Abstract
Abstract
Poor adherence to antipsychotics, which affects outcome, is frequent in first episode psychosis (FEP). Most randomized studies demonstrate no superiority of long-acting injectable antipsychotics (LAI-AP) over oral antipsychotics (OAP). However, participants in these studies represent a minority of patients who may benefit from LAI-AP. Mirror and naturalistic studies generally demonstrate efficacy of LAI-AP on more representative samples, but studies on FEP are scarce.
Aim
To describe LAI-AP’s utilization and impact on FEP outcome in a naturalistic setting.
Methods
A 3-year longitudinal prospective and retrospective descriptive study of all consecutive admissions from two Early Intervention Services for psychosis (EIS) in Montréal, Canada, compared the characteristics and evolution of patients who received LAI-AP for at least 12 months to those who received OAP only.
Results
From 375 FEP patients included, 26,7% received LAI-AP during their follow-up. They were more likely to have poor prognostic factors (male gender, lower premorbid functioning, homelessness, substance use disorder and schizophrenia spectrum diagnoses). Despite a more severe illness and lower functioning in the LAI-AP group, at admission and study endpoint, clinical and functional improvements were observed.
Conclusion
Early prescription of LAI-AP seems beneficial in FEP with poor prognostic factors.
Keywords: schizophrenia spectrum, psychotic disorders, observational study, first-episode psychosis, clinical outcomes, long-acting injectable antipsychotics
Introduction
Despite high response rates in first episode psychosis (FEP), relapses are frequent – 40%–50% of patients relapse within 2 years1 – mainly because of poor adherence to medication (more than 50% of patients during the first year),2–4 and of comorbid substance use disorder (SUD)5 in 30 to 70% of patients,6,7 SUD being a culprit of poor adherence.8
Long-acting injectable antipsychotics (LAI-AP), by removing the need for daily intake of medication, represent a promising tool to overcome poor medication adherence in FEP, therefore improving the poor outcome associated with it. Indeed, in schizophrenia and FEP patients, LAI-AP have been shown to reduce the risk of relapse9–12 and also of rehospitalization by about 20%–60%13–15 in comparison to oral antipsychotics (OAP). However, other studies and meta-analyses report no superiority of LAI-AP over OAP.16–19 These contradictions in terms of relapse rate or symptom reduction2 probably stem from distinct methodologies including different measurement tools (e.g., definitions of relapse vary from one study to another, scales used), heterogeneous inclusion/exclusion criteria and unsimilar study designs, i.e., randomized controlled trials (RCTs) vs naturalistic studies.20 Of note, RCTs tend to create a cohort bias by usually including stabilized patients with a less severe sickness, better cognitive capacities, and good adherence to medication or good engagement toward health professionals in order to improve homogeneity of their sample and maximize the probability of finding results.9,18 Also, in many clinical trials, particularly RCTs, there is alteration of the therapeutic experience: more attention, financial compensation, free medication, more frequent visits with physician and appointment reminders.9,18,20 Several studies exclude patients with poor adherence and SUD, creating a favorable bias toward OAP and a lower degree of external validity.20 Hence, they do not reflect the reality of everyday clinical practice.2,17
Furthermore, there is an absence of data on the impact of LAI-AP on the outcome of young adults with FEP,9,10 but also a lack of knowledge regarding the characteristics of individuals to whom are prescribed LAI-AP in everyday clinical practice.
In this context, we first sought to describe, in a naturalistic setting, the utilization of LAI-AP and the characteristics of FEP patients who are prescribed LAI-AP compared to those who are prescribed OAP. Secondly, we compared the symptomatic and functional outcome, and service use of FEP patients treated early in the course of their illness with LAI-AP versus those who received OAP.
Method
Study Design and Subjects
A prospective and retrospective longitudinal naturalistic 3-year study took place in two urban early intervention services (EIS) in the University of Montreal’s network of early psychosis specialized intervention programs in Montréal, Québec, Canada.21 Both programs offer services to all FEP patients in their respectively defined catchment areas. The EIS were Programme PEP of the Institut Universitaire en Santé Mentale de Montréal, covering a population of 340,000 inhabitants in the eastern part of the city, and Clinique JAP of the CHUM, located in the city center and covering a catchment area of 225,000 inhabitants. The 5-year programs provide specialized treatment based on early psychosis intervention guidelines, including an array of psychosocial treatments, case management and pharmacological treatment.22
The study was approved by the institutional ethics and scientific committees. Recruitment started in fall 2005 until April 30th 2012. Patients were approached when stable and apt to provide written informed consent. In order to avoid major biases and to better represent the whole population, file reviews of FEP patients who refused to participate or who had not been approached for different reasons, i.e., not able to provide consent, or lost to follow-up (LTF) early, were authorized by the ethics and scientific committees and the Hospital Direction. Indeed, the description of the entire cohort, including those who are more suspicious or reluctant to collaborate to care or research, is of primary importance since many of these FEP patients were good candidates for LAI-AP.
The inclusion criteria were age 18–30 and a primary diagnosis of untreated psychotic disorder or within 1 year of treatment initiation. Patients with FEP and a comorbid disorder (e.g., SUD or cluster B personality) were included. Patients with a primary intellectual disability were excluded. A subject was also excluded if he received an antipsychotic medication from another institution within a year of admission in our EIS and if we did not have the necessary information on his treatment and could not assess with precision his adherence to medication.
Group Categorization
Eligible patients were classified in two study groups. The first consisted of patients who received LAI-AP medication for at least 12 months during their 36 months follow-up (f/u). This group might have received OAP medication during their f/u (both before, after or during LAI-AP). The second group consisted of FEP patients who were only prescribed OAP or who were prescribed OAP and a trial of 3 months or less of LAI-AP (since their exposition to the LAI-AP was insufficient to consider its impact on outcome). Those who took LAI-AP for >3 months, but <12 months were excluded (as it was difficult to evaluate the impact of LAI-AP because of insufficient but still significant exposition to LAI-AP), making this group different from the one receiving only OAP.
Assessments
Socio-demographics, symptoms and functional data were collected at admission and annually for three years. A research assistant trained in the administration of psychometric scales interviewed the patients and reviewed files for the collection of socio-demographic data, Positive and Negative Syndrome Scale) (PANSS),23 Calgary Depression Scale for Schizophrenia (CDS),24 Quality of Life Scale (QLS),25 Drug Use Scale (DUS), Alcohol Use Scale (AUS),26 and types of substances abused. Inspired from Childhood trauma questionnaire items (CTQ),27 childhood trauma history was collected from file review or clinicians and patient interview (when possible) on the different types of trauma experienced before the age of eighteen: negligence, physical, psychological and sexual abuse, foster care, bullying, parents’ separation/divorce, separation from caregiver and caregiver’s death. If one of these types of trauma was present, history of childhood trauma was considered positive.
DSM-IV-TR28 diagnoses of psychotic disorder, SUD and Cluster B personality traits or disorder were determined by the best-estimate consensus method29 with all available data considered by 2 raters (one psychiatrist and either a psychiatry resident, a medical doctor or another psychiatrist). Although all Cluster B personality traits were noted, the most frequent presentation was borderline and antisocial personality traits not better explained by a DSM-IV axis I disorder.
The Social and Occupational Functioning Assessment Scale (SOFAS),30 the Global Assessment of Functioning (GAF) Scale31 and Clinical Global Impression-Severity sub-scale (CGI-S)32 were administered by the research psychiatrists, as was the assessment of AUS, DUS and type of substance used. At admission, a retrospective assessment of the best in lifetime premorbid functioning was estimated with the GAF and SOFAS according to the information available in patients’ files. If patient was transferred or LTF at the date of evaluation, we extrapolated the data up to 3 months prior to exit from study if data was easily estimable (e.g., stable patient since many months/years in the same occupation and housing).
For LAI-AP medication, the following was recorded by file reviews: type of LAI-AP prescribed, reason for prescription, reason for cessation and type of cessation. Based on recommendations in the field,33 medication compliance was assessed by multiple sources of information: patients’ self-reports, patients’ file reviews (including information from family reports), laboratory measures, and patients’ case manager and psychiatrist reports. Based on these 5 sources of information, for OAP medication, adherence was recorded and defined as good (90%–100% adherence), and poor adherence (partial or none).
For both LAI-AP and OAP, information on the number of medication trials and community treatment order (CTO) was collected.
Statistical Analyses
Analyses were performed using IBM SPSS software version 20.34 Sample representativeness was determined by comparing subjects LTF at 36 months to those still followed on independent variables (known to be associated with outcome) at baseline. To determine whether there were differences in outcome between the two treatment groups, the baseline, 12th month, 24th month and 36th month clinical and functional outcome measures were compared. Analysis of variance (ANOVA) were used for group comparisons for continuous variables. For discrete variables, Pearson chi-square were performed. Type I error was fixed at p = 0,05. The same outcome analyses were also run between the OAP group and a hypothetical LAI-AP group where only patients exposed to LAI-AP for >3 months but <6 months (instead of <12 months) would be excluded. These analysis were performed in order to ascertain whether the decision to exclude those with >3 months but <12 months LAI-AP would bias results. Since baseline characteristics and outcome results were very similar in terms of representativity of those still followed at 36 months, it was decided to present the data for the group with a significant LAI-AP exposition of 12 months or more.
Results
Of the 416 eligible patients, 2 groups were created: the LAI-AP group and the OAP group. Of the latter, 10 patients had received OAP for most of the study and LAI-AP for 3 months or less. Those who took LAI-AP for >3 months, but <12 months were excluded (n = 41), leaving a total of 375 patients for analyses. In the LAI-AP group, 24% of patients received LAI-AP only, i.e., they had no trial of OAP during f/u.
Among the 375 patients included (LAI-AP (n = 100), OAP (n = 275)), 62,9% accepted to participate, 13,3% refused and 23,7% had not been approached because of inability to consent or early loss to f/u (i.e., before being approached to participate in the research). Of the 375 patients, 34,4% (n = 129) were LTF at 36 months. Those who were still followed compared to those LTF (Supplemental Material Table S1) had previous work income in greater proportion, had a lower best premorbid GAF during adulthood, were more likely to have a schizophrenia spectrum diagnosis, to be single, to live with their parents at admission and throughout their adult life, and to be in the LAI-AP group (15% were LTF) than the OAP group (41,5% were LTF).
