Abstract
As the final output of the somatic nervous system, the neuromuscular junction (NMJ) is essential for all voluntary movements. The NMJ is also necessary for connected cells to function and survive. Because of this central role, much effort has been devoted to understanding the effects of aging, diseases, and injuries on the NMJ. These efforts have revealed a close relationship between aberrant changes at NMJs and its three cellular components - the presynaptic site on motor axons, the postsynaptic region on muscle fibers and perisynaptic Schwann cells. Here, we review the morphological and molecular changes associated with aging NMJs in rodents and humans. We also provide an overview of factors with potential roles in maintaining and repairing adult and aged NMJs.
Keywords: NMJ, aging, z-agrin, LRP4, FGFBP1, injury, regeneration, synaptic cleft, cholinergic transmission, sarcopenia, exercise, caloric restriction
1. Introduction
The NMJ is the interface between the nerve ending of α-motor neurons (the presynapse), a specialized region on extrafusal muscle fibers (the postsynapse) and perisynaptic Schwann cells (PSCs). These cellular components come together early in development, with muscle fibers first forming rudimentary postsynaptic sites that are then approached and innervated by growing motor axons. As development proceeds, PSCs join in to modulate NMJ function and stabilize the presynaptic and postsynaptic sites. In adulthood, these cellular components remain essential for the NMJ to function and remain viable [1]. Thus, it is not surprising that the loss or dysfunction of motor neurons, muscle fibers and PSCs invariably causes deleterious changes at NMJs. For example, the NMJ degenerates in amyotrophic lateral sclerosis (ALS), as well as in the spectrum of myasthenia gravis and muscular dystrophies [2,3]. These are diseases that invariably compromise the function and health of motor neurons, muscle fibers and PSCs. The NMJ also falls apart in conditions that affect tissue homeostasis, such as diabetes, vascular exclusion and nerve injuries [4–7]. Additionally, the morphological integrity and function of the NMJ is intimately linked to sympathetic nerves that innervate skeletal muscles, which are also affected by a variety of diseases and injuries [8]. This review, however, will detail the morphological and molecular changes associated with aging NMJs in rodents and humans. It will also summarize published findings implicating exercise, caloric restriction, and synapse-associated molecules in the maintenance and repair of adult NMJs.
2. Overview of aging NMJs
The NMJ has been examined in aging rodents and humans since the 1970s. These studies have deployed a variety of tools to assess the integrity of NMJs in an array of skeletal muscles. Several general conclusions can be drawn from these studies. 1) NMJs acquire deleterious morphological, functional, and molecular features with advancing age, and ultimately degenerate [9–12]. 2) While NMJs acquire age-related features, the underlying cause of such changes remains debated. In particular, it remains unknown whether NMJs are a focal site of pathology or degenerate due to deleterious changes elsewhere in skeletal muscles, motor neurons or other cells [13–16]. 3) The rate of aging among NMJs located in different muscles, within the same muscle, and even in the same motor unit varies considerably [17].
2.1. Rodent NMJs and aging
The NMJ has now been well examined in aging rodents, and particularly in mice. These studies have revealed that aging alters the morphology of each cellular component of the NMJ: postsynaptic muscle fibers, presynaptic axon terminals, and PSCs (Figure 1). As NMJs age, the postsynaptic site fragments into smaller, non-contiguous regions, [18] and junctional folds disappear [19]. The presynapse also exhibits a number of age-related morphological features. It is often found with sprouts that go beyond the postsynapse, with massive blebs at or adjacent to the postsynapse, and vacating and missing from the postsynaptic site (Figure 2). Additionally, the presynapse loses its privilege as the only site where synaptic vesicles concentrate, since synaptic vesicles are found aggregated along non-synaptic regions of aged motor axons [20]. Furthermore, aging also reduces the density of active zones [21], altering the ability of motor neurons to release acetylcholine appropriately. In parallel with these changes, PSCs are also affected in old age (Figure 3). These cells fail to completely wrap around the presynaptic and postsynaptic regions [22], and instead vacate the NMJ or protrude branches into the synaptic cleft [23].
Figure 1.
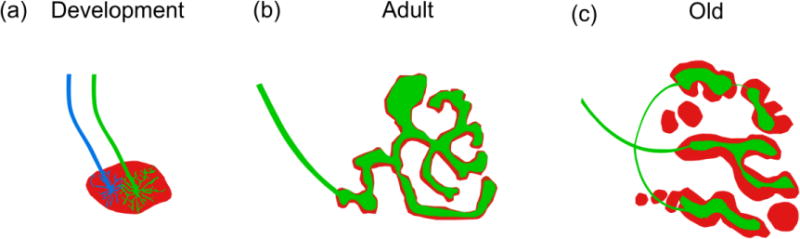
NMJ morphology in development and aging. (a) The developing NMJ is characterized by a plaque of nAChRs (red) on the postsynapse that is often innervated by multiple motor axons (blue and green) that compete for establishment at the postsynaptic site in an activity-dependent manner. (b) As the NMJ matures into adulthood, the postsynapse forms complex junctional folds giving the NMJ its pretzel-like appearance. The postsynapse is innervated by a single motor axon which forms branches along postsynaptic folds to create a uniform apposition to the postsynapse. (c) With increasing age, the continuous junctional folds of the postsynapse become fragmented into a series of islands. The area over which the motor axon terminal covers the postsynapse is reduced resulting in a loss of uniform apposition between pre-and postsynapse.
Figure 2.
