nontrained muscle. Cortical microstimulation in animals showed a shift toward the motor territory of the adjacent nontrained body parts or an increased overlap with neighboring representations of adjacent nontrained body parts (Nudo et al. 1996; Kleim et al. 1998; Molina-Luna et al. 2008). Our findings significantly extend these studies, showing that a convergence of cortical motor representations also occurs within the cortical motor area presenting the same body part. The distances between peak representations of single fingers as revealed by functional MRI range from 6 to 10 mm (e.g., Volkmann et al., 1998; Lotze et al. 2000; Dechent and Frahm 2003; Beisteiner et al. 2004). Using sulcus-based TMS mapping, the mean distance between peak cortical representations of the FDI and ADM muscles at preinterventional baseline was 9.5 (±0.1) mm in our study. The mean distance drastically decreased in the “training-only” group to 5.5 (±0.2) mm, showing an increased overlap in cortical representation.
The mapping results indicate that learning transfer of motor skills may at least partially be mediated within the primary motor cortex, possibly through a stronger overlap of functional representations. The prevailing notion is that learning transfer is mainly mediated through intermediate motor representations in premotor and parietal areas, which encode general knowledge of visuomotor predictions and skills (Grafton et al. 1998; Romei et al. 2009; Diedrichsen and Kornysheva 2015). Our finding raises the possibility that learning transfer can occur at the executive level in the M1HAND through shared cortical motor representations. This hypothesis is in line with a recent study showing that the “trained” motor representation may contribute to intermanual transfer by “educating” the untrained motor representation or supporting the exchange of information between them (Gabitov et al. 2015). However, the exact neural mechanisms that underpin the observed skill transfer from one finger of the hand to another remain to be clarified.
Paired-pulse TMS of gamma-aminobutyric acid (GABA)-mediated, intracortical inhibition revealed an attenuation of intracortical inhibition in contralateral M1HAND after one week of training. Sulcus shape-based TMS mapping revealed that training-induced intracortical disinhibition was not confined to a distinct cortical site or to a specific muscle representation. On the contrary, the reduction in SICI was evenly expressed across all stimulation sites in M1HAND and comprised the representation of the nontrained muscle. These observations significantly extend previous paired-pulse TMS studies which found training-induced reductions in intracortical inhibition (Rosenkranz et al. 2007; Cirillo et al. 2011; Coxon et al. 2014; Stavrinos and Coxon 2017), showing that selective motor skill training with a single finger produces widespread disinhibition in M1HAND. Previous studies have shown that a reduction of GABA-mediated, intracortical inhibition promotes synaptic plasticity in motor cortex and hereby, motor skill learning (Jacobs and Donoghue 1991; Hess and Donoghue 1994; Castro-Alamancos et al. 1995; Rioult-Pedotti et al. 1998). However, in the present study, the individual magnitude of SICI reduction did not scale with overall improvement in tracking performance after one week of training. The amount of disinhibition also did not predict the amount of skill transfer to the nontrained muscle. We therefore conclude that although GABAergic disinhibition, as measured with the SICI paradigm, may facilitate the expression of synaptic plasticity, it might not determine the final level of visuomotor tracking skill that can be acquired during one week of training. As we will discuss in more detail below, this might be different during early motor skill training, during which the focality and magnitude of intracortical inhibition might be more relevant.
Combining Visuomotor Finger Training with Immobilization
The combination of visuomotor finger training with immobilization of adjacent fingers produced a more confined reorganization pattern compared with finger training alone. Training enhanced the corticomotor representation of the trained muscle but not the nontrained, immobilized muscle without producing any spatial shifts (Fig. 7, groups C1 and C2). Like the increase in corticospinal excitability, training-induced cortical disinhibition was only expressed in the trained muscle. At the behavioral level, the magnitude of acquired tracking skill in the trained muscle was not enhanced after one week of training as opposed to finger training alone. Training also produced no learning transfer to the nontrained muscle, when the nontrained muscle was immobilized. The effects of immobilization on training-induced plasticity and skill learning clearly speak against the notion that cortical motor representations are competing with each other for neural resources in the human M1HAND. If this was the case, immobilization-induced sensorimotor deprivation would have promoted an expansion of the trained muscle representation into the “deprived cortex” and hereby boosted the learning success of the trained finger.
