Abstract
The levels of several brain metabolites were investigated in the anterior (ACC) and posterior cingulate cortex (PCC) in 13 healthy controls (HC) and 13 patients with mild cognitive impairment (MCI) using single-voxel magnetic resonance spectroscopy (MRS) at 7T. Levels of γ-aminobutyric acid (GABA), glutamate (Glu), glutathione (GSH), N-acetylaspartylglutamate (NAAG), N-acetylaspartate (NAA), and myo-inositol (mI) were quantified relative to total creatine (tCr). The effect of diagnosis on metabolite levels, and relationships between metabolite levels and memory and executive function, correcting for age, were investigated. MCI patients showed significantly decreased GABA/tCr (ACC, PCC), Glu/tCr (PCC), and NAA/tCr (PCC), and significantly increased mI/tCr (ACC). In the combined group, worse episodic verbal memory performance was correlated with lower Glu/tCr (PCC), lower NAA/tCr (PCC), and higher mI/tCr (ACC, PCC). Worse verbal fluency performance was correlated with lower GSH/tCr (PCC). In summary, MCI is associated with decreased GABA and glutamate, most consistently in the PCC. Further studies in larger patient samples should be undertaken to determine the utility of 7T MRS in detecting MCI-related neurochemical changes.
Keywords: mild cognitive impairment, magnetic resonance spectroscopy, 7T, anterior cingulate cortex, posterior cingulate cortex, glutamate, GABA
1. Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease. The cognitive decline and neuropsychiatric symptoms associated with AD lead to increased disability, mortality and caregiver burden (Alzheimer’s Association, 2015; Reitz et al., 2011). The economic impact of AD is estimated to be $100B per year in the US alone. The estimated global AD prevalence of 24 million in 2011 is projected to double every 20 years until 2040 due to the world’s aging population, challenging health care systems and societies in unprecedented ways (Brookmeyer et al., 2007; Reitz et al., 2011). Mitigating the future impact of AD will depend on identifying individuals at risk, determining predictors of progression, and providing early preventive treatment in preclinical stages with high conversion rates to AD, such as mild cognitive impairment (MCI).
The complex pathophysiology of MCI/AD includes deposition of amyloid-β plaques in multiple brain regions including the anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC) (Pike et al., 2007; Small et al., 2006), deposition of tau tangles in the medial-temporal lobe (Pike et al., 2007; Small et al., 2006), and increased oxidative stress (Butterfield, 2002; Lin and Beal, 2006; Mecocci, 2004; Wang et al., 2014) and mitochondrial dysfunction (Devi et al., 2006; Lin and Beal, 2006; Reddy and Beal, 2008) that may lead to disruptions in several neurotransmitter systems and neuronal dysfunction. The regions affected by beta-amyloid and tau play an integral role in cognitive processes such as memory and learning, but the role of other aspects of pathophysiology in cognitive decline and the transition from MCI to AD is poorly understood.
Hypotheses with a focus on the relationships between AD pathology, mitochondrial dysfunction and oxidative stress can be tested in vivo due to advances in molecular imaging methods. The spatial distribution patterns of protein deposition and associations with cognitive decline and disease progression have been studied with positron emission tomography (PET). As a complementary molecular quantification technique, proton magnetic resonance spectroscopy (MRS) can be used to determine levels of endogenous brain metabolites non-invasively in the living brain. At lower field strength (<3 T), only metabolites with high signal-to-noise ratio (SNR) can be reliably determined (without advanced separation methods such as J-difference editing), including the neuronal marker N-acetylaspartate (NAA) (Rae, 2014), and myo-inositol (mI) which acts as an osmolyte (Rae, 2014) and is thought to reflect demyelination, inflammation, and glial activation (Chang et al., 2013). Decreased levels of NAA and increased levels of mI are a consistent finding in MCI and AD (Gao and Barker, 2014), and have been suggested as an early biomarker for diagnosis and progression (Waragai et al., 2017).
At high field strengths (7T and above), the increased spectral resolution and SNR of MRS permits the assessment of several biochemically important molecules with enhanced sensitivity compared to 3T due to their low concentrations and coupled, often overlapped resonances (Mekle et al., 2009; Pradhan et al., 2015). These metabolites include the inhibitory neurotransmitter γ-aminobutyric acid (GABA), the redox compound glutathione (GSH), and the glutamatergic modulator N-acetylaspartylglutamate (NAAG). In the context of MCI, altered levels of GSH may indicate a challenge of the cellular redox regulation systems in response to the increased production of reactive oxygen species – e.g. in the form of a depletion of the antioxidant defense line, resulting in decreased GSH levels (Mandal et al., 2015), or an adaptive upregulation, represented by increased GSH levels (Duffy et al., 2014). Abnormal levels of GABA and Glu may reflect disturbed inhibitory and excitatory neurotransmission, which primarily shape cortical information processing, and may underlie the observed loss of cognitive and executive function in MCI (Huang et al., 2017). Lastly, altered levels of NAAG may exert an additional critical modulatory influence on glutamatergic neurotransmission (Jaarsma et al., 1994; Labak et al., 2010). Determining the levels of multiple low-concentration compounds in a single MRS experiment at 7T is a promising avenue to probe several critical mechanisms of MCI/AD pathophysiology (oxidative stress, disturbed neurotransmission) in vivo, and whether they are related to quantitative measures of cognitive performance.
In this study, MRS data were acquired at 7T in individuals with MCI and healthy elderly controls in two cortical regions relevant to MCI/AD pathophysiology: the ACC and PCC. The ACC and PCC regions were chosen as these regions have been implicated in MCI/AD in studies of neural circuitry and neuropathology that show associations between pathology in these regions and cognitive deficits and neuropsychiatric symptoms (Arnold et al., 1991; Hirao et al., 2015). Levels of the neurotransmitters GABA and glutamate (Glu), the antioxidant GSH, the neuromodulator NAAG, the neuronal marker NAA and the osmolyte and glial marker mI were tested for differences between healthy controls and individuals with MCI. In addition, associations between metabolite levels and cognitive measures of episodic verbal memory and verbal fluency that are affected in MCI were investigated.
2. Material and Methods
2.1. Subjects
2.1.1. Recruitment
Thirteen healthy control subjects (HC; 7 female; mean age 63.6 ± 7.8 y) and 13 patients with MCI (3 female; mean age 69.6 ± 7.7 y: p < 0.05 compared to HC) were included in this study.