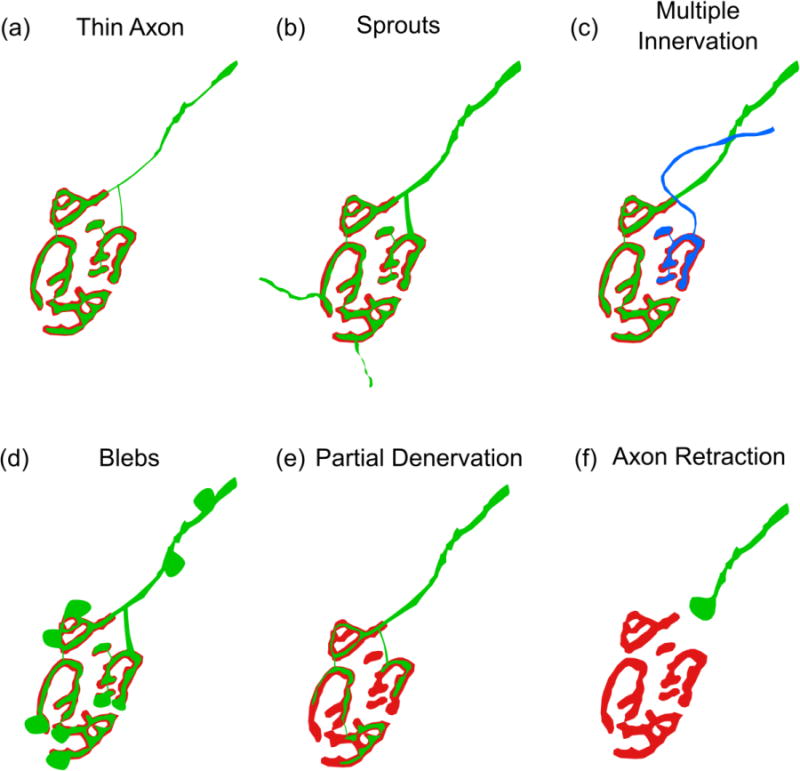
Pre-synaptic morphological alterations of the aged NMJ. (a) The diameter of the axon is reduced as it approaches the NMJ. (b) The motor axon terminal forms extensions that do not correspond with the postsynapse. (c) A single postsynaptic site is innervated by two or more motor axons. (d) Blebs form along the motor axon near the NMJ as well as on the motor axon terminal. (e) The area of coverage by the synaptic terminal is reduced, resulting in incomplete coverage of the postsynapse. (f) The connection between the presynaptic terminal and the postsynapse is lost as the motor axon pulls away from the muscle fiber forming a retraction bulb.
Figure 3.
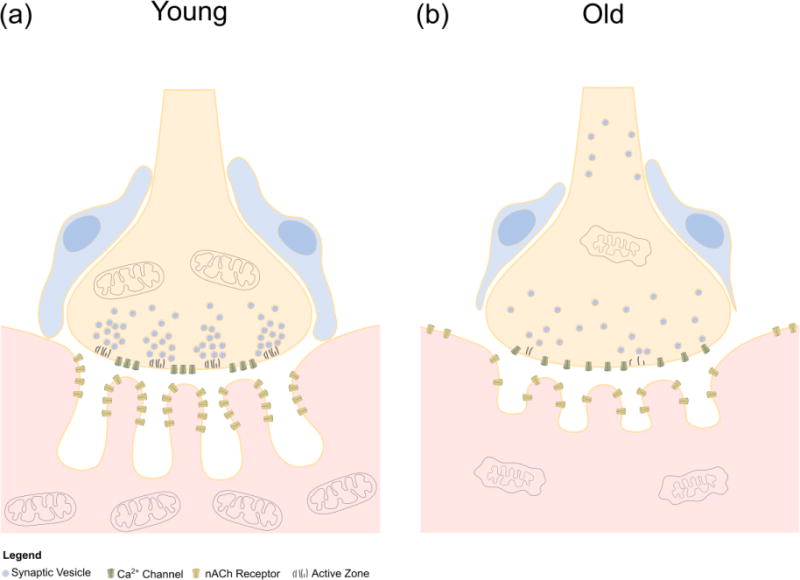
Cellular and molecular changes in the aged NMJ. (a) The young NMJ is characterized by deep junctional folds along the postsynapse containing high concentrations of nAChRs at the peaks. Active zones are organized on the motor axon terminal to lie in direct apposition to the junctional folds of the postsynapse. Vesicles containing acetylcholine aggregate at active zones where they are available to release acetylcholine in close proximity to nAChRs. Mitochondria are abundant throughout the pre- and postsynapse to support the high energy demands of cholinergic transmission. (b) With aging, the junctional folds of the postsynapse become shallow, while nAChRs are less concentrated along the peaks of the folds. In addition to postsynapses, nAChRs are found in extra-synaptic areas of the muscle fiber membrane. Mitochondria are damaged and fewer in the postsynaptic region of the muscle fiber. Active zones are lost in the motor axon terminal. Aggregation of vesicles containing acetylcholine near the terminal membrane is lost and vesicles are present away from the terminal along the axon. Dysfunctional megamitochondria are present in the motor axon terminal. Ensheathment of the NMJ by perisynaptic Schwann cells is lost as they migrate away from the motor axon terminal.