When training was combined with immobilization, sulcus shape-based TMS mapping of SICI revealed a more selective disinhibition of intracortical GABAergic circuits in the M1HAND (Fig. 7, groups B1 and B2). Relative reduction in SICI was limited to the trained muscle, while the immobilized muscle showed no consistent change. We hypothesize that the muscle-specific attenuation of intracortical disinhibition in the trained muscle might have contributed to a faster learning rate during the first days of learning in the combined learning–immobilization group. This hypothesis is supported by the observation that the rapid increase in tracking performances correlated with the reduction in SICI obtained after one week. Although speculative, it is possible that SICI reduction facilitates skill acquisition especially at the early phase of learning, while its functional role becomes less prominent during continued learning. This is in accordance with a recent study showing a transient early decrease in SICI after one day of learning (Spampinato and Celnik 2017). Furthermore, rapid GABAergic disinhibition can be induced acutely in M1HAND by ischemic nerve block and has been shown to locally boost the expression long-term potentiation-like plasticity (Ziemann et al. 1998).
The modulatory influence of concurrent immobilization of the adjacent fingers on training-induced plasticity in M1HAND can only be fully understood, when one considers the effects of immobilization alone on the corticomotor representations and visuomotor tracking skill (Fig. 7; groups B1 and B2). Finger immobilization led to a selective downregulation of corticomotor excitability with an increase in SICI, which was confined to the corticomotor representation of the immobilized muscle. The immobilized finger also showed a degradation of visuomotor tracking performance relative to preimmobilization baseline. The findings indicate that one week of reduced sensorimotor experience is sufficient to weaken the deprived cortical representation and to deteriorate associated sensorimotor skills.
These detrimental effects of finger immobilization were prevented by concurrent training of the nonimmobilized fingers. Visuomotor tracking training of the neighboring sensorimotor representation stabilized the pre-existing excitability and skill level of the immobilized muscle. In line with previous animal data suggesting that the recovery of a lesioned area depends on the activity of the adjacent cortical regions (Castro-Alamancos and Borrel 1995), our findings provide additional support for a collaborative and synergistic mode of interaction between motor representations within M1HAND: The combined intervention resulted in a relative “upscaling” of both muscle representations in M1HAND, increasing the trained muscle representation and preserving the immobilized muscle representation. Likewise, the net effect of finger training on dexterity was synergistic, improving the tracking skill in the trained muscle and maintaining the pre-existing skill level in the nontrained muscle despite of immobilization-induced deprivation.
Strengths and Weaknesses of the Study
Seeking conceptual within-study replication, we trained or constrained the index and ring finger in different groups. All three interventions induced analogous changes at the behavioral and representational levels regardless of whether the index or little finger was trained or constrained. Hence, the observed plasticity and learning patterns can be generalized, because they were consistently expressed in two intrinsic hand muscles. Another methodological strength is that we assessed intracortical changes in inhibition in addition to mapping the magnitude and spatial properties of corticomotor representations. This enabled us to demonstrate a consistent impact of motor experience (training or immobilization) on the level of intracortical inhibition that is exerted on the corresponding corticomotor representations. Yet, the experience-dependent modulation of intracortical inhibition, as reflected by SICI, did not determine the overall improvement in tracking performance. Future studies need to clarify in more detail how dynamic changes in intracortical inhibition contribute to the acquisition of manual skills and its transfer to other motor representations within the M1HAND.
A weakness of the study is the relatively small sample sizes, which could have limited the reliability of our results. Yet it should be noted that the FDI and ADM target groups showed highly coherent patterns of results in terms of within-area plasticity. This conceptual within-study replication confirms the robustness of the results despite of the small sample size. Another limitation is that home-based training did not include EMG recordings of FDI and ADM activities during training. Hence, subjects might have performed correlated (i.e., in-phase) tracking movements with the nontrained finger during home-based tracking training. We exclude this possibility for several reasons. First, the wrist and the nontrained fingers were fixed to the platform with Velcro strap during all home-based training sessions. Hence, the position of the nontrained fingers was fully constrained, making it impossible to make correlated tracking movements with the nontrained fingers. Second, we recorded the EMG when subjects were familiarized with the tracking task at baseline. EMG revealed no evidence for overt or covert (isometric) engagement of the nontrained finger during the tracking task. Third, the biomechanics of the index and little finger render a covert (isometric) coactivation of the nontrained finger during training highly unlikely. In everyday life, coactivation usually involves concurrent activation of the ADM and FDI muscles when spreading the fingers. In this situation, the ADM and FDI muscles produce opposite movements with respect to the axis of the finger. In contrast, it is very difficult to voluntarily produce correlated (i.e., in-phase) abduction movements with the index and little finger. Thus, it is highly unlikely that participants covertly performed correlated (i.e., in-phase) tracking movements with the constrained fingers to support tracking performance. Accordingly, all subjects reported that they selectively engaged the target finger during tracking. The pattern of representational plasticity in M1HAND associated with learning a visuomotor tracking skill may depend on the nature of the motor task. Different principles may apply for learning other types of manual skills that do not require continuous mapping of sensory input onto motor output. For instance, sequential finger tapping skills, which rely on specific effector-dependent representation and motor coordinates hardly transfer to other motor effectors (Park and Shea 2005; Panzer et al. 2009; Shea et al. 2011). Future studies need to determine how much synergistic and competitive interactions among motor presentations are expressed in M-HAND during the learning of other manual skills.