Participants were recruited from advertisements in the community or from the Johns Hopkins University Alzheimer’s Disease Research Center (2 controls and 2 MCI subjects). All subjects underwent the same diagnostic procedures. Psychiatric and cognitive evaluations included a structured clinical interview by a clinical psychologist (SCID) (First et al., 1997), Clinical Dementia Rating scale (CDR) (Morris, 1993), and Mini Mental State Examination (MMSE) (Folstein et al., 1975). All participants also underwent a physical and neurological examination, laboratory testing (including complete blood count and blood chemistries) and toxicology screening (psychotropic drugs and drugs of abuse) prior to the MR scans. Participants were excluded from the study if they had a history of or active neurological or Axis I psychiatric disorders including substance abuse, if they were not medically stable (i.e. if they had poorly controlled hypertension and/or insulin dependent diabetes), after a positive toxicology screening for psychotropic drugs or medications with central nervous system effects, or if they used prescription or over-the-counter medications with potential central nervous system effects (e.g. antihistamines, cold medications) within the past two weeks prior to enrollment. The MCI patients were required to have a CDR global score of 0.5, whereas the controls were required to have a CDR global score of 0 (normal). Further, all participants also underwent beta-amyloid imaging with positron emission tomography (PET) to determine whether the MCI patients had evidence of a biomarker associated with AD, in addition to meeting clinical criteria for MCI (see section 2.1.3). The study protocol and consent forms were approved by the Institutional Review Board of the Johns Hopkins University School of Medicine. Written informed consent was obtained.
2.1.2. Cognitive testing
Three measures sensitive to global cognition, executive function and memory were selected from a comprehensive, multi-domain neuropsychological assessment battery to be tested for correlations with MRS measures of metabolite levels: the MMSE; the letter fluency test included in the Delis-Kaplan Executive Function System (D-KEFS) test (Delis et al., 2001) (letter-total words recalled); and the California Verbal Learning Test (CVLT; sum of the first five recall trials) (Delis et al., 2000). The Brief Visuospatial Memory Test-Revised was also administered to assess memory deficits (Benedict, 1997).
2.1.3. Beta-amyloid PET data acquisition and processing
The radiotracer (N-methyl-[11C])2-(4’-methylaminophenyl)-6-hydroxybenzothiazole ([11C]-PiB) was used to measure beta-amyloid deposition in all subjects and was synthesized as previously described (Klunk et al., 2004). PET scans were acquired at the PET Center, Russell H. Morgan Department of Radiology, Johns Hopkins University School of Medicine. The scanner used was a second-generation High Resolution Research Tomograph scanner (HRRT, Siemens Healthcare, Knoxville, TN), a cerium-doped lutetium oxyorthosilicate (Lu25i05[Ce] or LSO) based, dedicated brain PET scanner with 2 mm resolution (Sossi et al., 2005). Dynamic scanning began immediately upon a 15 mCi ± 10% radiotracer injection and lasted for 90 minutes. [11C]-PiB was analyzed using the simplified reference tissue model (SRTM) method with the cerebellum as the input function that has been validated against arterial blood sampling (Price et al., 2005; Zhou et al., 2003).
MRS data acquisition and processing
2.2.1. MRS acquisition
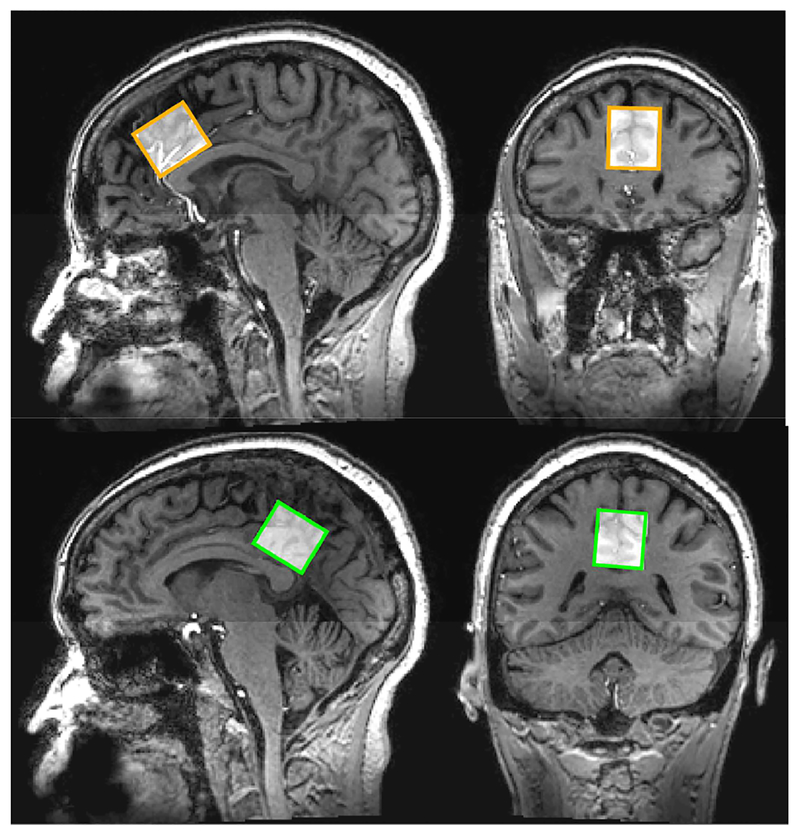
All data were acquired on a 7T Philips Achieva scanner (Philips Healthcare, Best, The Netherlands) with a 32-channel receive and quadrature transmit head coil (Nova Medical, Wilmington, MA). After a high-resolution (0.96 mm isotropic) anatomical T1-weighted magnetization prepared gradient echo (MP-RAGE) scan, MRS voxels were prescribed in two brain regions: the anterior cingulate (ACC) and the posterior cingulate cortex (PCC, as shown in Figure 1). For both regions, the MRS voxels had dimensions of 28 mm (anterior-posterior) × 20 mm (left-right) × 16 mm (caudal-cranial). Voxels were centered at the midline with the anterior-posterior edge tangential to the corpus callosum. The ACC voxel was placed in dorsal ACC with the caudal-cranial edge perpendicular to the genu of the corpus callosum, and the PCC voxel was placed in PCC with the caudal-cranial edge perpendicular to the splenium of the corpus callosum. Prior to data acquisition, shimming was performed up to 2nd order using a FASTMAP-based routine, and RF power was optimized on the localized volume. Data were acquired using the Stimulated Echo Acquisition Mode (STEAM) sequence with the following parameters: TR = 3000 ms; 96 averages; 2048 data points; 3 kHz spectral width; VAPOR (Tkac et al., 1999) water suppression. Four water-unsuppressed average per voxel were recorded with the same settings. TE was set to the shortest possible value (14 ms in ACC, 15 ms in PCC).