Aging also alters the expression and distribution of several molecules that are integral to the stability and function of the NMJ. This includes loss of nicotinic acetylcholine receptors (nAChRs) from the postsynaptic site, and accumulation of nAChRs in non-synaptic regions of muscle fibers [24]. Additionally, the gamma subunit of the nAChR pentamer increases in old age [25] even though the epsilon subunit is already highly abundant. Thus, it is possible that mixed nAChRs pentamers composed of the gamma or the epsilon subunit accumulate at NMJs with advancing age. Interestingly, the gamma and epsilon nAChR subunits are co-expressed during the first few postnatal days, which is a time when the NMJ also undergoes dramatic structural and functional changes [1]. An increase in expression of the gamma nAChR subunit is also associated with other pathological conditions in which NMJs are damaged, and has been proposed as a mechanism of muscle regeneration and NMJ repair. Several other NMJ-associated genes are also increased in skeletal muscles of aged mice, including the muscle-specific kinase (MuSK) and low-density lipoprotein receptor-related protein 4 (Lrp4) [13]. Interestingly, these NMJ-associated genes invariably increase in denervated skeletal muscles of young adult mice [7], further suggesting that aging causes denervation of NMJs. Aging also affects the expression of genes known to have important functions at the NMJ, but are not critical for its formation or function. For example, levels of laminin-α4 [26] and of the fibroblast growth factor binding protein 1 (FGFBP1) [27] decrease in old skeletal muscles. The dysregulated expression of these synaptic molecules, in addition to altered cholinergic transmission, has been shown to precipitate age- and disease-related morphological and functional changes at NMJs [28,29].
2.2. Human NMJs and aging
In contrast to rodents and other species, there is much less known about human NMJs at any stage of life. The limited studies carried out to date have revealed that, compared to rodents, human NMJs are significantly smaller and exhibit a simpler topology [23,30,31]. Although the differences in NMJ morphology are established, the effect of aging on human NMJs is less clear. In two studies [23,30], light and electron microscopy revealed age-related features on NMJs in postmortem intercostal muscles. Similar to those found in rodents, postsynapses were fragmented and had fewer junctional folds. Indicative of degenerating NMJs and muscle fibers, nAChRs were also found in non-synaptic in addition to synaptic regions in old human muscle fibers. These studies also revealed that aging affects the presynapse and causes PSCs to extend branches into the synaptic cleft. However, a recent study called these findings into question. Jones RA and colleagues examined the NMJs of individuals aged 34–92 years-old. Rather than using postmortem tissue, this study obtained four different muscles from each patient immediately after amputating the lower limb, most due to peripheral vascular disease and diabetes mellitus [31]. Using confocal and super-resolution microscopy, the morphology of NMJs was found unchanged at all ages examined, including in 92 year-old individuals. The size of the NMJ, the degree of fragmentation, and the overlap between the presynaptic and postsynaptic regions were all preserved even in very old individuals. Moreover, the distribution of synaptic vesicles and the number of active zones were unaffected in old age. Furthermore, it was found that aging does not alter the diameter of muscle fibers and of motor axons. These findings are surprising for a couple of reasons. Firstly, this study examined skeletal muscles following amputation below and above the knee due to complications caused by peripheral vascular disease and diabetes mellitus, conditions that cause widespread muscle necrosis and nerve damage. The specific muscles analyzed were the soleus, extensor digitorum longus, peroneus longus and peroneus brevis, all of which extend from just below the knee to the heel or front of the ankle. These muscles are therefore expected to be affected by both diseases, whether directly or indirectly due to ongoing swelling and necrosis throughout the lower limb. Secondly, there is extensive literature, from studies using a variety of approaches including muscle biopsy and MRI, demonstrating that muscle fibers and motor axons degenerate as humans advance into old age [32–37]. Regardless, it is clear that additional studies of aged human NMJs are needed to resolve these discrepancies.
3. Lifestyle and molecular factors that preserve the NMJ into old age
There is sufficient published data indicating that the structural and functional integrity of NMJs can be preserved into old age. The two lifestyles best known to protect the motor system, a calorically restricted diet and exercise [18], have been shown to attenuate and even reverse age-related changes at NMJs. NMJs also maintain their youthful architecture in mice fed resveratrol and metformin [38], two small pharmacological agents that increase metabolic activity. These and other discoveries, together with the central role of the NMJ in the somatic motor system have ushered, investigations aimed at uncovering molecular mechanisms that maintain and preserve the NMJ into old age.
3.1. Lifestyle factors and downstream mechanisms that attenuate NMJ aging
A calorically restricted diet and exercise are regarded as two lifestyle factors that are effective in combating aging in general, including attenuating and reversing age-related changes at NMJs [18,38–42]. Mice placed under a calorically restricted diet beginning at 4 months of age showed significant attenuation of NMJ degradation at 24 months of age. Structural defects of NMJs including postsynaptic receptor fragmentation, denervation and axonal sprouting were reduced by more than 50% in the tibialis anterior, gastrocnemius and gracilis muscles of aged mice placed under lifelong calorie restriction [18]. These findings were corroborated in a follow up study that demonstrated that resveratrol, a caloric restriction memetic, similarly attenuates aging of NMJs. In this study, mice were fed a diet supplemented with resveratrol beginning at 12 months of age and examined at 24 months of age. The addition of resveratrol significantly reduced the number denervated and fragmented NMJs in 24 month-old mice [38]. Providing further evidence that resveratrol directly influences the NMJ, C2C12 myotubes treated with resveratrol contained more postsynaptic sites marked by nAChR clusters. Studies over the years have suggested that the anti-aging effects of both caloric restriction and resveratrol occur partly through the sirtuin1 (SIRT1), peroxisome proliferator-activated receptor-gamma coactivator alpha (PGC1α) and mechanistic target of rapamycin (mTor) signaling pathways [43–46], among other mechanisms [47]. SIRT1 activation particularly influences a number of molecular and cellular functions with important implications for NMJ health in aged muscle. It enhances mitochondrial function [48], increases the number of myonuclei in muscle fibers, augments the proliferation of satellite cells [49], fine-tunes autophagy [50], and reduces oxidative stress [48].