Table S1. Baseline Sociodemographic, Symptomatic And Functional Characteristics Of Sample (N = 375) Of Young Adults With First Episode Psychosis (Fep) According To Follow-Up Status At 3 Years (Representativity Analyses Of Residual Sample).
| LTFa AT 3 YEARS (N = 129) | STILL FOLLOWED AT 3 YEARS (N = 246) | P-VALUE | ||||
|---|---|---|---|---|---|---|
| Sociodemographics | ||||||
| Age, mean (s.d) | 23,3 (3,5) | 23,3 (3,6) | 0,849 | |||
| Male, n (%) | 94 (72,9) | 193 (78,5) | 0,249 | |||
| Diagnosis*, n (%)b | <0,001 | |||||
| - Schizophrenia spectrum | 58 (45,0) | 172 (69,9) | ||||
| - Affective psychosis | 43 (33,3) | 56 (22,8) | ||||
| - Psychosis NOS/delusional disorder/reactive psychosis | 28 (21,7) | 18 (7,3) | ||||
| Cluster B personality disorders, n (%) | 43 (33,3) | 61 (24,9) | 0,090 | |||
| Immigration status, n (%) | 0,469 | |||||
| - Non-immigrant | 72 (55,8) | 131 (53,3) | ||||
| - FGIc | 35 (27,1) | 60 (24,4) | ||||
| - SGId | 22 (17,1) | 55 (22,4) | ||||
| Last completed diploma, n (%) | 0,276 | |||||
| - No diploma | 48 (37,8) | 98 (40,5) | ||||
| - High school, completed | 40 (31,5) | 88 (36,4) | ||||
| - Post high school diploma | 39 (30,7) | 56 (23,1) | ||||
| Years of education, n (%) | 0,912 | |||||
| - Less than 11 years | 70 (55,1) | 136 (56,2) | ||||
| - More than 11 years | 57 (44,9) | 106 (43,8) | ||||
| Employment best in lifetime, n (%) | 0,068 | |||||
| - Full time/Part time/Stay-at-home parent/Rehabilitation | 101 (80,2) | 215 (87,4) | ||||
| - None | 25 (19,8) | 31 (12,6) | ||||
| Income**, best in lifetime, n (%) | 0,008 | |||||
| - Independent income | 92 (74,8) | 212 (86,5) | ||||
| - Government income security/Dependent of family | 31 (25,2) | 33 (13,5) | ||||
| Living arrangements best in lifetime, n (%) | 0,005 | |||||
| - Living independently | 98 (77,8) | 150 (61,2) | ||||
| - Living with parents | 28 (22,2) | 94 (38,4) | ||||
| - Homelessness | 0 (0) | 1 (0,4) | ||||
| History of childhood trauma, n (%) | 28 (50,9) | 64 (50,0) | 1,000 | |||
| Homelessness history, n (%) | 16 (12,8) | 46 (18,9) | 0,145 | |||
| History of legal problems before admission, n (%) | 35 (29,2) | 58 (23,7) | 0,306 | |||
| Symptomatology | ||||||
| PANSSe, mean (s.d) | 76,9 (13,9) | 78,0 (15,3) | 0,649 | |||
| - Positive symptoms | 20,0 (5,1) | 19,6 (6,2) | 0,660 | |||
| - Negative symptoms | 21,7 (5,2) | 22,3 (5,9) | 0,516 | |||
| - General symptoms | 35,2 (6,8) | 36,1 (6,8) | 0,403 | |||
| CDSf, mean (s.d) | 6,1 (3,9) | 6,4 (3,6) | 0,635 | |||
| CGI-Sg, mean (s.d) | 4,8 (1,0) | 4,8 (0,9) | 0,638 | |||
| Substance use disorder, n (%) | 67 (51,9) | 127 (51,6) | 1,000 | |||
| - alcohol | 23 (17,8) | 47 (19,1) | 0,889 | |||
| - cannabis | 58 (45,0) | 110 (44,7) | 1,000 | |||
| - cocaine | 11 (8,5) | 15 (6,1) | 0,397 | |||
| - amphetamines | 16 (12,4) | 34 (13,8) | 0,751 | |||
| - others | 3 (2,3) | 6 (2,4) | 1,000 | |||
| Functioning | ||||||
| GAFhbest in lifetime, mean (s.d.) | 63,2 (10,3) | 60,8 (11,4) | 0,046 | |||
| GAF, mean (s.d.) | 29,3 (9,2) | 30,4 (10,1) | 0,337 | |||
| SOFASi, best in lifetime, mean (s.d.) | 62,7 (11,0) | 61,4 (10,7) | 0,252 | |||
| SOFAS, mean (s.d) | 33,3 (13,2) | 33,9 (12,4) | 0,629 | |||
| QLSj, mean (s.d) | 50,1 (21,2) | 50,1 (22,4) | 0,990 | |||
| Marital status, n (%) | 0,040 | |||||
| - Single | 101 (78,3) | 212 (86,2) | ||||
| - Boyfriend/Girlfriend | 19 (14,7) | 21 (8,5) | ||||
| - Separated/Divorced | 5 (3,9) | 2 (0,8) | ||||
| - Married/Common-law partner | 4 (3,1) | 11 (4,5) | ||||
| Studying, n (%) | 0,075 | |||||
| - Full-time/Part-time | 29 (22,6) | 41 (16,7) | ||||
| - None | 99 (77,3) | 205 (83,3) | ||||
| Employment***, n (%) | 0,115 | |||||
| - Full time/Part time/Work rehabilitation | 42 (33,1) | 62 (25,2) | ||||
| - None | 85 (66,9) | 184 (74,8) | ||||
| Income**, n (%) | 0,489 | |||||
| - Independent income | 83 (67,5) | 156 (63,7) | ||||
| - Government income security/Dependent of family | 40 (32,5) | 89 (36,3) | ||||
| Living arrangements****, n (%) | 0,001 | |||||
| - Living independently | 85 (65,9) | 110 (44,7) | ||||
| - Living with parents | 37 (28,7) | 112 (45,5) | ||||
| - Supervised living, hospital, family | 1 (0,8) | 10 (4,1) | ||||
| - Homelessness/other | 6 (4,7) | 14 (5,7) | ||||
| Service use | ||||||
| Community treatment order, n (%) | 9 (7,0) | 52 (21,1) | <0,001 | |||
aLTF: Lost to follow-up.
bDiagnosis has been revised at 3 years with all the information available.
cFGI: First generation immigrant.
dSGI: Second generation immigrant.
ePANSS: Positive and negative syndrome scale.
fCDS: Calgary depression scale.
gCGI: Clinical global impression - severity subscale.
hGAF: General assessment of functioning.
iSOFAS: Social and occupational functioning assessment scale.
jQLS: Quality of Life scale.
*Diagnosis has been revised at 3 years will all the information available.
**Work/Bank loan/Employment insurance benefits/Scholarship.
***“Full- or part-time work” included competitive work, work rehabilitation programs or sheltered work, and stay-at-home parents taking care of their own children full time.
****Living arrangements were rated according to scales adapted from Ciompi,1980: “Independent” regrouped all patients living on their own alone, with their partner and/or their children; “With parents” regrouped all patients living with any family members; “Supervised” regrouped patients living in supported housing (supervised apartment, group home, foster home, hospital
Baseline Characteristics
The LAI-AP group were more likely to be males with lower education and a history of childhood trauma, homelessness and legal problems. Compared to the OAP group who was more likely to live on its own at baseline, the LAI-AP group lived in greater proportion with their parents. Their premorbid and baseline functioning was lower (e.g., best in lifetime GAF and SOFAS, baseline GAF, baseline employment and autonomous living arrangements) (Table 1). The level of illness severity (CGI-S) was higher in the LAI-AP group, but there was no difference in PANSS and CDS scores. The LAI-AP group was more likely to be diagnosed with schizophrenia spectrum disorders and to suffer from comorbid SUD (Table 1).
Table 1. Baseline Sociodemographic Characteristics, Symptomatic And Functional Outcome, And Service Use Of Young Adults With A First-Episode Psychosis (Fep) And Treated With A Long-Acting Injectable Antipsychotic (Lai-Ap) For At Least 12 Months During 3-Year Follow-Up Compared To Those Treated With An Oral Antipsychotic Medication (Oap).
| BASELINE | 12 MONTHS | 24 MONTHS | 36 MONTHS | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAI-AP N = 100 | OAP N = 275 | P-VALUE | LAI-AP N = 97 | OAP N = 210 | P-VALUE | LAI-AP N = 92 | OAP N = 188 | P-VALUE | LAI-AP N = 85 | OAP N = 161 | P-VALUE | |||||||||||||
| Baseline sociodemographics | ||||||||||||||||||||||||
| Age, mean (s.d) | 22,9 (3,4) |
23,4 (3,6) |
0,170 | – | – | – | – | – | – | – | – | – | ||||||||||||
| Male, n (%) | 84 (84,0) |
203 (73,8) |
0,040 | – | – | – | – | – | – | – | – | – | ||||||||||||
| Diagnosis*, n (%) | <0,001 | – | – | – | – | – | – | – | – | – | ||||||||||||||
| - Schizophrenia spectrum | 86 (86,0) |
144 (52,4) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - Affective psychosis | 10 (10,0) |
89 (32,4) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - Psychosis NOS/delusional disorder/reactive psychosis | 4 (4,0) |
42 (15,3) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| Cluster B personality disorders, n (%) | 30 (30,0) |
74 (27,0) |
0,603 | – | – | – | – | – | – | – | – | – | ||||||||||||
| Immigration status, n (%) | 0,445 | – | – | – | – | – | – | – | – | – | ||||||||||||||
| - Non-immigrant | 50 (50,0) |
153 (55,6) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - FGIa | 30 (30,0) |
65 (23,6) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - SGIb | 20 (20,0) |
57 (20,7) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| Last completed diploma, n (%) | <0,001 | – | – | – | – | – | – | – | – | – | ||||||||||||||
| - No diploma | 57 (57,0) |
89 (33,1) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - High school, completed | 30 (30,0) |
98 (36,4) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - Post high school diploma | 13 (13,0) |
82 (30,5) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| Years of education, n (%) | <0,001 | – | – | – | – | – | – | – | – | – | ||||||||||||||
| - Less than 11 years | 71 (71,0) |