Figure 1.
Voxel placement in the ACC (upper panel) and PCC (lower panel). For both voxels, dimensions were 28 mm (anterior-posterior) × 20 mm (left-right) × 16 mm (caudal-cranial).
2.2.2. Data processing
Spectroscopic data were analyzed with LCModel v6.3–0D (Provencher, 2001, 1993), using TE-specific simulated basis sets including alanine (Ala), aspartate (Asp), creatine (Cr), GABA, glucose (Glc), Glu, glutamine (Gln), GSH, glycerophosphocholine (GPC), glycine (Gly), lactate (Lac), mI, NAA, NAAG, phosphocholine (PCh), phosphocreatine (PCr), phosphoethanolamine (PE), serine (Ser), scyllo-inositol (sI), taurine (Tau), and resonances from lipids (Lip09, Lip13a-d, Lip20) and macromolecules (MM09, MM12, MM14, MM17, MM20) as internally simulated by LCModel. The levels of GABA, Glu, GSH, NAA, NAAG, and mI (estimated with respect to the total creatine signal tCr = Cr + PCr) were used for further analysis. Individual metabolite measures with Cramér-Rao lower bounds (CRLB, as determined by LCModel) higher than 15% were excluded. Detection of gross outliers was performed by calculating the mean Cook’s distance across both groups (HC, MCI) and regions (ACC, PCC) for each metabolite (Stevens, 1984). Individual data points with more than 5 times the mean Cook’s distance were regarded as gross outliers and discarded.
The T1-weighted structural images were segmented with SPM12 (Friston, 2007) into separate gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) probability maps. Using an in-house-developed MATLAB (The MathWorks, Natick, MA) routine based on the Gannet 3.0 toolbox (Edden et al., 2014), the MRS voxels for each participant were co-registered to the structural image, and the relative fractions of voxel tissue composition for GM, WM, and CSF were extracted. GM tissue fraction was subsequently used as a covariate (see below) to account for the individual degree of gray matter loss, which may underlie changes in metabolite levels.
2.2.3. Statistical analysis
All statistical analyses were performed in R (R core team, 2017). For each metabolite, a two-way ANCOVA was performed on the entire dataset after exclusion and outlier rejection. Metabolite levels (expressed as ratios to tCr) were defined as dependent variables, group (HC, MCI) and region (ACC, PCC) as independent variables. To investigate effects of group, post-hoc pairwise contrasts between HC and MCI groups were tested using the least-squares means method for each region. Tukey-adjusted comparisons were tested for significance at a single-test alpha level of 0.05. To assess relationships between local metabolite levels and cognitive scores, separate linear regression analyses were performed between each metabolite and cognitive score in ACC and PCC, respectively, and a single-test alpha level of 0.05.
For the two-way ANCOVA modeling and the linear regression analyses, models were designed with the following covariates: a) without covariates; b) age; c) age and gray matter tissue fraction; d) age and sex; e) age, sex, and gray matter tissue fraction. The linear regression analyses showed the best adjusted R2 (adjusted for the number of covariates) for the model only including age as a covariate (see results for all models in the Supplementary Material), which was subsequently chosen for analysis.
To investigate whether changes in metabolites correlated with the extent of neuronal loss characteristic of MCI, relationships between metabolite levels and levels of NAA (which is generally interpreted as a general indicator of neuronal integrity) were assessed separately for each voxel using Pearson’s product-moment correlation analysis. The rationale for this additional analysis was to assess whether potential changes in levels of neurotransmitters or oxidative compounds can be linked to the specific mechanism of neuronal loss, or whether they appear independent, e.g. as a result of altered metabolic synthesis or recycling mechanisms.
In order to scrutinize whether disease effects of the reference compound (tCr) are driving changes in metabolite-to-tCr ratios, the signal ratios of tCr to internal tissue water in institutional units (as returned by LCModel with the water relaxation correction parameter ‘atth2o’ = 0.742) were compared between groups (separately for ACC and PCC) with a two-tailed t-test at a single-test alpha level of 0.05.
3. Results
All of the MCI participants demonstrated a distribution volume ratio for [11C]-PiB that is associated with cognitive decline; 1.2 or higher for anterior (anterior cingulate or middle frontal cortex) and/or posterior cortical regions (Resnick et al., 2010) (superior temporal cortex, precuneus or posterior cingulate; data not shown). In addition, all of the MCI patients performed at least one and a half standard deviations below the normative value on either the CVLT or the BVMT (data not shown). Thus, based on the beta-amyloid imaging and cognitive performance, this MCI sample is likely to demonstrate further cognitive decline.
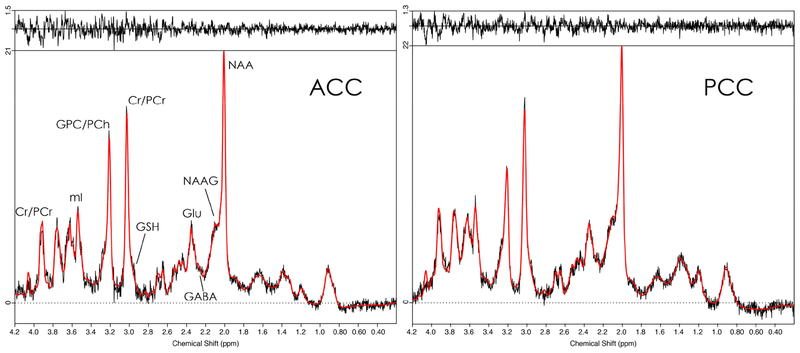
Out of 52 datasets (26 subjects × 2 voxels), the following numbers were discarded prior to statistical analysis: 6 for GABA (1 outlier); 2 for Glu (2 outliers); 5 for GSH (2 outliers); 2 for NAA (2 outliers); 21 for NAAG (2 outliers); 1 for mI (1 outlier). Representative spectra and LCModel fits for ACC and PCC are shown in Fig. 2, indicating good SNR, low linewidth, and successful water suppression.