Exercise, even late in life, also has beneficial effects on NMJs (Figure 4). Exercise has been shown to significantly reduce the number of NMJs with age-related structural features in several hind limb muscles in 23-month-old mice. In this study, mice were given access to running wheels starting at 22 months of age and examined 1 month later. This short exercise regimen was sufficient to decrease the incidence of fragmented and denervated NMJs. Because the exercise regimen began late in life, the findings suggested that exercise not only slowed but reversed the deleterious effects of aging on NMJs. Live imaging of the same NMJ, between 22 and 23 months, confirmed that exercise partially reverses damages at NMJs caused by aging [18]. A separate study also found that exercise benefits aged NMJs. The morphology as well as the function of NMJs were improved in 25-month-old mice following 12 weeks of endurance exercise [51].
Figure 4.
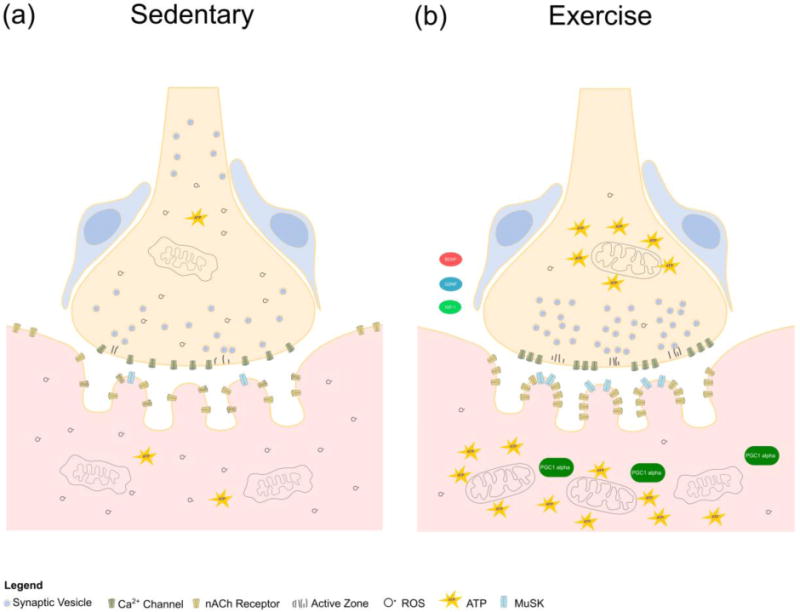
Cellular and molecular changes associated with exercise in the aged NMJ. Exercise improves the health of the aging NMJ by boosting mitochondrial function, increasing ATP levels and decreasing ROS. Postsynaptically, exercise elevates MuSK and increases PGC1α signaling which is associated with improved autophagy and more efficient recycling of nAChRs on the postsynaptic membrane. Exercise elevates the number of active zones on the presynapse and increases levels of beneficial growth factors including BDNF, GDNF and IGF-1.
While exercise affects a wide variety of molecular pathways, PGC1α is one of the most promising molecules for relaying the beneficial effects of exercise to skeletal muscles and NMJs. PGC1α promotes mitochondrial function, which becomes severely compromised with increasing age. Through its interaction with transcription factors such as NRF-1 and 2, and mitochondrial transcription factor A (TFAM) and B2 (TFB2M) [52], PGC1α mitigates the deleterious effects of dysfunctional mitochondria. It reduces the release of apoptotic mediators, intracellular reactive oxygen species (ROS), and promotes ATP generation and calcium buffering [52,53]. Recently, Garcia S. and colleagues [54] showed that these positive actions of PGC1α can be extended to old muscle and their NMJs. In 22-month-old transgenic mice overexpressing PGC1α, skeletal muscles have more mitochondria with fewer DNA deletions compared to age-matched control mice. Overexpression of PGC1α also increased expression of genes that are important for NMJ function, including those involved in metabolic processes, autophagy and satellite cell proliferation. These findings thus extend on previous studies showing that PGC1α remodels the NMJ [55,56], and also converts muscle fibers from a fast to slow subtype [55]. Additionally, exercise boosts production of a number of local and systemic growth factors that have positive benefits on aging muscles and NMJs, including insulin like growth factor-1 (IGF1), brain derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) [57,58].