135 (50,2) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - More than 11 years | 29 (29,0) |
134 (49,8) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| Employment best in lifetime, n (%) | 0,051 | – | – | – | – | – | – | – | – | – | ||||||||||||||
| - Full time/Part time/Stay-at-home parent/Rehabilitation | 91 (91,0) |
225 (82,7) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - None | 9 (9,0) |
47 (17,3) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| Income**, best in lifetime, n (%) | 0,021 | – | – | – | – | – | – | – | – | – | ||||||||||||||
| - Independent income | 90 (90,0) |
214 (79,9) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - Government income security/Dependent of family | 10 (10,0) |
54 (20,1) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| Living arrangements best in lifetime***, n (%) | 0,830 | – | – | – | – | – | – | – | – | – | ||||||||||||||
| - Living independently | 66 (66,7) |
182 (66,9) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - Living with parents | 33 (33,3) |
89 (32,7) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| - Homelessness | 0 (0) | 1 (0,4) |
– | – | – | – | – | – | – | – | – | |||||||||||||
| History of childhood trauma, n (%) | 40 (62,5) |
52 (43,7) |
0,020 | – | – | – | – | – | – | – | – | – | ||||||||||||
| Homelessness history, n (%) | 33 (33,0) |
29 (10,8) |
<0,001 | – | – | – | – | – | – | – | – | – | ||||||||||||
| History of legal problems before admission, n (%) | 36 (36,0) |
57 (21,5) |
0,007 | – | – | – | – | – | – | – | – | – | ||||||||||||
| Symptomatology PANSSc, mean (s.d) |
79,6 (11,8) |
77,1 (15,6) |
0,358 | 59,1 (17,3) |
54,5 (14,6) |
0,120 | 55,2 (14,6) |
48,5 (13,1) |
0,013 | 54,3 (2,5) |
45,6 (12,1) |
0,002 | ||||||||||||
| - Positive symptoms | 20,1 (5,2) |
19,6 (6,1) |
0,673 | 13,0 (5,2) |
12,1 (4,9) |
0,353 | 12,0 (4,3) |
10,0 (3,6) |
0,011 | 11,4 (4,6) |
9,4 (3,4) |
0,015 | ||||||||||||
| - Negative symptoms | 23,2 (4,7) |
21,8 (5,9) |
0,201 | 17,5 (5,8) |
15,7 (4,8) |
0,063 | 16,5 (5,0) |
14,0 (4,9) |
0,010 | 16,2 (4,7) |
13,0 (4,6) |
0,001 | ||||||||||||
| - General symptoms | 36,4 (5,9) |
35,6 (7,0) |
0,562 | 28,6 (7,2) |
26,7 (6,2) |
0,128 | 26,8 (6,3) |
24,5 (5,9) |
0,058 | 26,7 (6,8) |
23,1 (5,3) |
0,004 | ||||||||||||
| CDSd, mean (s.d) | 6,0 (3,5) |
6,4 (3,8) |
0,544 | 3,3 (3,0) |
3,0 (2,5) |
0,618 | 3,3 (3,6) |
2,3 (2,6) |
0,086 | 2,9 (3,1) |
1,6 (2,4) |
0,029 | ||||||||||||
| CGIe, mean (s.d) | 5,0 (0,7) |
4,8 (1,0) |
0,022 | 3,7 (1,3) |
2,8 (1,2) |
<0,001 | 3,4 (1,1) |
2,7 (1,3) |
<0,001 | 3,6 (1,1) |
2,9 (1,2) |
<0,001 | ||||||||||||
| Substance use disorder, n (%) | 63 (63,0) |
131 (47,6) |
0,008 | 59 (60,2) |
63 (30,4) |
<0,001 | 51 (56,0) |
51 (27,6) |
<0,001 | 44 (52,4) |
42 (26,6) |
<0,001 | ||||||||||||
| - alcohol | 18 (18,0) |
52 (18,9) |
0,882 | 21 (21,4) |
28 (13,5) |
0,095 | 17 (18,7) |
26 (14,1) |
0,378 | 15 (17,9) |
23 (14,6) |
0,578 | ||||||||||||
| - cannabis | 57 (57,0) |
111 (40,4) |
0,005 | 49 (50,0) |
52 (25,1) |
<0,001 | 43 (47,3) |
37 (20,0) |
<0,001 | 38 (45,2) |
32 (20,3) |
<0,001 | ||||||||||||
| - cocaine | 6 (6,0) |
20 (7,3) |
0,820 | 9 (9,2) |
10 (4,8) |
0,202 | 9 (9,9) |
8 (4,3) |
0,107 | 4 (4,8) |
7 (4,4) |
1,000 | ||||||||||||
| - amphetamines | 19 (19,0) |
31 (11,3) |
0,059 | 16 (16,3) |
11 (5,3) |
0,004 | 15 (16,5) |
8 (4,3) |
0,001 | 14 (16,7) |
10 (6,3) |
0,013 | ||||||||||||
| - others | 3 (3,0) |
6 (2,2) |
0,705 | 2 (2,0) |
3 (1,4) |
0,658 | 2 (2,2) |
2 (1,1) |
0,601 | 0 (0) |
3 (1,9) |
0,553 | ||||||||||||
| GAFf best in lifetime, mean (s.d.) | 58,4 (10,6) |
62,8 (11,0) |
<0,001 | – | – | – | – | – | – | – | – | – | ||||||||||||
| GAF, mean (s.d.) |
28,3 (8,5) |
30,6 (10,2) |
0,041 | 45,4 (12,4) |
53,0 (13,3) |
<0,001 | 47,6 (11,8) |
53,2 (13,9) |
0,001 | 46,7 (11,5) |
53,6 (13,2) |
<0,001 | ||||||||||||
| SOFASg, best in lifetime, mean (s.d.) | 59,6 (9,1) |
62,7 (11,3) |
0,013 | – | – | – | – | – | – | – | – | – | ||||||||||||
| SOFAS, mean (s.d) | 32,1 (10,9) |
34,3 (13,2) |
0,130 | 48,5 (11,9) |
54,5 (12,9) |
<0,001 | 51,0 (11,1) |
55,2 (13,6) |
0,011 | 51,1 (10,6) |
56,5 (11,8) |
0,001 | ||||||||||||
| QLSh, mean (s.d) | 45,1 (19,7) |
51,7 (22,4) |
0,102 | 60,5 (27,6) |
73,9 (27,2) |
0,011 | 65,5 (22,5) |
82,2 (25,5) |
0,001 | 64,2 (20,1) |
82,6 (23,8) |
<0,001 | ||||||||||||
| Marital status, n (%) | 0,730 | 0,290 | 0,246 | 0,286 | ||||||||||||||||||||
| - Single | 84 (84,0) |
229 (83,3) |
85 (85,9) |
170 (82,1) |
78 (85,7) |
142 (78,0) |
75 (86,2) |
124 (77,5) |
||||||||||||||||
| - Boyfriend/Girlfriend | 9 (9,0) |
31 (11,3) |
7 (7,1) |
26 (12,6) |
9 (9,9) |
26 (14,3) |
8 (9,2) |
22 (13,8) |
||||||||||||||||
| - Separated/Divorced | 3 (3,0) |
4 (1,5) |
3 (3,0) |
2 (1,0) |
2 (2,2) |
2 (1,1) |
2 (2,3) |
3 (1,9) |
||||||||||||||||
| - Married/Common-law partner | 4 (4,0) |
11 (4,0) |
4 (4,0) |
9 (4,3) |
2 (2,2) |
12 (6,6) |
2 (2,3) |
11 (6,9) |
||||||||||||||||
| Studying, n (%) | 0,132 | 0,249 | 0,090 | 0,028 | ||||||||||||||||||||
| - Full-time/Part-time | 12 (12,0) |
58 (21,2) |
18 (18,2) |
55 (26,5) |
17 (18,7) |
63 (29,1) |
9 (10,3) |
39 (24,4) |
||||||||||||||||
| - None | 88 (88,0) |
216 (78,8) |
81 (81,8) |
152 (73,4) |
74 (81,3) |
129 (70,9) |
78 (89,7) |
121 (75,6) |
||||||||||||||||
| Employment****, n (%) | 0,009 | 0,072 | 0,036 | 0,013 | ||||||||||||||||||||
| - Full time/Part time/Work rehabilitation | 18 (18,0) |
86 (31,5) |
27 (27,3) |
79 (38,2) |
27 (29,7) |
78 (42,9) |
23 (26,4) |
69 (43,1) |
||||||||||||||||
| - None | 82 (82,0) |
187 (68,5) |
72 (72,7) |
128 (61,8) |
64 (70,3) |
104 (57,1) |
64 (73,6) |
91 (56,9) |
||||||||||||||||
| Employment and/or studying, n (%) | 0,002 | 0,007 | 0,003 | 0,000 | ||||||||||||||||||||
| - Full time/Part time | 29 (29,0) |
129 (47,1) |
40 (40,4) |
118 (57,0) |
39 (42,9) |
113 (62,1) |
31 (35,6) |
96 (60,0) |
||||||||||||||||
| - None | 71 (71,0) |
145 (52,9) |
59 (59,6) |
89 (43,0) |
52 (57,1) |
69 (37,9) |
56 (64,4) |
64 (40,0) |
||||||||||||||||
| Income, n (%) | <0,001 | 0,015 | 0,001 | 0,021 | ||||||||||||||||||||
| - Independent outcome | 49 (49,0) |
190 (70,9) |
26 (26,3) |
82 (40,6) |
24 (26,4) |
83 (46,9) |
26 (29,9) |
72 (45,3) |
||||||||||||||||
| - Government income security/Dependent of family | 51 (51,0) |
78 (29,1) |
73 (73,7) |
120 (59,4) |
67 (73,6) |
94 (53,1) |
61 (70,1) |
87 (54,7) |
||||||||||||||||
| Living arrangements****, n (%) | 0,045 | 0,035 | 0,011 | 0,001 | ||||||||||||||||||||
| - Living independently | 44 (44,0) |
151 (54,9) |
34 (34,3) |
89 (42,8) |
27 (29,7) |
87 (47,8) |
28 (32,2) |
71 (44,4) |
||||||||||||||||
| - Living with parents | 42 (42,0) |
107 (38,9) |
35 (35,4) |
86 (41,3) |
38 (41,8) |
67 (36,8) |
25 (28,7) |
63 (39,4) |
||||||||||||||||
| -Supervised living, hospital, family | 6 (6,0) |
5 (1,8) |
27 (27,3) |
30 (14,4) |
24 (26,4) |
24 (13,2) |
32 (36,8) |
24 (15,0) |
||||||||||||||||
| - Homelessness/other | 8 (8,0) |
12 (4,4) |
3 (3,0) |
3 (1,4) |
2 (2,2) |
4 (2,2) |
2 (2,3) |
2 (1,2) |
||||||||||||||||
| History of legal problems during follow-up, n (%) | – | – | – | – | – | – | – | – | – | 21 (21,0) |
25 (10,0) |
0,008 | ||||||||||||
| Homelessness during follow-up, n (%) | – | – | – | – | – | – | – | – | – | 23 (23,0) |
20 (7,8) |
<0,001 | ||||||||||||
| Service use during 3-year follow-up | ||||||||||||||||||||||||
| Community treatment order at some point during follow-up*****, n (%) | – | – | – | – | – | – | – | – | – | 50 (50,0) |
11 (4,0) |
<0,001 | ||||||||||||
aFGI: First generation immigrant.
bSGI: Second generation immigrant.
cPANSS: Positive and negative syndrome scale.
dCDS: Calgary depression scale.
eCGI: Clinical global impression - severity subscale.
fGAF: General assessment of functioning.
gSOFAS: Social and occupational functioning assessment scale.
hQLS: Quality of Life scale.
*Diagnosis has been revised at 3 years will all the information available.
**Work/Bank loan/Employment insurance benefits/Scholarship.
***Living arrangements were rated according to scales adapted from Ciompi,1980: “Independent” regrouped all patients living on their own alone, with their partner and/or their children; “With parents” regrouped all patients living with any family members; “Supervised” regrouped patients living in supported housing (supervised apartment, group home, foster home, hospital).
****“Full- or part-time work” included competitive work, work rehabilitation programs, sheltered work, and stay-at-home parents taking care of their own children full time.