Figure 2.
Representative spectra (black) and LCModel fits (red) for STEAM data from ACC (left panel) and PCC (right panel). Fit residuals are shown at the top of the respective panel.
Mean MMSE were 28.7 ± 1.2 for HC and 27.5 ± 1.7 for MCI (p < 0.01). Mean D-KEFS scores were 42.8 ± 12.6 for HC and 36.4 ± 12.5 for MCI (p = 0.073). Mean CVLT (sum of the first five trials) were 55.5 ± 8.3 for HC and 38.5 ± 10.3 for MCI (p < 0.001).
Mean GM tissue fractions were 64 ± 7 % for HC and 59 ± 7 % for MCI in ACC (p = 0.12), and 64 ± 12 % for HC and 60 ± 6 % for MCI in PCC (p = 0.38). Mean WM tissue fractions were 11 ± 5 % for HC and 15 ± 9 % for MCI in ACC (p = 0.23), and 21 ± 6 % for HC and 24 ± 3 % for MCI in PCC (p = 0.09). Mean CSF tissue fractions were 25 ± 9 % for HC and 26 ± 10 % for MCI in ACC (p = 0.83), and 16 ± 10 % for HC and 16 ± 4 % for MCI in PCC (p = 0.96).
Mean tCr/water was 3.97 ± 0.35 i.u. for HC and 3.95 ± 0.38 i.u. for MCI in ACC (p = 0.89), and 4.44 ± 0.40 i.u. for HC and 4.63 ± 0.39 % for MCI in PCC (p = 0.25).
Group comparisons between the HC and MCI groups revealed statistically significant differences in metabolite-to-tCr ratios (Table 1). MCI patients had significantly lower levels of GABA/tCr (ACC and PCC, p < 0.01 each), Glu/tCr (PCC, p < 0.05) and NAA/tCr (PCC, p < 0.05), and significantly higher levels of mI/tCr (ACC, p < 0.01). If no covariates were included in the models, Glu/tCr in the ACC was significantly lower in MCI patients (p < 0.05). No additional significant results were observed when including sex or gray matter content as covariates (see Supplementary Material).
Table 1.
Quantitative results of post-hoc group comparisons after two-way ANCOVA analysis, with metabolite levels as dependent variable, group and region as independent variables, and age, as covariate. Results were tested for statistical significance at a single-test alpha level of 0.05. The table shows the group mean with the standard error of the mean (SEM) in parentheses.
| Metabolite [/tCr] |
ACC | PCC | ||||
|---|---|---|---|---|---|---|
| HC | MCI | p | HC | MCI | p | |
| GABA | 0.418 (0.018) | 0.343 (0.017) | 0.005 | 0.361 (0.017) | 0.295 (0.016) | 0.009 |
| Glu | 1.418 (0.025) | 1.340 (0.023) | 0.096 | 1.292 (0.023) | 1.215 (0.023) | 0.024 |
| GSH | 0.215 (0.011) | 0.208 (0.010) | 0.685 | 0.233 (0.011) | 0.230 (0.010) | 0.835 |
| NAA | 1.440 (0.028) | 1.374 (0.027) | 0.095 | 1.542 (0.028) | 1.435 (0.027) | 0.010 |
| NAAG | 0.209 (0.015) | 0.197 (0.013) | 0.551 | 0.230 (0.012) | 0.206 (0.010) | 0.163 |
| mI | 0.662 (0.018) | 0.750 (0.018) | 0.001 | 0.685 (0.018) | 0.732 (0.018) | 0.072 |
MMSE test scores showed a negative correlation with mI/tCr in the ACC (p < 0.01) and the PCC (p < 0.05). D-KEFS scores were positively correlated with GSH/tCr in the PCC (p < 0.01). CVLT test scores were positively correlated with Glu/tCr (PCC, p < 0.05) and NAA/tCr (PCC, p < 0.05), and negatively correlated with mI/tCr (ACC and PCC, p < 0.05 each). Results of the analyses of relationships between metabolite levels and cognitive scores are summarized in Table 2. Without covariates included in the models, positive correlations between CVLT test scores and Glu/tCr and NAA/tCr were observed in the ACC (p < 0.05 each), and mI/tCr did not correlate with any test score. None of the additional correlations were observed after inclusion of gray matter and sex as covariates. (see Supplementary Material).
Table 2.
Results of linear regression analysis for relationships between metabolite levels and cognitive test measures for the combined group (both control and MCI subjects). P values represent partial correlations between metabolite levels (or age) and cognitive test measures. R2 is the adjusted value for the complete linear regression model including age as covariate. Partial correlations between metabolite levels and cognitive test measures were considered significant at a single-test alpha level of 0.05.
| Metabolite [/tCr] |
ACC | PCC | ||||
|---|---|---|---|---|---|---|
| MMSE | DKEFS | CVLT | MMSE | DKEFS | CVLT | |
| GABA | R2 = 0.403 | R2 = −0.069 | R2 = 0.109 | R2 = 0.180 | R2 = −0.035 | R2 = 0.093 |
| page = 0.001 | page = 0.458 | page = 0.315 | page = 0.014 | page = 0.301 | page = 0.117 | |
| Glu | R2 = 0.064 | R2 = 0.006 | R2 = 0.155 | R2 = 0.063 | R2 = 0.105 | R2 = 0.207 |
| page = 0.114 | page = 0.234 | page = 0.558 | page = 0.458 | page = 0.160 | page = 0.611 | |
| GSH | R2 = 0.209 | R2 = 0.013 | R2 = 0.030 | R2 = 0.253 | R2 = 0.361 | R2 = 0.074 |
| page = 0.011 | page = 0.326 | page = 0.137 | page = 0.010 | page = 0.065 | page = 0.079 | |
| NAA | R2 = 0.085 | R2 = −0.037 | R2 = 0.139 | R2 = 0.050 | R2 = −0.037 | R2 = 0.180 |
| page = 0.087 | page = 0.337 | page = 0.276 | page = 0.146 | page = 0.296 | page = 0.364 | |
| NAAG | R2 = 0.209 | R2 = 0.021 | R2 = −0.131 | R2 = 0.234 | R2 = 0.023 | R2 = −0.065 |
| page = 0.067 | page = 0.445 | page = 0.417 | page = 0.019 | page = 0.187 | page = 0.383 | |
| mI | R2 = 0.346 | R2 = 0.102 | R2 = 0.191 | R2 = 0.241 | R2 = −0.028 | R2 = 0.155 |
| page = 0.005 | page = 0.072 | page = 0.035 | page = 0.014 | page = 0.279 | page = 0.059 | |
Glu/tCr was positively correlated with NAA/tCr in ACC and PCC (p = 0.001 each). No other relationships of metabolite levels with NAA/tCr were observed (Table 3).