3.2. Role of synaptic molecules at aging NMJs
The viability of the NMJ requires active communication through molecular mechanisms between all three cellular components: the presynapse, the postsynapse and PSCs. Exemplifying this point, the loss of the presynaptic or postsynaptic site in adulthood resulting from diseases and injuries causes the NMJ to degenerate [59]. The NMJ fragments and malfunctions within a few days after Schwann cells, including PSCs, are ablated [60]. Conversely, these three cellular components collaborate through synapse-associated molecules to regenerate adult NMJs following injuries that cause severing of motor axons, loss of PSCs and atrophy of muscle fibers [27]. These examples highlight the interdependence between all three cellular components of the NMJ, and roles of synapse-associated molecules in the maintenance and repair of adult NMJs. In this regard, studies over the last few years have shown that synaptic molecules agrin, Lrp4, the muscle-specific kinase (MuSK) receptor, and Dok7 remain essential at adult NMJs [61–64]. For example, deletion of agrin in a subset of adult motor neurons causes the disintegration of the postsynapse and degeneration of motor axons [63]. Likewise, adult NMJs degenerate in the absence of LRP4, MuSK, or Dok7 [61,62,64]. Thus, molecules required for the formation of the NMJ continue to play integral functions in adulthood. There is also evidence suggesting that targeting these molecules could mitigate age-related changes at NMJs. In old age, the C-terminal agrin fragment is elevated in the serum of individuals with sarcopenia [65,66], suggesting the possibility that degradation of z-agrin during aging contributes to NMJ pathology. Supporting this possibility, the Sonderegger group has shown that agrin is cleaved by neurotrypsin, an enzyme that motor neurons secrete at the NMJ, and overexpressing neurotrypsin causes severe fragmentation of the NMJ and induces sarcopenia in young rodents [57]. Evidence indicating that agrin and other integral components of the NMJ act to repair age-related damages comes from studies assessing their therapeutic potential following injury and in disease. Following sciatic nerve crush surgery in young animals, introduction of biologically active agrin fragments into skeletal muscles accelerates the rate of NMJ reformation [68]. Dok-7 has also been shown to repair damaged NMJs. In congenital myasthenic syndromes and NMJ disease, Dok-7 overexpression preserves the structural and functional integrity of the NMJ. Similarly, increasing Dok-7 levels slows degeneration of NMJs and extends lifespan in the SOD1G93A mouse model for ALS [69], an age-associated neurodegenerative disease.
There is increasing evidence that other NMJ-associated molecules, not required for its formation, also have roles in maintaining and repairing adult NMJs. In the absence of α-syntrophin and α-dystrobrevin, two members of the dystrophin complex, or collagen XIII, NMJs are severely fragmented [70–72]. Interestingly, Zainul Z and colleagues recently showed that the transmembrane domain of collagen XIII promotes regeneration of NMJs following injury to motor axons [71]. NMJs have also been found to progressively degenerate, characterized by increased fragmentation and denervation, in mice lacking laminin-α4 [26]. These findings suggest that augmenting levels and activity of collagen XIII and laminin-α4 may slow and reverse damages at NMJs that occur with advancing age. Our group recently discovered that the fibroblast growth factor binding protein 1 (FGFBP1), a molecule that enhances the function of FGFs [73], may help protect the NMJ from age-related degeneration. FGFBP1 is enriched in the synaptic region of young adult skeletal muscles but significantly decreases in aged skeletal muscles. Indicating important functions at adult NMJs, deletion of FGFBP1 results in the premature appearance of age-related morphological features at NMJs. Mice lacking FGFBP1 also exhibit motor deficits earlier in life. Further indicating important functions at NMJs, deletion of FGFBP1 in a mouse model for ALS accelerates degeneration of NMJs [27]. These published findings demonstrate that genes with important roles at NMJs may also hold therapeutic potential for slowing, preventing and reversing age-related changes at NMJs.
4. Concluding Remarks
Research over the last few decades has provided significant insights regarding the impact of aging on NMJs in rodents and humans. They have shown that NMJs undergo a myriad of deleterious morphological alterations in old age, similar to those caused by diseases that affect motor neurons and skeletal muscles. There is also increasing evidence that aging alters levels and function of NMJ-associated molecules. While it remains unknown whether alterations at NMJs cause or are a consequence of sarcopenia, the function and health of skeletal muscles is intimately linked to the NMJ. This relationship highlights the importance of identifying molecular mechanisms that function to prevent and repair age-related damages at NMJs. This information could lead to treatments for sarcopenia and other conditions that impair motor function.
Highlights.
Impact of aging on NMJs
Effects of exercise and a caloric restricted diet on aging NMJs
Molecules that maintain and repair NMJs
Abbreviations
- NMJ
neuromuscular junction
- PSC
perisynaptic Schwann cells
- MuSK
muscle-specific kinase
- nAChR
nicotinic acetylcholine receptor
- LRP4
low-density lipoprotein receptor-related protein 4
- ALS
amyotrophic lateral sclerosis
- SOD1
superoxide dismutase 1
- FGFBP1
fibroblast growth factor binding protein 1
- BDNF
brain derived neurotrophic factor
- GDNF
glial derived growth factor
- IGF-1
insulin like growth factor 1
- SIRT1
sirtuin 1
- mTor
mechanistic target of rapamycin
- PGC1-α