*****Community treatment orders duration varied from 1 to 3 years.
LAI-AP and OAP Treatment
Medication utilization profile is described in Table 2 and Figures 1–2. Relapse due to non-adherence was the most frequent reason (56,4%) for LAI-AP prescription. Interestingly, 14,3% of patients preferred a LAI-AP to an OAP after physician’s explanations of the advantages/disadvantages of this formulation (Figure 1).
Table 2. LAI-AP and OAP Utilization Profile.
| Delay between admission and first prescription of LAI-AP (months) (mean (s.d.)/median) | 6,0 (7,1)/3,2 | |
| Total exposure to LAI-AP (months) (mean (s.d.)) | 25,4 (7,6) | |
| Duration of first trial of LAI-AP (months) (mean (s.d.)) | 22,8 (10,0) | |
| Duration of first trial of OAPa (for OAP group, n = 275) (months) (mean (s.d.)) | 15,2 (14,9) | |
| Proportion of good adherence (>90% of adherence) to first trial of OAP group (%) | 57,2 | |
| Type of LAI-AP prescribed during 3-year follow-up, all trials combined (%) | – | |
| - Long-acting risperidone | 44,3 | |
| - Monthly paliperidone palmitate | 32,1 | |
| - Long-acting zuclopenthixol | 12,1 | |
| - Long-acting haloperidone | 7,9 | |
| - Long-acting flupenthixol | 2,1 | |
| - Long-acting aripiprazole | 0,0 | |
| - Fluphenazine decanoate | 0,7 | |
| - Pipothiazine palmitate | 0,7 | |
| Number of trials of LAI-AP for the LAI-AP group (n = 100) during 3-year follow-up (%) | – | |
| - 1 trial | 65,0 | |
| - 2 trials | 30,0 | |
| - 3 trials | 5,0 | |
| - 4 trials | 0 | |
| Number of trials of OAP for the OAP group (n = 275) during 3-year follow-up (%) | – | |
| - 1 trial | 70,5 | |
| - 2 trials | 23,6 | |
| - 3 trials | 5,1 | |
| - 4 trials | 0,7 | |
| Reasons for LAI-AP cessation (%) | – | |
| - Desire to see if medication is needed | 50,8 | |
| - Desire to stop LAI-AP but accepts OAP | 28,8 | |
| - Adverse effects | 10,2 | |
| - No efficacy/switch to clozapine | 10,2 | |
| Proportion of LAI-AP group receiving LAI-AP and OAP concomitantly (%) | 13,0 |
aIncluding lost to follow-up (23,6% at 12 months, 31,6% at 24 months and 41,5% at 36 months.
Figure 1.
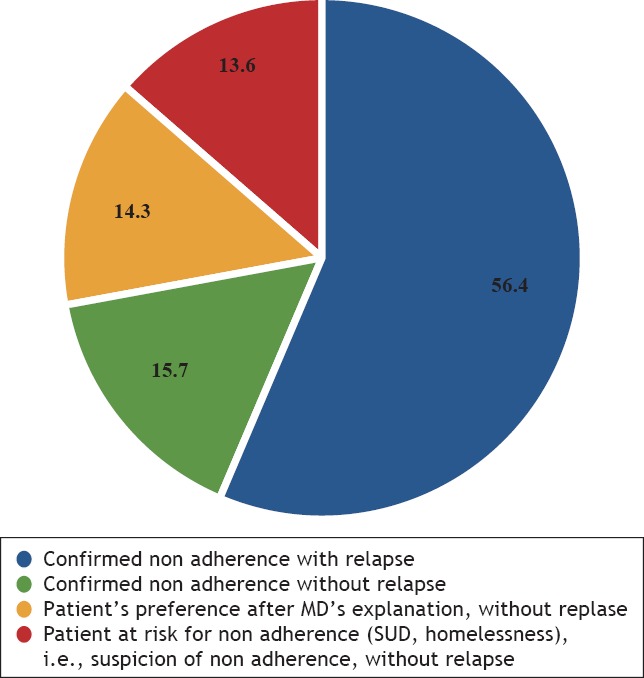
Reasons Justifying The Prescription of LAI-AP (%)
Figure 2.
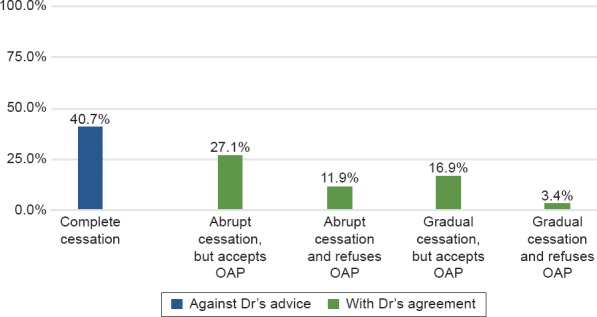
Type of Cessation of LAI-AP (%)
Sixty-five percent of patients in the LAI-AP group had only one trial of LAI-AP. Second generation LAI-AP were mostly prescribed (76,4%). More than half (50,8%) of the LAI-AP group ceased medication because of a desire to see if medication was needed (Table 2).
Although 40,7% stopped the LAI-AP against psychiatrist’s advice, 59,3% of those who ceased did so in context of a collaborative treatment plan with their treatment team (insert Figure 2). Moreover, 16,3% (n = 61) of the whole cohort (n = 375) have been the object of a CTO, of which 82% (n = 50) were prescribed LAI-AP, 18% (n = 11) an OAP and none were prescribed clozapine. Fifty percent of the LAI-AP group (n = 50) were the object of a CTO.
Outcome
Symptomatology
Although symptom severity improved in equal proportions (PANSS, CGI) throughout the 3-year f/u for both groups, at 36 months, the LAI-AP group had more psychotic (PANSS) and depressive (CDS) symptoms, and were considered more severely ill (CGI-S) (Table 1; Supplementary Table S2).
Table S2. Improvement Between Admission And 36 Months (Mean Or %) For Both Study Groups.
| CHANGE AT 36 MONTHS COMPARED TO BASELINE | ||||||||
|---|---|---|---|---|---|---|---|---|
| VARIABLES | EVOLUTION | LAI-AP GROUP | OAP GROUP |
P-VALUE | ||||
| PANSS-Ta(mean) | Less total sx | –26,9 | –31,7 | 0,998 | ||||
| PANSS-Pb(mean) | Less positive sx | –8,1 | –9,7 | 0,671 | ||||
| PANSS-Nc(mean) | Less negative sx | –8,1 | –9,0 | 0,142 | ||||
| PANSS-Gd(mean) | Less general sx | –10,7 | –13,0 | 0,790 | ||||
| CDSe(mean) | Less symptoms | –3,1 | –5,0 | 0,022 | ||||
| CGIf(mean) | Lower illness severity | –1,4 | –1,9 | 0,429 | ||||
| SUDg(%) | Prevalence reduction | –25,0 | –47,8 | 0,009 | ||||
| SUD-cannabis (%) | Prevalence reduction | –31,4 | –47,3 | 0,094 | ||||
| GAFh(mean) | Improvement | 18,0 | 22,5 | 0,089 | ||||
| SOFASi(mean) | Improvement | 18,6 | 21,8 | 0,221 | ||||
| QLSj(mean) | Improvement | 20,6 | 30,6 | 0,536 | ||||
| Studying and/or employment (%) | Improvement | 8,0 | 16,2 | <0,001 | ||||
| Homelessness (%) | Prevalence reduction | –6,9 | –2,5 | 0,533 | ||||
aPANSS-T: Positive and negative syndrome scale - total symptoms.
bPANSS-P: Positive and negative syndrome scale - positive symptoms subscale.
cPANSS-N: Positive and negative syndrome scale - negative symptoms subscale.
dPANSS-G: Positive and negative syndrome scale - general symptoms subscale.
eCDS: Calgary depression scale.
fCGI: Clinical global impression.
gSUD: Substance use disorder.
hGAF: General assessment of functioning scale.
iSOFAS: Social and occupational functioning assessment scale.
jQLS: Quality of life scale.
SUD rate decreased in both groups during the study, but the proportion of patients with SUD was larger in the LAI-AP group at study endpoint (Table 1; Supplemental Table S2). Patients prescribed LAI-AP misused cannabis and amphetamines in a greater proportion (Table 1).
Functioning
Both groups show a marked improvement of similar amplitude in functioning (GAF, SOFAS, rate of homelessness) and quality of life (QLS) at the end of the study (Table 1; supplemental Table S2). However, GAF, SOFAS and QLS scores remain significantly lower for the LAI-AP group at 36 months. The LAI-AP group was less likely to be studying or working at the end of the study. At the end of the study, a greater proportion of the OAP group lived independently or with their families compared to the LAI-AP group (83,8% vs 60,9%), while the LAI-AP group was more likely to be living in a supervised setting than the OAP group (36,8% vs 15,0%). A greater proportion of the LAI-AP group experienced homelessness during f/u as well as legal problems.
Discussion
Our study highlights the improvement in symptomatic and functional outcomes, and of quality of life over the 36 months f/u for both treatment groups. It is nonetheless difficult to conclude on LAI-AP’s impact since both groups are very dissimilar at baseline. According to our results, in real life setting, clinicians are indeed more likely to prescribe LAI-AP to FEP patients with poor prognostic factors associated with poor adherence and, consequently, relapse: history of childhood trauma, homelessness, and legal problems, male gender, low level of education, poor premorbid functioning, unemployment, higher illness severity, as well as schizophrenia spectrum diagnosis and comorbid SUD.
Even if there is great improvement at the end of the study compared to baseline, the final outcome levels for the LAI-AP group are poorer than those of the OAP group. Since individuals with poorer prognostic factors seem more likely to be prescribed LAI-AP,6,35,36 this group was possibly more severely ill than the other. It is then important to keep in mind a realistic prognosis; a patient having a low premorbid level of functioning can’t be expected to reach much higher levels of functioning than premorbidly despite intensive treatment and therapies.37–39
Baseline Characteristics
The differences between the two groups on baseline socio-demographics and clinical characteristics were not noted in two observational studies on FEP12,40 (Supplemental Table S3). This could be explained by the exclusion of patients with SUD in both studies, hence excluding patients who have factors of poor prognosis and possibly poorer adherence to medication, while in the present study 51,7% have a SUD at baseline. Our results corroborate previous ones6,35,36 suggesting that patients with poor prognostic factors are more likely to be prescribed LAI-AP. Therefore, the evidence suggests that clinicians tend to prescribe LAI-AP to patients with factors of poor adherence to antipsychotics or at risk thereof (severe psychopathology, SUD and legal antecedents).41–43
Table S3. Summary Of the Literature Review Of Observational Studies On First Episode Psychosis (Fep) And Randomized Controlled Trials (Rct) On Recent-Onset Psychosis Studying the Impact Of Lai-Ap On Symptomatic and Functional Outcomes, Relapse and Service Use.