Conclusions
Our findings have practical implications for preserving or recovering manual motor skills. In patients, in whom the upper limb has to be partially immobilized, intensive motor training of the nonimmobilized part of the limb may help to minimize a functional degradation of motor skills relying on the immobilized muscles. Besides, immobilization of the non-affected limb is a commonly used strategy to boost motor function of the affected limb in patients with chronic motor stroke (Taub et al. 1993; Morris et al. 1997; Taub and Morris 2001; Taub and Uswatt 2006). While constraint-induced movement therapy may improve motor function of the affected limb, immobilization of the non-affected limb is likely to weaken the “immobilized” corticomotor representations in the healthy non-lesioned hemisphere. Future studies are warranted which systematically assess the effects of constraint-induced movement therapy on the motor representations in the healthy non-lesioned hemisphere and how this might affect skilled hand function of the non-affected limb.
Notes
The authors would like to thank Arkadiusz Stopczynski for coding the smartphone application and all the volunteers for their participation. Conflict of Interest: None declared.
Funding
This work was supported by a Grant of Excellence sponsored by The Lundbeck Foundation Mapping, Modulation & Modelling the Control of Actions (ContAct, R59-A5399) to H.R.S.
References
- Alaverdashvili M, Paterson PG. 2017. Mapping the dynamics of cortical neuroplasticity of skilled motor learning using micro X-ray fluorescence and histofluorescence imaging of zinc in the rat. Behav Brain Res. 318:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awiszus F. 2003. TMS and threshold hunting. Suppl Clin Neurophysiol. 56:13–23. [DOI] [PubMed] [Google Scholar]
- Bagce HF, Saleh S, Adamovich SV, Krakauer JW, Tunik E. 2013. Corticospinal excitability is enhanced after visuomotor adaptation and depends on learning rather than performance or error. J Neurophysiol. 109:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisteiner R, Gartus A, Erdler M, Mayer D, Lanzenberger R, Deecke L. 2004. Magnetoencephalography indicates finger motor somatotopy. Eur J Neurosci. 19:465–472. [DOI] [PubMed] [Google Scholar]
- Beisteiner R, Windischberger C, Lanzenberger R, Edward V, Cunnington R, Erdler M, Gartus A, Streibl B, Moser E, Deecke L. 2001. Finger somatotopy in human motor cortex. Neuroimage. 13:1016–1026. [DOI] [PubMed] [Google Scholar]
- Boudreau SA, Lontis ER, Caltenco H, Svensson P, Sessle BJ, Andreasen Struijk LN, Arendt-Nielsen L. 2013. Features of cortical neuroplasticity associated with multidirectional novel motor skill training: a TMS mapping study. Exp Brain Res. 225:513–526. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Borrel J. 1995. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 68:793–805. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Donoghue JP, Connors BW. 1995. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 15:5324–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J, Todd G, Semmler JG. 2011. Corticomotor excitability and plasticity following complex visuomotor training in young and old adults. Eur J Neurosci. 34:1847–1856. [DOI] [PubMed] [Google Scholar]
- Classen J, Knorr U, Werhahn KJ, Schlaug G, Kunesch E, Cohen LG, Seitz RJ, Benecke R. 1998. Multimodal output mapping of human central motor representation on different spatial scales. J Physiol. 512(Pt 1):163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. 1998. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 79:1117–1123. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Peat NM, Byblow WD. 2014. Primary motor cortex disinhibition during motor skill learning. J Neurophysiol. 112:156–164. [DOI] [PubMed] [Google Scholar]
- Dechent P, Frahm J. 2003. Functional somatotopy of finger representations in human primary motor cortex. Hum Brain Mapp. 18:272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Kornysheva K. 2015. Motor skill learning between selection and execution. Trends Cogn Sci. 19:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbioso R, Raffin E, Karabanov A, Thielscher A, Siebner HR. 2017. Centre-surround organization of fast sensorimotor integration in human motor hand area. Neuroimage. 158:37–47. [DOI] [PubMed] [Google Scholar]