peroxisome proliferator-activated receptor-gamma coactivator
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- 1.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Xiong W-C, Mei L. Neuromuscular Junction Formation, Aging, and Disorders. Annu Rev Physiol. 2018;80:159–188. doi: 10.1146/annurev-physiol-022516-034255. [DOI] [PubMed] [Google Scholar]
- 3.Park KHJ. Mechanisms of Muscle Denervation in Aging: Insights from a Mouse Model of Amyotrophic Lateral Sclerosis. Aging Dis. 2015;6:380–9. doi: 10.14336/AD.2015.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu H, Zhang D, Corrick RM, Muelleman RL, Wadman MC, Li Y-L. Morphological Regeneration and Functional Recovery of Neuromuscular Junctions after Tourniquet-Induced Injuries in Mouse Hindlimb. Front Physiol. 2017;8:207. doi: 10.3389/fphys.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin KL, Mao XO, Greenberg DA, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahim MA, Hasan MY, Alshuaib WB. Early morphological remodeling of neuromuscular junction in a murine model of diabetes. J Appl Physiol. 2000;89:2235–40. doi: 10.1152/jappl.2000.89.6.2235. [DOI] [PubMed] [Google Scholar]
- 7.Dalkin W, Taetzsch T, Valdez G. The Fibular Nerve Injury Method: A Reliable Assay to Identify and Test Factors That Repair Neuromuscular Junctions. J Vis Exp. 2016 doi: 10.3791/54186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan MM, Lustrino D, Silveira WA, Wild F, Straka T, Issop Y, O’Connor E, Cox D, Reischl M, Marquardt T, et al. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc Natl Acad Sci. 2016;113:746–750. doi: 10.1073/pnas.1524272113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front Aging Neurosci. 2014;6:208. doi: 10.3389/fnagi.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of Neuromuscular Junction in Age and Dystrophy. Front Aging Neurosci. 2014;6:99. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Power GA, Dalton BH, Rice CL. Human neuromuscular structure and function in old age: A brief review. J Sport Heal Sci. 2013;2:215–226. doi: 10.1016/j.jshs.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willadt S, Nash M, Slater C. Age-related changes in the structure and function of mammalian neuromuscular junctions. Ann N Y Acad Sci. 2018;1412:41–53. doi: 10.1111/nyas.13521. [DOI] [PubMed] [Google Scholar]
- 13.Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, Vickers C, Wu Z, Clarke BA, Shi J, et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol. 2013;33:194–212. doi: 10.1128/MCB.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Lee Yi, Thompson WJ. Changes in Aging Mouse Neuromuscular Junctions Are Explained by Degeneration and Regeneration of Muscle Fiber Segments at the Synapse. J Neurosci. 2011;31:14910–14919. doi: 10.1523/JNEUROSCI.3590-11.2011. By modulating the satellite cell population to levels below or above normal using tamoxifen-induced system, Liu and colleagues demonstrate the importance of a viable satellite cell population for maintaining the health of the NMJ in aged muscles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortés-Lopéz M, Tan A, Flaherty M, Miura P, Dirksen RT, et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife. 2017;6 doi: 10.7554/eLife.26464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared Resistance to Aging and ALS in Neuromuscular Junctions of Specific Muscles. PLoS One. 2012;7:e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdez G, Tapia JC, Kang H, Clemenson GD, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurokawa K, Mimori Y, Tanaka E, Kohriyama T, Nakamura S. Age-related change in peripheral nerve conduction: compound muscle action potential duration and dispersion. Gerontology. 1999;45:168–73. doi: 10.1159/000022081. [DOI] [PubMed] [Google Scholar]
- 20.Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21:887–95. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol. 2012;520:434–52. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y-J, Zhou C-J, Shi Q, Smith N, Li T-F. Aging Delays the Regeneration Process following Sciatic Nerve Injury in Rats. J Neurotrauma. 2007;24:885–894. doi: 10.1089/neu.2006.0156. [DOI] [PubMed] [Google Scholar]
- 23.Wokke JH, Jennekens FG, van den Oord CJ, Veldman H, Smit LM, Leppink GJ. Morphological changes in the human end plate with age. J Neurol Sci. 1990;95:291–310. doi: 10.1016/0022-510x(90)90076-y. [DOI] [PubMed] [Google Scholar]
- 24.Smith DO, Williams KD, Emmerling M. Changes in acetylcholine receptor distribution and binding properties at the neuromuscular junction during aging. Int J Dev Neurosci. 1990;8:629–42. doi: 10.1016/0736-5748(90)90058-a. [DOI] [PubMed] [Google Scholar]
- 25.Xie F, Min S, Liu L, Peng L, Hao X, Zhu X. Advanced age enhances the sepsis-induced up-regulation of the γ- and α7-nicotinic acetylcholine receptors in different parts of the skeletal muscles. Arch Gerontol Geriatr. 2016;65:1–8. doi: 10.1016/j.archger.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Lee KM, Chand KK, Hammond LA, Lavidis NA, Noakes PG. Functional decline at the aging neuromuscular junction is associated with altered laminin-α4 expression. Aging (Albany NY) 2017;9:880–899. doi: 10.18632/aging.101198. FGFBP1, a modulator of FGF signaling, was found concentrated at the NMJs of young mice. With increasing age, FGFBP1 levels were found to decrease at the NMJ, and this loss was associated with the progressive degeneration that occurs at NMJs during aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taetzsch T, Tenga MJ, Valdez G. Muscle Fibers Secrete FGFBP1 to Slow Degeneration of Neuromuscular Synapses during Aging and Progression of ALS. J Neurosci. 2017;37:70–82. doi: 10.1523/JNEUROSCI.2992-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugita S, Fleming LL, Wood C, Vaughan SK, Gomes MPSM, Camargo W, Naves LA, Prado VF, Prado MAM, Guatimosim C, et al. VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions. Skelet Muscle. 