| AUTHOR/YEAR | METHOD/DURATION | POPULATION | SYMPTOMS | FUNCTIONING | RELAPSE/SERVICE USE | OTHER | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observational Studies on FEP1 | ||||||||||||
| Emsley et al., 2008 (South Africa)56 | 2-year, single site, prospective, 1 arm, open label, descriptive study, performed between Feb 2004–Dec 2006. | n = 50: RLAI remission (n = 32; 64%) vs RLAI non remission (n = 18) | ↓ total PANSS and sub-scales remission group >non remission group | ↑ SOFAS remission group >non remission group no difference in SF-12 between groups | Remission at 2 years achieved by 64% (97% maintained this status throughout study) | |||||||
| Assessment of rate, predictors and correlation of remission2 in FEP patients under RLAIa. | 32 males; mean age = 23 years; mean DUPj = 129 days; 54% AP naive. | ↓ CGI-S remission group >non remission group no difference in CDS between groups | Median time to remission = 301 days | |||||||||
| Inclusion: 16–45 years, in-outpatient, dxb ≤1 year of sczc/schizophreniform/sczaffd, ≤2 hospitalization for psychosis, ≤12 weeks lifetime exposition to APe. Exclusion: txf with mood stabilizers or ADg at baseline, SUDh in the month preceding the study, anterior use of LAI-APi, mental retardation, acute suicide risk. | At baseline, remission group has more women (53,1% vs 5,6%). No difference in race/ethnicity, mean age, dx, anterior exposition to AP, DUP, total PANSSk and sub-scales, CGI-S1, SF-12m, CDSn n = 36 (72%) completed study. | 92% have a clinical response3 | ||||||||||
| Hargarter et al., 2016 (Germany)45,57 | 1-year multicentre, retrospective, non interventional exploratory descriptive study based on file review; period when study was held is unknown. | n = 84; | PANSS: 79% have a clinical response ↓ total PANSS score (mean 31,6 points) from baseline to endpoint ↓ CGI 91% were deemed to have a clinically7 relevant improvement in illness severity at last PP injection. | ↑ PSPp from baseline to endpoint ↑ GAFq from baseline to endpoint 65% were deemed to have a clinically8 relevant improvement functioning at last PP injection | At PP initiation, 36 (42,9%) patients were in hospital. | Main reasons for initiating PP: relapse prevention (56%), partial/non-adherence to previous medication (20,2%), convenience (15,5%). | ||||||
| Evaluate impact of PPo on hospitalization and clinical outcome, and description of patterns of use; evaluation period from documentation of the first PP injection (baseline) to the first 12 months of continuous tx (endpoint). | Mean age = 24,1; | Overall, 81 (96,4%) had no new hospitalization during the 12-month documentation period. Three (3,6%) patients had one new hospitalization for management of an episode/relapse. | ||||||||||
| Inclusion: 18–29 years at time of first PP injection, dx scz ≤1 year before the first PP injection, regular continuous injections with not more than one missed injection within the 12-month documentation period (365 ± 31 days). | Male = 69,0%; | |||||||||||
| Exclusion: anterior tx with LAI-AP other than PP, tx with other LAI-AP during 12 months of PP | Mean age at FEP suggestive of scz = 23,8; Mean time between FEP suggestive of scz and first PP injection = 5,5 months; Mean age at first AP tx = 23,9 Mean time between first AP treatment and first PP injection = 4,8 months. |
|||||||||||
| Emsley et al., 2008 (South Africa)40 | 2-year same site post-hoc comparison of effects of 2 groups extracted each from 2 separate studies:6 | RLAI group: n = 50 (32 males); mean age = 25,4 OAP group: n = 47 (27 males); mean age = 25,9 | Higher ↓ PANSS total score for RLAI group (44%) vs OAP group (28,8%) | – | Lower relapse4 rate at 2 years for RLAI group (9,3%) vs OAP group (42,1%) among responders to medication. Lower proportion for all-cause discontinuation for RLAI vs OAP group (20% vs 48,9% at 1 year; 26% vs 70% at 2 years). | |||||||
| RLAI (Emsley et al., 2008): | At baseline: no difference between groups in age, gender, dx, proportion of AP naive and total PANSS scores. | Higher remission rate at 2 years for RLAI group (64%) vs OAP group (40,4%). | ||||||||||
| 2-year, single site, prospective, 1 arm, open label, descriptive study performed between Feb 2004–Dec 2006. | Higher positive PANSS score and more caucasiens in OAP group. | |||||||||||
| OAPr (Schooler et al., 2005): 2-year multisite, RCT, double-blind study performed between Nov 1996–January 2000, compares efficacy of oral risperidone to oral haloperidol. Inclusion: 16–45 y.o., dx ≤1 year scz/schizophreniform/sczaff; ≤2 hospitalization for psychosis, ≤12 weeks lifetime exposition to AP. Exclusion: tx with mood stabilizers or AD at baseline, SUD in the month preceding the study, anterior use of LAI-AP, mental retardation, acute suicide risk. | ||||||||||||
| Tiihonen et al., 2006 (Finland)13 | 6-year prospective cohort study using national central registers and performed between January 1st 1995–Dec 31st 2001. | 2230 consecutive adults hospitalization (1383 men) (many but not all had first episode psychosis at first hospitalization). Mean age = 30,7 | – | – | Clozapine, perphenazine depot and oral olanzapine are associated with lower risk of rehospitalization. Perphenazine depot had the lowest relative risk (0,32; IC 0,13–0,47). Rehospitalization incidence for perphenazine depot = 0,28. Rehospitalization incidence for oral perphenazine = 0,47. Rehospitalization incidence for OAP = 0,52. | Initial use of clozapine, perphenazine depot and oral olanzapine is associated to lower rate of discontinuation for any reason compared to other OAP. No difference in mortality rate between drugs. | ||||||
| Measures association between prescribed AP (perphenazine depot and diverse OAP) and rehospitalization after first admission for scz or sczaff Inclusion: dx scz or sczaff, patients hospitalized for the first time for scz/ sczaff, 15–45 y.o. at index hospitalization. Mean duration off /us = 3,6 years. | ||||||||||||
| Tiihonen et al., 2011 (Finland)14 | 7-year prospective cohort study using national central registers and performed between 2000 – 2007. Measures risk of rehospitalization between LAI-AP (haloperidol, risperidone, perphenazine zuclopenthixol) and diverse OAP or oral equivalent. | 2588 consecutive first hospitalization for scz (62% male). Mean age = 37,8. | – | – | Risk of rehospitalization for pooled LAI-AP = 1/3 of risk for OAP (adjusted HR = 0,36; IC 0,17–0,75). Rehospitalization incidence for LAI-AP = 0,12. | LAI-AP have 59% lower risk of discontinuation vs OAP. | ||||||
| Inclusion: dx scz, patients hospitalized for the first time for scz, 16–65 y.o. atindex hospitalization. | Rehospitalization incidence for OAP = 0,18. | |||||||||||
| Tiihonen et al., 2017 (Sweden)15 | Mean duration of f/u = 2 years. | 29 823 = prevalent cohort (57% male); 4603 = incident cohort (58,7% male). | – | – | 43.7% were rehospitalized (prevalent cohort) | Oral olanzapine = most frequently used drug, and zuclopenthixol = most frequently used LAI-AP. 71.7% experienced treatment failure (prevalent cohort). Clozapine (HR, 0.58) and all LAI-AP (HRs 0.65–0.80) associated with the lowest rates of treatment failure compared with oral olanzapine. | ||||||
| 7-year prospective cohort study using nationwide databases and performed between 2006 – 2013. Measures risk of rehospitalization and treatment failure (psychiatric rehospitalization, suicide attempt, discontinuation or switch of medication, death) between LAI-AP (fluphenazine, flupentixol, haloperidol, perphenazine, zuclopenthixol, risperidone, paliperidone, olanzapine) and diverse OAP (including clozapine) or oral equivalent. | SUD = 8,3% (prevalent); 7,5% (incident). | Lower risk of rehospitalization with LAI-AP vs equivalent OAP (HR, 0.78 prevalent cohort; HR, 0.68 incident cohort). | ||||||||||
| Inclusion: dx scz, 16–64 y.o. in 2006., drug prescribed in outpatient care only. | Mean age = 44,9 (prevalent); 36,5 (incident). | |||||||||||
| Mean duration of f/u = 5,7 years. | 9–12 years of education = 47,3% (prevalent); 44.6% (incident). | |||||||||||
| Toll et al., 2015 (Spain)12 | 6-month, single site, prospective and retrospective, 2 arms descriptive study performed between 2008–2014. | 108 consecutive FEP patients: 11 LAI-AP (25% PP, 25% zuclopenthixol, 50% RLAI); mean age = 22,18 | No difference between groups in total PANSS and sub-scales scores, or CDS. | No difference in GAF between groups. | Greater reduction of rehospitalization and emergency visits for LAI- AP vs OAP. | – | ||||||
| Comparison of outcome for LAI-AP vs OAP in a FEPt clinic. Inclusion: age 18–35, dx scz/schizophreniform/brief psychotic disorder/psychosis NOS/affectivepsychosis, <1 year of symptoms’ evolution, IQ >80, absence of abuse/dependance except to cannabis or tobacco. | 177 OAP; mean age = 24,92 No difference between groups in age, gender, substance use, dx, DUPu. | During f/u: no difference between groups in use of cannabis, tobacco, alcohol, cocaine, heroin, amphetamines. | ||||||||||
| RCT Studies on Recent-onset Psychosis1 | ||||||||||||
| Malla et al., 2013 (Canada)58 | 2-year multisite, exploratory, 2 arms, open label RCT, recruitment between 2004–2006. | RLAI: n = 42; mean age = 22,5 OAP: n = 35; mean age = 23 Mean time between dx and study = 9 months. | Between baseline and end of study, no difference in CGI-S and positive or negative PANSS score, except for greater reduction of total PANSS score for RLAI. | – | No difference in time to relapse4 between groups. | No difference in attitudes towards medication. | ||||||
| Explore comparative efficacy, safety and tolerability of RLAI relative to OAP 2nd generation. | No difference between groups on baseline sociodemographic characteristics | Higher number of relapse for RLAI (n = 11) vs OAP (n = 5). | ||||||||||
| Inclusion: dx scz/sczaff/schizophreniform <3 years, age 18–30, AP naive or under OAP 2nd gen., total PANSS 60–120, stable after stabilization phase. | n = 31 (40,3%) completed study. | |||||||||||
| Exclusion: mood stabilizers or AD, SUD, use of LAI-AP in the 3 months before study, AP resistance, suicide/violence risk at admission, drug use in the 30 days before study, anterior use of clozapine. | ||||||||||||
| 2 phases: stabilization (18 weeks) and maintenance (86 weeks). | ||||||||||||
| Subotnik et al., 2015 (USA)11 | 1-year, single site, 2 arms RCT, recruitment between March 16, 2005–Sept 27, 2012. | n = 83 (RLAI = 40 RPO = 43) | Higher ↓ BPRS in terms of hallucinations and unusual thought content for RLAI vs RPO and this is attribuable to better adherence. Those with the best adherence have a BPRS <4. | – | 16/83 (19%) relapse:5 RLAI = 2/40 (5%) vs RPO = 14/43 (33%) 86% of relapses in the first 6 months (relative risk reduction RLAI vs RPO = 84,7%) (effect not attributable to adherence). | Higher rate of discontinuation for RPO under HBT. | ||||||
| Compare clinical efficacy of RLAI to RPOu | No difference in sociodemographic characteristics between groups (age, gender, race/ethnicity, dx, time before dx, education, marital status, BPRSx (at baseline and at randomization) | Number of hospitalization: RLAI = 2/40 (5%) vs RPO = 8/43 (18,6%). Time without exacerbation/relapse is associated to adherence. Adherence predicts need for hospitalization. | Better adherence for RLAI vs RPO (95% excellent vs 33%). | |||||||||