- Gabitov E, Manor D, Karni A. 2015. Learning from the other limb’s experience: Sharing the “Trained” M1’s representation of the motor sequence knowledge. J Physiol. 594(1):169–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Classen J. 2006. Modular organization of finger movements by the human central nervous system. Neuron. 52:731–742. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Pellizzer G, Poliakov AV, Schieber MH. 1999. Neural coding of finger and wrist movements. J Comput Neurosci. 6:279–288. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. 1998. Abstract and effector-specific representations of motor sequences identified with PET. J Neurosci. 18:9420–9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. 1994. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol. 71:2543–2547. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. 1991. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 251:944–947. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. 1998. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 80:3321–3325. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. 2006. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 9:735–737. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Kleim ED, Cramer SC. 2007. Systematic assessment of training-induced changes in corticospinal output to hand using frameless stereotaxic transcranial magnetic stimulation. Nat Protoc. 2:1675–1684. [DOI] [PubMed] [Google Scholar]
- Koeneke S, Lutz K, Herwig U, Ziemann U, Jancke L. 2006. Extensive training of elementary finger tapping movements changes the pattern of motor cortex excitability. Exp Brain Res. 174:199–209. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Veerbeek JM, van Wegen EE, Wolf SL. 2015. Constraint-induced movement therapy after stroke. Lancet Neurol. 14:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. 1999. Neural control of dexterity: what has been achieved? Exp Brain Res. 128:6–12. [DOI] [PubMed] [Google Scholar]
- Liepert J, Tegenthoff M, Malin JP. 1995. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. 97:382–386. [DOI] [PubMed] [Google Scholar]
- Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. 2000. fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage. 11:473–481. [DOI] [PubMed] [Google Scholar]
- Mawase F, Uehara S, Bastian AJ, Celnik P. 2017. Motor learning enhances use-dependent plasticity. J Neurosci. 37:2673–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliken GW, Plautz EJ, Nudo RJ. 2013. Distal forelimb representations in primary motor cortex are redistributed after forelimb restriction: a longitudinal study in adult squirrel monkeys. J Neurophysiol. 109:1268–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Luna K, Hertler B, Buitrago MM, Luft AR. 2008. Motor learning transiently changes cortical somatotopy. Neuroimage. 40:1748–1754. [DOI] [PubMed] [Google Scholar]
- Morris DM, Crago JE, DeLuca SC, Pidikiti RD, Taub E. 1997. Constraint-induced movement therapy for motor recovery after stroke. NeuroRehabilitation. 9:29–43. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. 2001. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 136:431–438. [DOI] [PubMed] [Google Scholar]
- Muret D, Dinse HR, Macchione S, Urquizar C, Farne A, Reilly KT. 2014. Touch improvement at the hand transfers to the face. Curr Biol. 24:R736–R737. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. 1996. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 75:2144–2149. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. 1996. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 16:785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9:97–113. [DOI] [PubMed] [Google Scholar]
- Panzer S, Krueger M, Muehlbauer T, Kovacs AJ, Shea CH. 2009. Inter-manual transfer and practice: coding of simple motor sequences. Acta Psychol (Amst). 131:99–109. [DOI] [PubMed] [Google Scholar]
- Park JH, Shea CH. 2005. Sequence learning: response structure and effector transfer. Q J Exp Psychol A. 58:387–419. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Cohen LG, Brasil-Neto JP, Hallett M. 1994. Non-invasive differentiation of motor cortical representation of hand muscles by mapping of optimal current directions. Electroencephalogr Clin Neurophysiol. 93:42–48. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. 1995. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 74:1037–1045. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ. 2000. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 74:27–55. [DOI] [PubMed] [Google Scholar]