2016;6:31. doi: 10.1186/s13395-016-0105-7. This study examined NMJs in four hind limb skeletal muscles from young and old human patients following amputation due to complications caused by peripheral vascular diseases and diabetes mellitus. It showed that aging does not affect NMJs, muscles fibers and their innervating motor axons. This study also found similar molecular profiles between the synaptic and non-synaptic areas of human muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbour D, Tremblay E, Martineau E, Julien J-P, Robitaille R. Early and Persistent Abnormal Decoding by Glial Cells at the Neuromuscular Junction in an ALS Model. J Neurosci. 2015;35:688–706. doi: 10.1523/JNEUROSCI.1379-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oda K. Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J Neurol Sci. 1984;66:327–338. doi: 10.1016/0022-510x(84)90021-2. [DOI] [PubMed] [Google Scholar]
- 31.Jones RA, Harrison C, Eaton SL, Llavero Hurtado M, Graham LC, Alkhammash L, Oladiran OA, Gale A, Lamont DJ, Simpson H, et al. Cellular and Molecular Anatomy of the Human Neuromuscular Junction. Cell Rep. 2017;21:2348–2356. doi: 10.1016/j.celrep.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esposito A, Campana L, Palmisano A, De Cobelli F, Canu T, Santarella F, Colantoni C, Monno A, Vezzoli M, Pezzetti G, et al. Magnetic Resonance Imaging at 7T Reveals Common Events in Age-Related Sarcopenia and in the Homeostatic Response to Muscle Sterile Injury. PLoS One. 2013;8:e59308. doi: 10.1371/journal.pone.0059308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galban CJ, Maderwald S, Stock F, Ladd ME. Age-Related Changes in Skeletal Muscle as Detected by Diffusion Tensor Magnetic Resonance Imaging. Journals Gerontol Ser A Biol Sci Med Sci. 2007;62:453–458. doi: 10.1093/gerona/62.4.453. [DOI] [PubMed] [Google Scholar]
- 34.Azzabou N, Hogrel J-Y, Carlier PG. NMR based biomarkers to study age-related changes in the human quadriceps. Exp Gerontol. 2015;70:54–60. doi: 10.1016/j.exger.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016;23:1034–1047. doi: 10.1016/j.cmet.2016.05.007. This study showed that resveratrol added to regular chow slows aging of NMJs in mice. Resveratrol was also shown to directly affect the formation and topology of postsynaptic sites in culture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piasecki M, Ireland A, Piasecki J, Stashuk DW, Swiecicka A, Rutter MK, Jones DA, McPhee JS. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J Physiol. 2018 doi: 10.1113/JP275520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murgia M, Toniolo L, Nagaraj N, Schiaffino S, Reggiani C, Mann M, Ciciliot S, Vindigni V. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. CellReports. 2017;19:2396–2409. doi: 10.1016/j.celrep.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 38.Stockinger J, Maxwell N, Shapiro D, deCabo R, Valdez G. Caloric Restriction Mimetics Slow Aging of Neuromuscular Synapses and Muscle Fibers. Journals Gerontol Ser A. 2018;73:21–28. doi: 10.1093/gerona/glx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deschenes MR, Roby MA, Glass EK. Aging influences adaptations of the neuromuscular junction to endurance training. Neuroscience. 2011;190:56–66. doi: 10.1016/j.neuroscience.2011.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–90. doi: 10.1016/j.tins.2008.12.007. A link between elevated SIRT1 signaling and increased satellite cell number establishes a possible link between increases in SIRT1/PGC1α activity associated with caloric restriction/exercise and improvements in muscle fiber and NMJ health in aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillette-Guyonnet S, Vellas B. Caloric restriction and brain function. doi: 10.1097/MCO.0b013e328313968f. [date unknown] [DOI] [PubMed] [Google Scholar]
- 42.Messi ML, Li T, Wang Z-M, Marsh AP, Nicklas B, Delbono O. Resistance Training Enhances Skeletal Muscle Innervation Without Modifying the Number of Satellite Cells or their Myofiber Association in Obese Older Adults. Journals Gerontol Ser A Biol Sci Med Sci. 2016;71:1273–1280. doi: 10.1093/gerona/glv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu M, Wilk SA, Wang A, Zhou L, Wang R-H, Ogawa W, Deng C, Dong LQ, Liu F. Resveratrol Inhibits mTOR Signaling by Promoting the Interaction between mTOR and DEPTOR. J Biol Chem. 2010;285:36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen D, Bruno J, Easlon E, Lin S-J, Cheng H-L, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–7. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercken EM, Hu J, Krzysik-Walker S, Wei M, Li Y, McBurney MW, de Cabo R, Longo VD. SIRT1 but not its increased expression is essential for lifespan extension in caloric-restricted mice. Aging Cell. 2014;13:193–196. doi: 10.1111/acel.12151. This study explored how the benefits of PGC1α in aging skeletal muscle are mediated on a molecular level and identified changes in transcriptional patterns associated with longevity in PGC1α-overexpressing muscle using a genome-wide analysis study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim S-H, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boutant M, Kulkarni SS, Joffraud M, Raymond F, Métairon S, Descombes P, Cantó C. SIRT1 Gain of Function Does Not Mimic or Enhance the Adaptations to Intermittent Fasting. Cell Rep. 2016;14:2068–2075. doi: 10.1016/j.celrep.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, et al. Declining NAD+ Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross JA, Levy Y, Svensson K, Philp A, Schenk S, Ochala J. SIRT1 regulates nuclear number and domain size in skeletal muscle fibers. J Cell Physiol. 2018 doi: 10.1002/jcp.26542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228:2262–2270. doi: 10.1002/jcp.24399. [DOI] [PubMed] [Google Scholar]