| Inclusion: age 18–45, outpatient, FEP ≤2 years, dx scz/sczaff/schizophreniform Exclusion: substance abuse/dependance in the last 6 months, mental retardation Mean duration of f/u = 10,2 months (no difference between groups). Randomization of medication and psychosocial treatment (CRv ou HBTw). Run-in phase and stabilisation with RPO. | mean age = 21,5; males = 78%; singles = 96%; caucasians = 49%, scz = 55% | Adherence during the first 6 months is highly correlated to adherence in the last 6 months. | ||||||||||
| An intent-to-treat analysis was performed between October 4, 2012, and November 12, 2014. | ||||||||||||
| Schreiner et al., 2015 (Multinational)59 | 2-year, multisite, prospective, open label, rater blinded, 2 arms RCT, unknown years of study. Assessment of efficacy of PP vs OAP. | Phase 2-year: n PP = 352; n OAP = 363 | No differences between groups for PANSS and CGI-S. | No differences between groups in PSP, SF-36y and EQ-5Dz. | Time to relapse4 longer for PP. 14,8% of PP relapse vs 20,9% for OAP (p = 0,032) | – | ||||||
| Inclusion phase of 2 weeks: patients with acute episode of scz and a PANSS 70–120 | No difference in sociodemographic and clinical characteristics between groups. | No mention if presence of SUD. | Relative risk reduction of relapse for PP = 29,4% | |||||||||
| who might benefit from switch to PP, age 18–65, dx scz since 1–5 years, history of ≤2 relapses requiring hospitalization in the last 24 months. | Mean age = 32,6; mean age at dx = 30,1; | |||||||||||
| Inclusion phase 2-year: score ≤4 in at least 4 PANSS items (P1, P2, P3, P6, P7, G8) and CGI-S ≤4 Exclusion: AP naive, resistance to AP, clozapine in the last 3 months, psychotherapy program started in the 2 months before study, involuntary hospitalization. | mean time between dx and study = 3 years for PP and 2,9 years for OAP; mean time between first treatment and start of study = 4 years for PP and 3,8 years for OAP. | |||||||||||
| OAP dispensed at each visit, pill count, reminder to take pills. | ||||||||||||
| Kishi et al., 201617 | Systematic review and meta-analysis of the 3 RCTs above. | Only adults (no adolescent and no geriatric population). | Pooled LAI-AP: no difference between LAI-AP and OAP in improvement of total PANSS score and its sub-scales. | – | Pooled LAI-AP are not superior to OAP in terms of relapse reduction (RRaa = 0,67; 95% CIbb = 0,24–1,83, P = 0,43; N = 3, N = 875), but significant heterogeneity (I2 = 76%) [Malla 2013: RR=1,83; 95%CI = 0,70–4,77; N = 77] showing no superiority, whether Subotnik and Schreiner show superiority of LAI-AP vs OAP [Schreiner 2015: [RR = 0,71; 95%CI = 0,51–0,97; NNTcc = –17; n = 715] [Subotnik 2015: RR = 0,15; 95%CI = 0,04–0,63; NNT = –4; n = 83] | Pooled LAI-AP have higher rate of discontinuation secondary to non adherence or inefficacy. | ||||||
| Mean age = 30,7 62,7% male. | The statistical power of the outcome in each study was 50,8% (Malla 2013), 69,4% (Schreiner 2015), and 95,3% (Subotnik 2015). | |||||||||||
1Only significant results are reported.
2Remission definition according to criteria by Andreasen et al., 2005: patient should keep for at least 6 months a score ≤3 on all the following key PANSS items: P1: Delusions, P2: Conceptual disorganization, P3: Hallucinatory behavior, N1: Blunted affect, N4: Passive/apathetic social withdrawal, N6: Lack of spontaneity, G5: Mannerisms and posturing, G9: Unusual thought content.
3Clinical response: lowering of 20% or more of PANSS score.
4Relapse definition according to criteria by Csernansky et al., 2002: Any one of the following: psychiatric hospitalization; an increase in the level of psychiatric care (e.g., from clinic visits to day treatment) and an increase of 25% from baseline in the total PANSS score or an increase of 10 points if the baseline score was 40 or less (total possible scores range from 30 to 210); deliberate self-injury; suicidal or homicidal ideation that was clinically significant in the investigator’s judgment; violent behavior resulting in clinically significant injury to another person or property damage; or substantial clinical deterioration, defined as a change score of 6 (“much worse”) or 7 (“very much worse”) on the CGI.
5Exacerbation and/or relapse was identified based on increases in the BPRS items unusual thought content, hallucinations, or conceptual disorganization using computer scoring algorithms.
6Participants in the 2 groups come from the same site. No patients participate in both studies. Protocols are similar between studies in terms of selection criteria and instruments of evaluation.
7 ≥20% decrease in PANSS or BPRS total score; or CGI–S decrease of at least 2 points; or CGI–C improved, much improved, or very much improved; and with no PANSS, BPRS, CGI–S or CGI–C score indicating disease worsening.
8PSP total score increase ≥7 points; or GAF total score increase ≥20 points; or SOFAS total score increase ≥30% from baseline; and with no PSP, GAF, or SOFAS score indicating worsening of the disease.
aRLAI: risperidone long-acting injection.
bdx: diagnosis.
cscz: schizophrenia.
dsczaff: schizoaffective disorder.
eAP: antipsychotics.
ftx: treatment.
gAD: antidepressants.
hSUD: Substance use disorder.
iLAI-AP: Long-acting injectable antipsychotic.
jDUP: Duration of untreated psychosis.
kPANSS: Positive and negative syndrome scale.
lCGI-S: Clinical general impression - severity sub scale.
mSF-12: 12 items Short form survey.
nCDS: Calgary depression scale.
oPP: Paliperidone palmitate.
pPSP: Personal and social performance scale.
qGAF: General assessment of functioning scale.
rOAP: Oral antipsychotic.
sf/u: follow-up.
tFEP: First episode psychosis.
uRPO: Risperidone per os.
vCR: Cognitive remediation.
wHBT: Healthy-behaviours training.
xBPRS: Brief psychiatric rating scale.
ySF-36: 36 items Short form survey.
zEQ-5D: EuroQol (quality of life) 5 dimensions questionnaire.
aaRR: risk ratio.
bbCI: confidence interval.
ccNNT: number needed to treat.
Reasons for Prescription and Cessation of LAI-AP
The vast majority (70,5%) of the OAP group had only one trial of antipsychotics, suggesting good satisfaction (efficacy/tolerance) (Table 2). High non adherence rate in both groups to first trial of oral antipsychotics (LAI-AP group = 73,7%; OAP group = 34,2%; total sample = 42,8%) confirms previous studies.44
Similarly to the present study, previous research report that poor adherence with or without relapse is the main reason that motivates prescription of LAI-AP both in FEP45 or later in psychotic illness evolution,46–48 and about 15% of FEP patients were prescribed LAI-AP because they preferred this formulation.45 This contrasts with many psychiatrists’ belief that patients perceive injectable medication as unacceptable.49,50
As the present study, previous investigations report that patient’s desire to stop medication is a major reason to cease LAI-AP in chronic psychosis.46,51 More than a third of them46 and half of FEP patients52 do so against medical advice.
Finally, in this study (spanning from 2005 to 2012), long-acting injectable risperidone was the LAI-AP most frequently used because it was the only 2nd generation LAI-AP available in Canada from 200553 until July 2010, when paliperidone palmitate became available.54 Long-acting aripiprazole became available only in March 201455 (a year before study’s end).
Symptoms and Functioning
The LAI-AP group show symptomatic and functional improvement, as described in many studies,11,40,56–58 as well as a reduction in SUD rate. However, at study endpoint, symptom severity is higher and functioning is lower in the LAI-AP group compared to OAP group, which is in contrast with observational studies12,40 or RCTs and their meta-analysis.11,17,58,59 Indeed, these studies demonstrated that patients taking LAI-AP have a better outcome than the OAP group11,40 or that there were no differences between groups.12,17,58,59 These differences might result from the high prevalence of poor prognostic factors in the LAI-AP group versus the OAP group in the present study, which is not the case in previously mentioned trials.11,12,40,58,59 Indeed, previous studies had similar groups at baseline,11,12,40,58,59 a consequence of selection bias induced by the exclusion of patients with a SUD,11,12,40,58 or severe psychopathology (e.g., resistance to antipsychotics, high suicidal risk, involuntary hospitalization).40,58,59
Strengths and Limitations
A strength of the present study is its naturalistic design in a “real life” setting assuring representativeness of the whole FEP population since no patient was excluded. Indeed, in the Quebec province (Canada), the public and universal healthcare system is sectorized for mental health services delivery, thus all individuals living in a specific catchment area are treated in a designated hospital. Additionally, we had access, by file review, to data on all FEP patients even those who refused to participate (13,3%) or who could not be recruited (23,7%). Among these patients, 25,2% were on LAI-AP, a similar proportion to the whole sample. Furthermore, compared to the present naturalistic study, in the three RCTs on recent-onset psychosis,11,58,59 patients who are susceptible to benefit from LAI-AP are under-represented or even excluded (e.g., unstable patients, those with poor adherence, SUD, aggressivity, suicide risk, AP-resistant symptoms, involuntary hospitalization, etc). More than half of the patients included in this study would have been excluded from recruitment if the selection criteria of many other studies had been applied, e.g., 51,7% of the present study participants had comorbid SUD. Of the latter, 12,4% refused to participate and 22,2% could not be recruited. Since 63% of them were prescribed LAI-AP, this indicates that an important proportion of FEP patients likely to benefit from LAI-AP are not included in most previous studies. Nevertheless, contrary to what could have been anticipated, the rate of refusal or impossibility of recruitment are alike between patients with and without a SUD. Other characteristics render our sample representative of the whole FEP population, such as a broad definition of psychosis (e.g. affective psychosis, delusional disorder), and inclusion of patients being the object of a CTO (16,3%).
Compared to other studies,11,40,58 a larger sample size, and a significant proportion of them taking LAI-AP, conferred greater statistical power. Many dimensions of recovery were considered, since different dimensions of outcome may have different evolution.60
More than a third of the cohort is LTF, the majority (88,4%) being in the OAP group. Hence, those with poor prognostic factors and on LAI-AP or CTO are more likely to remain in the study (Table 1). Therefore, the sample is possibly biased towards an over-estimation of the benefits of OAP. The lower rate of relapse per person for the OAP group may be influenced by a higher proportion of LTF in this group, since a shorter period of f/u does not allow to observe many relapses. On the other hand, the rate of LTF of the present study is similar to the ones of previous observational61,62 and randomized11,59 FEP studies, including two RCTs who also find a higher rate of LTF for their OAP groups compared to their LAI-AP groups.11,59
Another limit is that the introduction or change of medication does not occur simultaneously to the appointed time in which outcome measures were taken (baseline, 12, 24, 36 months). However, most participants in the LAI-AP group were receiving LAI-AP at the time measurements were taken (72,2%–89,4%).