Table 3.
Results of linear regression analysis for relationships between metabolite levels and NAA levels as marker of neuronal integrity for the combined group (both control and MCI subjects). R represents the Pearson product moment correlation coefficient. Correlations between metabolite levels and were considered significant at a single-test alpha level of 0.05. Note that the lack of general correlation indicates that effects are not primarily driven by changes in the tCr reference signal.
| Metabolite [/tCr] |
Correlation with NAA/tCr | Correlation with NAA/tCr |
|---|---|---|
| GABA | R = 0.098 | R = 0.167 |
| Glu | R = 0.615 | R = 0.595 |
| GSH | R = 0.302 | R = −0.199 |
| NAAG | R = 0.050 | R = 0.254 |
| mI | R = 0.011 | R = −0.014 |
4. Discussion
This study investigated differences in the levels of several brain metabolites between healthy elderly healthy control subjects and MCI patients, as well as their relationships with measures of cognitive functioning. To the best of our knowledge, this is the first study to employ MRS at 7T field strength to study metabolic changes in MCI patients, including quantification of the low-concentration metabolites GABA, GSH, and NAAG in a single session. In addition to reproducing well known findings from lower field strengths (i.e. decreased NAA and increased mI), this study also found selective reductions in the neurotransmitters GABA and Glu. The MCI patients studied are likely to demonstrate subsequent cognitive decline based on memory deficits and cortical beta-amyloid levels. Further, the MCI patients were carefully screened for psychiatric and medical comorbidities that could be associated with cognitive impairment, rather than AD.
One of the interesting significant findings was a reduction of ~16% in both ACC and PCC GABA/tCr levels in MCI patients compared to controls. No relationships between GABA/tCr levels and cognitive scores were found, however. These observations are in line with several previous findings from studies of MCI and AD patients that used J-difference-edited MRS at 3T. Most prominently, decreased posterior cingulate GABA+macromolecules (GABA+) levels were found in patients with amnestic MCI (Riese et al., 2015). In that study, no relationship between GABA+ and MMSE (but with the CERAD word learning score) or the uptake of the beta-amyloid radiotracer Pittsburgh-B (PiB) PET compound was observed, indicating that GABA+ decrease is not directly related to the extent of beta-amyloid deposition. Similarly, another study found significantly lower levels of GABA+ in AD patients for the posterior, but not the anterior cingulate region (Bai et al., 2014). As in the present study, levels of GABA were not correlated with MMSE. Another study did not find significant group differences for GABA+ or Glu + Gln (Glx) between healthy controls, MCI and AD patients in ACC and right hippocampus, nor were correlations with MMSE or the Montreal Cognitive Assessment score revealed (Huang et al., 2017). In a study of healthy elderly subjects and MCI patients using J-PRESS at 3T, it was found that administration of human growth hormone factor led to significant increases of GABA levels in dorsolateral frontal, posterior cingulate, and posterior parietal regions, which coincided with, but was not correlated with, improved cognitive functioning (Friedman et al., 2013). The finding in the current study of decreased GABA in ACC suggests that disturbed GABA homeostasis in MCI may also occur in cortical regions other than the PCC, with potential impact on cognitive function. The discrepancy to previous studies may be explained by the increased specificity of GABA detection in unedited MRS at 7T, compared to J-difference-edited methods at 3T that usually report combined GABA and macromolecules in a larger measurement volume.
Glu (or Glx) levels in MCI/AD have been more frequently studied, as the relatively strong combined Glx signal is easily detectable even with standard MRS techniques at lower field strengths. As the present study benefits from the higher field strength of 7T, the discrimination between Glu and Gln is improved, and the Glu estimates can be assumed to be more reliable and specific than at 1.5–3T, revealing reduced Glu/tCr levels in MCI (by ~5–6%). The observed correlation between PCC Glu/tCr and CVLT scores may suggest that disturbed Glu neurotransmission is at least partly contributing to memory impairment in MCI. A recent study found reduced posterior cingulate Glu levels in patients with amnestic MCI, which also inversely correlated with global PiB uptake (Zeydan et al., 2017). Previous studies reported decreased Glx levels in the posterior cingulate in MCI compared to controls (Hattori et al., 2002); no decrease in MCI, but in AD patients in the posterior cingulate (Fayed et al., 2011) and hippocampus (Rupsingh et al., 2011); no differences between MCI and controls, but longitudinal changes over time in atypical MCI patients in the posterior cingulate (Olson et al., 2008).
Reduced GABA or Glu levels may indicate loss of GABA- or glutamatergic neurons per se, disturbances in GABA/Glu synthesis, and/or changes in the Glu/Gln/GABA cycle between astrocytes and neurons. While changes in Glu/tCr levels in this study were closely linked to changes in levels of the neuronal marker NAA/tCr, GABA/tCr levels did not show a relationship with NAA/tCr. This finding may suggest that Glu changes are largely caused by the specific loss of glutamatergic neurons. In contrast, the notable decrease in bulk GABA/tCr levels may rather be attributed to MCI-induced alterations in GABA synthesis or recycling.
Due to the inherent difficulties in reliably differentiating NAAG from NAA, NAAG has not been extensively studied to date. Even at 7T, the separation of the two compounds relies on optimal measurement conditions, as evidenced by the relatively large fraction of datasets that were excluded due to high CRLB values for NAAG. The present study is the first study to explicitly compare NAAG levels between healthy controls and MCI patients at 7T, observing no effects of disease or correlations with cognitive measures (albeit in a substantially reduced cohort for aforementioned data quality reasons). A post-mortem study revealed decreased NAAG levels in AD brains (Jaarsma et al., 1994), and increased NAAG levels were reported in dorsolateral prefrontal cortex after human growth hormone factor treatment (Friedman et al., 2013). However, the functional significance of NAAG remains not well understood, beyond a putative role as neuromodulator activating secondary messenger pathways via glutamatergic metabotropic activity (Neale, 2011), and as a potential source of glutamate. Further, the decrease in NAAG may be observed in AD, rather than in its preclinical stages such as MCI.