- 51.Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J Appl Physiol. 1997;83:59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- 52.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–66. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rygiel KA, Picard M, Turnbull DM. The ageing neuromuscular system and sarcopenia: a mitochondrial perspective. J Physiol. 2016;594:4499–4512. doi: 10.1113/JP271212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia S, Nissanka N, Mareco EA, Rossi S, Peralta S, Diaz F, Rotundo RL, Carvalho RF, Moraes CT. Overexpression of PGC-1α in aging muscle enhances a subset of young-like molecular patterns. Aging Cell. 2018;17:e12707. doi: 10.1111/acel.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakkalakal JV, Nishimune H, Ruas JL, Spiegelman BM, Sanes JR. Retrograde influence of muscle fibers on their innervation revealed by a novel marker for slow motoneurons. Development. 2010;137:3489–99. doi: 10.1242/dev.053348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1 regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21:770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishimune H, Stanford JA, Mori Y. ROLE of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve. 2014;49:315–324. doi: 10.1002/mus.24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park J-S, Höke A. Treadmill Exercise Induced Functional Recovery after Peripheral Nerve Repair Is Associated with Increased Levels of Neurotrophic Factors. PLoS One. 2014;9:e90245. doi: 10.1371/journal.pone.0090245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu W, Chakkalakal JV. The Composition, Development, and Regeneration of Neuromuscular Junctions. Current topics in developmental biology. 2018:99–124. doi: 10.1016/bs.ctdb.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Barik A, Li L, Sathyamurthy A, Xiong W-C, Mei L. Schwann Cells in Neuromuscular Junction Formation and Maintenance. J Neurosci. 2016;36:9770–81. doi: 10.1523/JNEUROSCI.0174-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eguchi T, Tezuka T, Miyoshi S, Yamanashi Y. Postnatal knockdown of ok-7 gene expression in mice causes structural defects in neuromuscular synapses and myasthenic pathology. Genes to Cells. 2016;21:670–676. doi: 10.1111/gtc.12370. [DOI] [PubMed] [Google Scholar]
- 62.Kong XC, Barzaghi P, Ruegg MA. Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference. EMBO Rep. 2004;5:183–188. doi: 10.1038/sj.embor.7400065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samuel MA, Valdez G, Tapia JC, Lichtman JW, Sanes JR. Agrin and synaptic laminin are required to maintain adult neuromuscular junctions. PLoS One. 2012;7:e46663. doi: 10.1371/journal.pone.0046663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barik A, Lu Y, Sathyamurthy A, Bowman A, Shen C, Li L, Xiong W-c, Mei L. LRP4 Is Critical for Neuromuscular Junction Maintenance. J Neurosci. 2014;34:13892–13905. doi: 10.1523/JNEUROSCI.1733-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bondoc I, Cochrane SK, Church TS, Dahinden P, Hettwer S, Hsu F-C, Stafford RS, Pahor M, Buford TW, Life Study Investigators Effects of a one-year physical activity program on serum C-terminal Agrin Fragment (CAF) concentrations among mobility-limited older adults. J Nutr Health Aging. 2015;19:922–927. doi: 10.1007/s12603-015-0474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narici M, Conte M, Salvioli S, Franceschi C, Selby A, Dela F, Rieder F, Kösters A, Müller E. Alpine Skiing With total knee ArthroPlasty (ASWAP): impact on molecular and architectural features of musculo-skeletal ageing. Scand J Med Sci Sports. 2015;25:33–39. doi: 10.1111/sms.12458. [DOI] [PubMed] [Google Scholar]
- 67.Bütikofer L, Zurlinden A, Bolliger MF, Kunz B, Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 2011;25:4378–4393. doi: 10.1096/fj.11-191262. This study assessed the role of collagen XIII at regenerating NMJs following nerve injury. While deletion of collagen XIII slowed the reformation of NMJs, introducing its transmembrane domain accelerated the regeneration of NMJs. [DOI] [PubMed] [Google Scholar]
- 68.Hettwer S, Lin S, Kucsera S, Haubitz M, Oliveri F, Fariello RG, Ruegg MA, Vrijbloed JW. Injection of a soluble fragment of neural agrin (NT-1654) considerably improves the muscle pathology caused by the disassembly of the neuromuscular junction. PLoS One. 2014;9:e88739. doi: 10.1371/journal.pone.0088739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyoshi S, Tezuka T, Arimura S, Tomono T, Okada T, Yamanashi Y. DOK7 gene therapy enhances motor activity and life span in ALS model mice. EMBO Mol Med. 2017;9:880–889. doi: 10.15252/emmm.201607298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Latvanlehto A, Fox MA, Sormunen R, Tu H, Oikarainen T, Koski A, Naumenko N, Shakirzyanova A, Kallio M, Ilves M, et al. Muscle-Derived Collagen XIII Regulates Maturation of the Skeletal Neuromuscular Junction. J Neurosci. 2010;30:12230–12241. doi: 10.1523/JNEUROSCI.5518-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zainul Z, Heikkinen A, Koivisto H, Rautalahti I, Kallio M, Lin S, Härönen H, Norman O, Rüegg MA, Tanila H, et al. Collagen XIII is required for neuromuscular synapse regeneration and functional recovery after peripheral nerve injury. J Neurosci. 2018 doi: 10.1523/JNEUROSCI.3119-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin–glycoprotein complex. Neuron. 2000;25:279–93. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- 73.Tassi E, Al-Attar A, Aigner A, Swift MR, McDonnell K, Karavanov A, Wellstein A. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J Biol Chem. 2001;276:40247–53. doi: 10.1074/jbc.M104933200. [DOI] [PubMed] [Google Scholar]