About a third of FEP patients of the EIS where the study was conducted were prescribed LAI-AP, though in this study the proportion of FEP on LAI-AP is 26,7% since the 41 patients who received LAI-AP for a too short period (4–11 months) were excluded from analysis. At study’s end, 51,2% (n = 21) of these 41 patients were still on LAI-AP, so a longer study duration might capture LAI-AP’s effect on those with later initiation of LAI-AP in their evolution, since exposition would have been sufficient to evaluate its impact. Those patients might have a specific profile, since they were prescribed LAI-AP later in the first three years of f/u.
Although several sources of information were used to estimate adherence, there was no objective measure for medication adherence (e.g., pill counts) therefore possibly overestimating adherence. However, sample’s total rate of adherence to first trial of OAP was similar to the one reported previously.44
Like all naturalistic studies, since there is no random allocation of treatment, it seems obvious that treatment selection bias explains the worse outcome of patients prescribed LAI-AP because they had risk factors of poor prognosis and poor adherence. Nevertheless, this study design provides a higher external validity with a more representative sample2,20 that allows the description of the population that receives LAI-AP and its evolution. A mirror-image study63,64 could have been a better design to estimate the impact of LAI-AP for each outcome dimension and determine if LAI-AP contribute to modify the outcome trajectory positively. However, real-time measurements are complex in terms of evaluation timing and patient recruitment for interviews.
Conclusion
In FEP patients, LAI-AP are prescribed mainly to patients presenting risk factors of poor adherence and poor outcome. Nevertheless, FEP patients receiving LAI-AP improve on a symptomatic, functional and quality of life level as the OAP group, but to a lesser extent. These results suggest that FEP patients may benefit from early prescription of LAI-AP in the course of their illness, as recommended by different guidelines.65–71
Acknowledgments
SP is holder of the Eli Lilly Canada Chair in Schizophrenia Research.
Footnotes
Funding
Funding sources for this study were: Fondation IUSMM, Fondation CHUM, Eli Lilly Canada Chair in Schizophrenia Research at Université de Montréal, Research funds from the Department of Psychiatry of CHUM, Bristol-Myers-Squibb Canada, Janssen Ortho, and Otsuka-Lundeck.
Conflict of Interest
AAB has received speaker honoraries for continuing medical education talks from Janssen Canada, and received an unrestricted research grant from Janssen Canada, Otsuka Pharmaceuticals (for student grant) and Bristol-Myers Squibb. SP is holder of a grant from Otsuka Pharmaceuticals. ES is on the advisory board of Janssen Canada, Lundbeck and Otsuka Canada, and has given lectures with Janssen in France and Belgium, and with Otsuka and Lundbeck Canada. SM declares no conflict of interest.
Supplemental Materials
References
- 1.Bradford Daniel W, Perkins Diana O, Lieberman Jeffrey A. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63:2265–2283. doi: 10.2165/00003495-200363210-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kane John M, Kishimoto Taishiro, Correll Christoph U. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. Journal of clinical epidemiology. 2013;66:S37–S41. doi: 10.1016/j.jclinepi.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leclerc Emilie, Noto Cristiano, Bressan Rodrigo A, Brietzke Elisa. Determinants of adherence to treatment in first-episode psychosis: a comprehensive review. Revista Brasileira de Psiquiatria. 2015;37:168–176. doi: 10.1590/1516-4446-2014-1539. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovitch Mark, Béchard-Evans Laura, Schmitz Norbert, Joober Ridha, Malla Ashok. Early predictors of nonadherence to antipsychotic therapy in first-episode psychosis. The Canadian Journal of Psychiatry. 2009;54:28–35. doi: 10.1177/070674370905400106. [DOI] [PubMed] [Google Scholar]
- 5.Malla Ashok, Norman Ross, Schmitz Norbert et al. Predictors of rate and time to remission in first-episode psychosis: a two-year outcome study. Psychological medicine. 2006;36:649–658. doi: 10.1017/S0033291706007379. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Baki Amal, Ouellet-Plamondon Clairélaine, Salvat Émilie, Grar Kawthar, Potvin Stéphane. Symptomatic and functional outcomes of substance use disorder persistence 2 years after admission to a first-episode psychosis program. Psychiatry Research. 2017;247:113–119. doi: 10.1016/j.psychres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Ouellet-Plamondon C, Abdel-Baki A, Salvat É, Potvin S. Specific impact of stimulant, alcohol and cannabis use disorders on first-episode psychosis: 2-year functional and symptomatic outcomes. Psychological Medicine. 2017:1–11. doi: 10.1017/S0033291717000976. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Baki Amal, Ouellet-Plamondon Clairélaine, Malla Ashok. Pharmacotherapy challenges in patients with first-episode psychosis. Journal of affective disorders. 2012;138:S3–S14. doi: 10.1016/j.jad.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto Taishiro, Nitta Masahiro, Borenstein Michael, Kane John M, Correll Christoph U. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. The Journal of clinical psychiatry. 2013;74:957–965. doi: 10.4088/JCP.13r08440. [DOI] [PubMed] [Google Scholar]
- 10.Lafeuille Marie-Hélène, Dean Jason, Carter Valerie et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Current medical research and opinion. 2014;30:1643–1655. doi: 10.1185/03007995.2014.915211. [DOI] [PubMed] [Google Scholar]
- 11.Subotnik Kenneth L, Casaus Laurie R, Ventura Joseph et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia: a randomized clinical trial. JAMA psychiatry. 2015;72:822–829. doi: 10.1001/jamapsychiatry.2015.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toll Privat Alba, Berge Baquero Daniel, Mane Santacana Anna, Perez Sola Victor. Decreased Incidence of Readmissions in First Episode Psychosis in Treatment with Long-Acting Injectable Antipsychotics. Current Psychopharmacology. 2015;4:52–57. [Google Scholar]
- 13.Tiihonen Jari, Walhbeck Kristian, Lönnqvist Jouko et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. British Medical Journal. 2006;333:224. doi: 10.1136/bmj.38881.382755.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiihonen Jari, Haukka Jari, Taylor Mark, Haddad Peter M, Patel Maxine X, Korhonen Pasi. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. American Journal of Psychiatry. 2011;168:603–609. doi: 10.1176/appi.ajp.2011.10081224. [DOI] [PubMed] [Google Scholar]
- 15.Tiihonen Jari, Mittendorfer-Rutz Ellenor, Majak Maila et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA psychiatry. 2017;74:686–693. doi: 10.1001/jamapsychiatry.2017.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fusar-Poli Paolo, Kempton Matthew J, Rosenheck Robert A. Efficacy and safety of second generation long-acting injections in schizophrenia: a meta-analysis of randomized-controlled trials. International clinical psychopharmacology. 2013;28:57–66. doi: 10.1097/YIC.0b013e32835b091f. [DOI] [PubMed] [Google Scholar]
- 17.Kishi Taro, Oya Kazuto, Iwata Nakao. Long-acting injectable antipsychotics for the prevention of relapse in patients with recent-onset psychotic disorders: A systematic review and meta-analysis of randomized controlled trials. Psychiatry Research. 2016;246:750–755. doi: 10.1016/j.psychres.2016.10.053. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto Taishiro, Robenzadeh Alfred, Leucht Claudia et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophrenia bulletin. 2012:sbs150. doi: 10.1093/schbul/sbs150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenheck Robert A, Krystal John H, Lew Robert et al. Long-acting risperidone and oral antipsychotics in unstable schizophrenia. New England Journal of Medicine. 2011;364:842–851. doi: 10.1056/NEJMoa1005987. [DOI] [PubMed] [Google Scholar]
- 20.Bossie Cynthia A, Alphs Larry D, Correll Christoph U. Long-acting injectable versus daily oral antipsychotic treatment trials in schizophrenia: pragmatic versus explanatory study designs. International clinical psychopharmacology. 2015;30:272. doi: 10.1097/YIC.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicole L, Abdel-Baki A, Lesage A, Granger B, Stip E, Lalonde P. Study of the follow-up of early psychosis at the Université de Montréal L’Étude de Suivi des Psychoses Émergentes de l’Université de Montréal (ESPEUM): context, objectives and methodology. Sante mentale au Quebec. 2006;32:317–331. doi: 10.7202/016523ar. [DOI] [PubMed] [Google Scholar]
- 22.Early Psychosis Guidelines Writing Group, National Support Program EPPIC. Australian clinical guidelines for early psychosis. Melbourne: Orygen, The National Centre of Excellence in Youth Mental Health2nd ed; 2016. [Google Scholar]
- 23.Kay Stanley R, Flszbein Abraham, Opfer Lewis A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13:261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 24.Addington Donald, Addington Jean, Schissel Bernard. A depression rating scale for schizophrenics. Schizophrenia research. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- 25.Heinrichs Douglas W, Hanlon Thomas E, Carpenter William T. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia bulletin. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 26.Drake RE, Mueser KT, McHugo GJ. Clinician rating scales: alcohol use scale (AUS), drug use scale (DUS), and substance abuse treatment scale (SATS) Outcomes assessment in clinical practice. 1996:113–116. [Google Scholar]
- 27.Bernstein David P, Fink Laura. Childhood trauma questionnaire: A retrospective self-report: Manual. Harcourt Brace & Company; 1998. [Google Scholar]
- 28.American Psychiatric Association, others. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) Washington, DC: 2000. Author. [Google Scholar]
- 29.Roy M-A, Lanctôt G, Macute C et al. Clinical and methodological factors related to reliability of the best-estimate diagnostic procedure. American Journal of Psychiatry. 1997 doi: 10.1176/ajp.154.12.1726. [DOI] [PubMed] [Google Scholar]
- 30.Goldman Howard H, Skodol Andrew E, Lave Tamara R. Revising axis V for DSM-IV: a review of measures of social functioning. American Journal of Psychiatry. 1992;149:1148–1156. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- 31.Hall Richard CW. Global assessment of functioning: a modified scale. Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- 32.Padhi Ashwini, Fineberg Naomi. Clinical Global Impression Scales in Encyclopedia of Psychopharmacology. 2010 Springer;:303–303. [Google Scholar]