In contrast, decreased levels of NAA in several brain areas in MCI/AD have been long established (Gao and Barker, 2014), which is primarily thought of as an (unspecific) indicator of neuronal loss. However, reduced NAA seems to be more closely related to clinical disease progression than is cerebral atrophy (Adalsteinsson et al., 2000). Similarly, increased mI levels are a classic hallmark of MCI/AD pathophysiology, likely indicating glial activation, gliosis and inflammation, and providing a link to protein-related AD neuropathology. While the data in this study confirm the previously observed increase of mI/tCr in MCI patients in the ACC, the group comparison did not reach significance (p = 0.07) for the PCC. It is possible that investigations with a large sample size might yield significant differences for the PCC as well, in particular since mI/tCr was associated with cognitive scores in both regions of interest. The combined NAA/mI ratio has been suggested as an early biomarker of individuals at risk for developing MCI and progressing to AD (Waragai et al., 2017), and both compounds have been closely linked to increased accumulation of amyloid (Kantarci et al., 2011; Nedelska et al., 2017) and tau (Murray et al., 2014). In this light, the effects of NAA and mI that were observed in this study are in line with these findings; although, it is notable that mI was the only metabolite to be associated with the MMSE score, suggesting that it may serve as a functionally unspecific indicator of general disease severity. Interestingly, no direct correlation between NAA/tCr and mI/tCr levels was observed, indicating that the respective pathophysiological processes are not immediately linked.
Lastly, while this study showed no group differences in GSH/tCr levels between MCI patients and controls, it revealed a relationship between PCC GSH/tCr levels and verbal fluency. Previous in vivo MRS studies of GSH are rare: one study found significantly decreased GSH levels in MCI and AD in hippocampus, but only in AD in prefrontal areas (Mandal et al., 2015). In contrast to the present study, close relationships were found between prefrontal GSH and MMSE as well as CDR scores. Recently, associations between decreased temporal and parietal GSH levels and local PiB uptake measures were found, indicating relationships between oxidative stress and amyloidosis (Chiang et al., 2017). Taken together, these findings indicate a promising link between several pathophysiological MCI/AD mechanisms and cognitive outcome, and certainly warrant further investigation in terms of regional and functional specificity.
4.1. Limitations
This study features comparably small sample sizes with 13 individuals in each cohort. This limitation in power may have prevented the detection of significant effects of disease (e.g. ACC Glu). It should also be noted that there was a significant difference in age between the patient and control groups (with the MCI group being older). Age was therefore included as a covariate in the group analysis of metabolite levels and in the analysis of correlation between metabolite levels and cognitive scores. Finally, the strict requirements concerning spectral quality resulted in frequent rejection of quantitative NAAG measures, suggesting that the respective negative findings should be regarded with caution due to the low statistical power. Future studies investigating MCI-related changes of NAAG may require dedicated spectral editing efforts or optimized sub-echo times for unambiguous detection (Choi et al., 2010; Edden et al., 2007).
5. Conclusions
Results of this 7T MRS study revealed several region-specific effects of MCI on brain metabolite levels. Specifically, MCI was associated with decreased GABA and glutamate, most consistently in the PCC, the region implicated by studies of cerebral glucose metabolism and beta-amyloid deposition in MCI. Interactions between metabolite levels and cognitive scores were observed for specific brain regions. This suggests that key mechanisms in MCI/AD pathophysiology, like oxidative stress and disturbed neurotransmission, may contribute to cognitive deficits in a highly region- and function-specific manner. Future studies of MCI and AD patients should employ 7T MRS in various implicated brain regions including medial-temporal lobe / hippocampus to improve the understanding of the regional specificity of brain metabolite changes and their effects on different domains of cognitive function.
Supplementary Material
ACC and PCC GABA levels are reduced in patients with mild cognitive impairment
Levels of glutamate are reduced in the PCC in patients with mild cognitive impairment
Levels of Glu, NAA, mI, GSH show region-specific associations with cognitive test scores
6. Acknowledgements
This work was supported by National Institute of Health grants 2P50AG005146, AG038893, AG041633, UL1 TR 001079, and P41EB015909. RAEE and GO received salary support from R01EB016089, R01EB023963, R01MH106564, and R21MH098228.