- Pruitt DT, Schmid AN, Danaphongse TT, Flanagan KE, Morrison RA, Kilgard MP, Rennaker RL 2nd, Hays SA. 2016. Forelimb training drives transient map reorganization in ipsilateral motor cortex. Behav Brain Res. 313:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt F, Reichert C, Hinrichs H, Heinze HJ, Knight RT, Rieger JW. 2012. Single trial discrimination of individual finger movements on one hand: a combined MEG and EEG study. Neuroimage. 59:3316–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffin E, Pellegrino G, Di Lazzaro V, Thielscher A, Siebner HR. 2015. Bringing transcranial mapping into shape: Sulcus-aligned mapping captures motor somatotopy in human primary motor hand area. Neuroimage. 120:164–175. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. 1998. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1:230–234. [DOI] [PubMed] [Google Scholar]
- Romei V, Thut G, Ramos-Estebanez C, Pascual-Leone A. 2009. M1 contributes to the intrinsic but not the extrinsic components of motor-skills. Cortex. 45:1058–1064. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Williamon A, Rothwell JC. 2007. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci. 27:5200–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. 2003. Two phases of short-interval intracortical inhibition. Exp Brain Res. 151:330–337. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S. 1995. Shared neural substrates controlling hand movements in human motor cortex. Science. 268:1775–1777. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Deuel RK. 1997. Primary motor cortex reorganization in a long-term monkey amputee. Somatosens Mot Res. 14:157–167. [DOI] [PubMed] [Google Scholar]
- Shea CH, Kovacs AJ, Panzer S. 2011. The coding and inter-manual transfer of movement sequences. Front Psychol. 2:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. 2003. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 148:1–16. [DOI] [PubMed] [Google Scholar]
- Spampinato D, Celnik P. 2017. Temporal dynamics of cerebellar and motor cortex physiological processes during motor skill learning. Sci Rep. 7:40715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrinos EL, Coxon JP. 2017. High-intensity Interval Exercise Promotes Motor Cortex Disinhibition and Early Motor Skill Consolidation. J Cogn Neurosci. 29:593–604. [DOI] [PubMed] [Google Scholar]
- Sun ZY, Pinel P, Riviere D, Moreno A, Dehaene S, Mangin JF. 2016. Linking morphological and functional variability in hand movement and silent reading. Brain Struct Funct. 221:3361–3371. [DOI] [PubMed] [Google Scholar]
- Svensson P, Romaniello A, Arendt-Nielsen L, Sessle BJ. 2003. Plasticity in corticomotor control of the human tongue musculature induced by tongue-task training. Exp Brain Res. 152:42–51. [DOI] [PubMed] [Google Scholar]
- Taub E, Miller NE, Novack TA, Cook EW 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. 1993. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 74:347–354. [PubMed] [Google Scholar]
- Taub E, Morris DM. 2001. Constraint-induced movement therapy to enhance recovery after stroke. Curr Atheroscler Rep. 3:279–286. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatt G. 2006. Constraint-induced movement therapy: answers and questions after two decades of research. NeuroRehabilitation. 21:93–95. [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Mastaglia FL. 1999. Methodology and application of TMS mapping. Electroencephalogr Clin Neurophysiol. 51:48–54. [PubMed] [Google Scholar]
- Tyc F, Boyadjian A. 2006. Cortical plasticity and motor activity studied with transcranial magnetic stimulation. Rev Neurosci. 17:469–495. [DOI] [PubMed] [Google Scholar]
- Vangheluwe S, Puttemans V, Wenderoth N, Van Baelen M, Swinnen SP. 2004. Inter- and intralimb transfer of a bimanual task: generalisability of limb dissociation. Behav Brain Res. 154:535–547. [DOI] [PubMed] [Google Scholar]
- Vangheluwe S, Wenderoth N, Swinnen SP. 2005. Learning and transfer of an ipsilateral coordination task: evidence for a dual-layer movement representation. J Cogn Neurosci. 17:1460–1470. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Schnitzler A, Witte OW, Freund H. 1998. Handedness and asymmetry of hand representation in human motor cortex. J Neurophysiol. 79:2149–2154. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG. 1992. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 85:1–8. [DOI] [PubMed] [Google Scholar]
- Wilson SA, Thickbroom GW, Mastaglia FL. 1993. Transcranial magnetic stimulation mapping of the motor cortex in normal subjects. The representation of two intrinsic hand muscles. J Neurol Sci. 118:134–144. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. 1997. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 120(Pt 1):141–157. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. 1998. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 18:1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]