- 33.Haddad Peter M, Brain Cecilia, Scott Jan. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Related Outcome Measures. 2014;5:43–62. doi: 10.2147/PROM.S42735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.IBM Corporation. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA: IBM Corporation. 2011 [Google Scholar]
- 35.Abdel-Baki Amal, Lévesque Isabelle-Sarah, Ouellet-Plamondon Clairelaine, Nicole Luc. Should we care about homelessness in first episode psychosis?: impact on outcome. Early Intervention in Psychiatry. 2014;8:85. [Google Scholar]
- 36.Shi Lizheng, Ascher-Svanum Haya, Zhu Baojin et al. Characteristics and use patterns of patients taking first-generation depot antipsychotics or oral antipsychotics for schizophrenia. Psychiatric Services. 2007 doi: 10.1176/ps.2007.58.4.482. [DOI] [PubMed] [Google Scholar]
- 37.Rabinowitz Jonathan, De Smedt Goedele, Harvey Philip D, Davidson Michael. Relationship between premorbid functioning and symptom severity as assessed at first episode of psychosis. American Journal of Psychiatry. 2002;159:2021–2026. doi: 10.1176/appi.ajp.159.12.2021. [DOI] [PubMed] [Google Scholar]
- 38.Rabinowitz Jonathan, Harvey Philip D, Eerdekens Marielle, Davidson Michael. Premorbid functioning and treatment response in recent-onset schizophrenia. The British Journal of Psychiatry. 2006;189:31–35. doi: 10.1192/bjp.bp.105.013276. [DOI] [PubMed] [Google Scholar]
- 39.Strous Rael D, Alvir Jose Ma J, Robinson Delbert et al. Premorbid functioning in schizophrenia: relation to baseline symptoms, treatment response, and medication side effects. Schizophrenia Bulletin. 2004;30:265. doi: 10.1093/oxfordjournals.schbul.a007077. [DOI] [PubMed] [Google Scholar]
- 40.Emsley Robin, Oosthuizen Petrus, Koen Liezl, Niehaus Dana JH, Medori Rossella, Rabinowitz Jonathan. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clinical therapeutics. 2008;30:2378–2386. doi: 10.1016/j.clinthera.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 41.Bhanji Nadeem H, Chouinard Guy, Margolese Howard C. A review of compliance, depot intramuscular antipsychotics and the new long-acting injectable atypical antipsychotic risperidone in schizophrenia. European Neuropsychopharmacology. 2004;14:87–92. doi: 10.1016/S0924-977X(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 42.Fenton Wayne S, Blyler Crystal R, Heinssen Robert K. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophrenia bulletin. 1997;23:637–651. doi: 10.1093/schbul/23.4.637. [DOI] [PubMed] [Google Scholar]
- 43.Rossi Giuseppe, Frediani Sonia, Rossi Roberta, Rossi Andrea. Long-acting antipsychotic drugs for the treatment of schizophrenia: use in daily practice from naturalistic observations BMC. psychiatry. 2012;12:122. doi: 10.1186/1471-244X-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassidy Clifford M, Rabinovitch Mark, Schmitz Norbert, Joober Ridha, Malla Ashok. A comparison study of multiple measures of adherence to antipsychotic medication in firstepisode psychosis. Journal of clinical psychopharmacology. 2010;30:64–67. doi: 10.1097/JCP.0b013e3181ca03df. [DOI] [PubMed] [Google Scholar]
- 45.Hargarter L, Bergmans P, Cherubin P, Schreiner A. Early schizophrenia patients treated with once-monthly paliperidone palmitate over a 12-month period-a retrospective observational study. European Psychiatry. 2016;33:S251. [Google Scholar]
- 46.Bret P, Heil M, Queuille E, Bret M-C, Pic Réseau. Enquête des prescriptions de rispéridone injectable à libération prolongée dans neuf centres hospitaliers du réseau PIC. L’Encéphale. 2011;37:S58–S65. doi: 10.1016/j.encep.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Gándara Jesús, San Molina Luis, Rubio Gabriel, Rodriguez-Morales Alexander, Hidalgo Borrajo Rebeca, Burón José Antonio. Experience with injectable long-acting risperidone in longterm therapy after an acute episode of schizophrenia: the SPHERE Study. Expert review of neurotherapeutics. 2009;9:1463–1474. doi: 10.1586/ern.09.96. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler Amanda, Vanderpyl J, Carswell C, Stojkovic M, Robinson E. Explicit review of risperidone long-acting injection prescribing practice. Journal of clinical pharmacy and therapeutics. 2011;36:651–663. doi: 10.1111/j.1365-2710.2010.01219.x. [DOI] [PubMed] [Google Scholar]
- 49.Brissos Sofia, Veguilla Miguel Ruiz, Taylor David, Balanzá-Martinez Vicent. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Therapeutic advances in psychopharmacology. 2014;4:198–219. doi: 10.1177/2045125314540297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Borah, Lee Sang-Hyuk, Yang Yen Kuang, Park Jong-Il, Chung Young-Chul. Longacting injectable antipsychotics for first-episode schizophrenia: the pros and cons. Schizophrenia research and treatment. 2012:2012. doi: 10.1155/2012/560836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peuskens Joseph, Olivares JM, Pecenak J et al. Treatment retention with risperidone longacting injection: 24-month results from the Electronic Schizophrenia Treatment Adherence Registry (e-STAR) in six countries. Current medical research and opinion. 2010;26:501–509. doi: 10.1185/03007990903488670. [DOI] [PubMed] [Google Scholar]
- 52.Verdoux H, Lengronne J, Liraud F et al. Medication adherence in psychosis: predictors and impact on outcome. A 2-year follow-up of first-admitted subjects. Acta Psychiatrica Scandinavica. 2000;102:203–210. doi: 10.1034/j.1600-0447.2000.102003203.x. [DOI] [PubMed] [Google Scholar]
- 53.Santé Canada. Renseignement sur le produit Risperdal Consta de Santé Canada. [Wednesday, March 29, 2017]; https://produits-sante.canada.ca/dpd-bdpp/info.do?code=74091&lang=en2017. [Google Scholar]
- 54.Santé Canada. Renseignement sur le produit Invega Sustenna de Santé Canada. [Wednesday, March 29, 2017]; https://produits-sante.canada.ca/dpd-bdpp/info.do?code=83941&lang=fr2017. [Google Scholar]
- 55.Santé Canada. Renseignement sur le produit Abilify Maintena de Santé Canada. [Wednesday, March 29, 2017]; https://produits-sante.canada.ca/dpd-bdpp/info.do?code=90545&lang=fr2017. [Google Scholar]
- 56.Emsley Robin, Oosthuizen Petrus, Koen Liezl, Niehaus Dana JH, Medori Rossella, Rabinowitz Jonathan. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. International clinical psychopharmacology. 2008;23:325–331. doi: 10.1097/YIC.0b013e32830c2042. [DOI] [PubMed] [Google Scholar]
- 57.Hargarter L, Bergmans P, Cherubin P, Schreiner A. Clinical and functional response to paliperidone palmitate in early schizophrenia-A retrospective observational study in newly diagnosed patients treated over a 12-month period. European Psychiatry. 2016;33:S250–S251. [Google Scholar]
- 58.Malla Ashok, Chue Pierre, Jordan Gerald et al. An Exploratory, Open-Label, Randomized Trial Comparing Risperidone Long-Acting Injectable with Oral Antipsychotic Medication in the Treatment of Early Psychosis. Clinical schizophrenia & related psychoses. 2016;9:198–208. doi: 10.3371/CSRP.MACH.061213. [DOI] [PubMed] [Google Scholar]
- 59.Schreiner Andreas, Aadamsoo Kaire, Altamura A Carlo et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophrenia research. 2015;169:393–399. doi: 10.1016/j.schres.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Carpenter JR, William T, Strauss John S. The Prediction of Outcome in Schizophrenia IV: Eleven-Year Follow-Up of the Washington IPSS Cohort. The Journal of nervous and mental disease. 1991;179:517–525. doi: 10.1097/00005053-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Amminger G Paul, Henry Lisa P, Harrigan Susy M et al. Outcome in early-onset schizophrenia revisited: findings from the Early Psychosis Prevention and Intervention Centre long-term follow-up study. Schizophrenia research. 2011;131:112–119. doi: 10.1016/j.schres.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 62.McGorry Patrick D, Edwards Jane, Mihalopoulos Cathrine, Harrigan Susan M, Jackson Henry J. EPPIC: an evolving system of early detection and optimal management. Schizophrenia bulletin. 1996;22:305. doi: 10.1093/schbul/22.2.305. [DOI] [PubMed] [Google Scholar]
- 63.Carswell Christopher, Wheeler Amanda, Vanderpyl Jane, Robinson Elizabeth. Comparative Effectiveness of Long-Acting Risperidone in New Zealand. Clinical drug investigation. 2010;30:777–787. doi: 10.2165/11537680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Lachaine Jean, Lapierre Marie-Eve, Abdalla Nadine, Rouleau Alice, Stip Emmanuel. Impact of switching to long-acting injectable antipsychotics on health services use in the treatment of schizophrenia. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 2015;60:S40. [PMC free article] [PubMed] [Google Scholar]
- 65.Correll Christoph U, Citrome Leslie, Haddad Peter M et al. The Use of Long-Acting Injectable Antipsychotics in Schizophrenia: Evaluating the Evidence. The Journal of clinical psychiatry. 2016;77:1. doi: 10.4088/JCP.15032su1. [DOI] [PubMed] [Google Scholar]
- 66.Galletly Cherrie, Castle David, Dark Frances et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Australian & New Zealand Journal of Psychiatry. 2016;50:410–472. doi: 10.1177/0004867416641195. [DOI] [PubMed] [Google Scholar]
- 67.Samalin L, Abbar M, Courtet P, Guillaume S, Lancrenon S, Llorca P-M. Recommandations Formalisées d’Experts de l’AFPBN: prescription des neuroleptiques et antipsychotiques d’action prolongée. L’Encéphale. 2013;39:189–203. doi: 10.1016/S0013-7006(13)70121-0. [DOI] [PubMed] [Google Scholar]
- 68.National Institute for Clinical Excellence (NICE) Psychosis and schizophrenia in adults: prevention and management; National Clinical Practice Guidelines Number CG178. [November 15, 2016]; https://www.nice.org.uk/guidance/cg178/chapter/1-Recommendations#first-episode-psychosis-2 2014. [Google Scholar]
- 69.Azorin J-M. Guidelines sur les antipsychotiques atypiques d’action prolongée (APAPs) dans les premiers épisodes psychotiques. L’Encéphale. 2013;39:S121–S123. doi: 10.1016/S0013-7006(13)70107-6. [DOI] [PubMed] [Google Scholar]
- 70.Gardner Kristen N, Nasrallah Henry A. Rationale and Evidence for Nonstandard First-Line Treatments for Schizophrenia: Consider Long-Acting Injectable Antipsychotics; Use Clozapine as Second-or Third-Line Therapy. Only Current Psychiatry. 2015;14:33. [Google Scholar]
- 71.Stip Emmanuel, Abdel-Baki Amal, Bloom David, Grignon Sylvain, Roy Marc-André. Les antipsychotiques injectables à action prolongée: avis d’experts de l’Association des médecins psychiatres du Québec. The Canadian Journal of Psychiatry. 2011;56:367–376. doi: 10.1177/070674371105600608. [DOI] [PubMed] [Google Scholar]