Abbreviations:
- AD
Alzheimer’s disease
- ACC
anterior cingulate cortex
- CVLT
California Verbal Learning Test
- CSF
cerebrospinal fluid
- CDR
Clinical Dementia Rating scale
- CRLB
Cramér-Rao lower bounds
- D-KEFS
Delis-Kaplan Executive Function System
- Glu
glutamate
- Gln
glutamine
- Glx
glutamate plus glutamine
- GSH
glutathione
- GM
gray matter
- HC
healthy controls
- MRS
magnetic resonance spectroscopy
- MP-RAGE
magnetization prepared gradient echo
- MCI
mild cognitive impairment
- MMSE
Mini Mental State Examination
- mI
myo-inositol
- NAA
N-acetylaspartate
- NAAG
N-acetylaspartylglutamate
- PiB
Pittsburgh-B
- PET
positron emission tomography
- PCC
posterior cingulate cortex
- STEAM
Stimulated Echo Acquisition Mode
- SCID
Structured Clinical Interview for DSM-IV
- tCr
total creatine
- WM
white matter
- GABA
γ-aminobutyric acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Adalsteinsson E, Sullivan E, Kleinhans N, Spielman D, Pfefferbaum A, 2000. Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer’s disease. Lancet 355, 1696–1697. doi:10.1016/S0140-6736(00)02246-7 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2015. 2015 Alzheimer’s disease facts and figures. Alzheimer’s Dement 11, 332–384. doi:10.1016/J.JALZ.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW, 1991. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with alzheimer’s disease. Cereb. Cortex 1, 103–116. doi:10.1093/cercor/1.1.103 [DOI] [PubMed] [Google Scholar]
- Bai X, Edden RA, Gao F, Wang G, Wu L, Zhao B, Wang M, Chan Q, Chen W, Barker PB, 2014. Decreased gamma-aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease. J Magn Reson Imaging doi:10.1002/jmri.24665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB, 1997. The Brief Visuospatial Memory Test - Revised. Psychol. Assess 145–153. doi:10.1037/1040-3590.8.2.145 [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM, 2007. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement 3, 186–191. doi:10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- Butterfield D, 2002. Amyloid β-peptide (1–42)-induced Oxidative Stress and Neurotoxicity: Implications for Neurodegeneration in Alzheimer’s Disease Brain. A Review. Free Radic. Res 36, 1307–1313. doi:10.1080/1071576021000049890 [DOI] [PubMed] [Google Scholar]
- Chang L, Munsaka SM, Kraft-Terry S, Ernst T, 2013. Magnetic Resonance Spectroscopy to Assess NeuroInflammation and Neuropathic Pain. J. Neuroimmune Pharmacol 8, 576–593. doi:10.1007/s11481-013-9460-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang GC, Mao X, Kang G, Chang E, Pandya S, Vallabhajosula S, Isaacson R, Ravdin LD, Shungu DC, 2017. Relationships among cortical glutathione levels, brain amyloidosis, and memory in healthy older adults investigated in vivo with 1H-MRS and Pittsburgh compound-B PET. Am. J. Neuroradiol 38, 1130–1137. doi:10.3174/ajnr.A5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Dimitrov IE, Douglas D, Patel A, Kaiser LG, Amezcua CA, Maher EA, 2010. Improvement of resolution for brain coupled metabolites by optimized 1H MRS at 7 T. NMR Biomed. 23, 1044–1052. doi:10.1002/nbm.1529 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH, 2001. Delis-Kaplan Executive Function System®(DKEFS®): Examiner’s Manual: Flexibility of Thinking, Concept Formation, Problem Solving, Planning, Creativity, Impluse Control, Inhibition. Pearson. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA, 2000. CVLT-II: California verbal learning test: adult version. Psychological Corporation. [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK, 2006. Accumulation of Amyloid Precursor Protein in the Mitochondrial Import Channels of Human Alzheimer’s Disease Brain Is Associated with Mitochondrial Dysfunction. J. Neurosci 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SL, Lagopoulos J, Hickie IB, Diamond K, Graeber MB, Lewis SJG, Naismith SL, 2014. Glutathione relates to neuropsychological functioning in mild cognitive impairment. Alzheimers. Dement 10, 67–75. doi:10.1016/j.jalz.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Edden RA, Pomper MG, Barker PB, 2007. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med 57, 977–982. doi:10.1002/mrm.21234 [DOI] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ, 2014. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging 40, 1445–1452. doi:10.1002/jmri.24478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed N, Modrego PJ, Rojas-Salinas G, Aguilar K, 2011. Brain Glutamate Levels Are Decreased in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dementiasr 26, 450–456. doi:10.1177/1533317511421780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Benjamin L, 1997. User’s guide for the structured clinical interview for DSM-IV axis II personality disorders: SCID-II.
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12, 189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Friedman SD, Baker LD, Borson S, Jensen JE, Barsness SM, Craft S, Merriam GR, Otto RK, Novotny EJ, Vitiello MV, 2013. Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging. JAMA Neurol 70, 883–90. doi:10.1001/jamaneurol.2013.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, 2007. Statistical parametric mapping the analysis of funtional brain images. Elsevier/Academic Press, Amsterdam; Boston. [Google Scholar]
- Gao F, Barker PB, 2014. Various MRS Application Tools for Alzheimer Disease and Mild Cognitive Impairment. Am. J. Neuroradiol 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Abe K, Sakoda S, Sawada T, 2002. Proton MR spectroscopic study at 3 Tesla on glutamate/glutamine in Alzheimer’s disease. Neuroreport 13, 183–6. doi:10.1097/00001756-200201210-00041 [DOI] [PubMed] [Google Scholar]
- Hirao K, Pontone GM, Smith GS, 2015. Molecular imaging of neuropsychiatric symptoms in Alzheimer’s and Parkinson’s disease. Neurosci. Biobehav. Rev 49. doi:10.1016/j.neubiorev.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H, 2017. Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur. Radiol 27, 2698–2705. doi:10.1007/s00330-016-4669-8 [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Veenma-van der Duin L, Korf J, 1994. N-Acetylaspartate and N-acetylaspartylglutamate levels in Alzheimer’s disease post-mortem brain tissue. J. Neurol. Sci 127, 230–233. doi:10.1016/0022-510X(94)90077-9 [DOI] [PubMed] [Google Scholar]
- Kantarci K, Lowe V, Przybelski SA, Senjem ML, Weigand SD, Ivnik RJ, Roberts R, Geda YE, Boeve BF, Knopman DS, Petersen RC, Jack CR, 2011. Magnetic resonance spectroscopy, -amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology 77, 951–958. doi:10.1212/WNL.0b013e31822dc7e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang G-F, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B, 2004. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol 55, 306–319. doi:10.1002/ana.20009 [DOI] [PubMed] [Google Scholar]
- Labak M, Foniok T, Kirk D, Rushforth D, Tomanek B, Jasiński A, Grieb P, 2010. Metabolic Changes in Rat Brain Following Intracerebroventricular Injections of Streptozotocin: A Model of Sporadic Alzheimer’s Disease, in: Acta Neurochirurgica. Supplement pp. 177–181. doi:10.1007/978-3-211-98811-4_32 [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF, 2006. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. doi:10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- Mandal PK, Saharan S, Tripathi M, Murari G, 2015. Brain Glutathione Levels – A Novel Biomarker for Mild Cognitive Impairment and Alzheimer’s Disease. Biol. Psychiatry 78, 702–710. doi:10.1016/J.BIOPSYCH.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Mecocci P, 2004. Oxidative stress in mild cognitive impairment and Alzheimer disease: A continuum. J. Alzheimer’s Dis 6, 159–163. doi:10.3233/JAD-2004-6207 [DOI] [PubMed] [Google Scholar]
- Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R, 2009. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn. Reson. Med 61, 1279–1285. doi:10.1002/mrm.21961 [DOI] [PubMed] [Google Scholar]
- Morris J, 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology [DOI] [PubMed] [Google Scholar]
- Murray ME, Przybelski SA, Lesnick TG, Liesinger AM, Spychalla A, Zhang B, Gunter JL, Parisi JE, Boeve BF, Knopman DS, Petersen RC, Jack CR, Dickson DW, Kantarci K, 2014. Early Alzheimer’s Disease Neuropathology Detected by Proton MR Spectroscopy. J. Neurosci 34, 16247–16255. doi:10.1523/JNEUROSCI.2027-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale JH, 2011. N-acetylaspartylglutamate is an agonist at mGluR₃ in vivo and in vitro. J. Neurochem 119, 891–5. doi:10.1111/j.1471-4159.2011.07380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelska Z, Przybelski SA, Lesnick TG, Schwarz CG, Lowe VJ, Machulda MM, Kremers WK, Mielke MM, Roberts RO, Boeve BF, Knopman DS, Petersen RC, Jack CR, Kantarci K, 2017. 1 H-MRS metabolites and rate of β-amyloid accumulation on serial PET in clinically normal adults. Neurology 10.1212/WNL.0000000000004421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson BLB, Holshouser BA, Britt W, Mueller C, Baqai W, Patra S, Petersen F, Kirsch WM, 2008. Longitudinal metabolic and cognitive changes in mild cognitive impairment patients. Alzheimer Dis. Assoc. Disord 22, 269–77. doi:10.1097/WAD.0b013e3181750a65 [DOI] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC, 2007. -amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130, 2837–2844. doi:10.1093/brain/awm238 [DOI] [PubMed] [Google Scholar]
- Pradhan S, Bonekamp S, Gillen JS, Rowland LM, Wijtenburg SA, Edden RAE, Barker PB, 2015. Comparison of single voxel brain MRS AT 3T and 7T using 32-channel head coils. Magn. Reson. Imaging 33, 1013–1018. doi:10.1016/j.mri.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA, 2005. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow Metab 25, 1528–1547. doi:10.1038/sj.jcbfm.9600146 [DOI] [PubMed] [Google Scholar]
- Provencher SW, 2001. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14, 260–4. [DOI] [PubMed] [Google Scholar]
- Provencher SW, 1993. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med 30, 672–9. [DOI] [PubMed] [Google Scholar]
- R core team, 2017. R: A language and environment for statistical computing R Found. Stat. Comput Vienna, Austria: R Foundation for Statistical Computing. doi:http://www.R-project.org/ [Google Scholar]
- Rae CD, 2014. A Guide to the Metabolic Pathways and Function of Metabolites Observed in Human Brain 1H Magnetic Resonance Spectra. Neurochem. Res 39, 1–36. doi:10.1007/s11064-013-1199-5 [DOI] [PubMed] [Google Scholar]
- Reddy PH, Beal MF, 2008. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med 14, 45–53. doi:10.1016/j.molmed.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Brayne C, Mayeux R, 2011. Epidemiology of Alzheimer disease. Nat. Rev. Neurol 7, 137–52. doi:10.1038/nrneurol.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, Dannals RF, Mathis CA, Klunk WE, Ferrucci L, Kraut MA, Wong DF, 2010. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology 74, 807–815. doi:10.1212/WNL.0b013e3181d3e3e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese F, Gietl A, Zölch N, Henning A, O’Gorman R, Kälin AM, Leh SE, Buck A, Warnock G, Edden RAE, Luechinger R, Hock C, Kollias S, Michels L, 2015. Posterior cingulate γ-aminobutyric acid and glutamate/glutamine are reduced in amnestic mild cognitive impairment and are unrelated to amyloid deposition and apolipoprotein E genotype. Neurobiol. Aging 36, 53–59. doi:10.1016/j.neurobiolaging.2014.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R, 2011. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol. Aging 32, 802–810. doi:10.1016/J.NEUROBIOLAGING.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang S-C, Satyamurthy N, Phelps ME, Barrio JR, 2006. PET of Brain Amyloid and Tau in Mild Cognitive Impairment. N. Engl. J. Med 355, 2652–2663. doi:10.1056/NEJMoa054625 [DOI] [PubMed] [Google Scholar]
- Sossi V, De Jong HWAM, Barker WC, Bloomfield P, Burbar Z, Camborde ML, Comtat C, Eriksson LA, Houle S, Keator D, Knöß C, Krais R, Lammertsma AA, Rahmim A, Sibomana M, Teräs M, Thompson CJ, Trébossen R, Votaw J, Walker M, Wienhard K, Wong DF, 2005. The second generation HRRT - A multi-centre scanner performance investigation, in: IEEE Nuclear Science Symposium Conference Record pp. 2195–2199. doi:10.1109/NSSMIC.2005.1596770 [Google Scholar]
- Stevens JP, 1984. Outliers and influential data points in regression analysis. Psychol. Bull 95, 334–344. doi:10.1037/0033-2909.95.2.334 [Google Scholar]
- Tkac I, Starcuk Z, Choi IY, Gruetter R, 1999. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 41, 649–656. doi:10.1002/(SICI)1522-2594(199904)41:4<649::AID-MRM2>3.0.CO;2-G [pii] [DOI] [PubMed] [Google Scholar]
- Wang X, Wang W, Li L, Perry G, Lee H, Zhu X, 2014. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta - Mol. Basis Dis 1842, 1240–1247. doi:10.1016/j.bbadis.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waragai M, Moriya M, Nojo T, 2017. Decreased N-Acetyl Aspartate/Myo-Inositol Ratio in the Posterior Cingulate Cortex Shown by Magnetic Resonance Spectroscopy May Be One of the Risk Markers of Preclinical Alzheimer’s Disease: A 7-Year Follow-Up Study. J. Alzheimers. Dis 60, 1411–1427. doi:10.3233/JAD-170450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeydan B, Deelchand DK, Tosakulwong N, Lesnick TG, Kantarci OH, Machulda MM, Knopman DS, Lowe VJ, Jack CR, Petersen RC, Öz G, Kantarci K, 2017. Decreased Glutamate Levels in Patients with Amnestic Mild Cognitive Impairment: An sLASER Proton MR Spectroscopy and PiB-PET Study. J. Neuroimaging doi:10.1111/jon.12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Endres CJ, Brašić JR, Huang SC, Wong DF, 2003. Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. Neuroimage 18, 975–989. doi:10.1016/S1053-8119(03)00017-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.